Abstract
Study Objectives:
Our aim was to estimate heritability in phenotypic insomnia and the association between insomnia and mortality.
Design:
Representative follow-up study.
Participants:
1990 survey of the Finnish Twin Cohort (N = 12502 adults; 1554 monozygotic and 2991 dizygotic twin pairs).
Measurements:
Current insomnia-related symptoms (insomnia in general, difficulty in initiating sleep, sleep latency, nocturnal awakening, early morning awakening, and non-restorative sleep assessed in the morning and during the day) were asked. Latent class analysis was used to classify subjects into different sleep quality classes. Quantitative genetic modelling was used to estimate heritability. Mortality data was obtained from national registers until end of April 2009.
Results:
The heritability estimates of each symptom were similar in both genders varying from 34% (early morning awakening) to 45% (nocturnal awakening). The most parsimonious latent class analysis produced 3 classes: good sleepers (48%), average sleepers (up to weekly symptoms, 40%), and poor sleepers (symptoms daily or almost daily, 12%). The heritability estimate for the cluster was 46% (95% confidence interval 41% to 50%). In a model adjusted for smoking, BMI, and depressive symptoms, the all-cause mortality of poor sleepers was elevated (excess mortality 55% in men and 51% in women). Further adjustment for sleep length, use of sleep promoting medications, and sleep apnea-related symptoms did not change the results.
Conclusions:
Insomnia-related symptoms were common in both genders. The symptoms and their clusters showed moderate heritability estimates. A significant association was found between poor sleep and risk of mortality, especially in those with somatic disease.
Citation:
Hublin C; Partinen M; Koskenvuo M; Kaprio J. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. SLEEP 2011;34(7):957-964.
Keywords: Insomnia, heritability, twins, mortality, population
INTRODUCTION
Sleep is influenced by genetic and environmental factors, and it is regulated at many levels that are likely to be genetically controlled. In humans, EEG has been found to be one of the most heritable characteristics.1 Both waking and sleep EEG show remarkable similarity in monozygotic (MZ) twins but only familial relatedness in dizygotic (DZ) twins.2,3
Genetic factors are thought to play a role in most sleep disorders, including insomnia.1,4 High rates of familial insomnia have been reported in 3 studies based on patient series,5–7 but in a population-based study the family history rates were not significantly different when individuals with current insomnia symptoms or syndrome were compared with self-defined good sleepers.8 Twin studies have suggested a role of genetic effects in sleep and sleep disorders. An early study from the Finnish Twin Cohort indicated a significant hereditary effect on sleep length and sleep quality—heritability for both traits was 44%.9 Similar figures on insomnia-related symptoms have been reported in 3 other reports based on large twin data sets, with modest to moderate heritability estimates (21% to 57%) for insomnia in general, sleep latency, difficulty in falling asleep, nocturnal awakenings, and waking up feeling tired and worn out.10–12
Our aim in the present study is to assess the genetic effects in phenotypic insomnia in a large population-based twin cohort, using self-report of 7 insomnia-related symptoms, for which heritability estimates were obtained. Based on cluster analysis, we classified the study population into good sleepers, average sleepers, and poor sleepers, and estimated the heritability of the underlying susceptibility to poor quality sleep. Furthermore, to indicate the clinical effects of this classification we analyzed risk of overall mortality in these groups during a twenty year follow-up.
METHODS
Population Sample
The Older Finnish Twin Cohort is a longitudinal study of Finnish twin pairs of the same gender born before 1958 with both co-twins alive in 1975. These pairs were selected from the Central Population Registry of Finland in 1974.13 The third questionnaire survey in 1990 was mailed to pairs born 1930-57 with both co-twins resident in Finland in 1987, and 16,179 twin individuals could be contacted (response rate 77.3%; N = 12,502; 54.4% women; mean age in 1990 43.9 years). This study population included 1554 MZ and 2991 DZ twin pairs. The questionnaire included 103 multiple-choice questions, of which 22 were sleep- and vigilance-related. The study has been approved by the ethical committee of the Department of Public Health, University of Helsinki. Informed consent was obtained from all respondents. Zygosity was determined using an accurate and validated questionnaire method.14
Questionnaire and Register Data
The questionnaire included 7 questions on current insomnia-related symptoms (wordings and response alternatives are given in Table 1). One question assessed the frequency of insomnia in general, and the rest more specific insomnia-related symptoms: frequency of difficulty in initiating sleep, nocturnal awakenings, and feeling of non-restorative sleep separately in the morning and during the day. Additionally there were questions on the length of sleep latency in the evening and occurrence of early morning awakenings (this question is one item from the 21-item Beck Depression Inventory (BDI).15,16 Additionally, we asked smoking status (never, occasional, ex-, or current smoker), weight and height to compute BMI (body mass index, kg/m2), sleep length (categorized as short [ < 7 h], average [7-8 h], and long [ > 8 h]), use of hypnotics and/or tranquilizers (no use of either hypnotics or tranquilizers, infrequent use = 1–59 days per year of either medication; frequent use = 60 or more days per year of either medication),17 habitual snoring (≥ 3 nights per week), and apneas (≥ 1 night per week).
Table 1.
Distributions of self-reported insomnia-related symptoms (N = 12,502)
| Question and response alternatives* | Men (%) | Women (%) |
|---|---|---|
| INSOMNIA-SYMPTOM | ||
| How often do you suffer from insomnia? | ||
| never or less than once a month | 50.8 | 51.4 |
| less than one night per week | 30.2 | 28.9 |
| 1–2 nights per week | 12.2 | 12.3 |
| 3 nights or more per week | 6.8 | 7.4 |
| DIFFICULTY IN INITIATING SLEEP | ||
| Do you have difficulties in falling asleep in the evening? | ||
| never or less than once a month | 55.4 | 56.0 |
| less than once a week | 25.8 | 25.0 |
| 1–2 evenings per week | 11.8 | 11.1 |
| 3 evenings or more per week | 7.1 | 7.9 |
| SLEEP LATENCY | ||
| How fast do you usually fall asleep in the evening? | ||
| in less than 10 minutes | 33.0 | 30.1 |
| in 10–20 minutes | 43.9 | 44.3 |
| in 21–30 minutes | 14.3 | 15.9 |
| more than 30 minutes | 8.8 | 9.7 |
| NOCTURNAL AWAKENINGS | ||
| How often on average are you awaken during the night? | ||
| usually I do not wake during the night | 30.5 | 25.8 |
| at most during a couple nights a week | 25.1 | 22.7 |
| once a night | 23.5 | 25.2 |
| twice a night | 13.6 | 17.1 |
| 3 times or more a night | 7.3 | 9.3 |
| EARLY MORNING AWAKENING | ||
| I sleep as well as before | ||
| when I wake up in the morning I am much more sleepy/tired than before | 79.4 | 73.4 |
| “I wake up 1–2 hours earlier than before and it is difficult to fall asleep again ”or | 11.4 | 17.2 |
| “I wake up early every morning, and I cannot sleep more than 5 hours” | 9.2 | 9.4 |
| NON-RESTORATIVE SLEEP (MORNING) | ||
| How often do you feel sleepy/tired when you wake up in the morning? | ||
| never or less than once a month | 32.8 | 29.8 |
| less than one morning per week | 33.1 | 29.2 |
| 1–2 mornings per week | 20.2 | 21.5 |
| 3–5 mornings per week | 6.7 | 7.7 |
| every or almost every morning | 7.2 | 11.8 |
| NON-RESTORATIVE SLEEP (DAY) | ||
| Are you sleepy/tired during the daytime? | ||
| never or less than once a month | 33.0 | 28.9 |
| less than one day per week | 34.3 | 31.2 |
| 1–2 days per week | 19.6 | 22.2 |
| 3–5 days per week | 5.9 | 6.7 |
| every or almost every day | 7.1 | 11.1 |
Range of missing data on each question 0.3–1.7%
Vital status (alive in Finland on April 30, 2009, date of death or date of migration from Finland) was obtained from the Population Register Centre of Finland. The follow-up for all-cause mortality was from the exact date of response (date 1990 questionnaire returned) to April 30, 2009.
Statistical Methods and Modeling
Basic statistics were computed using the Stata program (version 9.2) (StataCorp, USA). Latent class analysis (LCA) was used to classify subjects with differing patterns of symptoms. LCA is a data reduction technique that can be used to summarize categorical data. The assumption behind LCA is that subjects can be grouped into a small number of distinct clusters known as latent classes based on their symptom profile. Modeling aims to obtain the smallest number of clusters that accounts for all the associations between variables, so that within clusters the variables are as uncorrelated as possible. We fitted models successively, starting with a one-cluster model and then adding another cluster for each successive model. The optimal number of clusters can be determined in a variety of ways18–20 based on a variety of fit indices and some subjective assessment of the underlying biology and pathophysiology. We explored the possibilities of 2 to 5 latent class solutions. The 3-class model fit much better than 2 classes, but for 4 and 5 classes the main difference compared with the 3-class solution was a split of the “intermediate” category into 2 or 3. Therefore we concluded that 3 latent classes could classify the subjects best, and we termed the classes as class 1: no or occasional symptoms (good sleepers); class 2: infrequent symptoms (average sleepers); and class 3: frequent symptoms (poor sleepers). LCA was done using the Latent Gold18 statistical analysis program.
Genetic modeling can be used to estimate the contribution of genetic effects to the susceptibility to a trait, based on the polygenic multifactorial model.21 In twins reared together, it is possible to model 4 parameters: an additive genetic component (parameter A), genetic effects of dominance (non-additive; parameter D), shared environmental (family) components (parameter C), and non-shared environmental components (parameter E) in the variation of the underlying liability to the trait (in this study, insomnia-related symptoms). One can fit models based on the different combinations of these parameters ACE, ADE, AE, CE, DE, and E. The effects caused by dominance and shared environmental effect (DC) cannot be simultaneously modeled with data limited to that from twins reared together. These models are estimated under the usual assumptions of twin analyses, expecting no gene-environment interaction or correlation, no assortative mating, and environments relevant to sleep etiology to be similar in MZ and DZ twins.22
Before we started to model variance components, we confirmed the assumption that first and second twins and twins of both zygosities all represent the same population. The distribution of each insomnia-related symptom was studied using the method of full information maximum likelihood estimation for raw data. This method utilizes all available information, including that from pairs in which only one twin has responded. An initial fully saturated model, in which all the distributions for the first and second twins in both zygosities were free to vary was compared to successively more constrained models by likelihood ratio tests. The distributions were first set equal for the first- and the second-born co-twins and then set equal for MZ and DZ pairs. Standard model fitting methods were employed using Mx, a program for analysis of twin and family data.22,23 Chi-square goodness-of-fit statistics were used to assess how well the models fit the data. The superiority of alternative, hierarchically nested models was assessed by the difference in χ2 values of the models, which is itself χ2 distributed with degrees of freedom equal to the difference in degrees of freedom of the models to be compared. Men and women were modeled together, and we tested that the magnitude of variance components could be set to be equal in men and women.
Cox proportional hazard models were used to obtain hazard ratios (HR) and their 95% CI for all-cause mortality by sleep quality class (good sleepers, average sleepers, or poor sleepers; see below). In the 1990 questionnaire, the subjects were asked if they had ever had any chronic disease diagnosed by a physician (a list containing 20 diseases). The subject was considered to have a somatic disease if he/ she had any self-reported (1) disease diagnosed by a physician, (2) life event of serious injury/ illness, or (3) permanent work disability. Other subjects were classified as healthy.24 Examination of the validity of self-reported chronic illness in a population study confirmed that the agreement between questionnaire data and the individual's medical records was very good for well-known chronic diseases with clear diagnostic criteria that are easily communicated to the patient.25
RESULTS
Self-report of weekly insomnia in general was reported by 19% of men and 20% of women (≥ 3 nights, 6.8 and 7.4%, respectively; Table 1). The most common insomnia-related symptom was waking up during the sleep period: weekly it occurred in the majority of the respondents (70% of men and 74% of women), and waking up at least twice a night also was frequent (reported by 21% of men and 26% of women). The second most common symptom was non-restorative sleep, defined as sleepiness or tiredness at least weekly in the morning or during the daytime. It occurred in the morning in 34.1% of men and 41.0% of women (≥ 3 mornings, 7.2 and 11.8%), and during the daytime 32.6 and 40.0% (≥ 3 days, 7.1 and 11.1%), respectively. The third in frequency was difficulty in initiating sleep: weekly it was reported by 19% of both men and women (≥ 3 evenings, 7.1 and 7.9%, respectively). The usual sleep latency in the evening was > 30 min in 8.8% of men and 9.7% of women. The least common insomnia-related symptom was early morning awakening (9.2% of men and 9.4% of women).
The intraclass (pairwise) polychoric correlations of each insomnia-related symptom by sex and zygosity are given in Table 2. They were about twice as high in MZ (around 0.4) than in DZ (around 0.1-0.2) twin pairs in both genders, suggesting that genetic effects may be present. The correlations were highest in MZ pairs in nocturnal awakenings (both genders), and lowest in non-restorative sleep (in men) and early morning awakenings (in women).
Table 2.
Pairwise similarity of self-reported insomnia-related symptoms: polychoric correlations (r) and their standard errors (SE) of occurrence in twin pairs (N given in each column)
| Men |
Women |
|||
|---|---|---|---|---|
| MZ (N = 628) | DZ (N = 1295) | MZ (N = 926) | DZ (N = 1696) | |
| Insomnia-Symptom | 0.396 ± 0.045 | 0.110 ± 0.035 | 0.424 ± 0.035 | 0.208 ± 0.030 |
| Difficulty in Initiating Sleep | 0.377 ± 0.046 | 0.146 ± 0.036 | 0.436 ± 0.037 | 0.245 ± 0.030 |
| Sleep Latency | 0.397 ± 0.041 | 0.159 ± 0.032 | 0.442 ± 0.034 | 0.212 ± 0.028 |
| Nocturnal Awakening | 0.448 ± 0.039 | 0.215 ± 0.030 | 0.451 ± 0.030 | 0.238 ± 0.026 |
| Early Morning Awakening | 0.370 ± 0.067 | 0.035 ± 0.055 | 0.327 ± 0.052 | 0.146 ± 0.040 |
| Non-Restorative Sleep (Morning) | 0.343 ± 0.042 | 0.103 ± 0.033 | 0.384 ± 0.033 | 0.183 ± 0.028 |
| Non-Restorative Sleep (Day) | 0.334 ± 0.044 | 0.171 ± 0.032 | 0.362 ± 0.034 | 0.176 ± 0.027 |
The best fitting genetic model of each symptom by gender is given in Table 3. The best model was ADE in insomnia-symptom, early morning awakening, and non-restorative sleep assessed in the morning; and AE in the remaining 4 insomnia-related symptoms. Fit of model (p-value) was good (around 0.9) in nocturnal awakening and non-restorative sleep assessed during the day, satisfactory (around 0.3) in sleep latency and early morning awakening, and acceptable (around 0.1) in difficulty in insomnia symptom, initiating sleep, and non-restorative sleep assessed in the morning. The gender differences in the percentages of each model component were small, and the broad sense heritability estimates (i.e., proportion of all genetic variance out of total variance) were similar in men and women, from 34% (early morning awakening) to 45% (nocturnal awakening).
Table 3.
Best fitting model of each insomnia-related symptom
| Model* | Fit of model probability | A | D | E | Broad sense heritability (A+D) | |
|---|---|---|---|---|---|---|
| Insomnia-Symptom | ADE (both) | 0.099 (both) | ||||
| Men | 3 (0-32) | 39 (6-50) | 58 (52-64) | 42 (33-50) | ||
| Women | 40 (16-47) | 2 (0-28) | 58 (50-67) | 42 (36-48) | ||
| Difficulty in Initiating Sleep | AE | 0.130 | 41 (36-46) | N/A | 59 (54-64) | 41 (36-46) |
| Sleep Latency | AE | 0.341 | 41 (37-46) | N/A | 59 (54-63) | 41 (37-46) |
| Nocturnal Awakening | AE | 0.945 | 45 (41-49) | N/A | 55 (51-59) | 45 (41-49) |
| Early Morning Awakening | ADE | 0.302 | 7 (0-33) | 27 (0-42) | 66 (58-74) | 34 (26-42) |
| Non-Restorative Sleep (Morning) | ADE | 0.128 | 22 (5-38) | 15 (0-34) | 63 (58-68) | 37 (32-42) |
| Non-Restorative Sleep (Day) | AE | 0.973 | 35 (31-39) | N/A | 65 (61-69) | 35 (31-39) |
For each component given percentage and 95% confidence interval, which were the same in both genders except for insomnia symptom which are given separately for men and women.
A, additive genetic component; D, genetic effects of dominance; E, non-shared environmental component.
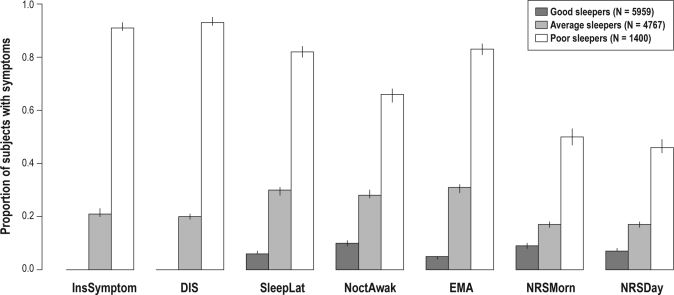
The most parsimonious cluster analysis with 3 classes classified 48.3% of the study population into good sleepers (class 1), 39.7% into average sleepers (class 2), and 12.0% into poor sleepers (class 3). The responses corresponding to the mean values in the cluster analysis in good sleepers were: insomnia symptom never or less than monthly, difficulty in falling asleep less often than weekly, sleep latency ≤ 20 min, nocturnal awakenings maximally 2 nights a week, no early morning awakening, and feeling of non-restorative sleep more seldom than weekly. In poor sleepers, these responses were: insomnia symptom ≥ 3 nights a week, difficulty in falling asleep every or almost every evening, sleep latency ≥ 40 min, nocturnal awakenings ≥ 3 times a night, current early morning awakening, and feeling of non-restorative sleep ≥ 3 days weekly. Figure 1 shows the proportions of those with insomnia symptoms in each of the 3 clusters when the response alternatives are dichotomized according to the mean responses of good sleepers. Characteristics of the 3 clusters are given in Table 4. There are major differences in the proportion of healthy subjects (good vs. poor sleepers 51% vs. 24%), normal BDI (94% vs. 47%), short sleepers (14% vs. 40%), and frequent use of sleep promoting medications (2% vs. 21%). Notably, there are much smaller differences in habitual snoring (22% vs. 28%) and weekly apneas (1% vs. 4%).
Figure 1.
Proportions (95% confidence intervals; y-axis range 0–1) of insomnia-related symptoms of the three sleeper categories in cluster analysis. This figure shows the proportions of those with insomnia symptoms in each of the three clusters when the response alternatives are dichotomized according to the mean responses of good sleepers. Low values indicate absence or infrequent occurrence of each symptom (in sleep latency short time) and high values increasing frequency of the symptom (long sleep latency) N = 12,126. InsSymptom, insomnia-symptom; DIS, difficulty in initiating sleep; SleepLat, sleep latency; NoctAwak, nocturnal awakenings; EMA, early morning awakening; NRSMorn, non-restorative sleep (morning); NRSDay, non-restorative sleep (day).
Table 4.
Characteristics of the three clusters grouped by the frequency of insomnia-related symptoms
| Total sample N = 12 126 | Clusters |
|||
|---|---|---|---|---|
| Good sleepers N = 5959 | Average sleepers N = 4767 | Poor sleepers N = 1400 | ||
| Age in years: mean (SD) | 43.9 (7.8) | 42.7 (7.3) | 44.5 (7.9) | 46.3 (8.3) |
| Women (%) | 54.4 | 54.0 | 53.3 | 59.7 |
| Healthy (%) | 43.6 | 50.7 | 40.4 | 24.3 |
| Current smokers (%) | 26.0 | 25.2 | 25.4 | 31.4 |
| BDI normal (%)* | 83.0 | 93.6 | 80.2 | 47.2 |
| Obese (BMI > 30; %)** | 7.7 | 6.4 | 8.0 | 11.9 |
| Short sleepers (%) | 17.9 | 14.2 | 16.0 | 40.1 |
| Long sleepers (%) | 14.2 | 14.5 | 14.8 | 11.1 |
| Frequent use of hypnotics and/or tranquilizers (%) | 4.7 | 1.5 | 4.4 | 20.5 |
| Habitual snoring (%) | 23.1 | 21.7 | 23.4 | 27.7 |
| Apneas ≥ 1 night per week (%) | 1.9 | 1.4 | 1.7 | 4.3 |
BDI, Beck Depression Inventory;
BMI, body mass index
When assessing the single question on the frequency of insomnia in general (“How often do you suffer from insomnia?”) the mean response value in good sleepers (1.1) corresponds to the alternative “never or less than monthly,” in average sleepers (2.1) “less than once a week,” and in poor sleepers (3.6) between “1–2 nights per week” and “3–5 nights per week” (first 3-bar set to the left in Figure 1). The correlation of this single general question to the cluster was 0.94 (similar in both genders). Of those with the response alternative indicating fewest insomnia symptoms, 88.6% are included in good sleepers, and of those with most frequent symptoms 91.2% in the poor sleepers. Similarly, regarding difficulties in falling asleep, 84.7% of subjects with the fewest symptoms were classified good sleepers, and 94.6% of subjects with most frequent symptoms were classified poor sleepers. In nocturnal awakenings, the corresponding figures were 78.5% and 66.9%; length of sleep latency, 77.4% and 87.8%; early morning awakening, 61.6% and 70.7%; non-restorative sleep in the morning, 49.2% and 38.9%; and non-restorative sleep during the day, 71.8% and 39.4%.
The genetic modelling of the cluster result was based on the assumption that the clusters represented one dimension with 3 categories (good, average, and poor sleepers), which was confirmed by a test for underlying bivariate normality of the unmeasured susceptibility to insomnia symptoms in twin pairs (i.e., based on a 3 × 3 classification of twin A vs. twin B). The maximum likelihood ratio test had a P-value of 0.49, indicating that the data fits well a model of bivariate normality in all pairs. The results were the same for both genders, and AE model had the best fit (probability 0.54, Akaike's Information Criterion −28.396, and χ2 fit of model 25.604). The heritability estimate was 46% (95% CI 41% to 50%) for the underlying liability to the insomnia symptom cluster distribution.
We also assessed the effect of age on the cluster distribution. Among 33- to 39-year-old subjects (N = 4387), 54.7% were good sleepers, 36.7% average sleepers, and 8.6% poor sleepers. Among 40- to 49-year-old subjects (N = 4653), the corresponding figures were 50.8%, 38.9%, and 10.3%; and among 50- to 60-year-old subjects (N = 3086) 38.7%, 43.7%, and 17.7%.
Risk of all-cause mortality in average and poor sleepers compared to good sleepers is shown in Table 5. In a model adjusted for smoking status, BMI, and depression (using the BDI), mortality of average sleepers compared to good sleepers was increased 22% in men and 7% in women. In poor sleepers, there was a significant increase (55% in men and 51% in women). Mortality in poor sleepers showed no evidence of heterogeneity between MZ and DZ twin individuals (data not shown). Additionally, we adjusted the analyses of risk of mortality with 4 sleep covariates (sleep length, use of hypnotics and/ or tranquilizers, habitual snoring, and weekly apneas), and the risks were essentially unchanged in men (with loss of statistical significance in average sleepers) and slightly increased in women.
Table 5.
Risk of all-cause mortality (hazard ratio [95 % confidence interval]) in good sleepers, average sleepers, and poor sleepers from date of response in 1990 to April 30, 2009
| Crude | Adjusted | Adjusted additionally with sleep covariates | |
|---|---|---|---|
| Men | N = 5530, n = 675 | N = 5267, n = 630 | N = 4949, n = 576 |
| Good sleepers | 1.00 | 1.00 | 1.00 |
| Average sleepers | 1.33 (1.12, 1.58) | 1.22 (1.02, 1.46) | 1.20 (0.99, 1.45) |
| Poor sleepers | 2.17 (1.74, 2.71) | 1.55 (1.19, 2.02) | 1.55 (1.16, 2.06) |
| Women | N = 6590, n = 423 | N = 6234, n = 386 | N = 5711, n = 342 |
| Good sleepers | 1.00 | 1.00 | 1.00 |
| Average sleepers | 1.13 (0.90, 1.40) | 1.07 (0.85, 1.35) | 1.15 (0.90, 1.48) |
| Poor sleepers | 1.76 (1.36, 2.29) | 1.51 (1.12, 2.03) | 1.63 (1.16, 2.30) |
Number of subjects (N) and cases (n) in analyses given in each column. Crude estimates take age into account, while adjusted models take into account also smoking status, body mass index (BMI), and BDI depression scores. In addition to them, sleep covariates (sleep length, use of hypnotics and/ or tranquilizers, snoring, and apneas) are included in the third set of analyses.
Risk of all-cause mortality (adjusted for sex, BMI, BDI, and smoking status) was also computed for a healthy subgroup (5042 subjects with 269 deaths) and those with somatic disease (6462 subjects with 747 deaths). Among healthy persons with average sleep, the risk was in men 1.35 and in females 1.12; with poor sleep, the risk in men was 1.40 and in females 1.38 (all statistically nonsignificant). Among those with baseline somatic disease, the figures were for average sleep in men 1.35 and in females 1.01 (nonsignificant), and for poor sleep in men 1.44 (95% CI 1.08, 1.92) and in females 1.43 (1.02, 2.02).
DISCUSSION
Our results show that insomnia-related symptoms are common in working-age population in both genders, there are significant genetic effects in insomnia symptoms, and frequent symptoms (several times weekly) may be associated with increased risk of all-cause mortality. Although some of the insomnia-related symptoms occurred weekly in a high percentage of the respondents (e.g., waking up during sleep in about 70% and non-restorative sleep in about one-third), only about one-fifth considered themselves suffering from insomnia. Pairwise similarity of each symptom was significantly higher in MZ than in DZ pairs, indicating significant genetic effects. The broad sense heritability varied between 34% and 45%, being lowest in early morning awakenings and highest in nocturnal awakenings in both genders. In cluster analysis, three groups were identified: good sleepers (48.3%) with each symptom mostly less than weekly, average sleepers (39.7%), and poor sleepers (12.0%) with each symptom 3 times or more weekly.
To test the validity and usefulness of the cluster analysis, we assessed the age effect on the distribution and the association with mortality. The percentage of good sleepers decreased with age and correspondingly the percentage of poor sleepers increased with age as expected. Risk of total mortality (adjusted for smoking, BMI, and depression compared to good sleepers) was increased by 22% in men and 7% in women among average sleepers, and was significantly increased in poor sleepers about 1.5-fold in both genders. The risk remained essentially unchanged when sleep length, use of hypnotics and/ or tranquilizers, habitual snoring, and weekly apneas were taken into account. When the risk was computed separately for healthy subjects at baseline and for those with somatic disease, the increase remained significant only for the latter group (point estimates being very similar—about 1.40).
There are considerable differences in the prevalence studies of insomnia in general and of individual insomnia symptoms, most probably due to the heterogeneity of definitions and methods.26 The figures of the present study are well in the range of previous studies. In a study with telephone interview, a representative sample of participants (N = 993) aged 18 years or older were classified in three groups according to a algorithm based on a combination of insomnia diagnostic criteria from the DSM-IV27 and ICD-10,28 and on the utilization of sleep-promoting products.8 Those with insomnia syndrome met all the diagnostic criteria for insomnia with, e.g., symptoms at least 3 nights per week and psychological distress or daytime improvement, and their prevalence was 15.5%. Those classified as having insomnia symptoms could present frequent but less severe symptoms not fulfilling all the diagnostic criteria of an insomnia syndrome, and their prevalence was 32.5%. The remaining 52.0% of participants were classified as good sleepers.8 Although profound methodological differences, the figures of our classification using cluster analysis of self-reported symptoms into good (about half), average (about two-fifths), and poor (12%) sleepers are similar, indicating a relevant categorizing by the cluster analysis.
There are only a few studies giving heritability estimates of insomnia. For insomnia in general, heritability has ranged from 28%11 to 57%12; it was 42% in the present study. For difficulty in initiating sleep, the range has been from 28%11 to 32%10; our figure was 41%. Similarly, the heritability for sleep latency was 44% for men and 32% for women in the study of Heath and co-workers,10 and the result in the present study was 41%. For nocturnal awakenings, the figures have varied between 26% and 42%10,11; our figure was 45%. In the study of McCarren et al.11 the heritability of waking up feeling tired and worn out was 21%, and in the present study that of the corresponding symptom (non-restorative sleep/morning) was 37%. In two of these studies the study population included twins of both genders, aged 17-88 years10 and 18 years or more,12 and one study male-male twins aged 33-51 years.12 Thus, the heritability estimates are of same order as in published studies, and given that heritability estimates reflect both genetic and environmental differences between populations at different times, these are remarkably consistent.
These results indicate a significant role of genetic effects in insomnia, but they are also in accordance with the cognitive and behavioral models of insomnia, suggesting significant non-genetic factors in genesis of insomnia and especially in chronic insomnia. For example, Spielman's model posits that insomnia occurs acutely in relation to both traits (predisposing factors) and life stresses (precipitating factors), and that the chronic form is maintained by maladaptive coping strategies (perpetuating factors).29,30 The genetic effects probably act mainly through the predisposing factors, but also through how an individual reacts in and copes with stress.
We used in the present paper questions assessing the main manifestations of insomnia described in the current International Classification of Sleep Disorders31 as the general criteria of insomnia, namely “a complaint of difficulty initiating sleep, difficulty maintaining sleep, or waking up too early, or sleep that is chronically non-restorative or poor in quality.” Therefore data on sleep length were not used. This is also in accordance with the proposed DSM-5 criteria for primary insomnia (code 307.42, www.dsm5.org/ProposedRevisions). As insomnia—especially when based on a self-report—must be considered as a symptom with many possible underlying causes, there certainly is some overlap in our phenotypic insomnia with other sleep disorders, e.g., insomnia related to sleep disturbed breathing or restless legs syndrome. This is the case in previous similar studies cited above, and should be kept in mind when interpreting the results. However, this is not a major limitation, as the focus has been phenotypic insomnia (self-reported symptoms regardless of their cause).
In the analysis of risk of mortality we used as covariates well established risk factors for mortality (BMI, Beck Depression Inventory score, and smoking status). We made additional analyses including two aspects of sleep behavior as covariates, of which sleep length has been shown in a meta-analysis to have a significant association with mortality,32 and there are over a dozen studies reporting excess mortality hazards among hypnotic users.33 Both these associations have also been shown in the present study population.17 There was no indication of possible effect of sleep apnea as there were only fairly minor differences in habitual snoring and weekly occurring apneas between the three clusters. However, there are only a few studies on the association between insomnia and all-cause mortality. The previous two studies have indicated no increase34 or even a decrease35 in risk of mortality. However, a very recent study showed a significant increase in risk of mortality (1.70) for nearly every-day insomnia,36 being of the same order as in the present study. The varying results may at least partly be explained by the differences in the follow-ups (6 years in those with no increase, and > 15 years in the study by Chien et al.36 and our study).
Our study has several strengths. The study population is large and the follow-up is long. The different insomnia-related symptoms have been surveyed with a comprehensive set of questions covering the main manifestations of insomnia. These questions are based on the widely used Basic Nordic Sleep Questionnaire.37 In modeling of each insomnia-related symptom, the fit of model has been good, and the heritability figures (with narrow 95% CIs) can be considered reliable. The categorizing into good, average, and poor sleepers obtained using cluster analysis and self-reports of the insomnia-related symptoms seems to give similar proportions to the more clinical approach using telephone interview and categorizing into insomnia syndrome or symptom, or good sleepers.8 Thus, our result that risk of mortality is increased in poor sleepers may have also clinical relevance, stressing the importance to identify and treat these subjects. However, some limitations in our study need to be considered. In a questionnaire-based study, it is not possible to make exact clinical diagnoses or differentiate between primary and secondary insomnia. Our sample consists of twins, but it is representative of general population, and as far as we know there are no studies indicating significant differences in sleep and its disorders between twins and general population. Also, the mortality of twins from age six until the other end of the lifespan is the same as in the overall population.38 Although our questionnaire data was surveyed in 1990 we think that the results well reflect the current situation, as there have been no major changes in sleep length and occurrence of insomnia symptoms in Finland during 1972–2005.39
In conclusion, insomnia-related symptoms are common in the working-age population in both genders. When occurring every night or almost every night, these symptoms may be associated with an increased risk of mortality, especially in those with somatic disease. This is one of the first studies to suggest a possible association between insomnia and increased risk of all-cause mortality, although the association between insomnia and mental health problems affecting risk of mortality has long been known. More prospective studies with representative populations and long follow-ups are needed. There are moderate genetic effects in phenotypic insomnia. On practical level, it is important to identify in the health care system persons with insomnia as it is associated with increased risk of depression and decrease in quality of life. Our results suggest that screening can probably be done reliably using a single question on insomnia symptom in general, as it appears to capture the essential features compared to a series of questions assessing in more detail the individual symptoms of insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Partinen has participated in clinical trials sponsored by Actelion, MSD, Nycomed-Leiras, Servier, Boehringer Ingelheim, Schwartz Pharma, and UCB and is on the advisory boards of Boehringer Ingelheim, GlaxoSmithKline, MSD, Nycomed, UCB, Somnomedics, and Vitalmed Res Center (Chief of Board). He has participated in speaking engagements for Boehringer Ingelheim, GlaxoSmithKline, Nycomed-Leiras, Servier, and UCB. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work has been performed at Department of Public Health, University of Helsinki, Helsinki, Finland, and the Finnish Institute of Occupational Health, Helsinki, Finland. This work was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics.The authors thank D.Sc. (Tech.) Matti Grohn for making Figure 1.
REFERENCES
- 1.Tafti M. Genetic aspects of normal and disturbed sleep. Sleep Med. 2009;10(Suppl 1):S17–21. doi: 10.1016/j.sleep.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–73. [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosius U, Lietzenmaier S, Wehrle R, et al. E. Heritability of sleep electroencephalogram. Biol Psychiatry. 2008;64:344–8. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Dauvilliers Y, Maret S, Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9:91–100. doi: 10.1016/j.smrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Hauri P, Olmstead E. Childhood-onset insomnia. Sleep. 1980;3:59–65. doi: 10.1093/sleep/3.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Bastien CH, Morin CM. Familial incidence of insomnia. J Sleep Res. 2000;9:49–54. doi: 10.1046/j.1365-2869.2000.00182.x. [DOI] [PubMed] [Google Scholar]
- 7.Dauvilliers Y, Morin C, Cervena K, et al. Family studies in insomnia. J Psychosom Res. 2005;58:271–8. doi: 10.1016/j.jpsychores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu-Bonneau S, LeBlanc M, Merette C, Dauvilliers Y, Morin CM. Family history of insomnia in a population-based sample. Sleep. 2007;30:1739–45. doi: 10.1093/sleep/30.12.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 10.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 11.McCarren M, Goldberg J, Ramakrishnan V, Fabsitz R. Insomnia in Vietnam era veteran twins: influence of genes and combat experience. Sleep. 1994;17:456–61. doi: 10.1093/sleep/17.5.456. [DOI] [PubMed] [Google Scholar]
- 12.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 13.Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the Older Finnish Twin Cohort. Twin Research. 2002;5:358–65. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 14.Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28:241–54. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.Varjonen J, Romanov K, Kaprio J, Heikkila K, Koskenvuo M. Self-rated depression in 12,063 middle-aged adults. Nord J Psychiatry. 1997;51:331–8. [Google Scholar]
- 17.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermunt JK, Magidson J. Latent gold 4.0 user's guide. Belmont, MA: Statistical Innovations Inc; 2005. [Google Scholar]
- 19.Dunn KM, Jordan K, Croft PR. Characterizing the course of low back pain: a latent class analysis. Am J Epidemiol. 2006;163:754–61. doi: 10.1093/aje/kwj100. [DOI] [PubMed] [Google Scholar]
- 20.Markkula R, Jarvinen P, Leino-Arjas P, Koskenvuo M, Kalso E, Kaprio J. Clustering of symptoms associated with fibromyalgia in a Finnish Twin Cohort. Eur J Pain. 2009;13:744–50. doi: 10.1016/j.ejpain.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Posthuma D, Beem AL, de Geus EJ, et al. Theory and practice in quantitative genetics. Twin Res. 2003;6:361–76. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- 22.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic; 1992. [Google Scholar]
- 23.Neale MC, Lubke G, Aggen SH, Dolan CV. Problems with using sum scores for estimating variance components: contamination and measurement noninvariance. Twin Res Hum Genet. 2005;8:553–68. doi: 10.1375/183242705774860231. [DOI] [PubMed] [Google Scholar]
- 24.Romanov K, Varjonen J, Kaprio J, Koskenvuo M. Life events and depressiveness - the effect of adjustment for psychosocial factors, somatic health and genetic liability. Acta Psychiatr Scand. 2003;107:25–33. doi: 10.1034/j.1600-0447.2003.01419.x. [DOI] [PubMed] [Google Scholar]
- 25.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145:762–9. doi: 10.1093/aje/145.8.762. [DOI] [PubMed] [Google Scholar]
- 26.Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Elsevier Saunders; 2011. pp. 694–715. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- 28.World Health Organization. 10th ed. Geneva: WHO; 1992. The ICD-10 classification of mental and behavioral disorder: diagnostic criteria for research. [Google Scholar]
- 29.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 30.Perlis ML, Smith MT, Pigeon WR. Etiology and pathophysiology of insomnia. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. WB Saunders; 2005. [Google Scholar]
- 31.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. [Google Scholar]
- 32.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 33.Kripke DF. Do hypnotics cause death and cancer? The burden of proof. Sleep Med. 2009;10:275–6. doi: 10.1016/j.sleep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28:965–71. doi: 10.1093/sleep/28.8.965. [DOI] [PubMed] [Google Scholar]
- 35.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 36.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;(4):150–5. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 38.Christensen K, Wienke A, Skytthe A, Holm NV, Vaupel JW, Yashin AI. Cardiovascular mortality in twins and the fetal origins hypothesis. Twin Res. 2001;4:344–9. doi: 10.1375/1369052012506. [DOI] [PubMed] [Google Scholar]
- 39.Kronholm E, Partonen T, Laatikainen T, et al. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: A comparative review and re-analysis of Finnish population samples. J Sleep Res. 2008;17:54–62. doi: 10.1111/j.1365-2869.2008.00627.x. [DOI] [PubMed] [Google Scholar]