Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most common cancers in China. The lower survival rate of ESCC is attributed to late diagnosis and poor therapeutic efficacy; therefore, the identification of tumor-associated proteins as biomarkers for early diagnosis, and the discovery of novel targets for therapeutic intervention, seems very important for increasing the survival rate of ESCC. To identify tumor-associated proteins as biomarkers in ESCC, we have analyzed ESCC tissues and adjacent normal tissues by two-dimensional electrophoresis (2DE) and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis. The results showed that a total of 104 protein spots with different expression levels were found on 2DE, and 47 proteins were eventually identified by MALDI-TOF MS. Among these identified proteins, 33 proteins including keratin 17 (KRT17), biliverdin reductase B (BLVRB), proteasome activatorsubunit 1 (PSME1), manganese superoxide dismutase (MnSOD), high-mobility group box-1(HMGB1), heat shock protein 70 (HSP70), peroxiredoxin (PRDX1), keratin 13 (KRT13), and so on were overexpressed, and 14 proteins including cystatin B (CSTB), tropomyosin 2 (TPM2), annexin 1 (ANX1), transgelin (TAGLN), keratin 19 (KRT19), stratifin (SFN), and so on were down-expressed in ESCC. Biological functions of these proteins are associated with cell proliferation, cell motility, protein folding, oxidative stress, and signal transduction. In the subsequent study using immunoassay on ESCC serum samples and tissue-array slides, two representative proteins, HSP70 and HMGB1, were selected as examples for the purpose of validation. The results showed that both HSP70 and HMGB1 can induce autoantibody response in ESCC sera and have higher expression in ESCC tissues. Especially, the frequency of antibodies to HSP70 in ESCC sera was significantly higher than that in normal human sera. The preliminary results suggest that some of these identified proteins might contribute to esophageal cell differentiation and carcinogenesis, certain proteins could be used as tumor-associated antigen (TAA) biomarkers in cancer diagnosis, and further studies on these identified proteins should provide more evidence of how these proteins are involved in carcinogenesis of ESCC.
Keywords: esophageal squamous cell carcinoma (ESCC), tumor-associated proteins, biomarkers, proteomic approach, cancer autoantibody, cancer diagnosis
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is one of the leading causes of cancer in China. Its incidence varies remarkably among geographic regions.1 Among these regions, Anyang of Henan Province is one of the regions with the highest incidence, especially Linzhou city (formerly known as Linxian county,) which geologically belongs to the Taihang mountain region in northern China.1,2 ESCC has a dismal outcome, with an overall 5-year survival rate after surgery and radiation therapy of only 10–20%.3 The poor outcome of ESCC is attributed to multiple reasons including its aggressive nature, distant metastasis, and largely unknown molecular mechanism of its progression. According to the report from literatures, if surgery is preformed in the early stage of ESCC, the overall 5-year survival rate is over 90%.4 Since tumor markers that are currently utilized for the detection of ESCC in clinical practice lack the sensitivity and specificity to detect potentially curable lesions, developing an improved early detection strategy may offer the great hope to cure this malignant disease and save lives.
Esophageal cancer is composed of two main histological types, adenocarcinoma and squamous cell carcinoma of the esophagus. The incidence, prevalence and biological behavior between these two histological types are uniquely different from each other. Given such differences, future therapy options may be different for each type of esophageal cancer. At present, many tumorigenic molecules such as p16, p21, p53, Rb, cyclin D1, bcl-2, Cox 2, Annexin 1, and so on were found to be associated with ESCC.5–7 However, the molecular difference between ESCC and normal squamous mucosa is not clear. The molecular mechanism underlying tumor formation and progression is still not illuminated. It has been demonstrated that many genes and proteins work together and form a reticular network in carcinogenesis, and so far this network is still not completely understood. In this network, difference in protein expression is essential to understand the carcinogenesis. Since proteomics is an approach more similar to network than single protein research method, using this approach to study the molecular mechanism and disease pathogenesis by globally examining the different protein expressions has been successfully applied to identify tumor-associated proteins as biomarkers in various cancers.8–10 Here we report a global analysis of protein expression profiles in human ESCC tissues and adjacent normal tissues using two-dimensional electrophoresis (2DE) and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analysis. This study resulted in identification of a group of differentially expressed proteins, belonging to broad functional categories related to cell differentiation, tumor growth, transformation, apoptosis, invasion and carcinogen metabolism. These differences in protein expression represent the major alterations in physiology during ESCC development and provide insight into the molecular mechanisms underlying carcinogenesis.
MATERIALS AND METHODS
Tissue Specimens and Serum Samples
Tissue specimens used for proteomic analysis in this study were obtained from Anyang Tumor Hospital of Henan Province in China. A total of 30 human ESCC tissues and paired adjacent normal tissues were collected immediately after isolation of surgically resected tissues from patients with ESCC. Tissue samples were snap-frozen in liquid nitrogen and then kept in a −80 °C freezer before experiments. The histology for all 30 ESCC tissues and adjacent normal tissues was confirmed by two independent pathologists following fixation, embedding, sectioning and H&E staining. All tissue specimens comprised more than 80% of target cells (normal epithelial cells or cancer cells) without necrosis. Sera from 69 patients with ESCC and 76 normal human sera were obtained from the serum bank of Cancer Autoimmunity and Epidemiology Research Laboratory at UTEP (University of Texas at El Paso), which were originally provided by Dr. X.-X. Peng in Sun Yat-sen University, Guangzhou, P.R. China. This study was approved by the Institutional Review Board of Zhengzhou University and collaborating institutions.
Sample Preparation for Proteomic Analysis
Fresh frozen ESCC tissue samples (50 mg for each sample) were cut into small pieces, ground within liquid nitrogen, followed by solubilizing in lyses buffer at the ratio of 1 mg of tissue per 10 μL of lyses buffer (8 M urea, 4% CHAPS, 10 mM PMSF, 20 g/L IPG buffer, pH 3–10). The protein mixtures were vortexed vigorously for 30 min with frequent breaks of ice-incubation, then RNase A and 1% DTT were added and placed at 4 °C for 1 h, followed by centrifuging at 10 000 rpm, for 30 min at 4 °C. The supernatants were stored in aliquots at −80 °C prior to use. The protein concentration was quantified by improved Bradford protein assay system.11
2DE Analysis
Proteins were separated by following protocol described previously.10 First-dimension isoelectric focusing (1D-IEF) was performed on Ettan IPGphor Isoelectric Focusing System. The samples containing about 100 μg of cytosolic proteins were dissolved in 450 μL of rehydration buffer, consisting of 8 M urea, 2% CHAPS, 0.5% IPG Buffer and 0.28% DTT. Dry IPG strips (24 cm, pH3–10) were rehydrated for 14 h and then focusing was at 8000 V. Total voltage was 81–82 kvh. After IEF, each IPG strip was soaked for 15 min with equilibration solution (6 M urea, 50 mM Tris, 30% v/v glycerol, 2% SDS) containing 1% DTT and 2.5% iodoacetamide, and then the IPG strip was placed in contact with the top surface of SDS-PAGE and sealed with 0.5% agarose. Separation in the second dimension gel was performed in Trisglycine buffer (0.1% SDS, 25 mM Tris, 192 mM glycine), at a power setting of 2.5 W/gel for the initial 0.5 h and 18 W/gel thereafter and a temperature of 15 °C. The protein spots were visualized in gel by silver staining (analytical) and Coomassie brilliant blue staining (preparative) when electrophoresis was ended. Stained 2D gels were captured by transmission scan (LabScan). Target gels were analyzed with ImageMaster 5.0 2D platinum analysis software including spot detection, background subtraction, matching, and so on.
In-Gel Digestion
Spots from 2DE were excised from the gel and placed in centrifuge tubes. Gel particles were washed with 25 mM NH4HCO3 for 20 min and destained with 30% v/v (twice, 30 min each) acetonitrile in 100 mM NH4HCO3, until all traces of Coomassie Brilliant Blue were removed, and washed with 25 mM NH4HCO3 for 20 min again. Proteins were in-gel reduced with 10 mM DTT for 60 min at 56 °C and S-alkylated with 55 mM iodoacetamide for 30 min in the dark, both in 100 mM NH4HCO3. Gel particles were washed with 25 mM NH4HCO3 and then dried under vacuum for 30 min, rehydrated with the digestion solution (40 μg/mL of trypsin in 25 mM NH4HCO3). After incubated for 30 min at 4 °C, supernatants were replaced by 25 mM NH4HCO3, and gel particles were incubated overnight at 37 °C.
MALDI-TOF MS Analysis for Protein Identification
The samples were mixed (1:1) with a saturated matrix solution (α-cyano-4-hydroxycinnamic acid prepared in 50% acetonitrile/0.1% formic acid). All mass spectra were obtained on a 4700 Proteomics analyzer with TOF/TOF optics in the positive ion reflector mode with a mass accuracy of about 50 ppm. The MALDI tandem mass spectrometer used a 200 Hz frequency-tripled Nd:YAG laser operating at a wavelength of 355 nm. MS spectra were obtained in the mass range between 800 and 4000 Da with ca. 1000 laser shots. MS/MS spectra were acquired with 2000 laser shots using air as the collision gas. The singly charged peaks were analyzed using an interpretation method present in instrument software, where the five most intense peaks were selected and MS/MS spectra were generated automatically, excluding those from the matrix, due to trypsin autolysis peaks. Spectra were processed and analyzed by the Global Protein Server Workstation, which uses internal Mascot v2.0 software for searching the peptide mass fingerprints and MS/MS data. Searches were performed against the NCBI nonredundant protein database (updated 1 August 2007). Identification with a GPS confidence interval (CI) of greater than 95% can be accepted.
Enzyme-Linked Immunosorbent Assay (ELISA)
Purified recombinant HSP70 and HMGB1 were commercially purchased (Abcam, Cambridge, MA). The protein purity was >95% by SDS-PAGE. Proteins were diluted in PBS to a final concentration of 0.5 μg/mL for coating polystyrene 96-well microtiter plates (Dynatech Laboratories, Alexandria, VA). A volume of 200 μL human serum samples at 1:200 dilution was added to the antigen-coated wells and incubated for 1.5 h at room temperature. Horseradish peroxidase-conjugated goat antihu-man IgG (Caltag Laboratories, San Francisco, CA) at 1:5000 dilution and the substrate 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (Boehringer Mannheim GmbH, Mannheim, Germany) were used as detecting reagents. Each sample was tested in duplicate, and the average OD at 405 nm was used for data analysis. The cutoff value designating positive reaction was the mean optical density (OD) of 76 normal human sera plus 3 standard deviations (SD). The detailed protocol of ELISA was used as described in our previous study.12
Immunohistochemistry (IHC) with Tissue Array
Superfrost plus tissue slides that contain 64 paraffin-embedded esophagus cancer tissue specimens and 3 normal esophagus tissue specimens were purchased commercially (Cybridi, Bethesda, MD) and used for HSP70 and HMGB1 antigen detection. Antigen retrieval was performed by microwave-heating in a citrate-based antigen retrieval solution (BioGenex, San Ramon, CA) according to the manufacturer's recommendation. Nonspecific protein binding sites were blocked by 1.5% normal horse sera for 30 min in a humidifier. Tissue sections were incubated with 1:500 diluted rabbit IgG polyclonal antibody (original concentration 6 μg/mL). Biotinylated secondary antibody and an ABC (Avidin–Biotinylated enzyme Complex) kit, and DAB (3,3′-diaminobenzi- dine) substrate kit were used as detecting reagents according to the manufacturer's recommendation (Vector Laboratories, Burlingame, CA). The slides were counter-stained with hematoxylin, fixed by Scott's solution, and dehydrolyzed with different concentrations of EtoH and Citrisolvent. Finally, the slides were mounted with permount mounting medium and observed under a microscope. All IHC results were read blindly by two independent researchers and further confirmed by a pathologist. A 4-level scoring system (–, negative; +, low expression level; ++, moderate expression level; +++, high expression level) was used to evaluate the staining intensity. Polyclonal antibodies to Hsp70 and HMGB1 were commercially purchased (Sigma, St Louis, MO).
Statistical Analysis
To determine whether the frequencies of autoantibody to TAAs in each cohort of patients' sera were significantly higher than that in sera from normal individuals and other controls, the frequencies of antibody were compared using the Chi-squared (χ2) test with Yate's correction, and two significant levels (0.05 and 0.01) were used.
RESULTS AND DISCUSSION
Identification of ESCC-Associated Proteins by MALDI-TOF MS
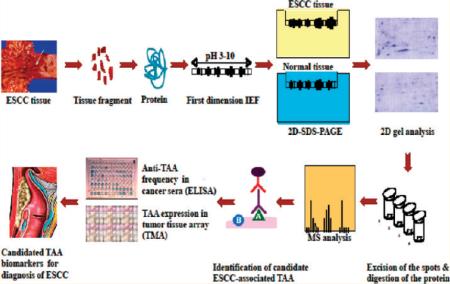
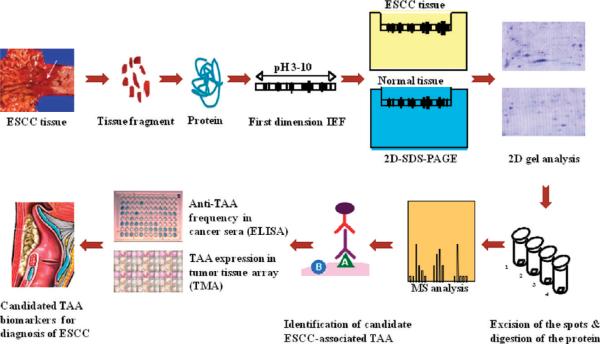
A brief description of the approach we have used to identify and characterize protein biomarkers in ESCC is shown in Figure 1. Briefly, proteins extracted from ESCC and adjacent tumor tissues were applied onto the first dimension isoelectro-focusing gel (1D-IEF) and subsequently loaded onto the second-dimension SDS-PAGE gel (2D-SDS-PAGE). The gel was visualized by silver staining or Coomassie blue staining. After comparing the gel from tumor tissues with the gel from control tissues, a number of protein spots of interest were excised from the 2D gels, digested by trypsin, and subsequently analyzed by MALDI-TOF MS. In subsequent studies, we have used two approaches (ELISA screening, tissue arrays) to comprehensively characterize and validate the identified tumor-associated proteins that are potentially useful for the early detection of ESCC and also for further developing “tumor-associated protein array” systems for cancer diagnosis, prediction, and for following the response of patients to treatment.
Figure 1.
Schematic representation of tumor-associated protein biomarker identification in ESCC. In brief, proteins extracted from ESCC tissues and adjacent normal tissues were applied onto the first dimension gel (isoelectrofocusing gel) and subsequently loaded onto the second-dimension SDS-PAGE gel (2D-SDS-PAGE). The gel was visualized by silver staining or Coomassie blue staining. After comparing the gel between ESCC tissues and normal tissues, a number of protein spots of interest were excised from the 2D gels, digested by trypsin, and analyzed by MALDI-TOF MS. In subsequent studies, we characterize the identified tumor-associated proteins that are potentially useful for the early detection of cancer, and then evaluate the sensitivity and specificity of different tumor markers in cancer for further developing “Tumor-Associated Protein Array” systems for cancer diagnosis and prediction and for following the response of patients to treatment.
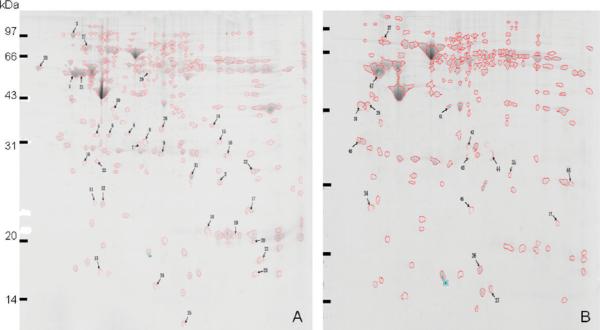
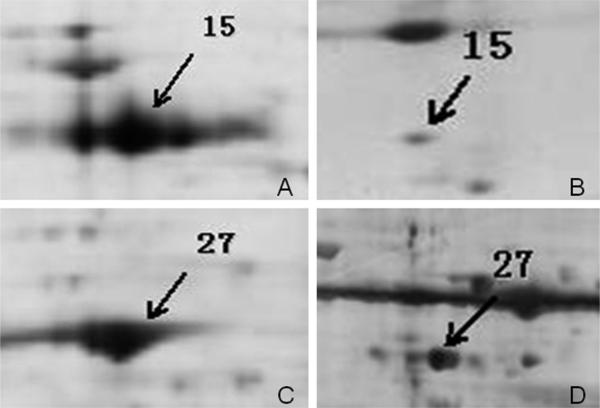
In the present study, a proteome-based approach was applied to identify potential cancer-associated protein biomarkers in ESCC. To obtain a global view of the proteins in ESCC tissues, proteins extracted from ESCC tissues and adjacent normal tissues were separated by 2DE, and subsequently analyzed by MALDI-TOF MS. We have compared the complexion of protein expression between ESCC tissues and adjacent normal tissues. As shown in Figure 2, a total of 104 protein spots with different expression levels were found on 2DE. Of the 104 protein spots, 47 proteins were eventually identified by MALDI-TOF-MS. Among these identified proteins, 33 proteins (shown in Table 1, spot #1 −33) such as keratin 17 (KRT17), biliverdin reductase B (BLVRB), proteasome activator subunit 1 (PSME1), manganese superoxide dismutase (MnSOD), high-mobility group box-1-(HMGB1), heat shock protein 70 (HSP70), peroxiredoxin (PRDX1), keratin 13 (KRT13), and so on were overexpressed, and 14 proteins (shown in Table 1, spot #34–47) such as cystatin B (CSTB), tropomyosin 2 (TPM2), annexin 1 (ANX1), transgelin (TAGLN), keratin 19 (KRT19), stratifin (SFN), and so on were down-expressed in ESCC. To clearly demonstrate the difference of differentially expressed proteins between ESCC and normal tissues, two representative protein spots were picked up from 2DE (see Figure 2) and amplified with a larger magnification (see Figure 3). These two representative proteins (spot #15 and #27) have much higher expression in ESCC tissues than in normal tissues.
Figure 2.
Total proteins from ESCC tissues and adjacent normal tissues were analyzed by 2DE. The proteins were separated by IEF (pH 3–10) and 15% SDS-PAGE and subsequently processed with Coomassie blue staining. The MALDI-TOF-MS analysis for the protein spots numbered on the gel is listed in Table 1. (A) Total proteins from ESCC tissues that were analyzed by 2DE; (B) total proteins from adjacent normal tissues that were analyzed by 2DE.
Table 1.
Proteins Identified in ESCC by MALDI-TOF MS
| spot no. | accession no. | identified proteins | score | no. of peptides | protein functions |
|---|---|---|---|---|---|
| 1 | gi|48735384 | Keratin 17 (KRT17) | 789 | 34 | Structural and binding protein |
| 2 | gi|32891807 | Biliverdin reductase B (BLVRB) | 236 | 9 | Converts biliverdin to bilirubin |
| 3 | gi|6470150 | Bip protein | 879 | 30 | Energy protein |
| 4 | gi|5453990 | Proteasome activator subunit 1 (PSME1) | 457 | 17 | Protein process |
| 5 | gi|4337097 | Chloride intracellular channel 1 (CLIC1) | 750 | 18 | Channel protein |
| 6 | gi|49456275 | Proteasome activator subunit 2 (PSME2) | 267 | 14 | Protein process |
| 7 | gi|21669669 | Immunoglobulin lambda light chain VLJ region | 471 | 8 | Antigen binding |
| 8 | gi|55925946 | Glutathione S-transferase omega 1 | 326 | 11 | Cell death/defense |
| 9 | gi|10835794 | Chain C, crystal structure of the Fab fragment of a human monoclonal IgM cold agglutinin | 264 | 10 | Antigen binding |
| 10 | gi|493940 | Carbonic anhydrase II complexed with the inhibitor Mts | 760 | 22 | Catalyzes reversible hydration of carbon dioxide |
| 11 | gi|15030212 | Glyoxalase 1 | 160 | 12 | Metabolism |
| 12 | gi|18204192 | Pituitary adenylate cyclase activating polypeptide (PACAP) | 516 | 10 | Stimulates adenylate cyclase and subsequently increases the cAMP level in target cells |
| 13 | gi|14249348 | Thioredoxin-like 5 | 276 | 6 | Activation of transcription factor |
| 14 | gi|7546412 | Manganese superoxide dismutase (Mn-SOD) | 594 | 12 | Detoxification |
| 15 | gi|55958715 | High mobility group box 1 (HMGB1) | 133 | 8 | Transcription/translation |
| 16 | gi|21669469 | Immunoglobulin kappa light chain VLJ region | 380 | 7 | Antigen binding |
| 17 | gi|15147369 | Cofilin 1 (nonmuscle) | 175 | 11 | Actin-depolymerizing factor |
| 18 | gi|2981750 | Chain F, secypa complexed with hagpia | 453 | 11 | Recognizing and binding site |
| 19 | gi|27923517 | Histone 3 | 106 | 6 | Involves with the structure of the nucleosomes of the `beads on a string' |
| 20 | gi|45767717 | Histone cluster 1, H2bm | 174 | 13 | DNA binding |
| 21 | gi|47077229 | Unknown protein | 147 | 13 | Unknown |
| 22 | gi|30584265 | Profilin 1 | 401 | 12 | Ubiquitous actin monomer-binding |
| 23 | gi|55662004 | FK506 binding protein 1A (FKBP1A) | 75 | 4 | Binding protein |
| 24 | gi|34616 | Beta-2 microglobulin | 96 | 5 | Major histocompatibility complex antigen |
| 25 | gi|1805696 | Polyubiquitin | 323 | 10 | Regulates many processes |
| 26 | gi|5902072 | Serine (or cysteine) proteinase inhibitor | 606 | 17 | Neutrophil elastase inhibitor |
| 27 | gi|109112231 | Heat shock protein 70 (HSP70) | 1110 | 32 | Protein folding |
| 28 | gi|62897681 | Calreticulin precursor variant | 563 | 16 | Protein folding |
| 29 | gi|6598323 | GDP dissociation inhibitor 2 | 566 | 23 | Regulate the GDP-GTP exchange reaction of members |
| 30 | gi|62898013 | F-actin capping protein alpha-1 subunit variant | 555 | 17 | Structural protein |
| 31 | gi|2780819 | Manganese-containing superoxide dismutase | 779 | 12 | Detoxification |
| 32 | gi|55959887 | Peroxiredoxin 1 (PRDX1) | 632 | 14 | Cell death/defense |
| 33 | gi|30377 | Keratin 13 (KRT13) | 96 | 6 | Intermediate filament |
| 34 | gi|55662177 | Translationally controlled tumor protein (TCTP) | 123 | 5 | A histamine-releasing factor, calcium binding protein |
| 35 | gi|1633065 | Carbonic anhydrase II mutant E117q, holo form | 178 | 9 | Reversible hydration of carbon dioxide |
| 36 | gi|179032 | Smooth muscle 22 (SM22) | 230 | 11 | A calponin-related protein |
| 37 | gi|7263012 | Cystatin B (CSTB) | 455 | 6 | Cysteine protease inhibitor |
| 38 | gi|19353393 | Tropomyosin 2 (TPM2) | 165 | 16 | Interact with Ras-related associated with diabetes, PDZ and LIM domain 7 and TPM1 |
| 39 | gi|55626262 | PREDICTED: similar to H2A histone family, member E | 204 | 6 | Involved with the structure of the nucleosomes of the `beads on a string' structure |
| 40 | gi|60835714 | Stratifin (SFN) | 191 | 8 | Stimulate the expression of matrix metallopeptidase 1 in fibroblasts |
| 41 | gi|442631 | Annexin 1 (ANX1) | 568 | 19 | Calcium ion binding |
| 42 | gi|20380766 | Carbonic anhydrase 1 (CA1) | 111 | 9 | Metalloenzyme |
| 43 | gi|13937950 | Triosephosphate isomerase 1 (TPI1) | 449 | 19 | Glycolytic enzyme |
| 44 | gi|1065006 | Unknown protein | 443 | 18 | Unknown |
| 45 | gi|62897565 | Transgelin variant (TAGLN) | 145 | 9 | Actin stress fibers |
| 46 | gi|2981750 | Chain F, secypa complexed with hagpia | 554 | 10 | Recognizing and binding site |
| 47 | gi|34783124 | Keratin 19 (KRT19) | 782 | 30 | Structural protein |
Figure 3.
Two representative protein spots were picked up from 2DE (see Figure 2), and amplified with a larger magnification. (A and C) Two representative proteins HMGB1 and HSP70 (#15 and #27) were analyzed on 2DE with ESCC tissues; (B and D) two representative proteins HMGB1 and HSP70 were analyzed on 2DE with normal tissues. These two representative proteins have much higher expression in ESCC tissues than in normal tissues.
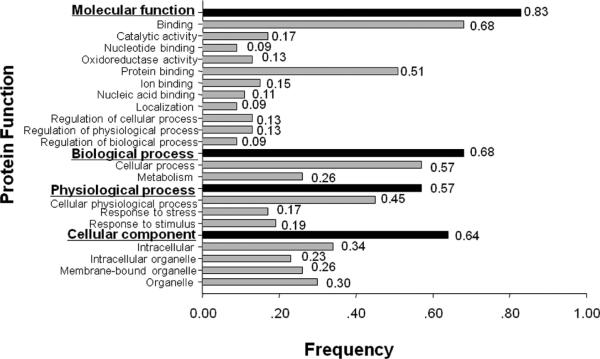
Functional Categorization and Cancer Association
The molecular and cellular functions of these identified proteins have been documented in the literature, and some of these proteins were reported relating to cell proliferation, cell motility, protein folding, oxidative stress and signal transduction. In order to understand the function of these identified proteins, the gene ontology (GO) analysis using Goblet algorithm and searching sequences against TrEMBL and Swiss-Prot databases (http://www.expasy.ch/sprot/) was carried out. As shown in Figure 4, the 47 identified proteins were categorized in four groups including molecular function (83%, 39/47), biological process (68%, 32/47), physiological process (57%, 27/47), and cellular component (64%, 30/47). Proteins in each group were further categorized in different subgroups. For example, proteins in the group of molecular function were divided into 11 subgroups such as catalytic activity, nucleotide binding, oxidoreductase activity, and so on, and proteins in the group of physiological process were divided into only three subgroups such as cellular physiological process, response to stress, and response to stimulus. For further exploring the cancer association of these identified proteins, a literature search was conducted with PubMed (http://www.ncbi.nlm.nih.gov/pubmed/). On the basis of the literature search, 33 of the 47 identified proteins have been reported clearly relating to cancer. For example, several proteins such as glutathione S-transferase omega,13 glyoxalase 1,14,15 HMGB1,16,17 profilin 1,18,19 HSP70,20,21 PRDX1,22,23 annexin 1,24,25 transgelin26 and keratin 1927,28 were reported relating to breast cancer; keratin 17 was involved in tumor angiogenesis;29 Biliverdin reductase B (BVRB) wasx over-expressed in liver cancer;30 smooth muscle protein 22 was reported relating to gastric cancer and renal cell carcinoma;31,32 cystatin B was characterized as either a tissue and urinary biomarker in bladder cancer33 or serological marker in liver cancer,34 and the reduced expression of stratifin may use as a marker for prognosis of ESCC.35 More recently, the overexpression of PRDX1 was also reported in prostate cancer cell lines compared with nontumor cells.36 While analyzing the difference of differentially expressed proteins between ESCC and normal tissues, it was found that two proteins HMGB1 and HSP70 (spot #15 and #27) have much higher expression in ESCC tissues compared with normal tissues (Figure 3). In the subsequent study, we mainly focus on these two representative proteins HSP70 and HMGB1 to further evaluate whether these two proteins can be used as TAA biomarkers in immunodiagnosis of ESCC. As described above that many of other identified proteins such as profilin 1, ANX1, PRDX1, and KRT19 are also related to cancer, further study to determine which, if any, might be authentic TAA in ESCC is still underway.
Figure 4.
Functional categorization of identified proteins. Forty-seven identified proteins were functionally categorized in four groups based on gene ontology (GO) annotation terms and searching sequences against the TrEMBL and Swiss-Prot databases. Four functional groups include molecular function, biological process, physiological process and cellular component. Proteins in each group were further categorized in different subgroups.
HSP70 and HMGB1 and Their Association with Cancer
HSP70 plays a central role in the processing of cytosolic and secretory proteins, and it is involved in cell proliferation, differentiation and tumorigenesis.37 Many studies have demonstrated that HSP70 is a cancer-relevant survival protein. The overexpression of HSP70 correlates with increased cell proliferation, poor differentiation, lymph node metastases and poor therapeutic outcome in human breast cancer.38–40 Cancer cells depleted of HSP70 can display strikingly different morphologies (detached and round vs flat senescent-like), cell cycle distributions (G2/M vs G1 arrest), and gene expression profiles.41 HSP70 may play an important role in tumorigenesis through its antiapoptotic activity and also via its role as cochaperon for HSP90.42 More recently, several studies have been performed to detect autoantibody against HSP70 in different types of cancer such as esophageal,43 liver10 and nasopharyngeal cancer44 as well as head and neck cancer.45 HMGB1 is a highly conserved nuclear protein, acting as a chromatin-binding factor that bends DNA and promoters access to transcriptional protein assemblies on specific DNA targets.46–50 In addition to its nuclear role, HMGB1 also functions as an extracellular signaling molecule during inflammation, cell differentiation, cell migration, and tumor metastasis.46–50 HMGB1 has been implicated in some disease states such as rheumatic arthritis,51 systemic lupus erythematosus (SLE)52,53 and especially in cancer.16,17,54,55 Overexpression of HMGB1 has been observed in many types of cancer, including breast, colon and lung cancer.16,54,55 HMGB1 also interacts with and enhances the activities of a group of transcription factors implicated in cancer development, including p53, p73, the retinoblastoma protein (Rb), and so on.50,56
Prevalence of Autoantibodies to HSP70 and HMGB1 in ESCC
Previous studies have demonstrated that cancer sera contain antibodies which react with a unique group of autologous cellular antigens called tumor-associated antigens (TAAs), which was closely related to the cancer development.57–60 Since ESCC is one of the most common cancers worldwide, especially in China, how to establish a methodology to identify the high-risk individuals for ESCC remains to be investigated. Most studies indicated that cancer has long been recognized as a multistep process that involves not only genetic changes conferring growth advantage but also factors that disrupt regulation of growth and differentiation. It is possible that some of these factors could be identified and their functions evaluated with the aid of autoantibodies against TAAs arising during tumorigenesis. Building on our extensive experience characterizing TAAs as biomarkers in cancer, it is necessary to determine which of these identified proteins, if any, might be authentic TAAs. For this purpose, we need to determine the frequency of antibodies to the identified ESCC-related proteins in patients with ESCC and controls. This analysis is essential to establish the sensitivity, specificity and positive predictive value of individual antibody–antigen systems as biomarkers for the detection of ESCC.
As described above, the cancer association of these two identified proteins HSP70 and HMGB1 has been extensively investigated by other groups. At the present study, these two proteins were commercially purchased and further used as coating antigens in ELISA for the detection of autoantibodies against these two proteins in sera from patients with ESCC and normal individuals. Table 2 shows the frequency of antibodies to HSP70 and HMGB1 in ESCC sera and normal human sera (NHS). The cutoff value designating positive reaction was established as the mean OD of 76 normal human sera (NHS) plus 3 standard deviations (SD). Of the 69 sera with ESCC analyzed, 27 (39.1%) were reactive with HSP70, only 1 (1.3%) was positive in normal human sera. Statistical analysis indicated that there was a significant difference (p < 0.01) of anti-HSP70 antibody frequency between ESCC and NHS. For anti-HMGB1 antibody, 5 (7.2%) were positive in ESCC, and 1 (1.3%) in NHS. There was no statistical difference between ESCC and NHS (p > 0.05). This preliminary data suggests that HSP70 may have potential possibility in use as marker in ESCC, which is consistent with results reported from other groups. As described above, autoantibody to HSP70 has been detected in many types of cancer, and autoantibody to HMGB1 in cancer has not been reported yet. In one of our recent studies, we have identified and characterized autoantibody to HSP70 in hepatocellular carcinoma (HCC) as a potential biomarker.10 Whether HMGB1 and anti-HMGB1 can be also used as a serological biomarker in cancer such as ESCC or HCC remains to be investigated.
Table 2.
Frequency of Autoantibody Responses to Two Representative Proteins HSP70 and HMGB1 in ELISA
| autoantibodies to |
|||
|---|---|---|---|
| serum samples | number of sera tested | HSP70 number of positive sera (%)a | HMGB1 number of positive sera (%) |
| ESCC | 69 | 27 (39.1)* | 5 (7.2) |
| normal controls | 76 | 1 (1.3) | 1 (1.3) |
Cutoff value, mean +3SD of 76 normal controls.
p-values relative to normal controls, p < 0.01.
Expression of HSP70 and HMGB1 in ESCC Tissues by Immunohistochemistry with Tissue Array
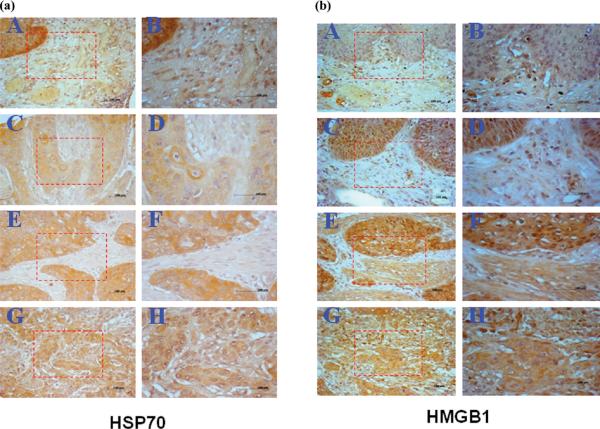
To further validate these two representative proteins as markers in ESCC, it is essential to examine their expression in ESCC specimens by immunohistochemistry (IHC). In this study, the expression profiles of HSP70 and HMGB1 in ESCC tissues were examined by IHC with tissue array slides. ESCC tissue array slides including 64 ESCC tissues and 3 adjacent normal esophageal tissues were commercially available for this study. The concentration of polyclonal anti-HSP70 and anti-HMGB1 used for immunostaining the tissue specimens were determined by initial IHC study on tumor tissue slides, and subsequently, the tissue-array slides were stained with polyclonal anti-HSP70 and anti-HMGB1, respectively. The results showed that 61 of 64 (95.3%) ESCC tissues were stained positive with anti-HSP70, and there was no positive in normal esophageal tissues. HSP70 was overexpressed in cytoplasm of invasive lesions of advanced esophageal cancer. The frequency of HSP70 positive staining in ESCC grade I, II and III were 87.5% (14/16), 96.4% (27/28), and 100% (20/20), respectively. In the IHC study with HMGB1, 63 of 64 ESCC tissues were positive with anti-HMGB1 antibody, and no normal esophageal tissue was positive. The frequency of HMGB1 positive staining in ESCC grade I, II and III were 100% (16/16), 100% (28/28), and 95% (19/20), respectively. Due to the small sample size of tissues with different grades in this tissue array slide, it is difficult to establish a statistical association between either HSP70 or HMGB1 and tumor grade. In this IHC study, we have also used a 4-level scoring system to evaluate the staining intensity. The frequency of HSP70 expression level (negative, lower, moderate and higher) in ESCC tissues was 4.7% (3/64), 12.5% (8/64), 45.3% (29/64) and 37.5% (24/64), respectively. Compared to HSP70, the frequency of HMGB1 expression level was 1.6% (1/64), 40.6 (26/64), 45.3% (29/64) and 12.5% (8/64), respectively. Of the interesting notion was that there was a significant difference of higher expression levels between HSP70 and HMGB1. This might be one of reasons why HSP70 could induce strong autoantibody response in ESCC patients compared to HMGB1. Figure 5a and B showed the representative positive and negative immunostaining patterns of HSP70 and HMGB1 in ESCC tissues and normal esophageal tissue. Because of lack of data regarding different clinical stages of ESCC tissues in these commercial tissue array slides, it was not able for us to establish a statistical correlation between HSP70 or HMGB1 expression and clinical stages in this study. In summary, our study demonstrates that both HSP70 and HMGB1 have significant higher immunogenicity in ESCC tissues compared with normal tissues. Whether HSP70 and HMGB1 can be used as diagnostic markers in ESCC detection, and the underlining mechanism of how these proteins induce humoral immune response in ESCC patients remains to be investigated. Since many of other identified proteins in the current study are also related to cancer based on literature report, further studies with a comprehensive analysis on these identified proteins may provide more insight into how these proteins are involved in the tumorigenesis of ESCC.
Figure 5.
(a) Expression of HSP70 in ESCC tissues examined by IHC. Tissue-array slide was stained with polyclonal anti-HSP70 antibody at a 1:500 dilution. A: A representative normal esophagus tissue was negatively stained with anti-HSP70 antibody; C, E and G: Representative ESCC grade I, II and III tissues were positively stained with anti-HSP70 antibody (magnification,×100). B, D, F and H: The corresponding area (rectangle) of A, C, E and G was enlarged (magnification, ×200). (b) Expression of HMGB1 in ESCC tissues examined by IHC. Tissue-array slide was stained with polyclonal anti-HMGB1 antibody at a 1:500 dilution. A: A representative normal esophagus tissue was negatively stained with anti-HMGB1 antibody; C, E and G: Representative ESCC grade I, II and III tissues were positively stained with anti-HMGB1 antibody (magnification, ×100). B, D, F and H: The corresponding area (rectangle) of A, C, E and G was enlarged (magnification, ×200).
CONCLUSIONS
In the present study, we have used a proteomic approach to analyze ESCC tissues and adjacent normal tissues to identify tumor-associated proteins as markers in ESCC. Of 104 protein spots with different expression levels found on 2DE, 47 proteins were eventually identified by MALDI-TOF MS. Among these identified proteins, 33 proteins including HSP70, HMGB1, PSME1, MnSOD, PRDX1, KRT17 and KRT13, and so on were overexpressed, and 14 proteins including CSTB, TPM2, ANX1, TAGLN, KRT19, and SFN, and so on were down-expressed in ESCC. Biological functions of these proteins are associated with cell proliferation, cell motility, protein folding, oxidative stress and signal transduction. In the subsequent study, two representative proteins, HSP70 and HMGB1, were used as examples for the validation purpose. Our results have indicated that both of HSP70 and HMGB1 can induce autoantibody response in ESCC and have a higher expression in ESCC tissues. The preliminary data suggest that some of the identified proteins might contribute to esophageal cancer cell differentiation and carcinogenesis, certain proteins could be used as TAA cancer markers, and further studies on these identified proteins should provide more evidence how these proteins are involved in carcinogenesis of esophageal cancer.
ACKNOWLEDGMENT
We thank Dr. Ping Wang for assistance with some of the proteomic techniques in this study. Tissue specimens used in this study were obtained from Tumor Hospital of Anyang, Henan, P. R. China, and serum samples were initially provided by Dr. Xuan-Xian Peng in Sun Yat-sen University, Guangzhou, P.R. China. We also thank the Biomolecule Analysis Core Facility and Analytical Cytology Core Facility at University of Texas at El Paso (UTEP) for their support. This work was supported by grants from China Natural Science Foundation (#30572115, #30872962), and also by grants from US-NIH (#2S06GM008012, #5G12RR008124).
REFERENCES
- (1).Li JY. Epidemiology of esophageal cancer in China. J. Natl. Cancer Inst. Monogr. 1982;62:113–120. [PubMed] [Google Scholar]
- (2).Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson L,L, Gail MH, Dong ZW, Yu B, Mark SD, Taylor PR. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J. Natl. Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kim T, Grobmyer SR, Smith R, Ben-David K, Ang D, Vogel SB, Hochwald SN. Esophageal cancer-the five year survivors. J.Surg. Oncol. 2011;103:179–183. doi: 10.1002/jso.21784. [DOI] [PubMed] [Google Scholar]
- (4).Law S, Wong J. The current management of esophageal cancer. Adv. Surg. 2007;41:93–119. doi: 10.1016/j.yasu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- (5).Kwong KF. Molecular biology of esophageal cancer in the genomics era. Surg. Clin. North Am. 2005;85:539–553. doi: 10.1016/j.suc.2005.01.004. [DOI] [PubMed] [Google Scholar]
- (6).McCabe ML, Dlamini Z. The molecular mechanisms of oesophageal cancer. Int. Immunopharmacol. 2005;5:1113–1130. doi: 10.1016/j.intimp.2004.11.017. [DOI] [PubMed] [Google Scholar]
- (7).Lin J, Beerm DG. Molecular biology of upper gastrointestinal malignancies. Semin. Oncol. 2004;31:476–486. doi: 10.1053/j.seminoncol.2004.04.019. [DOI] [PubMed] [Google Scholar]
- (8).Le Naour F, Brichory F, Misek DE, Bréchot C, Hanash SM, Beretta L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol. Cell. Proteomics. 2002;1:197–203. doi: 10.1074/mcp.m100029-mcp200. [DOI] [PubMed] [Google Scholar]
- (9).Wang KJ, Wang RT, Zhang JZ. Identification of tumor markers using two-dimensi- onal electrophoresis in gastric carcinoma. World J. Gastroenterol. 2004;10:2179–2183. doi: 10.3748/wjg.v10.i15.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Looi KS, Nakayasu ES, Diaz RA, Tan EM, Almeida IC, Zhang JY. Using proteomic approach to identify tumor-associated antigens as markers in hepatocellular carcinoma. J. Proteome Res. 2008;7:4004–4012. doi: 10.1021/pr800273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- (12).Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EKL, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol. Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- (13).Chariyalertsak S, Purisa W, Sangrajrang S. Role of glutathione S-transferase omega gene polymorphisms in breast-cancer risk. Tumori. 2009;95:739–743. doi: 10.1177/030089160909500617. [DOI] [PubMed] [Google Scholar]
- (14).Yiu CC, Sasano H, Ono K, Chow LW. Changes in protein expression after neoadjuvant use of aromatase inhibitors in primary breast cancer: a proteomic approach to search for potential biomarkers to predict response or resistance. Expert Opin. Investig. Drugs. 2010;19(Suppl 1):S79–89. doi: 10.1517/13543781003701011. [DOI] [PubMed] [Google Scholar]
- (15).Germanová A, Germanová A, Tesarová P, Jáchymová M, Zvára K, Zima T, Kalousová M. Glyoxalase I Glu111Ala polymorphism in patients with breast cancer. Cancer Invest. 2009;27:655–660. doi: 10.1080/07357900802350822. [DOI] [PubMed] [Google Scholar]
- (16).Flohr AM, Rogalla P, Meiboom M, Borrmann L, Krohn M, Thode-Halle B, Bullerdiek J. Variation of HMGB1 expression in breast cancer. Anticancer Res. 2001;21:3881–3885. [PubMed] [Google Scholar]
- (17).Gong H, Zuliani P, Komuravelli A, Faeder JR, Clarke EM. Analysis and verification of the HMGB1 signaling pathway. BMC Bioinform. 2010;11(Suppl 7):S10. doi: 10.1186/1471-2105-11-S7-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zou L, Hazan R, Roy P. Profilin-1 overexpression restores adherens junctions in MDA-MB-231 breast cancer cells in R-cadherin-dependent manner. Cell Motil. Cytoskeleton. 2009;66:1048–1056. doi: 10.1002/cm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zou L, Ding Z, Roy P. Profilin-1 overexpression inhibits proliferation of MDA-MB-231 breast cancer cells partly through p27kip1 upregulation. J. Cell Physiol. 2010;223:623–629. doi: 10.1002/jcp.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? J. Cell. Mol. Med. 2005;9:208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Karihtala P, Mäntyniemi A, Kang SW, Kinnula VL, Soini Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res. 2003;9:3418–3424. [PubMed] [Google Scholar]
- (23).Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J. Exp. Clin. Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Nair S, Hande MP, Lim LH. Annexin-1 protects MCF7 breast cancer cells against heat-induced growth arrest and DNA damage. Cancer Lett. 2010;294:111–117. doi: 10.1016/j.canlet.2010.01.026. [DOI] [PubMed] [Google Scholar]
- (25).Ang EZ, Nguyen HT, Sim HL, Putti TC, Lim LH. Annexin-1 regulates growth arrest induced by high levels of estrogen in MCF-7 breast cancer cells. Mol. Cancer Res. 2009;7:266–274. doi: 10.1158/1541-7786.MCR-08-0147. [DOI] [PubMed] [Google Scholar]
- (26).Xu SG, Yan PJ, Shao ZM. Differential proteomic analysis of a highly metastatic variant of human breast cancer cells using two-dimensional differential gel electrophoresis. J. Cancer Res. Clin. Oncol. 2010;136:1545–1556. doi: 10.1007/s00432-010-0812-0. [DOI] [PubMed] [Google Scholar]
- (27).Tjensvoll K, Oltedal S, Farmen RK, Shammas FV, Heikkilä R, Kvaløy JT, Gilje B, Smaaland R, Nordgård O. Disseminated tumor cells in bone marrow assessed by TWIST1, cytokeratin 19, and mammaglobin A mRNA predict clinical outcome in operable breast cancer patients. Clin. Breast Cancer. 2010;10:378–384. doi: 10.3816/CBC.2010.n.050. [DOI] [PubMed] [Google Scholar]
- (28).Bateman NW, Sun M, Hood BL, Flint MS, Conrads TP. Defining central themes in breast cancer biology by differential proteomics: conserved regulation of cell spreading and focal adhesion kinase. J. Proteome Res. 2010;9:5311–5324. doi: 10.1021/pr100580e. [DOI] [PubMed] [Google Scholar]
- (29).Xu Y, Zhang SZ, Huang CH, Liu XY, Zhong ZH, Hou WL, Su ZF, Wei YQ. Keratin 17 identified by proteomic analysis may be involved in tumor angiogenesis. BMB Rep. 2009;42:344–349. doi: 10.5483/bmbrep.2009.42.6.344. [DOI] [PubMed] [Google Scholar]
- (30).Melle C, Ernst G, Scheibner O, Kaufmann R, Schimmel B, Bleul A, Settmacher U, Hommann M, Claussen U, von Eggeling F. Identification of specific protein markers in microdissected hepatocellular carcinoma. J. Proteome Res. 2007;6:306–315. doi: 10.1021/pr060439b. [DOI] [PubMed] [Google Scholar]
- (31).Li N, Zhang J, Liang Y, Shao J, Peng F, Sun M, Xu N, Li X, Wang R, Liu S, Lu Y. A controversial tumor marker: is SM22 a proper biomarker for gastric cancer cells? J. Proteome Res. 2007;6:3304–3312. doi: 10.1021/pr0702363. [DOI] [PubMed] [Google Scholar]
- (32).Klade CS, Voss T, Krystek E, Ahorn H, Zatloukal K, Pummer K, Adolf GR. Identification of tumor antigens in renal cell carcinoma by serological proteome analysis. Proteomics. 2001;1:890–898. doi: 10.1002/1615-9861(200107)1:7<890::AID-PROT890>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- (33).Feldman AS, Banyard J, Wu CL, McDougal WS, Zetter BR. Cystatin B as a tissue and urinary biomarker of bladder cancer recurrence and disease progression. Clin. Cancer Res. 2009;15:1024–1031. doi: 10.1158/1078-0432.CCR-08-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lee MJ, Yu GR, Park SH, Cho BH, Ahn JS, Park HJ, Song EY, Kim DG. Identification of cystatin B as a potential serum marker in hepatocellular carcinoma. Clin. Cancer Res. 2008;14:1080–1089. doi: 10.1158/1078-0432.CCR-07-1615. [DOI] [PubMed] [Google Scholar]
- (35).Ren HZ, Pan GQ, Wang JS, Wen JF, Wang KS, Luo GQ, Shan XZ. Reduced stratifin expression can serve as an independent prognostic factor for poor survival in patients with esophageal squamous cell carcinoma. Dig. Dis. Sci. 2010;55:2552–2560. doi: 10.1007/s10620-009-1065-0. [DOI] [PubMed] [Google Scholar]
- (36).Basu A, Banerjee H, Rojas H, Martinez SR, Roy S, Jia Z, Lilly MB, De León M, Casiano CA. Differential expression of peroxiredoxins in prostate cancer: Consistent upregulation of PRDX3 and PRDX4. Prostate. 2010 doi: 10.1002/pros.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Cecconi D, Astner H, Donadelli M, Palmieri M, Missiaglia E, Hamdan M, Scarpa A, Righetti PG. Proteomic analysis of pancreatic ductal carcinoma cells treated with 5-aza-2-deoxycytidine. Electrophoresis. 2003;24:4291–4303. doi: 10.1002/elps.200305724. [DOI] [PubMed] [Google Scholar]
- (38).Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J. Natl. Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- (39).Lazaris A, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis B. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res. Treat. 1997;43:43–51. doi: 10.1023/a:1005706110275. [DOI] [PubMed] [Google Scholar]
- (40).Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int. J. Cancer. 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- (41).Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jaattela M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–582. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Powers MV, Clarke PA, Workman P. Death by chaperone: HSP90, HSP70 or both? Cell Cycle. 2009;8:518–526. doi: 10.4161/cc.8.4.7583. [DOI] [PubMed] [Google Scholar]
- (43).Fujita Y, Nakanishi T, Miyamoto Y, Hiramatsu M, Mabuchi H, Miyamoto A, Shimizu A, Takubo T, Tanigawa N. Proteomics-based identification of autoantibody against heat shock protein 70 as a diagnostic marker in esophageal squamous cell carcinoma. Cancer Lett. 2008;263:280–290. doi: 10.1016/j.canlet.2008.01.013. [DOI] [PubMed] [Google Scholar]
- (44).Tong YQ, Zhang ZJ, Liu B, Huang J, Liu H, Liu Y, Guo FJ, Zhou GH, Xie PL, Li YH, Zuo CH, Hu JY, Li GC. Autoantibodies as potential biomarkers for nasopharyngeal carcinoma. Proteomics. 2008;8:3185–3193. doi: 10.1002/pmic.200700651. [DOI] [PubMed] [Google Scholar]
- (45).Shukla S, Pranay A, D'Cruz AK, Chaturvedi P, Kane SV, Zingde SM. Immunoproteomics reveals that cancer of the tongue and the gingivobuccal complex exhibit differential autoantibody response. Cancer Biomark. 2009;5:127–135. doi: 10.3233/CBM-2009-0604. [DOI] [PubMed] [Google Scholar]
- (46).Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev., Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- (47).Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Dong Xda. E., Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, Zeh HJ., 3rd. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J. Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- (49).Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin. Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- (50).Tang D, Kang R, Zeh HJ, 3rd., Lotze MT. High-mobility group box 1 and cancer. Biochim. Biophys. Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim. Biophys. Acta. 2010;1799:141–148. doi: 10.1016/j.bbagrm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- (52).Hayashi A, Nagafuchi H, Ito I, Hirota K, Yoshida M, Ozaki S. Lupus antibodies to the HMGB1 chromosomal protein: epitope mapping and association with disease activity. Mod. Rheumatol. 2009;19:283–292. doi: 10.1007/s10165-009-0151-7. [DOI] [PubMed] [Google Scholar]
- (53).Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kusume A, Sasahira T, Luo Y, Isobe M, Nakagawa N, Tatsumoto N, Fujii K, Ohmori H, Kuniyasu H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76:155–162. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- (55).Shen X, Hong L, Sun H, Shi M, Song Y. The expression of high-mobility group protein box 1 correlates with the progression of non-small cell lung cancer. Oncol. Rep. 2009;22:535–539. doi: 10.3892/or_00000468. [DOI] [PubMed] [Google Scholar]
- (56).Gong H, Zuliani P, Komuravelli A, Faeder JR, Clarke EM. Analysis and verification of the HMGB1 signaling pathway. BMC Bioinform. 2010;11(Suppl 7):S10. doi: 10.1186/1471-2105-11-S7-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Zhang JY. Tumor-associated antigen array to enhance antibody detection for cancer diagnosis. Cancer Detect. Prev. 2004;28:114–118. doi: 10.1016/j.cdp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- (58).Casiano CA, Mediavilla-Varela M, Tan EM. Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol. Cell. Proteomics. 2006;5:1745–59. doi: 10.1074/mcp.R600010-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol. Rev. 2008;222:328–340. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zhang JY, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev. Mol. Diagn. 2010;10:321–328. doi: 10.1586/erm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]