SUMMARY
Complex neural circuits in the mammalian brain develop through a combination of genetic instruction and activity-dependent refinement. The relative role of these factors and the form of neuronal activity responsible for circuit development is a matter of significant debate. In the mammalian visual system, retinal ganglion cell projections to the brain are mapped with respect to retinotopic location and eye of origin. We manipulated the pattern of spontaneous retinal waves present during development without changing overall activity levels through the transgenic expression of β2-nicotinic acetylcholine receptors in retinal ganglion cells of mice. We used this manipulation to demonstrate that spontaneous retinal activity is not just permissive, but instructive in the emergence of eye-specific segregation and retinotopic refinement in the mouse visual system. This suggests that specific patterns of spontaneous activity throughout the developing brain are essential in the emergence of specific and distinct patterns of neuronal connectivity.
INTRODUCTION
The development of precise patterns of neural connectivity characteristic of the mammalian brain is thought to occur through a combination of molecular and neuronal activity-dependent mechanisms (Goodman and Shatz, 1993; Cline, 2003). During late stages of mammalian brain development, sensory driven neuronal activity profoundly shapes neural circuit structure and function so that manipulating sensory experience (e.g. through monocular deprivation) can produce dramatic shifts in neural response properties and corresponding changes in neural circuits during ‘critical periods’ of development. In contrast, during early stages of brain development, molecular factors directly regulate cell survival, neurite outgrowth and branch formation. While it is generally accepted that during these early stages of development neuronal activity can modulate brain development (Spitzer, 2006), it remains remarkably controversial whether this early neuronal activity acts only in a passive way to trigger downstream signaling pathways that promote neuron development (Chalupa, 2009; Sun et al., 2008; Huberman et al., 2003), or whether it can act in an instructive way to guide neural circuit formation through specific spatiotemporal patterns of neural activity (Feller 2009; Huberman et al., 2008).
These issues have been investigated in some detail in the mammalian visual system, where retinal ganglion cell (RGC) projections to the dorsal lateral geniculate nucleus (dLGN) and superior colliculus (SC) form two sensory maps, one reflecting eye of origin and the other retinotopic location (Huberman et al., 2008). Molecular factors are clearly involved in forming these neural circuits, directing RGC axons whether to cross at the optic chiasm (Petros et al., 2008) and where to branch in the dLGN and SC (Huberman et al., 2008; McLaughlin and O’Leary, 2005). Evidence concerning the role of neuronal activity in early visual map development is more equivocal, failing to distinguish whether neuronal activity acts in a passive way to promote cell survival and neurite outgrowth, or in an instructive way to guide neural circuit formation through specific spatiotemporal patterns of neural activity (Crair, 1999; Stellwagen and Shatz, 2002; Huberman et al., 2003). This fundamental question has been difficult to answer because manipulations that change the spatiotemporal pattern of ongoing spontaneous neuronal activity typically also alter the activity of individual neurons (their overall spike rate, or burst frequency, etc.). This completely confounds changes in inter-neuronal activity patterns with changes in single neuron activity levels, making it impossible to distinguish between a passive and active role for neuronal activity in visual map development (Chalupa, 2009; Feller 2009).
As in many parts of the developing brain and spinal cord (Meister et al., 1991; Bekoff et al., 1975; Feller, 1999), coordinated waves of spontaneous neuronal activity are found in the retina of all mammalian species examined (Wong, 1999; Warland et al., 2006), well before the onset of sensory experience. Maps for eye of origin and retinotopy emerge in neonatal mice in the first week after birth, a period in which spontaneous retinal activity is mediated by nicotinic acetylcholine receptors containing the β2 subunit (β2-nAChRs; Feller et al., 1996; Bansal et al., 2000). Genetic and pharmacologic manipulations that impair β2-nAChR mediated retinal waves cause deficits in visual system development, including defects in retinotopy and eye segregation (Stellwagen and Shatz, 2002; Chandrasekaran et al., 2005; Mrsic-Flogel et al., 2005; Rossi et al., 2001; Grubb et al., 2003; McLaughlin et al., 2003; Penn et al., 1998; Pfeiffenberger et al., 2005; Pfeiffenberger et al., 2006; Cang et al. 2005; Rebsam et al., 2009; Wang et al., 2009). However, these manipulations invariably change retinal activity levels in addition to disrupting retinal waves, making it ambiguous whether a threshold level of activity or specific patterns of spontaneous waves are important in map development. Moreover, genetic manipulations of spontaneous retinal waves have mainly utilized whole-animal knockouts (β2(KO) mice), leading to uncertainty about the retinal origin of the observed visual map phenotypes because of the broad expression of β2-nAChRs in the eye and brain.
Here we establish an instructive role for spontaneous activity in neural circuit development by investigating the emergence of retinotopy and eye-specific segregation in a line of transgenic mice (β2(TG) mice) with β2-nAChR expression that is limited to the ganglion cell layer of the retina. A detailed examination of spontaneous activity in β2(TG) mice shows that a wide range of single neuron RGC activity parameters are normal, but the spatiotemporal pattern (spread) of retinal waves is visibly truncated. Remarkably, this retinal wave manipulation completely disrupts the segregation of eye-specific inputs to the dLGN and SC, but has no influence on the development of retinotopic maps in the monocular zone of the dLGN and SC. These results demonstrate that the presence of normal levels of spontaneous retinal activity, including bursts of spikes and even ‘small’ retinal waves, is not sufficient to produce normal circuits. Rather, we identify specific spatiotemporal patterns of spontaneous retinal activity that are necessary for the emergence of eye-specific segregation, and distinct aspects of retinal activity that mediate the development of retinotopy. This shows that spontaneous retinal waves are not just permissive, but instructive in the development of the visual system, and suggests that specific and distinct patterns of spontaneous activity found throughout the developing brain are essential in the emergence of specific and distinct patterns of neuronal connectivity.
RESULTS
Inducible Expression of β2-nAChRs in the Retina
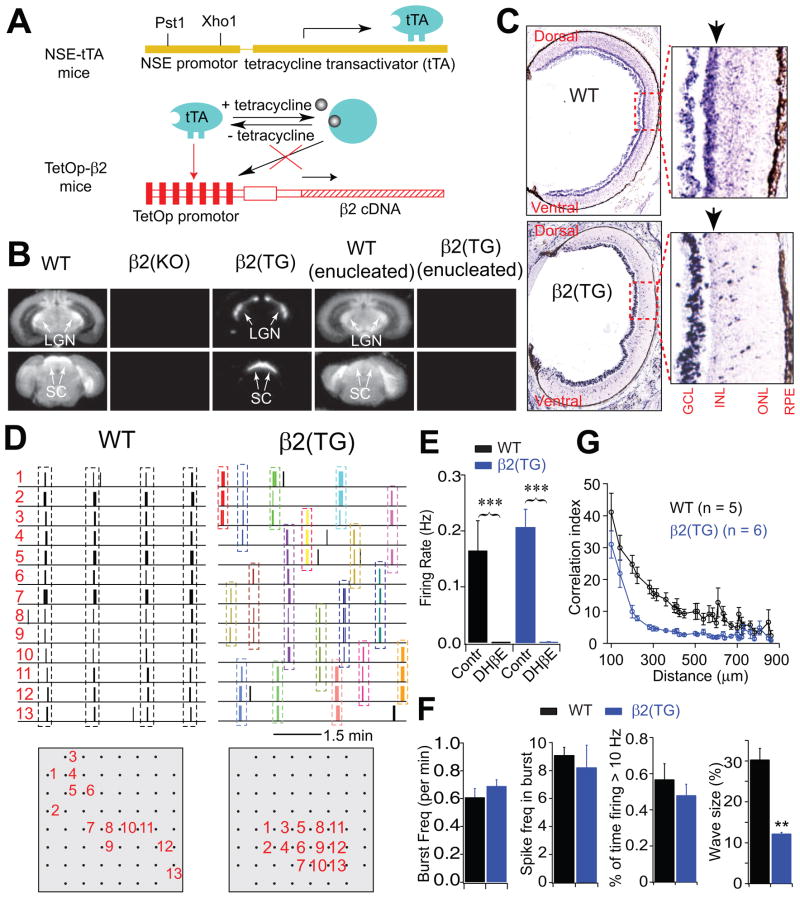
We examined the role of retinal β2-nAChRs and spontaneous waves in visual map development utilizing a line of transgenic mice with retina-specific expression of β2-nAChR expression. Retinal specificity is achieved in these transgenic mice, referred to here as β2(TG) mice, by expressing the tetracycline transactivator under control of the neuron-specific enolase promoter (NSE-tTA) and β2-nAChRs under the control of a tetracycline-regulated promoter (TetOp-β2) on a β2–null background (Fig. 1A, B; King et al., 2003). In this system (Shockett et al., 1995), in the absence of tetracycline, tTA binds to a promoter consisting of the tetracycline operator (TetOp) to drive the expression of β2-nAChRs. When tetracycline is present, tTA undergoes a conformational change that interferes with binding to the TetOp promoter and the transcription of β2-nAChRs is inhibited. Retina specific expression of β2-nAChRs in the β2(TG) mice was confirmed using [125I]A85380, a specific ligand for nicotinic receptors containing the β2 subunit (Mukhin et al., 2000). In WT mice (Fig 1B), [125I]A85380 binding is found throughout the brain, but is absent in β2(KO) mice. In β2(TG) mice, [125I]A85380 is found only in retino-recipient targets such as the dLGN and SC. This label is eliminated when both eyes are enucleated, confirming the retina-specific expression of β2-nAChRs in β2(TG) mice. Within the retina, expression of β2-nAChR mRNA at P4 normally spans all retinal lamina (Fig. 1C, top), but is strongest in the ganglion cell layer (GCL) and inner nuclear layer (INL) (Moretti et al., 2004). In β2(TG) mice, expression of β2-nAChR mRNA is largely absent from the INL, and is restricted to the GCL (Fig. 1C, bottom).
Figure 1. β2(TG) mice express β2-nAChRs only in the ganglion cell layer of the retina, have normal RGC firing properties when considered in isolation, but have small retinal waves.
(A) Expression of β2-nAChRs in the β2(TG) retina is controlled by a Tet-Off system, formed through the expression of both NSE-tTA and TetOp-β2 transgenes. (B) β2-nAChRs are broadly expressed in WT mice, with no [125I]A85380 binding in β2(KO) mice. In β2(TG) mice, binding is detected only in the optic tract, dLGN and SC. Enucleating both eyes completely eliminates binding in β2(TG) mice, demonstrating that β2-nAChRs in β2(TG) mice are expressed on RGC axon terminals. (C) In situ hybridization for β2-nAChR mRNA in P4 WT and β2(TG) mice. In WT mice, β2-nAChR mRNA expression is broad, but highest in the ganglion cell layer (GCL) and inner nuclear layer (INL, arrow in top panel). In β2(TG) mice, β2-nAChR mRNA expression is concentrated in the GCL and much weaker in other retinal layers (arrow in bottom panel). (D) Spontaneous RGC activity in P4 retina recorded in Ringer’s solution at 37°C. RGC activity is synchronous across the entire multielectrode recording array (shown in grey at bottom) in WT mice, while there are only local patches of synchronous activity in β2(TG) mice. (E) Retinal ganglion cell firing rates in WT and β2(TG) mice are similar (P = 0.51, two-tailed Student’s t-test) and sensitive to the β2-nAChR antagonist, DHβE. (F). A wide range of RGC firing parameters were compared between WT and β2(TG) mice under a range of conditions (see also Table 1 and Supplementary Table 2). Illustrated here are four of these parameters, including burst frequency, spike frequency in a burst, and percent of time firing greater than 10 Hz. Only parameters related to the spatiotemporal pattern of the waves, not spiking properties (independent of waves), differed between WT and β2(TG) mice. By far the largest difference between WT and β2(TG) mice is wave size (P < 0.002, two-tailed Student’s t-test). (G) Correlation index (cross correlation) of RGC activity is broad in WT mice, but falls off more steeply with separation in β2(TG) mice. dLGN, lateral geniculate nucleus; SC, superior colliculus; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Triasterisk, P < 0.001, two-tailed Student’s t-test. Error bars are s.e.m.
Normal Single Neuron Firing, But Altered Retinal Waves in β2(TG) Mice
Since cholinergic synapses between amacrine cells in the INL are thought to mediate wave propagation within the early neonatal retina (Blankenship and Feller, 2010) but are absent in β2(TG) mice, we used a multielectrode array in vitro to examine spontaneous RGC activity in β2(TG) and WT mice. We compared a wide range of RGC spontaneous activity properties, including firing rate (Fig. 1E), the prevalence of bursts and percent of spikes in bursts (Fig. 1F; Table 1). Normal levels of spontaneous retinal activity were observed in β2(TG) mice in comparison to WT mice (WT: 0.17 ± 0.12 Hz; β2(TG): 0.21 ± 0.08 Hz; mean ± SD, P = 0.54), and retinal expression of β2-nAChRs in β2(TG) mice was confirmed by the sensitivity of this spontaneous activity to the β2-nAChR-specific antagonist, Dihydro-beta-erythroidine (DHβE) (Fig. 1E). In fact, all spontaneous activity properties for RGCs considered in isolation were similar in β2(TG) mice and WT mice, but the spatiotemporal properties of retinal waves were visibly abnormal (Fig. 1D–G; Table 1 and Supplementary Movie 1, 2). While waves are clear, consistent and just as frequent in the retina of β2(TG) mice as WT mice, they are much smaller in spatial extent than normal (Fig. 1D, F), and activity correlations between RGCs fall off much more steeply with separation in comparison to WT mice (Fig. 1G). Thus, β2(TG) mice are a suitable model system for distinguishing between a permissive role and an instructive role of spontaneous retinal activity in the development of maps for eye-specific segregation and retinotopy in the mouse.
Table 1. Properties of spontaneous retinal activity in β2(TG) mice and WT mice.
A wide range of spontaneous retinal activity parameters were quantified and compared in β2(TG) mice and WT mice. Nearly all of these parameters are comparable in β2(TG) and WT mice, with the conspicuous exception of retinal wave size (spatial extent), which is 3–5 times smaller in β2(TG) mice than WT mice. Shown in green are parameters related to wave properties, such as wave size, wave duration, etc. Spiking properties that are independent of waves (shown in yellow), such as firing rate, burst frequency and ISI in bursts, are all comparable in β2(TG) and WT mice. Similar findings were observed when spontaneous retinal activity was examined in a variety of different recording media or at 31°C instead of 37°C (Supplementary Table 2).
| WT | β2(TG) | |
|---|---|---|
| Total firing rate (Hz) | 0.17 ± 0.12 | 0.21 ± 0.08 |
| % of firing time > 10 Hz | 0.57 ± 0.19 | 0.48 ± 0.15 |
| Wave Freq. (per min) | 0.81 ± 0.09 | 0.74 ± 0.17 |
| Wave duration (s) | 1.78 ± 0.28 | 1.81 ± 0.49 |
| Wave size (% of channels) | 30.36 ± 6.05 | 12.21 ± 0.78 ** |
| Wave firing rate (Hz) | 3.65 ± 0.38 | 3.90 ± 0.44 |
| % spikes in waves | 93.74 ± 1.75 | 83.79 ± 3.92 |
| % bursts in waves | 94.44 ± 3.45 | 77.67 ± 3.56 *** |
| Burst Freq (per min) | 0.61± 0.14 | 0.69 ± 0.11 |
| % Spikes in Bursts | 66.66 ± 10.60 | 73.15 ± 3.27 |
| Burst duration (s) | 2.37 ± 1.37 | 2.64 ± 1.03 |
| ISI in burst (s) | 0.54 ± 0.40 | 0.44 ± 0.19 |
| Spike freq. in Burst | 9.11 ± 1.23 | 8.23 ± 3.53 |
| Interburst Interval (s) | 112.69 ± 31.83 | 93.58 ± 18.35 |
means ± SD are reported;
P < 0.05;
P< 0.01;
P < 0.001.
Normal Retinotopy in the SC of β2(TG) Mice
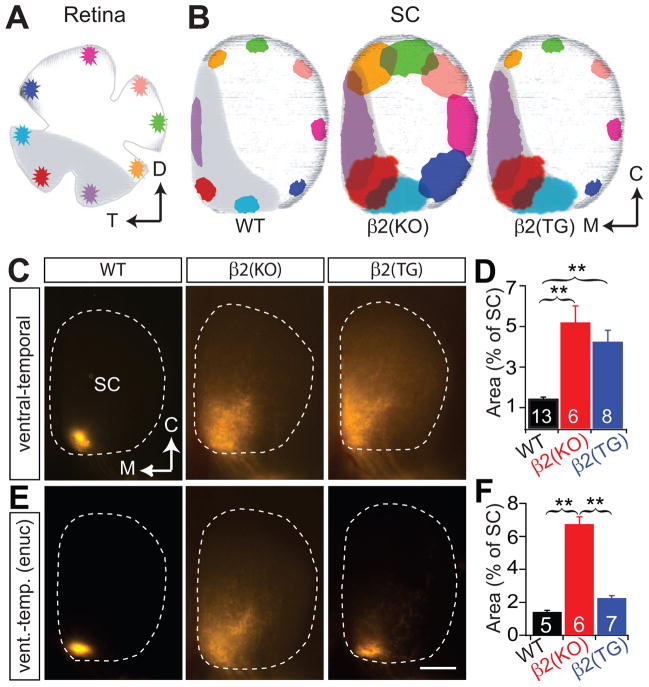
First, we examined the impact of spatially restricted (‘small’) retinal waves on the development of retinotopy in the SC of β2(TG) mice. Dorsal RGCs in β2(TG) mice, which project only to the contralateral SC in mice (Drager and Olsen, 1980), have retinotopic projections that are indistinguishable from WT mice (Fig. 2A, B). The size of the RGC target zone in the SC of β2(TG) mice (1.08 ± 0.48 %, mean ± SD) is no different than WT mice (1.05 ± 0.25 %, mean ± SD; P= 0.85), and much smaller than β2(KO) mice (3.78 ± 1.49 %, mean ± SD; P < 0.001 for both comparisons). The development of retinotopically refined projections in β2(TG) mice is clearly the consequence of transgene expression, as application of the tetracycline analog doxycycline, which suppresses β2-nAChRs expression in our TetOp-β2(TG) mice (Fig 1A), results in retinal projections that are as poorly refined as in β2(KO) mice (Fig. 2A, B; 3.43 ± 1.92 % with doxycycline, mean ± SD; P = 0.002 in comparison with β2(TG) and P = 0.66 in comparison with β2(KO)). This data demonstrates that ‘small’ retinal waves and the expression of β2-nAChRs in the retina, and not the SC, are sufficient for the development of normal retinotopy in mice.
Figure 2. Retinotopic map refinement, but not eye-specific segregation is rescued in the SC of β2(TG) mice.
(A, B) Focal DiI injections into dorsal retina result in a spot of label in the SC (whole mount, dorsal view). The target zone spot in β2(KO) mice and β2(TG) mice treated with doxycycline is much larger than in WT and β2(TG) mice. (C, D) Whole eye (vitreal) injections of Alexa-conjugated cholera toxin dye bulk label most RGC axon projections in the SC. Contralateral axons are green, ipsilateral red. Contralateral axons (green) project to the most superficial (SGS) layer of the SC (sagittal sections), ipsilateral eye axons (red) project to the SO layer just inferior to the contralateral axons. A large fraction of axons from the ipsilateral eye extend into the SGS layer in both β2(KO) and β2(TG) mice (D, top) and overlap with projections from the contralateral eye (D, bottom), indicating poor eye segregation. M, medial; C, caudal; R, rostral; SGS, stratum griseum superficial; SO, stratum opticum. Scale bars 500 μm for all figures. Biasterisk, P < 0.01 and triasterisk, P < 0.001, two-tailed Student’s t-test. Error bars are s.e.m.
Impaired Eye-Specific Segregation in the SC of β2(TG) Mice
While RGC projections in mice are mostly crossed, about 5% of RGCs project ipsilaterally (Drager and Olsen, 1980). Crossed projections in the SC form a retinotopic map and also segregate with respect to eye of origin, with a superficial layer (the SGS) in the SC that receives exclusive input from the contralateral eye, and a slightly deeper layer (the SO) that receives input from the ipsilateral eye (Fig. 2C, D). Remarkably, eye segregation is profoundly disturbed in β2(TG) mice (Fraction of SGS with ipsi: 3.17 ± 1.28 %, mean ± SD for WT; 33.01 ± 9.06 %, mean ± SD, for β2(TG); P < 0.001; % Overlap: 2.63 ± 1.69, mean ± SD, for WT; 32.82 ± 9.06, mean ± SD, for β2(TG); P < 0.001), and eye-specific lamina remain as poorly formed in the SC of β2(TG) mice as in mice completely lacking β2-nAChRs (β2(KO) mice; Fraction of SGS with ipsi: 37.31 ± 10.95 %, mean ± SD, for β2(KO); % Overlap: 37.19 ± 10.95, mean ± SD; P = 0.2361 and 0.2286 for comparison between β2(KO) and β2(TG)) (Fig. 2C, D, Supplementary Fig. 1).
Normal Retinotopy Only in the Absence of Binocular Competition in the SC of β2(TG) Mice
Due to the lateral position of their eyes, binocular projections in mice are limited to RGCs from the extreme ventral-temporal retina (Drager and Olsen, 1980; Godement et al., 1984). Curiously, retinotopic refinement in β2(TG) mice is normal in RGCs from throughout the retina with the exception of those from the ventral-temporal crescent (Fig. 3A–D, Supplementary Fig. 2, Supplementary Table 1); those RGC axons that fail to segregate with respect to eye of origin also lack retinotopic refinement. The failure of RGC axons from the binocular zone of the retina to refine in β2(TG) mice is not due to incomplete rescue of β2-nAChRs expression in ventral-temporal retina, as in-situ hybridization shows that β2-nAChR mRNA levels are indistinguishable in dorsal and ventral retina (Fig. 1C), and spontaneous retinal waves in ventral-temporal retina of β2(TG) mice are indistinguishable from dorsal-nasal retina (Supplementary Fig. 3). Furthermore, enucleating one eye at birth fully restores retinotopy of the ventral-temporal (binocular zone) RGC axons from the intact eye (Fig. 3E, F; Supplementary Table 1). This unambiguously demonstrates that small retinal waves even in ventral-temporal RGCs are completely capable of mediating retinotopic refinement, but RGC interactions between the two eyes impairs retinotopy in the binocular zone of the SC in β2(TG) mice.
Figure 3. Binocular competition interferes with retinotopic map refinement.
(A, B) Focal DiI injections around the periphery of the retina results in focal target spots in the SC of WT mice, but much larger target zones in β2(KO) mice (see also Supplementary Fig. 2). In β2(TG) mice, target zones are completely restored in regions of the SC that receive monocular input but remain enlarged in the regions that receive input from both eyes (shown in grey). (C, D) Focal DiI injections into ventral-temporal retina, which projects bilaterally, labels a spot in the rostro-medial portion of the contralateral SC in WT mice. A similar injection in β2(KO) and β2(TG) mice results in a much larger target zone. (E, F) Enucleation of one eye at birth restores retinotopic refinement of ventral-temporal RGCs in β2(TG) mice, but not in β2(KO) mice. M, medial; C, caudal; T, temporal; D, dorsal. Biasterisk, P < 0.01, two-tailed Student’s t-test. Error bars are s.e.m.
Normal Retinotopy but Impaired Eye-Specific Segregation in the dLGN of β2(TG) Mice
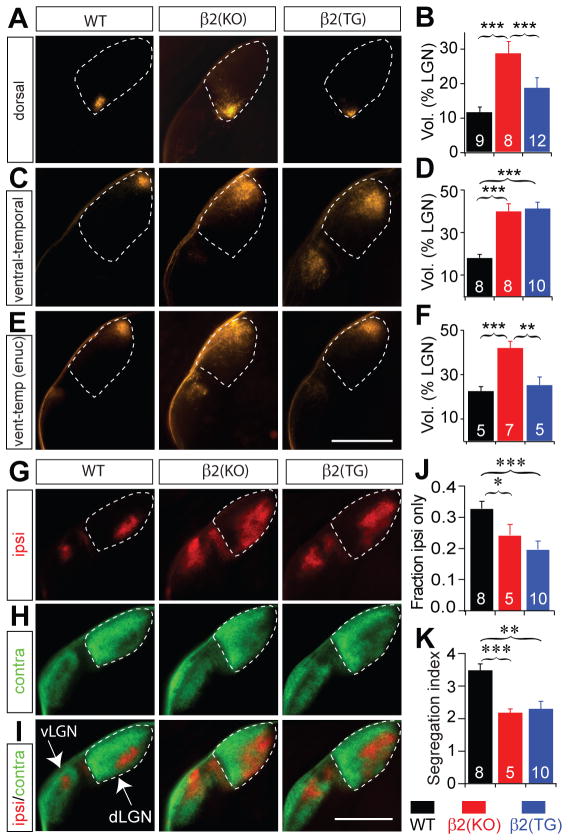
The SC and the dLGN are the dominant targets of retinal projections in mammals. Despite its relatively small size in rodents, RGC projections to the dLGN are segregated with respect to eye of origin and display sharp retinotopic organization (Lund et al., 1974; Godement et al., 1984; Pfeiffenberger et al. 2006). We examined retinotopy and eye segregation in the dLGN of β2(TG) mice and observed conditions analogous to that in the SC. In particular, we found that the retinotopy of projections to the dLGN from the dorsal monocular zone of the retina are normal (Fig. 4A, B; 12 ± 14 %, mean ± SD for WT; 29 ± 11 %, mean ± SD for β2(KO); 17 ± 9 %, mean ± SD for β2(TG); P < 0.001 for comparison between β2(KO) and both WT and β2(TG)), but RGC projections from the ventral-temporal binocular zone of the retina remain unrefined (Fig. 4C, D; 18 ± 5 %, mean ± SD for WT; 40 ± 10 %, mean ± SD for β2(KO); 41 ± 9 %, mean ± SD for β2(TG); P < 0.001 for comparison between WT and both β2(KO) and β2(TG)), unless binocular competition is removed through monocular enucleation (Fig. 4E, F, Supplementary Fig. 4; 22 ± 5 %, mean ± SD for WT; 42 ± 8 %, mean ± SD for β2(KO); 25 ± 8 %, mean ± SD for β2(TG); P < 0.001 for comparison between β2(KO) and WT; P = 0.005 between β2(KO) and β2(TG); P = 0.52 for comparison between β2(TG) and WT). Eye-specific segregation is also completely disrupted in the dLGN of β2(TG) mice, like in β2(KO) mice (Fig. 4G–K; Rossi et al., 2001; Muir-Robinson et al., 2002; Grubb et al., 2003; Pfeiffenberger et al., 2005; Pfeiffenberger et al., 2006). This data demonstrates that normal levels of spontaneous neuronal activity and ‘small’ retinal waves are not sufficient to mediate the segregation of retinal afferents with respect to eye of origin in the dLGN and SC, but are sufficient to mediate normal retinotopy (in the absence of binocular competition) throughout the dLGN and SC.
Figure 4. Retinotopic map refinement, but not eye-specific segregation is rescued in the dLGN of β2(TG) mice.
(A, B) Focal DiI injections into dorsal retina result in a large spot of label in the dLGN (coronal sections) of β2(KO) mice, but small spots in WT and β2(TG) mice. (C, D) Focal DiI injections into ventral-temporal retina labels a focal target spot in the contralateral dLGN of WT mice, but produces a much larger target zone in both β2(KO) and β2(TG) mice. (E, F) Enucleation of one eye improves retinotopic refinement of ventral-temporal RGC axons in the dLGN of β2(TG) mice, but not β2(KO) mice. (G, H, I) In the dLGN (coronal sections) of WT mice, RGC projections from the contralateral eye (green) are strictly excluded from the ipsilateral RGC axon terminal region (red). In β2(KO) and β2(TG) mice, ipsilateral eye projections have an expanded termination zone and intermingle with projections from the contralateral eye. (J, K) Two measures of eye-specific segregation in the dLGN show that eye segregation is much better in WT mice (0.33 ± 0.07, mean ± SD for Fraction ipsi only; 3.42 ± 0.51, mean ± SD, for Segregation index) than β2(KO) mice (0.24 ± 0.08, mean ± SD, for Fraction ipsi only; 2.11 ± 0.25, mean ± SD for Segregation index) or β2(TG) mice (0.20 ± 0.08, mean ± SD, for Fraction ipsi only; 2.27 ± 0.78, mean ± SD, for Segregation index). Biasterisk, P < 0.01 and triasterisk, P < 0.001, two-tailed Student’s t-test. Error bars are s.e.m. Scale bars 500 μm for all figures.
Chronic Binocular Application of CPT-cAMP Rescues Eye Segregation in β2(TG) Mice
We tested whether the abnormal spatiotemporal properties of waves in the β2(TG) mice are responsible for their visual map defects by manipulating β2(TG) retinal waves pharmacologically in vivo. Spontaneous retinal activity, retinal wave dynamics and size are modulated by cAMP levels (Stellwagen and Shatz, 2002; Stellwagen et al., 1999; Zheng et al., 2006). Acute application of CPT-cAMP and other cAMP signalling agonists increases retinal wave size and frequency (Stellwagen and Shatz, 2002; Stellwagen et al., 1999). Daily binocular intravitreal injection of CPT-cAMP, a nonhydrolyzable membrane-permeable analogue of cAMP, beginning at P2 in β2(TG) mice significantly improves eye-specific segregation in both the dLGN and SC in comparison to saline (control) injections (Fig. 5). This strengthens the assertion that the altered spatiotemporal properties of retinal waves in β2(TG) mice are responsible for their visual map defects, and demonstrates that expression of β2-nAChRs in the dLGN and SC is not necessary for eye-specific RGC axon segregation.
Figure 5. Daily binocular injections of CPT-cAMP rescue eye-specific segregation in β2(TG) mice.
(A) Example coronal sections show that binocular CPT-cAMP injections correct eye-specific segregation defects in the dLGN of β2(TG) mice compared to saline injection controls. Contralateral axons are labelled green, and ipsilateral axons are labelled red with whole eye (vitreal) injections of Alexa-conjugated cholera toxin. (B) The fraction of dLGN with segregated ipsi projections is larger in CPT-cAMP treated β2(TG) mice (0.31 ± 0.19, mean ± SD) than saline treated β2(TG) mice (0.16 ± 0.12, mean ± SD, 10% threshold shown, difference was consistent across a range of thresholds). (C) Eye-specific segregation in the dLGN measured with a segregation index was significantly improved in CPT-cAMP treated β2(TG) mice (2.46 ± 0.31, mean ± SD) in comparison to that of saline treated β2(TG) mice (1.70 ± 0.36, mean ± SD). (D) Eye-specific segregation in the SC improves significantly in β2(TG) mice when treated with daily binocular injections of CPT-cAMP. (E) Summary quantification of eye segregation measured as the fraction of the contralateral (SGS) layer with ipsi label (10% threshold shown, the difference was consistent across a range of thresholds). Fewer ipsilateral axons project to the contralateral (SGS) layer in CPT-cAMP treated β2(TG) mice (22.43 ± 5.29 %, mean ± SD) than in saline treated β2(TG) mice (37.03 ± 2.32 %, mean ± SD). (F) Summary quantification of binocular overlap of ipsi (red) projections with contralateral (green) projections in the SGS layer. In CPT-cAMP treated β2(TG) mice, the overlap was 22.15 ± 5.16 % (mean ± SD). In saline treated β2(TG) mice, the overlap was 35.95 ± 2.01 % (mean ± SD). Asterisk, P < 0.05; Biasterisk, P < 0.01; triasterisk, P < 0.001, two-tailed Student’s t-test. Error bars are s.e.m.
Computational Model for the Role of Spatiotemporal Retinal Wave Patterns in Visual Map Development
We constructed a computational model using activity-dependent Hebbian rules for synapse development to examine whether the mapping phenotype in β2(TG) mice can be explained based purely on the altered spatial properties of their retinal waves (Fig. 6). In the model (Fig. 6A), retinocollicular synapses develop according to a Hebbian plasticity rule, and compete with each other through the homeostatic regulation of total synaptic input to each SC neuron (see Experimental Procedures for more computational model details). At the beginning of each simulation, RGC projections to the SC are broad, and the binocular SC receives mixed input from the two eyes. During the simulation, retinal activity gradually modifies the pattern of retinocollicular connectivity through Hebbian synaptic plasticity rules so that after each retinal wave some of the synapses are potentiated and others are weakened, depending on the size, position and eye of origin of the wave.
Figure 6. A Hebbian model of visual map development recapitulates the anatomical phenotype observed in β2(TG) mice.
(A) Schematic of the computational model. RGCs and SC neurons are represented by a 1-dimensional array of spatially arranged computational units, and retinocollicular synaptic weights develop according to a standard Hebbian rule. (B) Each row in the diagrams displays the afferent connectivity to one SC neuron at the end of a simulation. The size of the boxes indicates the strength of the corresponding synaptic connections, while their color indicates ocularity (red ipsilateral and green contralateral, see scales at bottom). Large retinal waves result in both eye-specific segregation (red or green, not yellow) and refinement of axonal arbors (narrow diagonal bands). Small waves, in contrast, generate robust retinotopic refinement in the monocular zone but result in dramatically impaired eye segregation as well as poor retinotopic refinement in the binocular zone (yellow and broad connectivity patterns). (C–E) Quantification of simulation results for eye-specific segregation in the binocular SC and retinotopic refinement in the monocular and binocular SC. (C) Eye segregation is dramatically degraded by small waves in these simulations. (D) Retinotopic refinement is comparable for small and large waves in the monocular SC. (E) Retinotopic refinement is worse for small waves than large waves in the binocular SC. Eye segregation and retinotopic-refinement indices were averaged over SC neurons.
We simulated the difference in map development between WT and β2(TG) mice by varying the spatial extent of waves while maintaining the same level of overall retinal activity and the same frequency of waves per RGC, as observed experimentally. In simulations with large retinal waves (WT mice), inputs from the two eyes segregate so that neurons in the binocular SC become responsive to input from only one eye (Fig. 6B). Large waves also induce retinotopic refinement of retinocollicular projections, both in the monocular and binocular SC, by strengthening retinotopically correct projections and weakening spatially inappropriate ones. Notably, simulations with small retinal waves reproduce both the monocular and binocular mapping phenotype of β2(TG) mice. In the monocular SC (or throughout the SC in one-eye enucleated animals), small-wave simulations result in retinotopic refinement, but in the binocular SC, both eye segregation and retinotopic refinement are impaired (Fig. 6B – E).
Why, according to the model, is retinal wave size (spatial extent) important for proper formation of both visual maps? In the binocular zone of the SC/dLGN, afferents from the two eyes compete with each other so that during each retinal wave, inputs from the corresponding eye are strengthened while inputs from the opposing eye are weakened. With small retinal waves, the amount of cooperative activity among RGCs from one eye is correspondingly small, so the strengthening of a “waving” eye is greatly reduced compared to when the wave covers a large portion of the retina. Afferents from the two eyes still compete in the “small-wave” scenario, but competition in this case does a poor job distinguishing between afferents from the two eyes, resulting in degraded eye-specific segregation. The model also shows why impairing eye-specific segregation interferes with retinotopic refinement in the binocular zone of the SC/dLGN. Typically, as inputs from the two eyes segregate and strengthen, connections at retinotopically inappropriate locations are reduced through homeostatic regulation of the overall connectivity, but these spatially inappropriate connections persist in the absence of eye-specific segregation. If one eye is enucleated, interference from the other eye is eliminated, and small retinal waves are adequate to mediate retinotopic refinement even for ventral-temporal axons, as is normally the case in the monocular zone of the SC/dLGN. In sum, the model fully recapitulates the anatomical phenotypes observed in untreated and enucleated β2(TG) mice and demonstrates how specific spatiotemporal patterns of spontaneous retinal waves can dictate the emergence of specific patterns of neuronal connectivity during development.
DISCUSSION
There is a strong consensus in the field that during late stages of development (particularly in mammals), sensory driven neural activity profoundly shapes neural circuit structure and function. For instance, manipulating sensory experience (e.g. through monocular deprivation) produces dramatic shifts in neural response properties and corresponding changes in neural circuits during ‘critical periods’ of development (Morishita and Hensch, 2008). It is also generally accepted that even during early stages of development, neurons need to be active for the brain to develop normally (Spitzer, 2006). However, it remains remarkably controversial whether this early neuronal activity acts in a passive way by triggering downstream cellular signaling pathways to promote cell survival and neurite outgrowth (potentially through Ca2+ signaling), or in an instructive way, guiding neural circuit formation through specific spatiotemporal patterns of neural activity (Crair 1999; Crowley and Katz, 2000; Huberman et al., 2008; Chalupa 2009; Feller 2009). Patterns of spontaneous neuronal activity (‘waves’) have been described in a wide range of brain structures during early development, including the retina, thalamus, cortex, hippocampus, striatum and spinal cord (Feller, 1999). Still, nowhere has it been established whether this patterned spontaneous activity is ‘permissive’ or ‘instructive’ in guiding brain development. Why has this fundamental question been so hard to nail down? Simply put, manipulations that change the spatiotemporal pattern of spontaneous neuronal activity have invariably also altered the activity of individual neurons (their overall spike rate, or burst frequency, etc.). This completely confounds changes in inter-neuronal activity patterns with changes in single neuron activity levels. As a result this fundamental question, which permeates across a broad area of developmental neurobiology, remains unanswered.
Not Simply the Presence, But the Pattern of Retinal Waves Directs Visual Map Development
We demonstrated here that patterns of spontaneous neuronal activity instruct neural circuit development. We accomplished this with a novel line of transgenic mice (β2(TG)) in which we manipulated the expression of acetylcholine receptors responsible for the propagation of spontaneous waves in the inner retina. This genetic manipulation dramatically changed the spatiotemporal properties of spontaneous retinal waves (they become spatially restricted or ‘small’), but had no effect on spiking properties of retinal ganglion cells when considered in isolation (wave properties change, but the spiking properties of individual retinal ganglion cells are unchanged). This ‘small wave’ manipulation strikingly impaired the neural circuit that emerged between the retina and brain during development. This shows that not merely the presence, but the precise spatiotemporal pattern of spontaneous retinal activity instructs neural circuit development. These data are consistent with a body of literature arguing for an important role of activity-dependent competitive processes in mammalian brain development (Torborg et al., 2005; Chandrasekaran et al., 2005; Mrsic-Flogel et al., 2005; Penn et al., 1998; Cang et al., 2005; Katz and Shatz, 1996; Stryker and Harris, 1986; Cao et al., 2007), and demonstrate how even prior to sensory experience, patterned neuronal activity shapes developing brain circuits.
Retinotopic Refinement and Eye-Specific Segregation Rely on Different Aspects of Spontaneous Retinal Activity
β2(TG) mice have normal retinotopy but profoundly disturbed eye-specific segregation. To our knowledge, this is the first example of a distinction between the activity-dependent requirements for the development of these two visual maps, and may reflect a fundamental difference between the process of retinotopic refinement and eye-specific segregation. Eye-specific segregation involves expulsion of “wrong-eye” axons from the domain of the “correct-eye”. In an activity-dependent model, this process requires sufficient correlated intra-eye activity. Retinotopic refinement, in contrast, involves relative spatial correlations within an eye, where the activity of neighboring RGCs is more correlated than that of distant ones. Small retinal waves provide just these local correlations, and are therefore adequate for mediating retinotopic refinement in the absence of binocular competition. This interpretation is further supported by our computational model for retinotopy and eye segregation, which is based on axonal competition and a Hebbian, correlation-based synaptic plasticity rule. This model produces both eye-specific segregation and retinotopy for a wide range of parameters only if the waves are sufficiently large, but only retinotopy if the waves are spatially small.
Binocular Interactions Can Interfere With Retinotopic Refinement
In β2(TG) mice, retinotopic refinement is normal everywhere except for the binocular zone of the dLGN and SC. Why? We believe the reason is an interference effect between RGC axons from the two eyes caused by the persistent defects in eye-specific segregation. We demonstrated that the expression of β2-nAChR mRNA is similar in ventral-temporal (binocular projecting) and dorsal-nasal (monocular) retina of β2(TG) mice. Retinal waves are also similar in ventral-temporal and dorsal-nasal retina of WT mice and β2(TG) mice. This argues strongly that intrinsic differences in β2-nAChR expression or retinal waves across the retina are not responsible for the selective retinotopic refinement failure of binocular zone RGC axons in β2(TG) mice. Moreover, enucleation of one eye completely restores retinotopic refinement of ventral-temporal RGC axons from the remaining eye, clearly demonstrating that ventral-temporal RGC axons are fully capable of normal retinotopic refinement in β2(TG) mice, but binocular interactions prevent this refinement. Analogous results have been reported in the ferret (Huberman et al., 2006), where binocular pharmacological blockade of retinal waves with epibatidine significantly enlarged the receptive fields of neurons with binocular receptive fields in the visual cortex, but had no effect on the receptive fields of monocular neurons. These somewhat surprising results suggest that maps for retinotopy and eye-specific segregation are fundamentally linked; conditions that are appropriate for normal retinotopic refinement in the monocular zone may be inadequate to mediate retinotopic refinement in the presence of binocular competition. In the visual cortex, the plasticity of ocular dominance maps following monocular deprivation is linked to maps for stimulus orientation (Crair et al., 1997), but the current work specifically implicates the structure of spontaneous neuronal activity, not visual experience, in linking maps for retinotopy and eye of origin. Our Hebbian computational model recapitulates the link between eye-specific segregation and retinotopy. In simulations where binocular interactions persist due to poor eye segregation, retinotopic refinement is impaired as well. According to the model, if inputs from the two eyes do not segregate, the pattern of input activity to the SC and dLGN is fundamentally altered because it reflects activity from both eyes instead of one eye only. Normally, homeostatic regulation of the total synaptic input to neurons in the SC or dLGN favours the strengthening of highly correlated inputs from neighbouring RGCs. However, the persistence of conflicting inputs from the two eyes interferes with the process of RGC axon pruning from inappropriate retinotopic locations, and retinotopic refinement is impaired. By contrast, retinotopy develops normally in the monocular zone of β2(TG) mice, and throughout the SC in enucleated β2(TG) mice, because conflicting signals from the two eyes do not exist under these conditions.
Why are Retinal Waves Small in β2(TG) mice?
β2-nAChRs are normally expressed throughout the developing retina (Moretti et al., 2004; Fig. 1C), particularly in synapses amongst amacrine cells and between amacrine cells and ganglion cells (Blankenship and Feller, 2010). Retinal waves are thought to be nucleated by ChAT-positive intrinsically bursting starburst amacrine cells, and wave propagation across the retina mediated by β2-nAChR containing synapses between amacrine cells in the inner nuclear layer (Butts et al., 1999; Zheng et al., 2006). RGC firing during a wave is coupled to starburst amacrine cell bursting through synapses containing β2-nAChRs (Blankenship and Feller, 2010). However, little is known experimentally about specific mechanisms that regulate wave size. We reason that waves are small in the β2(TG) mice because β2-nAChR expression is largely limited to RGCs, which synaptically isolates starburst amacrine cells from each other and chokes off wave propagation across the inner retina. Since synaptic communication between amacrine cells in the inner nuclear layer and RGCs in the ganglion cell layer is preserved, RGCs in β2(TG) mice will faithfully relay the intrinsic bursting activity of underlying starburst amacrine cells, preserving overall activity levels but without the spatial spread typical of normal retinal waves. These data suggest that β2-nAChR expression is tightly regulated in the developing retina in order to promote the propagation of spontaneous waves with the appropriate spatiotemporal patterns that will drive eye segregation and retinotopic refinement.
What About β2(KO) Mice?
β2(KO) mice lack β2-nAChR expression throughout the brain and body, and both eye-specific segregation and retinotopic refinement are disturbed in the dLGN and SC (Rossi et al., 2001; Grubb et al., 2003; McLaughlin et al., 2003; Chandrasekaran et al., 2005). It is unlikely that these visual map deficits are due to the absence of β2-nAChR expression in the dLGN and SC because β2(TG) mice also lack expression in these RGC targets but retinotopy is normal in β2(TG) mice and eye-specific segregation can be rescued through the daily binocular application of CPT-cAMP. This demonstrates β2-nAChR expression in the dLGN and SC is not necessary for the development of retinotopy and eye-specific segregation in mice.
If β2-nAChR expression in the SC and dLGN is not required for retinotopic refinement or eye-specific segregation, why are visual maps disturbed in β2(KO) mice? Is it because waves are absent in β2(KO) mice, or very abnormal, or something else entirely? The precise effects of completely knocking out β2-nAChRs on retinal activity are controversial (Bansal et al., 2000; Sun et al.; 2008, Stafford et al., 2009). Spontaneous retinal activity in β2(KO) mice is very sensitive to the precise in vitro recording conditions used to examine activity (Bansal et al., 2000; Sun et al., 2008; Stafford et al., 2009). Variations in temperature, composition of the recording medium or even ambient light levels (Supplementary Fig. 5, data not shown) can dramatically affect whether waves are even present in β2(KO) mice. In contrast, retinal waves in WT and β2(TG) mice are very stable and quite insensitive to these variations (Supplementary Fig. 6; Supplementary Table 2). In particular, retinal wave size is consistently much smaller in β2(TG) mice relative to WT mice across all recording conditions, while other spontaneous retinal activity parameters are similar (Supplementary Fig. 6; Supplementary Table 2), reinforcing the conclusion that visual map defects in β2(TG) mice are the result of altered retinal waves. Ultimately, it will be necessary to examine retinal wave properties in vivo in awake mice to determine definitively what specific aspects of spontaneous retinal activity are disturbed in β2(KO) mice that may lead to their disturbed visual maps. Regardless, spontaneous retinal activity in β2(KO) mice is abnormal under all reported conditions (Bansal et al., 2000; Sun et al., 2008; Stafford et al., 2009), and in the interim we propose that even if waves are present in vivo in β2(KO) mice, the majority of RGC activity is likely to reside outside of waves (Stafford et al., 2009 observed only ~30% of RGC activity resided in retinal waves, whereas >80% of activity is in waves in β2(TG) and WT mice (Table 1)). In this case, our computational model predicts that retinal activity will fail to induce either eye segregation or retinotopic map refinement in β2(KO) mice (Supplementary Fig. 6).
Sperry and Hebb in Visual Map Development
We have presented compelling evidence that the development of visual maps in the dLGN and SC is dependent not simply on the presence, but the precise pattern of spontaneous ongoing activity in the retina. What are the mechanisms that mediate this activity-dependent development at retinofugal synapses? Hebbian synaptic plasticity is known to exist at retinal ganglion cell synapses onto neurons in the dLGN (Butts et al., 2006) and SC (Shah and Crair, 2008). Furthermore, our computational model, based on a synaptic learning rule that obeys Hebbs postulate, fully captures the experimental results observed in β2(TG) mice. Of course, this does not exclude an essential role for molecular targeting events in visual map development. We (Chandrasekaran et al., 2005) and many others (e.g., Goodman and Shatz, 1993; Cline, 2003; Feller, 2009) have long argued that both molecular patterning events and activity-dependent mechanisms work together to wire the vertebrate visual system. It is possible that a molecular process that is dependent on the pattern of spontaneous neuronal activity but independent of synaptic plasticity (Hebb) or even synaptic function is responsible for the refined development of visual maps in the dLGN and SC. For example, specific neural activity patterns in RGCs may drive distinct patterns of cAMP oscillations and associated second messenger cascades, which then regulate neurite outgrowth and development to achieve map refinement (Kumada et al., 2009; Shelly et al., 2010; Nicol et al., 2007; Carrillo et al., 2010). In this case, our data show that the precise spatiotemporal pattern of spontaneous retinal waves is still critical for normal map development, but the result may be achieved through as yet unknown molecular mechanisms that are dependent on patterned neuronal activity but don’t critically rely on synaptic function or Hebbian mechanisms at the synapse.
With the increasing power and ease of molecular-genetic techniques to identify molecules and genes involved in visual system development, it is tempting to focus on these signalling pathways at the exclusion of more ‘traditional’ activity-dependent processes. However, it seems clear that both molecules and activity play important roles in visual map development, and the expression of genes involved in visual system development is likely tightly regulated by activity-dependent processes and vice-versa. Indeed, several molecules and signalling pathways recently shown to be involved in visual map development were initially identified through differential screens for genes regulated by neuronal activity (e.g., Shatz, 2009). The results described here show that even rather subtle genetic manipulations that only alter patterns of spontaneous activity without changing the levels of activity can have a profound impact on brain development. This may have significant implications for diseases of multi-genetic origin, such as schizophrenia and autism, in which brain wiring may be negatively affected not because of direct effects of genes on neural circuits or synaptic function, but because of indirect effects on patterns of spontaneous or evoked activity during neural circuit development.
EXPERIMENTAL PROCEDURES
Animals
β2-nAChR subunit knockout β2(KO) and transgenic β2(TG) mice with retina-specific expression of β2-nAChRs were generated as described (King et al., 2003). Wild type (WT) mice (C57BL/6J) were obtained from Jackson Laboratory (Bar Harbor, ME). Doxycycline administration was provided through the mothers of experimental mice via water containing doxycycline (1 mg/ml) from E0 to P8. Animals were treated in compliance with the Yale IACUC, U. S. Department of Health and Human Services and Institution guidelines.
Eye Injections, fluorescent images and data analysis
Focal DiI injections (2.3 nl) for measurements of retinotopy were performed, imaged and quantified blind to genotype as described (Chandrasekaran et al., 2005). Injections were localized along the perimeter of the retina, using as a reference the insertion points of the four major eye muscles (Plas et al., 2005). Retinal injection size, quantified by measuring the area of fluorescent signal in the retina above one half of the maximum fluorescent signal after background subtraction, showed no difference across all genotypes and injection locations, and there was no relationship between TZ area and retinal injection area (Supplementary Fig. 7; McLaughlin et al., 2003).
Measurements of eye-specific segregation were performed with whole eye injections (1 μl into the vitreous) of Alexa Fluor 488-conjugated cholera toxin (left eye) and Alexa Fluor 594 (right eye) at P6, then returned to their mother for 24–48 hours to allow transport of tracer from the retina to the SC and dLGN. CPT-cAMP treated animals were injected daily with 500 nl of saline or CPT-cAMP (5 mM) into both eyes from P2 to P6, then received whole eye injections of Alexa dye at P7. Eye-specific segregation in the SC was quantified by measuring the fraction of fluorescence signal labelled from the ipsilateral eye in the SGS layer, and also by measuring the overlap (in % of pixels) of ipsilateral eye fluorescence signal with contralateral eye fluorescence signal in the SGS layer. Quantification of eye-specific segregation in the dLGN followed previously published methods (Stellwagen and Shatz, 2002; Huberman et al., 2003; Torborg et al., 2005).
[125I]A85380 binding assay
The [125I]A85380 binding assay was performed on 15 μm brain sections as previously described (King et al., 2003).
In situ hybridization
Expression patterns were determined by means of non-radioactive in situ hybridization (ISH) on frozen sagittal sections of P4 mouse brains by the in situ hybridization core at Baylor College of Medicine following published methods (Visel, Thaller et al. 2004).
Retinal wave recording and data analysis
Spontaneous RGC activity was recorded at P4 using a multielectrode array at 37°C in Ringer’s solution (unless otherwise noted) following previously published protocols (Tian and Copenhagen, 2003; Xu et al., 2010). Various retinal wave properties were measured, including firing rate, correlation index, wave frequency, wave size, burst frequency and burst duration. Wave size was defined as the fraction of all electrodes that were capable of recording spikes from at least one cell with a firing rate not less than 2 Hz during a wave. The correlation index was calculated as previously described (Torborg et al., 2004). Burst analysis was carried out using the burst analysis algorithm provided by Neuroexplorer (Nex Technologies, Lexington, MA) following previous published protocols (Sun et al., 2008; Stafford et al., 2009).
Computational model
We constructed a computational model of retinocollicular map development in which RGC projections to SC neurons develop through a Hebbian plasticity rule. The model simulates the essential aspects of retinocollicular circuitry while retaining a level of simplicity that generalizes across biological details but allows for examination of the consequences of varying retinal wave size on visual map development. The difference in map development between WT and β2(TG) mice is modelled by modifying the spatial extent and frequency of waves, keeping constant the overall level of retinal activity per RGC, as observed experimentally.
Supplementary Material
Acknowledgments
We would like to thank members of the Crair lab for valuable comments on the manuscript, particularly Onkar Dhande and James Ackman, and Yueyi Zhang for technical help. This work was supported by NIH grant P30 EY000785 to MCC, DZ, NT and ZJZ; R01 EY015788 to MCC, R01 EY012345 to NT; R01 EY014990 to DZ, R01 EY010894 and EY017353 to ZJZ, an RPB Challenge Grant to the Department of Ophthalmology and Visual Science and R01 DA14241 and DA10455 to MRP. MCC also thanks the family of William Ziegler III for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Stein P, Hamberger V. Coordinated motor output in the hindlimb of the 7-day chick embryo. Proc Nat Acad Sci USA. 1975;72:1245–1248. doi: 10.1073/pnas.72.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Feller MB, Shatz CJ, Rokhsar DS. Retinal waves are governed by collective network properties. J Neurosci. 1999;19:3580–3593. doi: 10.1523/JNEUROSCI.19-09-03580.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2006;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Rentería RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason CA, Gogos JA. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol. 2007;17:911–921. doi: 10.1016/j.cub.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo RA, Olsen DP, Yoon KS, Keshishian H. Presynaptic activity and CaMKII modulate retrograde semaphorin signaling and synaptic refinement. Neuron. 2010;68:32–44. doi: 10.1016/j.neuron.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM. Retinal waves are unlikely to instruct the formation of eye- specific retinogeniculate projections. Neural Dev. 2009;4:25. doi: 10.1186/1749-8104-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci. 2005;25:6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. Sperry and Hebb: oil and vinegar? Trends Neurosci. 2003;26:655–661. doi: 10.1016/j.tins.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Crair MC, Ruthazer ES, Gillespie DC, Stryker MP. Relationship between the ocular dominance and orientation maps in visual cortex of monocularly deprived cats. Neuron. 1997;19:307–318. doi: 10.1016/s0896-6273(00)80941-1. [DOI] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Curr. Opin Neurobiol. 1999;9(9):88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- Drager UC, Olsen JF. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol. 1980;191:383–412. doi: 10.1002/cne.901910306. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaün J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984;230:552–575. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;10:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, Meinertzhagen IA, Bellen HJ. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52:247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wang GY, Liets LC, Collins OA, Chapman B, Chalupa LM. Eye-specific retinogeniculate segregation independent of normal neuronal activity. Science. 2003;300:994–998. doi: 10.1126/science.1080694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- King SL, Marks MJ, Grady SR, Caldarone BJ, Koren AO, Mukhin AG, Collins AC, Picciotto MR. Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. J Neurosci. 2003;23:3837–3843. doi: 10.1523/JNEUROSCI.23-09-03837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Jiang Y, Kawanami A, Cameron DB, Komuro H. Autonomous turning of cerebellar granule cells in vitro by intrinsic programs. Dev Biol. 2009;326:237–249. doi: 10.1016/j.ydbio.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Lund JS, Wise RP. The organization of the retinal projection to the dorsal lateral geniculate nucleus in pigmented and albino rats. J Comp Neurol. 1974;158:383–403. doi: 10.1002/cne.901580403. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DDM. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–55. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg TCL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong ROL, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Moretti M, Vailati S, Zoli M, Lippi G, Riganti L, Longhi R, Viegi A, Clementi F, Gotti C. Nicotinic acetylcholine receptor subtypes expression during rat retina development and their regulation by visual experience. Mol Pharmacol. 2004;66:85–96. doi: 10.1124/mol.66.1.85. [DOI] [PubMed] [Google Scholar]
- Morishita H, Hensch T. Critical period revisited: impact on vision. Current Opinion in Neurobiology. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Creutzfeldt C, Cloëz-Tayarani I, Changeux JP, Bonhoeffer T, Hübener M. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. J Neurosci. 2005;25:6921–6928. doi: 10.1523/JNEUROSCI.1555-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Gündisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57:642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Nevin LM, Taylor MR, Baier H. Hardwiring of fine synaptic layers in the zebrafish visual pathway. Neural Dev. 2008;3:36. doi: 10.1186/1749-8104-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Nême N, Südhof TC, Miles R, Gaspar P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;3:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason CA. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci. 2008;31:295–315. doi: 10.1146/annurev.neuro.31.060407.125609. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Rentería RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DT, Lopez JE, Crair MC. Pretarget sorting of retinocollicular axons in the mouse. J Comp Neurol. 2005;49:305–319. doi: 10.1002/cne.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Petros TJ, Mason CA. Switching retinogeniculate axon laterality leads to normal targeting but abnormal eye-specific segregation that is activity dependent. J Neurosci. 2009;29:14855–14863. doi: 10.1523/JNEUROSCI.3462-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci USA. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RD, Crair MC. Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves. J Neurosci. 2008;28:292–303. doi: 10.1523/JNEUROSCI.4276-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. MHC Class I: An unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Stafford BK, Sher A, Litke AM, Feldheim DA. Spatial-temporal patterns of retinal waves underlying activity-dependent refinement of retinofugal projections. Neuron. 2009;64:200–212. doi: 10.1016/j.neuron.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller FB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–33. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller FB. Unbiased analysis of bulk axonal segregation patterns. J Neurosci Methods. 2004;135:17–26. doi: 10.1016/j.jneumeth.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Torborg L, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat Neurosci. 2005;8:72–78. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Südhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rangarajan KV, Lawhn-Heath CA, Sarnaik R, Wang BS, Liu X, Cang J. Direction-specific disruption of subcortical visual behavior and receptive fields in mice lacking the beta2 subunit of nicotinic acetylcholine receptor. J Neurosci. 2009;29:12909–12918. doi: 10.1523/JNEUROSCI.2128-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warland DK, Huberman AD, Chalupa LM. Dynamics of spontaneous activity in the fetal macaque retina during development of retinogeniculate pathways. J Neurosci. 2006;26:5190–5197. doi: 10.1523/JNEUROSCI.0328-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Chen H, Ding Q, Xie ZH, Chen L, Diao L, Wang P, Gan L, Crair MC, Tian N. The immune protein CD3zeta is required for normal development of neural circuits in the retina. Neuron. 2010;65:503–15. doi: 10.1016/j.neuron.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–66. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.