Abstract
Objectives:
Under simulated shift-work conditions, we investigated the efficacy of a restart break for maintaining neurobehavioral functioning across consecutive duty cycles, as a function of the circadian timing of the duty periods.
Design:
As part of a 14-day experiment, subjects underwent two cycles of five simulated daytime or nighttime duty days, separated by a 34-hour restart break. Cognitive functioning and high-fidelity driving simulator performance were tested 4 times per day during the two duty cycles. Lapses on a psychomotor vigilance test (PVT) served as the primary outcome variable. Selected sleep periods were recorded polysomnographically.
Setting:
The experiment was conducted under standardized, controlled laboratory conditions with continuous monitoring.
Participants:
Twenty-seven healthy adults (13 men, 14 women; aged 22–39 years) participated in the study.
Interventions:
Subjects were randomly assigned to a nighttime duty (experimental) condition or a daytime duty (control) condition. The efficacy of the 34-hour restart break for maintaining neurobehavioral functioning from the pre-restart duty cycle to the post-restart duty cycle was compared between these two conditions.
Results:
Relative to the daytime duty condition, the nighttime duty condition was associated with reduced amounts of sleep, whereas sleep latencies were shortened and slow-wave sleep appeared to be conserved. Neurobehavioral performance measures ranging from lapses of attention on the PVT to calculated fuel consumption on the driving simulators remained optimal across time of day in the daytime duty schedule, but degraded across time of night in the nighttime duty schedule. The 34-hour restart break was efficacious for maintaining PVT performance and other objective neurobehavioral functioning profiles from one duty cycle to the next in the daytime duty condition, but not in the nighttime duty condition. Subjective sleepiness did not reliably track objective neurobehavioral deficits.
Conclusions:
The 34-hour restart break was adequate for maintaining performance in the case of optimal circadian placement of sleep and duty periods (control condition) but was inadequate (and perhaps even detrimental) for maintaining performance in a simulated nighttime duty schedule (experimental condition). Current US transportation hours-of-service regulations mandate time off duty but do not consider the circadian aspects of shift scheduling. Reinforcing a recent trend of applying sleep science to inform policymaking for duty and rest times, our findings indicate that restart provisions in hours-of-service regulations could be improved by taking the circadian timing of the duty schedules into account.
Citation:
Van Dongen HPA; Belenky G; Vila BJ. The efficacy of a restart break for recycling with optimal performance depends critically on circadian timing. SLEEP 2011;34(7):917-929.
Keywords: Simulated shift work, circadian rhythms, recovery sleep, neurobehavioral functioning, cognitive performance, driving simulator, commercial truck drivers, drowsy driving, hours of service, fatigue risk management
INTRODUCTION
An important question in operational environments is how much off-duty time workers should be given to recuperate after a duty cycle, so that they will be able to perform optimally throughout the next duty cycle. In night and shift-work settings, a variety of off-duty or “restart break” paradigms have been implemented to provide time for recuperation between duty cycles, with varying degrees of effectiveness.1–10 The duty cycle and restart break patterns implemented in night- and shift-work operations are predominantly based on a combination of regulations, labor contracts, and traditions for which there is little empirical basis in the science of sleep and fatigue. Recent studies examining performance during a recovery period after a period of sleep restriction11–13 have started to build the necessary scientific foundation. What is urgently needed, however, are studies that systematically investigate the effect of a restart break on performance from one duty cycle to the next.14 The present study, motivated by hours-of-service (HOS) regulations for commercial motor vehicle (CMV) drivers in the United States, is the first published laboratory investigation of that type.
The current HOS regulations for property-carrying CMV drivers in the United States state that (1) drivers may drive up to 11 hours in a 14-hour period on duty following 10 hours off duty, (2) drivers may not drive after 60 hours/70 hours on duty in a duty cycle of 7/8 consecutive days, and (3) drivers may not recycle to the next duty cycle until after they are off duty for at least 34 hours. The latter provision is known as the 34-hour restart rule, and, as with all restart-break provisions, the scientific evidence supporting this rule is sparse. The 34-hour restart rule applies regardless of the start and end times of the prior duty schedule and, therefore, does not take into account the circadian rhythms in performance and sleep propensity.15–17 As such, the rule may be inadequate to maintain performance from one duty cycle to the next in the case of adverse circadian placement of sleep and work periods.
Recognizing this issue, the US Federal Motor Carrier Safety Administration (FMCSA) formally proposed a new rule on HOS requirements for CMV drivers (December 24, 2010, see footnote following acknowledgments), in which new evidence from the science of sleep and fatigue was brought to bear on the issue of the restart break. Our study provided a portion of the scientific research informing the debate leading to the new rule by investigating the efficacy of the 34-hour restart rule for maintaining neurobehavioral performance capability and driving performance on a high-fidelity driving simulator across duty cycles as a function of the circadian placement of the duty schedule.
METHODS
Subjects
Twenty-seven healthy subjects aged 22 to 40 years completed the study—3 more than the minimum sample size indicated by a power calculation performed in advance.18 Of the 27 subjects, 13 were randomized to a nighttime duty experimental condition, and 14 were randomized to a daytime duty control condition, as further explained under Study Design. The subjects in the nighttime duty condition were 6 men and 7 women, aged 22 to 39 years (mean ± SD: 27.0 ± 5.4). The subjects in the daytime duty condition were 7 men and 7 women, aged 23 to 38 years (27.5 ± 5.6). There was no significant difference in age distribution between the two groups (F1,25 = 0.06, P = 0.82).
Subjects were physiologically and psychologically healthy, with no current medical or drug treatment (excluding oral contraceptives), as determined by physical examination, blood chemistry, urinalysis, history, and questionnaires. They were free of traces of alcohol and drugs, as determined by breathalyzer and urine drug testing during screening and upon entering the study, and they reported to be nonsmokers. They were also asked to refrain from caffeine consumption during the week before the study. Subjects had no history of psychiatric illness or moderate to severe brain injury, no history of learning disability, no previous adverse reaction to sleep deprivation, no history of drug or alcohol abuse in the past year, and no history of methamphetamine abuse, as determined by history and questionnaires. They were not pregnant, had normal or corrected-to-normal vision, and were all native English speakers. They each had a valid driver's license, and were proficient in driving as determined by supervised test driving of one of our high-fidelity driving simulators, in which they demonstrated no signs of simulator adaptation syndrome (simulator sickness).
Subjects were good sleepers, with habitual sleep duration between 6 hours and 10 hours, and had regular bedtimes, habitually getting up between 06:00 and 09:00, as determined by questionnaire and verified with wrist actigraphy and diary in the week before the study. They exhibited no sleep or circadian disorders, as determined by baseline polysomnography, history, and questionnaires, and were neither extreme morning-types nor extreme evening-types, as determined by questionnaire. They reported no travel across time zones and no shift work within one month of entering the study.
The Institutional Review Board of Washington State University reviewed and approved the study, and each subject gave written informed consent.
Study Design
Subjects participated in a 14-day in-residence laboratory study with a simulated nighttime duty (SND) experimental condition and a simulated daytime duty (SDD) control condition. Figure 1 illustrates the sleep/wake and test schedules for the two conditions. Subjects' randomization to condition was announced on the morning of the first day the subjects were in the laboratory.
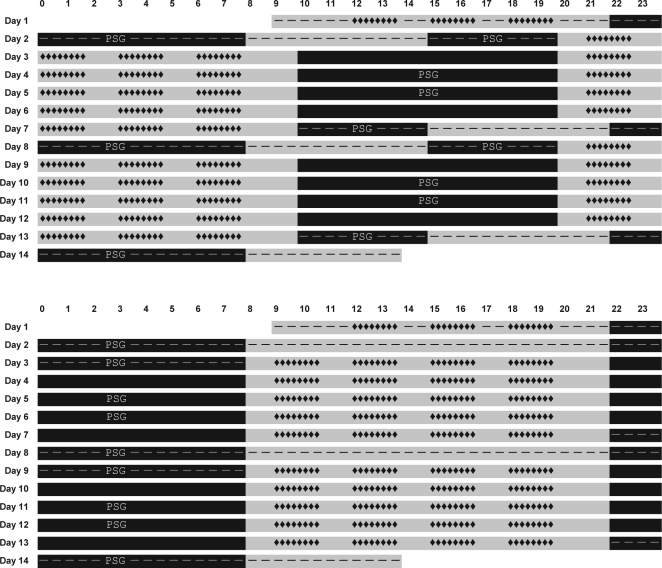
Figure 1.
Schematics of the laboratory study design in the simulated nighttime duty (SND) experimental condition (top) and the simulated daytime duty (SDD) control condition (bottom). Each bar in each schematic represents a day; time of day is indicated above the first bar. Gray bar sections indicate scheduled wakefulness, and black bar sections indicate scheduled sleep. Sleep periods recorded polysomnographically are marked PSG. Dashes indicate the adaptation/baseline period (starting on day 1), 34-hour restart break (starting on day 7), and recovery period (starting on day 13). Periods not marked with dashes represent the simulated duty cycles. Neurobehavioral performance and simulator driving test blocks are indicated by strings of diamonds: 20 test bouts in each of the two duty cycles and three practice bouts during adaptation. Total time scheduled for wakefulness, sleep, duty cycles, restart breaks, and test bouts is equal for the two conditions.
Subjects in the SND experimental condition began the study with an adaptation day (starting at 09:00), during which they practiced neurobehavioral tests and driving on the high-fidelity driving simulator. This was followed by 10 hours of time in bed (TIB) for baseline sleep (22:00–08:00). The second day in the laboratory included a 5-hour prophylactic nap opportunity (15:00–20:00) initiating a transition to a nighttime work schedule. Subjects then experienced the first of two 5-day nighttime duty cycles. The duty cycle consisted of five 14-hour simulated duty periods with performance testing and simulator driving, each separated by 10-hour diurnal periods for sleep (10:00–20:00). Consistent with the current HOS regulations for property-carrying CMV drivers in the US, the first duty cycle ended after a cumulative total of 70 hours of simulated duty time.
The first duty cycle was followed by a 34-hour restart break, during which there was no performance testing or simulator driving. The restart break began with a 5-hour nap opportunity (10:00–15:00). This allowed subjects to transition back to a daytime schedule, as has been found to be typical for night drivers during off-duty days.19,20 The restart break also contained a 10-hour nighttime sleep period (22:00–08:00), and ended with a 5-hour prophylactic nap opportunity (15:00–20:00) initiating a transition back to a nighttime work schedule. Subjects then experienced the second 5-day nighttime duty cycle, which was identical to the first and contained 10-hour diurnal periods for sleep (10:00–20:00). Following the second duty cycle, after another cumulative 70 hours of simulated duty time, the study ended with a 5-hour nap opportunity (10:00–15:00) to transition back to a daytime schedule and a 10-hour nighttime recovery sleep period (22:00–08:00). Subjects went home at 14:00 on the 14th day. See Figure 1, top panel.
Subjects in the SDD control condition likewise began the study with an adaptation day (starting at 09:00), during which they practiced neurobehavioral tests and driving on the simulator, followed by baseline sleep. The second day in the laboratory was a transition day as in the SND condition but, since there was no need to transition to a different schedule, this second day was similar to all other days in the SDD condition yet without performance testing and driving. The second day ended with a nocturnal sleep period. Subjects then experienced the first of two 5-day daytime duty cycles. The duty cycle consisted of five 14-hour simulated duty periods with performance testing and simulator driving, each separated by nocturnal sleep. Consistent with the HOS regulations, the first duty cycle ended after a cumulative total of 70 hours of simulated duty time. It was followed by a 34-hour restart break, during which there was no performance testing or simulator driving. The restart break in the SDD condition contained two nocturnal sleep periods. After the restart break, subjects experienced the second 5-day daytime duty cycle, which was identical to the first. Following the second duty cycle, after another cumulative 70 hours of simulated duty time, the study ended with a nighttime recovery sleep period. Subjects went home at 14:00 on the 14th day. All sleep periods in the SDD condition involved 10 hours of scheduled TIB (22:00–08:00). See Figure 1, bottom panel.
The total amounts of scheduled wakefulness and sleep and the cumulative performance testing and simulator driving loads were identical for the SND condition and the SDD condition. Furthermore, the 34-hour restart break contained 20 hours total of TIB for sleep and 14 hours total of scheduled wakefulness in both conditions. However, the 34-hour restart break in the SND condition involved transiently reverting to a daytime schedule, whereas the 34-hour restart break in the SDD condition involved no schedule shifting. The study design permitted comparison of performance and driving outcomes (see below) in the post-restart duty cycle versus the pre-restart duty cycle for the SND experimental condition, as contrasted with the SDD control condition. This allowed assessment of the efficacy of the 34-hour restart break for maintaining on-duty performance in the context of two opposing circadian phases of simulated time on duty.
The experiment was conducted in the isolated sleep and simulation laboratory of the Sleep and Performance Research Center at Washington State University Spokane. Subjects were assigned their own private rooms for performance testing and for sleep. During scheduled wakefulness, light levels were fixed at just below 50 lux. During scheduled sleep periods, lights were off, and subjects were not allowed to engage in activities other than sleeping (or quiet resting if they could not sleep). Ambient temperature was kept at 21°C ± 1°C throughout the laboratory. Meals were served in a central suite inside the laboratory at regular intervals; no caffeine intake was allowed. Breakfast, lunch, and dinner times were shifted by 12 hours during nighttime wake periods in the SND condition. Between meals and performance testing and simulator driving, subjects in both conditions were allowed to read, watch movies, construct jig-saw puzzles, play board games, and interact with laboratory staff, but no computer use, vigorous activities, or exercise were permitted. No visitors, phone calls, e-mail, Internet, radio, live television, or other external communication were allowed inside the laboratory; and only trained research staff interacted with the subjects. Throughout the study, subjects were monitored by direct observation or, when in their own rooms, through cameras (with infrared light for observation in darkness during scheduled sleep).
Measurements
During the 5-day duty cycles, neurobehavioral testing and simulator driving blocks were scheduled 4 times per duty day, as indicated in Figure 1. Each test block consisted of a 10-minute PVT, a 30-minute driving session on a high-fidelity driving simulator, and another 10-minute PVT, as well as a brief (less than 15-minute) neurobehavioral test battery. Two driving simulators were available in the laboratory, whereas up to four subjects could be participating in the study at one time. Therefore, subjects were randomly assigned to consistently either do the driving (preceded and followed by the PVT) first and undergo the neurobehavioral testing second, or the other way around. Either way, there was a 45-minute break between the PVT/driving/PVT bout and the neurobehavioral test bout in each block.
The PVT, a simple reaction-time task with a random response-stimulus interval of 2 to 10 seconds, is a well-validated and widely used standard assay of performance impairment from sleep loss and circadian misalignment.21 Performance lapses, defined as the number of reaction times greater than 500 milliseconds, were extracted for each 10-minute test, and a priori designated to serve as the primary outcome measure of the study.
During the driving sessions, subjects drove a 30-minute route on a PatrolSim IV driving simulator (MPRI, Salt Lake City, UT). This is a high-fidelity simulator widely used to train professional drivers. An additional hardware and software system was implemented (external to the simulators) to capture driving-performance data at high resolution (72 Hz), so as to turn this training device into a research tool.22 We developed a standardized driving scenario involving rural highway driving. There were 5 to 7 randomly located encounters with pedestrians or dogs crossing the road, and braking responses to these unexpected events were recorded to capture any lapses of attention. In addition, 10 straight, uneventful road segments in the scenario (“straightaways”) were used to extract data on lane deviation and other performance metrics potentially indicative of drowsy driving. The speed limit throughout the scenario was 55 mph, and subjects' compliance was monitored continuously.
Lane deviation (standard deviation of lateral lane position), a frequently used measure of performance in simulator driving,23–25 was calculated for the straightaways in each simulator driving session. The following additional driving variables were extracted: reaction time of braking for the pedestrian- or dog-crossing events; average and variability (standard deviation) of speed in the straightaways; and fuel consumption (as calculated by the simulator's internal engine simulation model) across the straightaways. Driving simulator data from two subjects in the SDD condition were missing as a result of data-capture problems with the simulators.
The neurobehavioral test battery included computerized versions of, in order of administration, the Karolinska Sleepiness Scale (KSS),26 a visual analog scale of mood (VASM),27 the Positive and Negative Affect Schedule (PANAS),28 the digit symbol substitution task (DSST),29 performance and effort rating scales (PERF and EFFR, respectively),30 and a cardinal direction decision task (CDDT).31 The KSS, VASM, PANAS, PERF, and EFFR yielded subjective assessments of sleepiness, mood, and effort. For each, an overall score was extracted, with the exception of the PANAS, for which both positive and negative affect scores were determined. For the DSST, the number of correct responses in the 3-minute task duration was extracted as a measure of cognitive throughput.
The CDDT, which is a mental orientation and rotation task, has only recently been introduced in sleep research32 and is therefore described here in more detail. The task requires subjects to make judgments about the location of a target. The stimulus consists of a first-person view, where a single target is highlighted in a set of 8 objects arranged in a circular target field. This view is presented adjacent to a rotated allocentric perspective (i.e., a map with the target field at the center), which indicates the direction of the first-person viewing perspective. The task is to identify the portion of the target field containing the target in the rotated allocentric perspective. Twenty-five trials are presented per test bout, and responses are self-paced. In this study, from the number of attempts needed to complete all trials, the number of error responses was calculated as the outcome measure for the CDDT.
During the adaptation day, subjects practiced performance testing and driving on the high-fidelity driving simulator (see Figure 1). These practice blocks were not used for data analysis. Other than during the adaptation day, PVT/driving/PVT and neurobehavioral test blocks were administered only during the two duty cycles. A few brief additional neurobehavioral test bouts took place at specific time points spread across the duration of the study (not shown in Figure 1). These tests, the scheduling of which was equivalent between the two study conditions, did not serve the purpose of evaluating the efficacy of the 34-hour restart break and, therefore, are not discussed further here.
Selected sleep periods were recorded polysomnographically (see Figure 1). The electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), and electrocardiogram (ECG) were recorded using digital equipment (Nihon Kohden, Foothill Ranch, CA). The EEG electrodes were placed at frontal (F3, F4), central (C3, C4), and occipital (O1, O2) locations and referenced against the mastoids (M1, M2). Sleep stages were scored using standard criteria promulgated by the American Academy of Sleep Medicine.33 Every third day, the recording of sleep periods was suspended, electrodes were removed, and subjects were given an opportunity to take a shower.
Statistical Analyses
The primary statistical design involved a within-subjects comparison of neurobehavioral functioning during the first 5-day duty cycle versus the second 5-day duty cycle, and between-groups comparison of the SND experimental condition versus the SDD control condition. We employed 2-way mixed-effects analysis of variance (ANOVA)34 and focused on the interaction of condition by duty cycle (collapsed over duty days and times of day within duty cycles). This interaction tests the null hypothesis that the 34-hour restart break was efficacious for maintaining performance across duty cycles in both conditions (regardless of any potential baseline differences). Additional analyses useful for interpretation of the main results involved 3-way mixed-effects ANOVA of condition by duty cycle by day (to examine changes over duty days within duty cycles) and 3-way mixed-effects ANOVA of condition by duty cycle by time of day (to examine the effects of circadian timing). These additional analyses were also used for graphic presentation of the study results. Analyses of driving-simulator data included subjects' assignment to simulator (#1 or #2) as a covariate, to control for any potential hardware differences.
Standard descriptive statistics were used to examine and graph the polysomnographic measures of sleep periods selected to be recorded (as indicated in Figure 1). One-way ANOVAs were performed for comparisons between conditions. Although total TIB across the days of the study was the same for the two conditions, the recorded sleep periods did not match exactly. The sleep variables were therefore compared between conditions (SND versus SDD, respectively) by study segment, as follows:
(1) the 10-hour nocturnal sleep and 5-hour prophylactic nap versus the two 10-hour nocturnal sleeps in the baseline segment (“Baseline”);
(2) the middle two 10-hour diurnal sleeps versus the middle two 10-hour nocturnal sleeps in the first duty cycle (“Cycle 1”);
(3) the 5-hour transition nap and 10-hour nocturnal sleep and 5-hour prophylactic nap versus the two 10-hour nocturnal sleeps in the restart break (“Restart”);
(4) the middle two 10-hour diurnal sleeps versus the middle two 10-hour nocturnal sleeps in the second duty cycle (“Cycle 2”); and
(5) the 5-hour transition nap and 10-hour nocturnal sleep versus the 10-hour nocturnal sleep in the recovery segment (“Recovery”).
RESULTS
Primary Outcome: PVT Lapses
Performance lapses on the PVT served as the primary outcome measure of the study, used to assess the efficacy of the 34-hour restart break for maintaining performance capability across duty cycles. The primary statistical analysis focused on the interaction of condition (SND vs SDD) by duty cycle (pre-restart vs post-restart), collapsed over duty days and times of day within duty cycles. For PVT lapses, this interaction was statistically significant (F1,2129 = 20.06, P < 0.001). There also was a significant main effect of duty cycle (F1,2129 = 21.79, P < 0.001) but no significant main effect of condition (F1,2129 = 1.31, P = 0.25). PVT performance did not vary significantly across the two duty cycles in the SDD condition (simple effect of duty cycle: F1,2129 = 0.02, P = 0.89) but deteriorated significantly from before to after the 34-hour restart break in the SND condition (simple effect of duty cycle: F1,2129 = 40.38, P < 0.001). Thus, the 34-hour restart break was efficacious for maintaining PVT performance from one duty cycle to the next in the daytime duty schedule but not in the nighttime duty schedule.
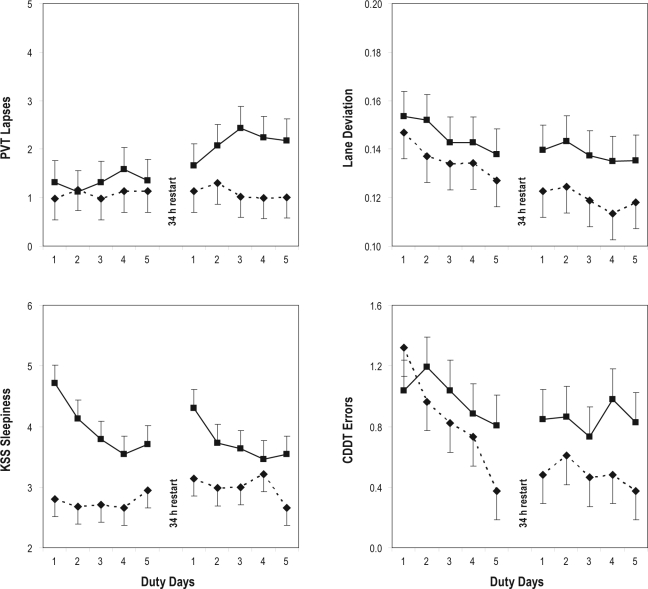
Figure 2 (top left panel) shows PVT lapses by duty day (collapsed over time of day). The 3-way interaction of condition by duty cycle by duty day was not statistically significant (F4,2113 = 0.79, P = 0.53) nor were the 2-way interaction of condition by duty day (F4,2113 = 1.50, P = 0.20), the 2-way interaction of duty cycle by duty day (F4,2113 = 0.69, P = 0.60), or the main effect of duty day (F4,2113 = 0.70, P = 0.59). It seemed graphically that PVT performance deteriorated progressively from the first to the third day of the second duty cycle in the SND condition (see Figure 2, top left panel), but this was not corroborated by the statistical results.
Figure 2.
Neurobehavioral performance during simulated duty days in the simulated nighttime duty (SND) experimental condition and the simulated daytime duty (SDD) control condition. The panels show group means (with standard errors) by duty day, collapsed over time of day, for the duty cycles before and after the 34-hour restart break. Boxes (solid lines) represent the SND condition; diamonds (dashed lines) represent the SDD condition. Top left: number of performance lapses on the psychomotor vigilance test (PVT). Top right: lane deviation (standard deviation of lane position), expressed in meters, in the straightaways during high-fidelity simulator driving. Bottom left: sleepiness score on the Karolinska Sleepiness Scale (KSS). Bottom right: number of error responses on the cardinal direction decision task (CDDT). Upwards on the ordinate indicates worse performance in each panel.
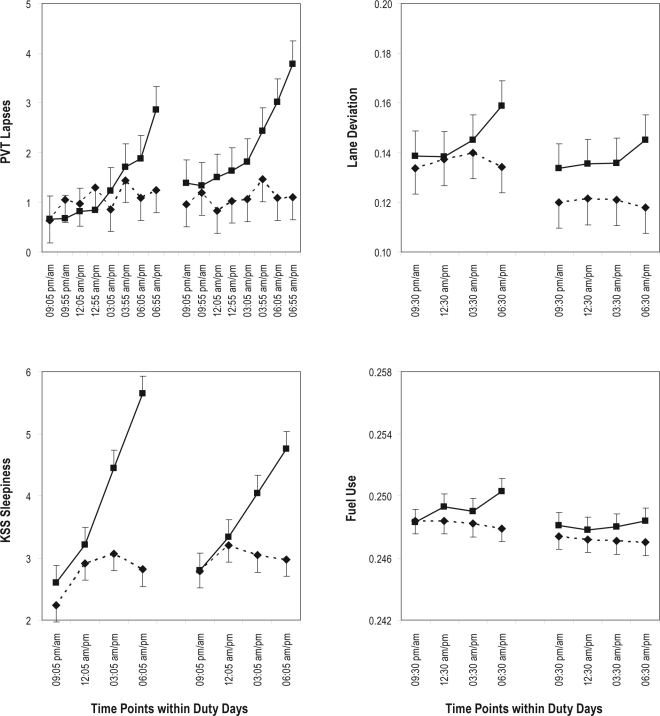
Figure 3 (top left panel) shows PVT lapses by time of day (collapsed over duty days) for the first and second duty cycles in each of the two conditions. The 3-way interaction of condition by duty cycle by time of day was not statistically significant (F7,2101 = 0.44, P = 0.88). Yet, there was a significant 2-way interaction of condition by time of day (F7,2101 = 10.59, P < 0.001) and a significant main effect of time of day (F7,2101 = 16.32, P < 0.001), although the 2-way interaction of duty cycle by time of day was not significant (F7,2101 = 0.22, P = 0.98). PVT performance was relatively stable over time of day in the SDD condition, but there was a pronounced time-of-day effect in the SND condition, with performance degrading progressively across time of night.
Figure 3.
Neurobehavioral performance as a function of time of day in the simulated nighttime duty (SND) experimental condition and the simulated daytime duty (SDD) control condition. The panels show group means (with standard errors) by time of day, collapsed over duty days within each duty cycle, for the duty cycles before the 34-hour restart break (left-hand curves) and after the 34-hour restart break (right-hand curves). Boxes (solid lines) represent the SND condition; diamonds (dashed lines) represent the SDD condition. Top left: Number of performance lapses on the psychomotor vigilance test (PVT); note that there were twice as many PVT administrations as driving sessions and neurobehavioral test bouts during each duty day. Top right: Lane deviation (standard deviation of lane position), expressed in meters, in the straightaways during high-fidelity simulator driving. Bottom left: sleepiness score on the Karolinska Sleepiness Scale (KSS). Bottom right (inset): calculated fuel consumption, in gallons, across the straightaways during high-fidelity simulator driving. Upward on the ordinate indicates worse performance in each panel. Times of day shown on the abscissa are through the night (from 09:05 pm = 21:05 until 06:55 am) in the SND condition, and through the day (from 09:05 am until 06:55 pm = 18:55) in the SDD condition. For all outcomes except the KSS, times of day are given for subjects doing the PVT/driving/PVT bout first in each test block; 1 hour should be added to each time point for subjects doing the PVT/driving/PVT bout second. For the KSS (which was part of the neurobehavioral test bout), times of day are given for subjects doing the neurobehavioral test bout first in each test block; 1.8 hour should be added to each time point for subjects doing the PVT/driving/PVT bout first and the neurobehavioral test bout second.
Driving Simulator Performance
For lane deviation in the straightaways during the simulator driving sessions, the primary statistical analysis of the interaction of condition by duty cycle (collapsed over duty days and times of day within duty cycles) yielded a significant interaction effect (F1,970 = 9.15, P = 0.003) and a significant main effect of duty cycle (F1,970 = 75.23, P < 0.001) but no significant main effect of condition (F1,970 = 0.88, P = 0.35). Figure 2 (top right panel) shows lane deviation by duty day (collapsed over time of day). The 3-way interaction of condition by duty cycle by duty day was not significant (F4,954 = 0.34, P = 0.85) nor was the 2-way interaction of condition by duty day (F4,954 = 0.40, P = 0.81). However, the 2-way interaction of duty cycle by day duty day was significant (F4,954 = 2.67, P = 0.031), as was the main effect of day (F4,954 = 10.47, P < 0.001). As seen in Figure 2 (top right panel), there was a practice effect over days, which was less precipitous in the second duty cycle than in the first, and which was more pronounced in the SDD condition than in the SND condition.
Figure 3 (top right panel) shows lane deviation by time of day (collapsed over duty days) for the first and second duty cycles in each of the two conditions. The 3-way interaction of condition by duty cycle by time of day was not significant (F3,958 = 0.63, P = 0.59), nor was the 2-way interaction of duty cycle by time of day (F3,958 = 1.20, P = 0.31). However, there was a significant 2-way interaction of condition by time of day (F3,958 = 10.25, P < 0.001) and a significant main effect of time of day (F3,958 = 5.98, P < 0.001). Lane deviation increased gradually across time of night in the SND condition but not in the SDD condition (see Figure 3, top right panel).
For conciseness, only significant effects and interactions of condition are reported for the other variables extracted from the high-fidelity simulator driving sessions. There were no such significant findings for reaction time of braking for the pedestrian- or dog-crossing events. There were also no significant findings for average speed—subjects in both conditions stayed close to the speed limit of 55 mph. In the analyses of the variability (standard deviation) of speed, though, the 3-way mixed-effects ANOVA of condition by duty cycle by time of day yielded a significant 2-way interaction of condition by time of day (F3,958 = 4.95, P = 0.002). Driving-speed variability increased over the hours of the night in the SND condition, whereas it decreased over the hours of the day in the SDD condition.
For calculated (simulated) fuel consumption, the 3-way mixed-effects ANOVA of condition by duty cycle by time of day yielded a significant 2-way interaction of condition by time of day (F3,958 = 3.89, P = 0.009). As shown in Figure 3 (inset bottom right panel), fuel consumption increased moderately from the beginning to the end of the night in the SND condition (by about 1%), whereas it decreased slightly from the beginning to the end of the day in the SDD condition.
Other Neurobehavioral Outcomes
For subjective sleepiness as measured with the KSS, the primary statistical analysis focusing on the interaction of condition by duty cycle yielded a significant interaction effect (F1,1050 = 8.34, P = 0.004) and a significant main effect of condition (F1,1050 = 8.11, P = 0.005), but no significant main effect of duty cycle (F1,1050 < 0.01, P = 0.99). Figure 2 (bottom left panel) shows subjective sleepiness scores by duty day (collapsed over time of day). The 3-way interaction of condition by duty cycle by duty day was not statistically significant (F4,1034 = 0.92, P = 0.45). However, the 2-way interaction of condition by duty day (F4,1034 = 4.01, P = 0.003) was significant, as was the main effect of duty day (F4,1034 = 5.72, P < 0.001), though not the 2-way interaction of duty cycle by duty day (F4,1034 = 0.87, P = 0.48). In the SND condition, subjective sleepiness diminished across days in the first 5-day duty cycle, increased immediately after the 34-hour restart break, and then again diminished across days in the second 5-day duty cycle. In the SDD condition, subjective sleepiness increased from the first to the second duty cycle, except for a small drop on the last day of the second duty cycle; and subjects reported overall less sleepiness than in the SND condition.
Figure 3 (bottom left panel) shows subjective sleepiness by time of day (collapsed over duty days) for the first and second duty cycles in each of the two conditions. The 3-way interaction of condition by duty cycle by time of day was not statistically significant (F3,1038 = 1.86, P = 0.13). Yet, there was a significant interaction of condition by time of day (F3,1038 = 49.28, P < 0.001), a significant interaction of duty cycle by time of day (F3,1038 = 5.91, P = 0.005), and a significant main effect of time of day (F3,1038 = 75.87, P < 0.001). There was a pronounced time-of-day effect in the SND condition, with subjective sleepiness worsening progressively across time of night, particularly in the duty cycle preceding the 34-hour restart break. In contrast, subjective sleepiness was relatively stable across time of day in the SDD condition.
For the subjective scales of mood (VASM), affect (PANAS) and effort (EFFR), and for cognitive throughput on the DSST, the primary analysis yielded no statistically significant interactions of condition by duty cycle (VASM mood score: F1,1051 = 0.01, P = 0.92; PANAS positive affect: F1,1051 = 1.26, P = 0.26; PANAS negative affect: F1,1051 = 0.05, P = 0.83; EFFR effort score: F1,1051 = 2.19, P = 0.14; DSST number correct: F1,1050 = 0.28, P = 0.60). In agreement with previously reported observations,35 DSST performance displayed a pronounced learning curve extending across all duty days in the two duty cycles in both conditions. This was reflected in a significant 2-way interaction of duty cycle by duty day (F4,1034 = 6.13, P < 0.001) and a significant main effect of duty day (F4,1034 = 29.84, P < 0.001).
For conciseness, only additional significant effects and interactions of condition are reported here for the VASM, PANAS, EFFR, and DSST. Significant interactions of condition by time of day were found for the VASM (F3,1039 = 6.02, P < 0.001), for PANAS positive affect (F3,1039 = 20.37, P < 0.001), for the EFFR (F3,1039 = 4.63, P = 0.003), and for DSST number correct (F3,1038 = 2.94, P = 0.032). Subjective mood, positive affect, subjective effort, and cognitive throughput gradually declined across time of night in the SND condition but were relatively stable across time of day in the SDD condition.
Subjects in both conditions showed low negative affect on the PANAS throughout the study. However, there was a significant 3-way interaction of condition by duty cycle by duty day (F4,1035 = 3.12, P = 0.014) and a significant 2-way interaction of condition by duty day (F4,1035 = 2.76, P = 0.027). During the first 2 days of the first duty cycle, relative to the other duty days, negative affect was slightly increased in the SND condition and slightly decreased in the SDD condition. Starting on the third day of the first duty cycle, negative affect no longer differed between the two conditions. PANAS negative affect did not vary significantly as a function of time of day in either study condition.
For the subjective performance ratings (PERF), the primary analysis yielded no statistically significant interaction of condition by duty cycle (F1,1051 = 0.14, P = 0.71), but there was a significant effect of condition (F1,1051 = 7.29, P = 0.007). Subjects in the SND condition rated their performance as overall poorer than did subjects in the SDD condition.
Lastly, for the number of error responses on the CDDT, the primary analysis focusing on the interaction of condition by duty cycle (collapsed over duty days and over times of day within duty cycles) yielded a trend for an interaction (F1,1051 = 3.35, P = 0.067) and a significant main effect of duty cycle (F1,1051 = 17.78, P < 0.001), but no significant main effect of condition (F1,1051 = 1.48, P = 0.22). Figure 2 (bottom right panel) shows CDDT error responses by duty day (collapsed over time of day). The 3-way interaction of condition by duty cycle by duty day was not statistically significant (F4,1035 = 1.09, P = 0.36), nor was the 2-way interaction of condition by day (F4,1035 = 1.24, P = 0.29). However, the 2-way interaction of duty cycle by duty day was significant (F4,1035 = 2.63, P = 0.033), as was the main effect of duty day (F4,1035 = 4.00, P = 0.003). In both conditions, mean performance on the CDDT improved steadily across days in the duty cycle preceding the 34-hour restart break and leveled off in the duty cycle following the 34-hour restart, where asymptotic performance tended to be better (fewer errors) in the SDD condition than in the SND condition.
Polysomnography
Polysomnographic measures of sleep periods selected to be recorded (see Figure 1) were compared between the SND experimental condition and the SDD control condition to help interpret the neurobehavioral findings. The sleep variables were compared between the two conditions by study segment (Baseline, Cycle 1, Restart, Cycle 2, Recovery), with each segment containing one or more recorded sleep periods, as described in the Methods section. Statistical comparisons focused primarily on Cycle 1, Restart, and Cycle 2, where combined TIB was the same for the two study conditions; and on the cumulative totals across all recorded sleep periods, where total TIB was likewise the same. The combined TIB durations in each of the study segments are shown in Figure 4 (top left panel).
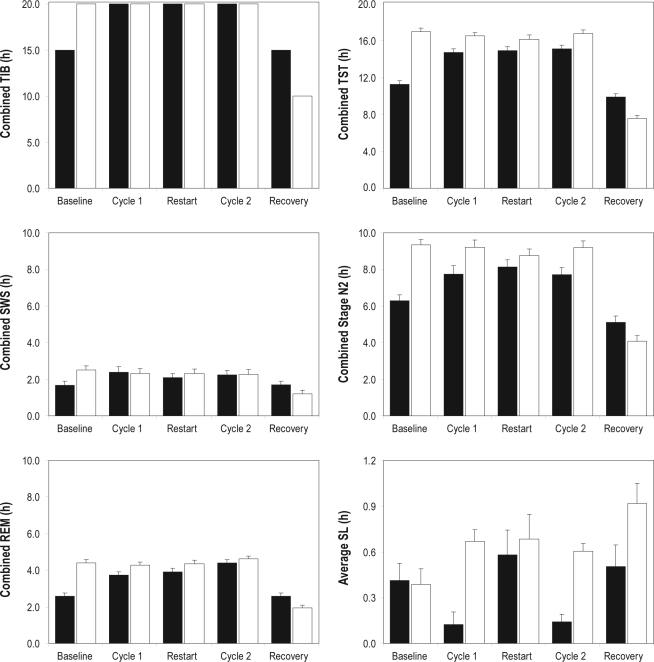
Figure 4.
Polysomnographically assessed sleep variables in the simulated nighttime duty (SND) experimental condition and the simulated daytime duty (SDD) control condition. The panels show group means (in hours) for combined time in bed (TIB), combined total sleep time (TST), combined slow-wave sleep (SWS), combined stage N2 sleep, combined rapid eye movement (REM) sleep, and average sleep latency (SL), in the polysomnographically recorded sleeps (see Figure 1) of the baseline segment, first duty cycle, restart break, second duty cycle, and recovery segment of the study. Black bars display the means for the SND condition; white bars display the means for the SDD condition; whiskers indicate standard errors (note that TIB means are exact). The differences between the two conditions in combined TST, SWS, N2, and REM during the baseline and recovery segments should be interpreted in the context of the differences in scheduled TIB (top left panel). Note that the vertical scale varies among the top panels, the middle and bottom left panels, and the bottom right panel.
Total sleep time (TST), shown in Figure 4 (top right panel), closely followed the pattern of TIB across study segments. However, TST in the 10-hour daytime sleep periods of the SND condition was significantly shorter than TST in the 10-hour nighttime sleep periods of the SDD condition for Cycle 1 (F1,24 = 11.99, P = 0.002) and Cycle 2 (F1,24 = 8.76, P = 0.007); average daily TST was 0.9 hours shorter during the nighttime duty days than during the daytime duty days. There was a trend for less combined TST in the SND condition during Restart (F1,24 = 3.61, P = 0.070). The cumulative TST across all polysomnographically recorded sleep periods (90 hours TIB in total) was 66.2 hours in the SND condition and 74.3 hours in the SDD condition, which was significantly different (F1,21 = 16.47, P < 0.001).
For slow-wave sleep (SWS; sleep stage N3), shown in Figure 4 (middle left panel), there was no significant difference between the two conditions during Cycle 1 (F1,24 = 0.03, P = 0.86), Restart (F1,24 = 0.49, P = 0.49), or Cycle 2 (F1,24 = 0.01, P = 0.91). The cumulative SWS across all polysomnographically recorded sleep periods was 10.1 hours in the SND condition and 10.5 hours in the SDD condition, which also did not constitute a significant difference (F1,21 = 0.03, P = 0.86).
Sleep stage N2, shown in Figure 4 (middle right panel), followed the pattern of TST (top right panel). Combined N2 was significantly shorter in the SND condition than in the SDD condition for Cycle 1 (F1,24 = 5.64, P = 0.026) and Cycle 2 (F1,24 = 7.39, P = 0.012). There was no statistically significant difference between conditions during Restart (F1,24 = 1.23, P = 0.28). Across all polysomnographically recorded sleep periods, the SND condition accumulated 35.3 hours of sleep stage N2, and the SDD condition accumulated 40.8 hours, which was significantly different (F1,21 = 5.29, P = 0.032).
For sleep stage N1 (not included in Figure 4), there were no statistically significant differences between the conditions during Cycle 1 (F1,24 = 0.73, P = 0.40), Restart (F1,24 = 0.13, P = 0.72), and Cycle 2 (F1,24 = 0.35, P = 0.56). Across all recorded sleep periods, the SND condition accumulated 3.6 hours of N1, and the SDD condition accumulated 3.3 hours of N1, which was not significantly different (F1,21 = 0.60, P = 0.45).
Rapid eye movement (REM) sleep, shown in Figure 4 (bottom left panel), exhibited a significant difference between the two conditions during Cycle 1 (F1,24 = 5.69, P = 0.025), with relatively reduced REM sleep during the daytime sleep periods of the SND condition. There was no significant difference between conditions during Restart (F1,24 = 2.48, P = 0.13) or Cycle 2 (F1,24 = 0.80, P = 0.38). In the aggregate across all recorded sleep periods, the SND condition accumulated 17.1 hours of REM sleep, and the SDD condition accumulated 19.7 hours of REM sleep, which entailed a statistically significant difference (F1,21 = 8.31, P = 0.009).
For sleep latency (SL), statistical comparisons between conditions focused on averages per study segment, which are more relevant than combined totals for this particular sleep variable. As can be seen in Figure 4 (bottom right panel), average SL was significantly shorter in the daytime sleep periods of the SND condition than in the nighttime sleep periods of the SDD condition for Cycle 1 (F1,24 = 23.61, P < 0.001) and Cycle 2 (F1,24 = 42.74, P < 0.001), as well as for Recovery (F1,24 = 4.60, P = 0.042). There was no significant difference between conditions for Restart (F1,24 = 0.20, P = 0.66) or Baseline (F1,25 = 0.04, P = 0.85).
The SND and SDD conditions were also compared for the sleep variables of just the first (10-hour) baseline night, which the two conditions had in common (see Figure 1). There were no statistically significant differences in TST (F1,25 = 2.72, P = 0.11), SWS (F1,25 = 0.12, P = 0.73), N2 (F1,25 = 1.72, P = 0.20), N1 (F1,25 = 1.55, P = 0.22), REM (F1,25 = 0.13, P = 0.72), or SL (F1,25 = 0.06, P = 0.81). Thus, the two subject groups were not significantly different in these indices of baseline sleep.
DISCUSSION
This is the first study to investigate the duration of the restart break needed in shift-work operations to maintain optimal performance across duty days when recycling from one duty cycle to the next—and the first to address this issue in the context of the circadian timing of work schedules. It is a priori unlikely that there would be a single minimal duration for the restart break that is neurobiologically, operationally, and economically optimal and guarantees recycling with stable performance; research on sustained sleep restriction11 suggests that off-duty time needed to recuperate depends on how much cumulative sleep loss was incurred during the prior duty period. The present study demonstrates that the issue is also critically dependent on the circadian timing of the duty schedule.
An innovative feature of our study was that performance outcomes were measured across two full duty cycles, one before and one after a 34-hour restart break. This enabled examination of impairment compounding across duty cycles, which would have remained unnoticed if our study had focused on recuperation during the restart period without measuring performance during the subsequent duty cycle. For subjects randomized to the simulated daytime duty (control) condition, we observed that performance lapses on the PVT (our primary outcome measure) were stable between the pre-restart and post-restart duty cycles, indicating that the 34-hour restart break was efficacious and perhaps not even necessary for maintaining optimal performance across daytime duty cycles. However, for subjects randomized to the simulated nighttime duty (experimental) condition, PVT lapses were increased in the post-restart duty cycle relative to the pre-restart duty cycle (Figure 2, top left panel), indicating that the 34-hour restart break was not efficacious for maintaining optimal performance across nighttime duty cycles.
There were equivalent findings for lane deviation on the driving simulator (with statistical significance) and errors on the CDDT (tending to significance)—see Figure 2 (right-hand panels). In the daytime duty condition, these secondary outcomes showed practice effects extending from the pre-restart into the post-restart duty cycle. In the nighttime duty condition, the practice effects were tempered, resulting in less performance improvement across duty cycles than seen in the daytime duty condition. Thus, the 34-hour restart was less recuperative in the nighttime duty condition than in the daytime duty condition, further demonstrating the dependence of the efficacy of the restart break on the circadian timing of the duty schedule.
The level of PVT performance impairment reached in the nighttime duty condition of our study (with group-average lapse counts not exceeding 4; see Figure 3, top left panel) was modest when compared with the documented effects on PVT performance during a night of acute total sleep deprivation or after a week of sustained sleep restriction to 6 hours per day (which are in the range of 6-8 lapses35). One reason for this may be the restriction of simulated duty time to 14 hours per day (in agreement with the current HOS regulations motivating this study), leaving as much as 10 hours per day for recuperation. Another reason may be the presence of nap opportunities preceding the transitions to nighttime wakefulness, which served as prophylactic naps known to be effective countermeasures for neurobehavioral impairment.36,37 Without strategic napping, performance in the nighttime duty condition likely would have been considerably worse. Moreover, performance impairment associated with insufficient recuperation appears to be cumulative.11,35,38 Thus, additional episodes of a 34-hour restart period followed by a nocturnal duty cycle could result in further impairment, compounding the impact of insufficient recuperation.
Not all secondary outcome variables showed increased deficits after the restart break. DSST performance and self-report measures of affect, mood, and effort did not vary significantly across duty cycles by condition, nor did measures drawn from the high-fidelity driving simulators other than lane deviation. There is evidence that the effects of sleep loss and circadian misalignment vary as a function of the components of cognition (e.g., sustained attention, working memory) involved in the performance task at hand,39–41 which may partially explain why the effects of the nighttime duty schedule and the 34-hour restart break varied among performance outcomes. Some of the performance measures also showed practice effects, which may have obscured differential condition effects. Furthermore, given the rather modest impact of the nighttime duty condition on neurobehavioral functioning (as indicated by the magnitude of PVT impairment discussed above), statistical power for secondary outcome variables may not have been sufficient to yield significant interaction effects.
The results for subjective sleepiness were incongruent with those for other outcome variables (Figure 2, bottom left panel). Although subjects in the nighttime duty condition reported overall greater subjective sleepiness relative to the daytime duty condition, they exhibited decreasing sleepiness across days (collapsed over time of day) in each of the two 5-day duty cycles. This pattern suggested subjective adaptation to the nighttime duty schedule, although the 34-hour restart break undid some of this subjective adaptation. Discrepancy between subjective and objective indices of neurobehavioral impairment has been observed previously in the context of chronic nocturnal sleep-restriction paradigms11,35 and individual differences in responses to total sleep deprivation.42,43 The present study extends this phenomenon to nighttime waking (i.e., daytime sleep) schedules, and highlights the operational problem of people not reliably gauging their own performance deficits resulting from sleep loss and circadian misalignment.
Performance deficits in the nighttime duty condition were most prominent in the later hours of the night and early morning (Figure 3). This was observed both before and after the restart break, suggesting that no substantive circadian phase-drifting occurred during the laboratory study. Nighttime performance decrements were also evident in calculated fuel consumption on the high-fidelity driving simulators—fuel consumption increased by up to about 1% across the night (Figure 3, bottom right panel). As such, night duty schedules may impact on driving performance not only in terms of road safety,44–46 but also in terms of direct economic cost.47
The progressive decline of neurobehavioral functioning across the night is consistent with established principles of sleep/wake regulation.48,49 Two major regulatory processes are involved in human sleep/wake regulation50: a homeostatic process that builds up pressure for sleep during wakefulness and dissipates that pressure during sleep, and a circadian process that exerts a net pressure for wakefulness during the daytime hours and withdraws that pressure during the night. For normal daytime waking and nighttime sleep schedules, the interaction of the two processes results in stable wakefulness during the day and consolidated sleep during the night.48 However, when wakefulness is placed during the night, the two processes become misaligned, resulting in progressive deterioration of performance during the nighttime waking period (Figure 3).
Furthermore, when wakefulness is placed during the night, sleep must be obtained during the day when the circadian pressure for wakefulness is high. This results in sleep curtailment, as observed in both duty cycles of the nighttime duty condition (see Figure 4, top right panel). Subjects in this condition obtained an average of 7.5 hours TST per duty day, which represented a daily sleep loss of 0.9 hours relative to the daytime duty condition. Given ample TIB (10 hours per duty day) and strict control over light exposure and other zeitgebers in the laboratory, this 0.9-hour difference would appear to represent the magnitude of the endogenous circadian effect (interacting with sleep homeostasis) on TST in night-shift versus day-shift schedules. Noting that SL was shortened during the duty days in the nighttime duty condition (Figure 4, bottom right panel), sleep curtailment was primarily due to difficulty maintaining sleep (rather than initiating sleep), as is consistent with the existence of a “wake maintenance zone”51 or forbidden zone for sleep52 in the early evening. Even so, the reduction of sleep to 7.5 hours per duty day that we observed is less substantial than what is reported for shift workers in real-world operations,53 who tend to obtain no more than 5 to 6 hours of sleep per day. This is not surprising, because shift workers in real-world settings face additional sleep challenges (such as ambient light and environmental noise), which make it difficult to obtain the amount of daily sleep their endogenous circadian rhythm would otherwise allow.
During the 34-hour restart break, the subjects in the nighttime duty condition transitioned back to a normal nighttime sleep schedule and had transition nap opportunities, yet they still tended to accumulate less sleep than did the subjects in the daytime duty control condition (despite equal amounts of TIB). The temporary transitioning back to a normal nighttime sleep schedule during the restart period may be one of the reasons why the restart break was not efficacious for mitigating the loss of sleep and consequent performance impairment—and, in fact, may even have contributed to the increased impairment in the post-restart duty cycle in the nighttime duty condition. If a nighttime wake schedule had been maintained through the restart break, it is possible that gradual circadian adjustment would have occurred, potentially making the restart period more efficacious.54 The real-world utility of this scenario is questionable, though, because few individuals would elect to maintain a permanent night-shift schedule if given the choice.19,20
Changes in the amounts of REM sleep and stage N2 sleep were concordant with those in TST in both study conditions (Figure 4). However, even though not all sleep periods were recorded polysomnographically, it appeared that SWS was systematically conserved in the nighttime duty condition compared with the daytime duty condition (Figure 4, middle left panel). Conservation of SWS has been found previously in the context of nighttime sleep-restriction paradigms,11,13,35,55 although not in all studies.56 Our finding that conservation of SWS extends to daytime sleep schedules (i.e., to opposite circadian phase) is new and could be important for models of sleep regulation.
In contrast to SWS, SL (Figure 4, bottom right panel) was significantly reduced in the daytime sleep periods of the experimental condition relative to the nighttime sleep periods of the control condition. This suggests that considerable homeostatic pressure for sleep was present and overcame the rising circadian pressure for wakefulness during the late morning. With TST reduced, SWS conserved, and SL shortened simultaneously, our simulated night-shift protocol dissociated three commonly used markers of sleep homeostasis, questioning their validity in circadian misalignment paradigms and contributing new data to the debate about whether or not sleep duration and sleep intensity are interrelated.13,35,57 As it stands, the most parsimonious assumption would seem to be that overall sleep curtailment (i.e., reduced TST) was the main effector of the observed performance impairments following the restart break in the nighttime duty condition, which would be consistent with conclusions drawn from other experiments.35,58
There are some noteworthy limitations to the interpretation of our findings in applied contexts. The study was conducted in a laboratory, which helped to eliminate environmental confounds, allowed for continuous behavioral and physiologic monitoring and use of sensitive laboratory performance measures, and moderated the sample-size requirement (as supported by a power calculation performed in advance of the study18). The laboratory setting was strictly controlled, and light levels during scheduled wakefulness were the same for the two study conditions. Outside the laboratory, light exposure would normally differ between nighttime and daytime duty schedules. Also, in view of the between-groups design of the study, we standardized the driving-simulator scenarios (roads, weather, light conditions). These scenarios did not, therefore, control for the increased traffic density typically encountered during daytime driving or the reduced visibility associated with nighttime driving. As such, the study findings should be interpreted in terms of human response rather than occupational response. A field study would be needed to determine how our results translate to real-world driving performance, safety, and cost.
It should also be noted that the research subjects were healthy young adults with no sleep disorders. Had we studied a sample of patients with sleep apnea—a common sleep disorder among CMV drivers59—or other medical conditions, the recuperative potential of the restart break would likely have been diminished. Thus, greater post-restart performance deficits would have been expected in the nighttime duty condition, strengthening our conclusion that the 34-hour restart break was not efficacious for maintaining optimal performance across repeated nighttime duty cycles.
In conclusion, we showed that a 34-hour restart break was adequate (and, pending further research, perhaps even unnecessary) for maintaining optimal performance in a daytime duty schedule, but was inadequate (and perhaps even detrimental) for maintaining performance in a nighttime duty schedule. Performance degradation was observed for sustained attention during the PVT, mental orientation in the CDDT, and driving in the high-fidelity driving simulator, all of which are relevant to real-world truck driving. Our study thus indicated that the 34-hour restart provision of the HOS regulations currently governing CMV drivers in the US could be improved by considering the circadian aspects of duty schedules. This was recognized in the notice of proposed rulemaking issued by the FMCSA on December 24, 2010, in which the 34-hour restart rule is proposed to be amended by requiring inclusion in the restart period of at least two “biological nights” (defined in the proposed amendment as periods between 00:00 and 06:00). For nighttime duty schedules, this implies that, in most cases, the duration of the restart break will need to be extended beyond 34 hours. (No complementary stipulation was made for daytime duty schedules to allow shortening of the restart break to less than 34 hours.) Follow-up research is underway to investigate the effectiveness of adding a second biological night for recovery sleep to the restart break to promote recycling with optimal performance.
DISCLOSURE STATEMENT
This was not an industry supported study. Prof. Van Dongen has received research funding from the Federal Motor Carrier Safety Administration, Regional Airline Association, Sleep Research Society, Air Force office of Scientific Research, Institutes for Behavior Resources, The Boeing Company, Pulsar Informatics, Battelle Center for Human Performance and Safety, Continental Airlines, Department of Defense, W M. Keck Foundation, Advanced Brain Monitoring, and the Office of Naval Research. Dr. Belenky has received research funding from Continental Airlines. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Thomas Balkin, Melissa Mallis, and Siobhan Banks, who served as a peer-review committee for the design and analysis of this study, for their valuable suggestions. We thank the laboratories of David Dinges and Glenn Gunzelmann for allowing us to use their neurobehavioral performance assays, Linda Ng Boyle for technical advice on operationalizing high-fidelity driving simulator metrics, and Pulsar Informatics for technical and programming support of data collection from the driving simulators. We also thank the laboratory staff and the volunteer subjects. This research was supported by the Federal Motor Carrier Safety Administration (FMCSA) under contract DTMC75-07-D-00006 (HVD); and in part by Office of Naval Research DURIP grant N00014-08-1-0802 (BV), Air Force Office of Scientific Research DURIP grant FA9550-06-1-0281 (GB), and CDMRP award W81XWH-05-1-0099.
FOOTNOTE
http://www.fmcsa.dot.gov/rules-regulations/topics/hos-proposed/hos-proposed.aspx (accessed January 8, 2011)
REFERENCES
- 1.Costa G, Ghirlanda G, Tarondi G, Minors D, Waterhouse J. Evaluation of a rapidly rotating shift system for tolerance of nurses to nightwork. Int Arch Occup Environ Health. 1994;65:305–11. doi: 10.1007/BF00405694. [DOI] [PubMed] [Google Scholar]
- 2.Bjorvatn B, Kecklund G, Åkerstedt T. Rapid adaptation to night work at an oil platform, but slow readaptation after returning home. J Occup Environ Med. 1998;40:601–8. doi: 10.1097/00043764-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bougrine S, Mollard R, Ignazi G, Coblentz A. Days off and bright light: effects on adaptation to night work. Int J Ind Ergonom. 1998;21:187–98. [Google Scholar]
- 4.Kandelaars KJ, Lamond N, Roach GD, Dawson D. The impact of extended leave on sleep and alertness in the Australian rail industry. Ind Health. 2005;43:105–13. doi: 10.2486/indhealth.43.105. [DOI] [PubMed] [Google Scholar]
- 5.Landrigan CP, Barger LK, Cade BE, Ayas NT, Czeisler CA. Interns' compliance with accreditation council for graduate medical education work-hour limits. JAMA. 2006;296:1063–70. doi: 10.1001/jama.296.9.1063. [DOI] [PubMed] [Google Scholar]
- 6.Viitasalo K, Kuosma E, Laitinen J, Härmä M. Effects of shift rotation and the flexibility of a shift system on daytime alertness and cardiovascular risk factors. Scand J Work Environ Health. 2008;34:198–205. doi: 10.5271/sjweh.1228. [DOI] [PubMed] [Google Scholar]
- 7.Karlson B, Eek F, Orbaek P, Osterberg K. Effects of sleep-related problems and self-reported health after a change of shift schedule. J Occup Health Psychol. 2009;14:97–109. doi: 10.1037/a0014116. [DOI] [PubMed] [Google Scholar]
- 8.Peach GM, Jay SM, Lamond N, Roach GD, Ferguson SA. The effects of different roster schedules on sleep in miners. Appl Ergon. 2010;41:600–6. doi: 10.1016/j.apergo.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Sallinen M, Kecklund G. Shift work, sleep, and sleepiness—differences between shift schedules and systems. Scand J Work Environ Health. 2010;36:121–33. doi: 10.5271/sjweh.2900. [DOI] [PubMed] [Google Scholar]
- 10.Orlando C, Levitan EB, Mittleman MA, Steele RJ, Shrier I. The effect of rest days on injury rates. Scand J Med Sci Sports. 2010 Jun 16; doi: 10.1111/j.1600-0838.2010.01152.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 11.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 12.Axelsson J, Kecklund G, Åkerstedt T, Donofrio P, Lekander M, Ingre M. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int. 2008;25:297–308. doi: 10.1080/07420520802107031. [DOI] [PubMed] [Google Scholar]
- 13.Banks S, Van Dongen HPA, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks S, Van Dongen H, Dinges D. Neurobehavioral response to sleep restriction is influenced by pre-existing sleep debt. Sleep Biol Rhythms. 2007;5(Suppl 1):A103. [Google Scholar]
- 15.Monk TH. The relationship of chronobiology to sleep schedules and performance demands. Work Stress. 1990;4:227–36. doi: 10.1080/02678379008256985. [DOI] [PubMed] [Google Scholar]
- 16.Van Dongen HPA. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol Int. 2006;23:1139–47. doi: 10.1080/07420520601100971. [DOI] [PubMed] [Google Scholar]
- 17.De Valck E, Quanten S, Berckmans D, Cluydts R. Simulator driving performance, subjective sleepiness and salivary cortisol in a fast-forward versus a slow-backward rotating shift system. Scand J Work Environ Health. 2007;33:51–7. doi: 10.5271/sjweh.1064. [DOI] [PubMed] [Google Scholar]
- 18.Van Dongen HPA, Belenky G. Washington, DC: Federal Motor Carrier Safety Administration; 2010. Investigation into motor carrier practices to achieve optimal commercial motor vehicle driver performance: phase I. Technical Report No. FMCSA-RRR-10-005. [Google Scholar]
- 19.Vespa S, Wylie D, Mitler M, Shultz T. Study of commercial vehicle driver rest periods and recovery of performance in an operational environment. In: Hartley L, editor. Managing Fatigue in Transportation. Oxford, UK: Pergamon; 1998. pp. 119–65. [Google Scholar]
- 20.Monk TH. What can the chronobiologist do to help the shift worker? J Biol Rhythms. 2000;15:86–94. doi: 10.1177/074873040001500202. [DOI] [PubMed] [Google Scholar]
- 21.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep Deprivation. Clinical Issues, Pharmacology, and Sleep Loss Effects. New York, NY: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 22.Moore JM, Van Dongen H, Belenky G, Mott CG, Huang L, Vila B. Use of a driving simulator to assess fuel inefficiency as a downstream effect of driver sleepiness in controlled laboratory experiments. Sleep. 2009;32:A387–8. [Google Scholar]
- 23.Boyle LN, Tippin J, Paula A, Rizzo M. Driver performance in the moments surrounding a microsleep. Transp Res Part F Traffic Psychol Behav. 2008;11:126–36. doi: 10.1016/j.trf.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Åkerstedt T, Ingre M, Kecklund G, et al. Reaction of sleepiness indicators to partial sleep deprivation, time of day and time on task in a driving simulator—the DROWSI project. J Sleep Res. 2010;19:298–309. doi: 10.1111/j.1365-2869.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 25.Jackson M. The Effects of Sleep Deprivation on Driving-Related Performance. Saarbrücken, Germany: Lambert Academic Publishing; 2010. [Google Scholar]
- 26.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Intern J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 27.Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 28.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Personality Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- 30.Dinges DF, Kribbs NB, Steinberg KN, Powell JW. Do we lose the willingness to perform during sleep deprivation? Sleep Res. 1992;21:318. [Google Scholar]
- 31.Gunzelmann G, Anderson JR, Douglass S. Orientation tasks with multiple views of space: strategies and performance. Spatial Cogn Comp. 2004;4:207–53. [Google Scholar]
- 32.Halverson T, Gunzelmann G, Moore LR, Jr, Van Dongen HPA. The effects of work shift and strategy on an orientation task. In: hlsson S, Catrambone R, editors. Proceedings of the 32nd Annual Meeting of the Cognitive Science Society; Austin, TX: Cognitive Science Society; 2010. pp. 2134–9. [Google Scholar]
- 33.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events. Rules, terminology and technical specifications. [Google Scholar]
- 34.Van Dongen HPA, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: Importance and techniques. Aviat Space Environ Med. 2004;75:A147–54. [PubMed] [Google Scholar]
- 35.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 36.Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: Contributions of circadian phase and prior wakefulness. Sleep. 1987;10:313–29. [PubMed] [Google Scholar]
- 37.Bonnet MH, Arand DL. Consolidated and distributed nap schedules and performance. J Sleep Res. 1995;4:71–7. doi: 10.1111/j.1365-2869.1995.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 38.McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPA. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–39. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond SPA, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res Neuroimaging. 2005;140:211–23. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Ratcliff R, Van Dongen HPA. Sleep deprivation affects multiple distinct cognitive processes. Psychon Bull Rev. 2009;16:742–51. doi: 10.3758/PBR.16.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 43.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 44.Gillberg M, Kecklund G, Åkerstedt T. Sleepiness and performance of professional drivers in a truck simulator – comparisons between day and night driving. J Sleep Res. 1996;5:12–5. doi: 10.1046/j.1365-2869.1996.00013.x. [DOI] [PubMed] [Google Scholar]
- 45.Åkerstedt T, Kecklund G, Hörte LG. Night driving, season, and the risk of highway accidents. Sleep. 2001;24:401–6. doi: 10.1093/sleep/24.4.401. [DOI] [PubMed] [Google Scholar]
- 46.Vester JC, Taillard J, Sagaspe P, Olivier B, Philip P. Prolonged nocturnal driving can be as dangerous as severe alcohol-impaired driving. J Sleep Res. 2011 Jan 12; doi: 10.1111/j.1365-2869.2010.00901.x. doi: 10.1111/j.1365-2869.2010.00901.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 47.Popp R, Rothe S, Jellentrup N, Geisler P, Zulley J, Hajak G. Sleepy truckers increase fuel consumption due to inefficient driving. J Sleep Res. 2010;19(Suppl 2):85–6. [Google Scholar]
- 48.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 49.Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24:237–49. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 51.Strogatz SH, Kronauer RE, Czeisler CA. Circadian pacemaker interferes with sleep onset at specific times each day: Role in insomnia. Am J Physiol. 1987;253:R172–8. doi: 10.1152/ajpregu.1987.253.1.R172. [DOI] [PubMed] [Google Scholar]
- 52.Lavie P. Ultrashort sleep-waking schedule. III. ‘Gates’ and ‘forbidden zones’ for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–25. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- 53.Kecklund G, Åkerstedt T. Effects of timing of shifts on sleepiness and sleep duration. J Sleep Res. 1995;4(Suppl 2):47–50. doi: 10.1111/j.1365-2869.1995.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 54.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep. 2004;27:1077–87. doi: 10.1093/sleep/27.6.1077. [DOI] [PubMed] [Google Scholar]
- 55.Brunner DP, Dijk DJ, Borbély AA. Repeated partial sleep deprivation progressively changes the EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 56.Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hor H, Tafti M. How much sleep do we need? Science. 2009;325:825–6. doi: 10.1126/science.1178713. [DOI] [PubMed] [Google Scholar]
- 58.Mollicone DJ, Van Dongen HPA, Rogers NL, Dinges DF. Response surface mapping of neurobehavioral performance: Testing the feasibility of split sleep schedules for space operations. Acta Astronautica. 2008;63:833–40. doi: 10.1016/j.actaastro.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pack AI, Maislin G, Staley B, et al. Impaired performance in commercial drivers. Role of sleep apnea and short sleep duration. Am J Respir Crit Care Med. 2006;174:446–54. doi: 10.1164/rccm.200408-1146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]