Summary
Autism is often described as a disorder of neural synchronization. However, it is unknown how early in development synchronization abnormalities emerge and whether they are related to the development of early autistic behavioral symptoms. Here, we show that disrupted synchronization is evident in the spontaneous cortical activity of naturally sleeping toddlers with autism, but not in toddlers with language delay or typical development. Toddlers with autism exhibited significantly weaker inter-hemispheric synchronization (i.e. weak “functional connectivity” across the two hemispheres) in putative language areas. The strength of synchronization was positively correlated with verbal ability, negatively correlated with autism severity, and enabled identification of the majority of autistic toddlers (72%) with high accuracy (84%). Disrupted cortical synchronization, therefore, appears to be a notable characteristic of autism neurophysiology that is evident at very early stages of autism development.
Keywords: Autism, Autism spectrum disorders, fMRI, Language, Language Delay, Language development, Developmental Disorders, Resting state, Sleep, Broca, Wernicke, Aphasia, Interhemispheric correlation, Functional connectivity
Introduction
Autism has been hypothesized to arise from the development of abnormal neural networks that exhibit irregular synaptic connectivity and abnormal neural synchronization (Belmonte et al., 2004; Courchesne et al., 2007; Geschwind and Levitt, 2007; Levy et al., 2009). Disrupted synchronization between neural networks located in particular brain areas may give rise to the specific cognitive, social, and sensory behavioral symptoms exhibited by individuals with autism. Supporting evidence for this hypothesis comes from genetic (Geschwind and Levitt, 2007), anatomical (Courchesne et al., 2007), and neuroimaging (Minshew and Keller) studies. Several key questions, however, remain unanswered: 1. How early in development does abnormal synchronization appear? 2. Is abnormal synchronization related to the behavioral symptoms exhibited during early autism development? 3. Is abnormal synchronization specific to a particular cortical system or widespread across multiple brain areas? 4. How consistent is the abnormality across different individuals with autism? Obtaining answers to these questions will not only advance our understanding of autism development, but will also enhance our understanding regarding the importance of synchronization for typical brain development. Here, we used functional magnetic resonance imaging (fMR) to examine these questions.
In the typical brain, neural activity is synchronized/correlated in time across functionally related cortical areas (e.g. visual cortex) not only during the completion of a task (e.g. watching a movie), but also in the complete absence of a task, during rest and sleep (Raichle, 2010). It has been suggested that the strength of spontaneous activity synchronization between two brain areas may offer a measure for the strength of their functional relationship. Indeed, the strongest synchronization is reliably found between areas belonging to a particular functional system (e.g. visual, auditory, motor, or “default mode”) rather than between areas belonging to different functional systems (Damoiseaux et al., 2006; Nir et al., 2008). Since the cortex is functionally organized in a symmetrical manner across the two hemispheres, the strongest synchronization is found between corresponding contralateral locations (e.g. right and left auditory cortex). This form of “inter-hemispheric” synchronization is evident even in newborn infants (Fransson et al., 2007; Gao et al., 2009). Recent studies in adults have suggested that reduced synchronization between particular cortical areas characterizes particular brain disorders such as Alzheimer’s disease (Greicius et al., 2004), schizophrenia (Bluhm et al., 2009), loss of consciousness (Vanhaudenhuyse et al.), and autism (Anderson et al., 2010; Cherkassky et al., 2006; Kennedy and Courchesne, 2008). These studies have suggested that the neural pathologies associated with each disorder may reveal themselves in particular synchronization abnormalities between specific brain areas, thereby offering possible insight into the characteristics of the underlying pathology and/or a possible biological marker that may aid in the diagnosis of the disorder.
It is challenging to measure brain activity in awake toddlers because of their inability to remain still. Several studies have successfully measured brain activity in typically developing toddlers under anesthesia (Kiviniemi et al., 2000), mild sedation (Fransson et al., 2007), or during natural sleep (Gao et al., 2009; Liu et al., 2008). Here, we report fMRI data acquired from 72 naturally sleeping toddlers (1-3.5 years old) who were either typically developing, language delayed, or autistic. Compared to both other groups, toddlers with autism exhibited significantly weaker inter-hemispheric correlations in inferior frontal gyrus (IFG) and superior temporal gyrus (STG), two areas commonly associated with language production and comprehension. Inter-hemispheric synchronization strength was positively correlated with verbal ability, negatively correlated with autism severity, and enabled accurate identification of autistic toddlers with high sensitivity (72%) and specificity (84%). These results suggest that poor neural synchronization is a notable neurophysiological characteristic that is evident at the earliest stages of autism development and is related to the severity of behavioral symptoms. Finally, the ability to measure this characteristic during sleep, where task compliance and subject cooperation are not required, suggests its utility as a possible diagnostic measure to aid growing efforts of identifying autism during infancy (Pierce et al., 2009; Zwaigenbaum et al., 2009).
Results
The data presented in this study were gathered from several studies performed at the Autism Center of Excellence (ACE) in San Diego. In all scans, toddlers were presented with blocks of soft auditory stimuli that were interleaved with silence. To ensure that the differences in synchronization between the groups were not due to differences in possible auditory evoked responses, we first “regressed out” the experiment structure from the data of each subject (see Experimental procedures). This ensured that there was zero correlation between each voxel’s timecourse and the experiment structure, effectively removing stimulus-evoked responses, while leaving spontaneous fMRI fluctuations in the data (see analyses below).
Spatial selectivity of inter-hemispheric synchronization
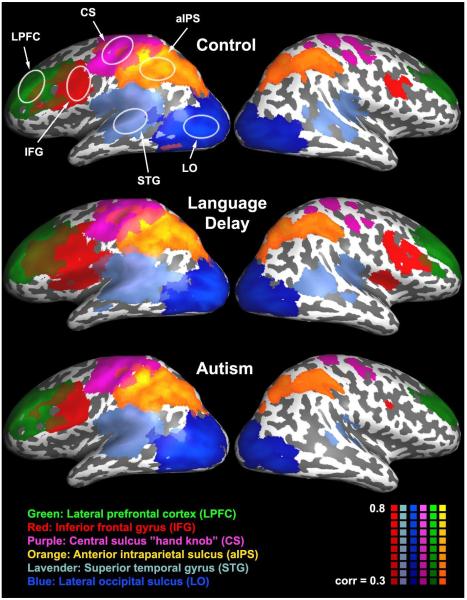
Spontaneous fMRI activity during natural sleep exhibited robust and spatially selective correlations between homologous locations across the two hemispheres. To demonstrate this, we sampled activity in six left hemisphere “seed” regions of interest (ROIs) and computed the correlation between each “seed” timecourse and the timecourse of every voxel in the cortex. These voxel-by-voxel correlation values were averaged across individuals of each group to generate six maps per group: one for each seed (Figure 1). The six seed ROIs selected for this analysis were defined in the left hemisphere according to anatomical criteria (see Experimental procedures, Figure S1, and Table S1) and included the lateral prefrontal cortex (LPFC), posterior part of inferior frontal gyrus (IFG), “hand knob” area of central sulcus (CS), anterior intraparietal sulcus (aIPS), posterior part of superior temporal gyrus (STG), and lateral occipital sulcus (LO). Selecting right hemisphere ROIs would have yielded a complementary analysis with equivalent findings.
Figure 1.
Correlation maps averaged across toddlers from the typically-developing (top), language delay (middle), and autism (bottom) groups. fMRI activity during natural sleep was sampled in six left hemisphere “seed” locations outlined by white ellipses: Lateral prefrontal cortex (LPLC), inferior frontal gyrus (IFG), “hand knob” area of central sulcus (CS), anterior intraparietal sulcus (aIPS), superior temporal gyrus (STG), and lateral occipital sulcus (LO). Each color represents voxels that exhibited strong correlation (above 0.3) with a particular seed. Note the spatial selectivity of the correlations in all groups. Only voxels located close to the seed’s location in the left hemisphere and the corresponding contralateral location in the right hemisphere exhibited strong correlation values.
Strong correlations with the seed timecourse were found in voxels adjacent to the location of the seed (white ellipses, Figure 1) and in voxels located in the homologous area of the contralateral right hemisphere. Note two important points. First, the voxels that exhibited correlation with each seed showed high spatial selectivity with very little overlap across seeds: this means that the spontaneous activity/fluctuations found for each seed and its corresponding contralateral location was relatively unique and different from that found for each of the other seeds and their contralateral locations. Second, the strength and spread of correlation in the contralateral locations are qualitatively similar across groups in all areas except for STG and IFG, which appear disproportionately reduced in the autism group.
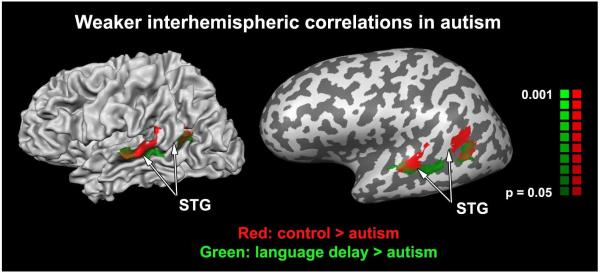
Poor inter-hemispheric synchronization in autism
Voxel-by-voxel comparisons showed that toddlers with autism exhibited significantly weaker inter-hemispheric correlations than both typically developing and language delayed toddlers in the STG, a cortical area commonly associated with language processing (Figure 2). The comparisons of the autism group to the two other groups were independent of one another, yet both revealed significant synchronization differences only in voxels located within the STG. This analysis was performed by first computing the correlation between the timecourse of each left hemisphere voxel with the timecourse of its corresponding contralateral right hemisphere voxel, in each subject. This gave us an inter-hemispheric correlation value for each pair of corresponding left/right voxels, which signified their synchronization strength. We then performed a t-test for each voxel, contrasting the correlation values across individuals of different groups. This analysis yields symmetrical results across the two hemispheres, hence the presentation of the voxel-wise group differences only on the left hemisphere. Presenting the results on the right hemisphere yields a reciprocal “mirror image”.
Figure 2.
Voxels exhibiting weaker inter-hemispheric correlations in the autism group as compared with the typically developing (red) and language delay (green) groups. The two independent comparison maps are overlaid on a folded (left) and inflated (right) left hemisphere of a single individual. Significantly weaker inter-hemispheric correlation was apparent in STG voxels in both comparisons. No voxels exhibited stronger inter-hemispheric correlation in children with autism. STG – superior temporal gyrus.
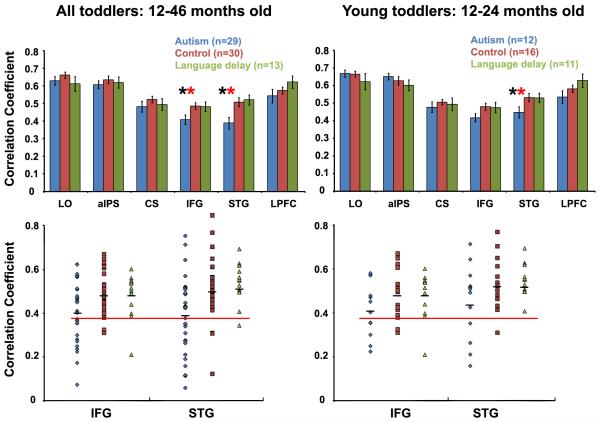
The results found in STG raised the possibility that poor inter-hemispheric synchronization may be a characteristic of the language system in toddlers with autism. To evaluate this further, we performed an ROI analysis in six anatomically defined ROIs that included two putative language areas, STG and IFG, and four control areas, LO, aIPS, CS, and LPFC. The ROI analysis was more sensitive than the voxel-wise analysis reported above since averaging across ROI voxels reduces any spatial noise inherent in the data. The results showed that inter-hemispheric synchronization was indeed significantly weaker in the autism group not only in STG, but also in IFG (p<0.05, randomization test and t-test, see Experimental procedures). None of the control ROIs exhibited significant differences between groups (Figure 3, top). Toddlers with language delay exhibited a trend for stronger synchronization in LPFC, as compared with autism and control groups (p<0.1, randomization test). Similar results were found when comparing only the youngest toddlers (Figure 3, right panels). Synchronization difference remained significant in STG (p<0.05) and was almost significant in IFG (p<0.07).
Figure 3.
Inter-hemispheric correlation strength between right and left ROIs in the autism (blue), typically developing (red), and language delay (green) groups when considering all subjects (left panels) or only the younger toddlers (right panels). Top panels: Average correlation strength in each toddler group for each of the six examined ROIs. The autism group exhibited significantly weaker inter-hemispheric correlation (p < 0.05) only in putative language areas (IFG and STG). When comparing younger toddler groups IFG correlation difference was almost significant (p < 0.07). Error bars: standard error across subjects. Black asterisk: significant difference between autism and control groups. Red asterisk: significant difference between autism and language delay groups. Bottom panels: Single subject correlation values in IFG and STG. The majority of toddlers with autism, but only a small minority of control (red) and language delay (green) toddlers, exhibited IFG or STG correlation values below 0.38 (red line). Black lines: mean correlation across the group.
The ROIs used in this analysis were selected manually in left and right hemispheres and the left hemisphere ROIs were identical to those used in the seed analysis described above (Figure 1). The anatomical criteria used for selection were identical in all groups and there was, therefore, no bias for any of the ROIs to exhibit stronger inter-hemispheric correlations in one group or another. This lack of bias was evident in the equivalent ROI sizes (Figure S1) and locations (Table S1) across groups.
Weak inter-hemispheric correlations in IFG and STG could be used to accurately identify the majority of toddlers with autism (Figure 3, bottom). We performed sensitivity-specificity and receiver operating characteristics (ROC) curve analyses to determine the usefulness of IFG and STG correlations for autism classification (Figure S2). In these analyses, toddlers who exhibited a below threshold correlation value in either IFG or STG were classified as autistic while those exhibiting above threshold correlation values in both IFG and STG were classified as non-autistic (control or language delay). The accuracy of the correlation-based classification was determined by comparing it with the actual clinical diagnosis performed by experienced psychologists. Selecting a correlation threshold/criterion of 0.38 enabled accurate classification of toddlers with autism yielding a sensitivity of 72% and specificity of 84%. In other words, 21 out of 29 toddlers in the autism group were correctly identified, while only 7 (5 control and 2 language delay) out of 43 non-autistic toddlers were mistakenly identified as autistic. When considering only the young toddlers, the same threshold yielded a sensitivity of 60% and specificity of 80%. Interestingly, different subsets of toddlers with autism exhibited poor inter-hemispheric correlation in IFG and in STG.
To ensure that weak inter-hemispheric correlation was not a consequence of our particular choice of ROI voxels, we examined single subject data in the toddlers with autism who exhibited the weakest inter-hemispheric correlations in IFG. We present the results for IFG, but equivalent results were found for STG in the autistic toddlers who exhibited the weakest STG correlations. Using a similar analysis to that described in Figure 1, we sampled the activity in left IFG and searched for correlated voxels throughout the brain (Figure S3). The toddlers did not show any correlated voxels, above a threshold of 0.3, in the vicinity of the contralateral right IFG. Weak inter-hemispheric correlations in these individuals were, therefore, not a consequence of particular IFG ROI location or size.
Synchronization strength and autism severity
There was a significant relationship between synchronization strength and expressive language scores as assessed using the Mullen test (r = 0.53, p < 0.005). This association held only in the autism group and was evident only in IFG (Figure 4), not in STG or any of the other ROIs. There was also a significant inverse relationship between synchronization strength and autism severity. IFG synchronization was significantly anti-correlated with the ADOS communication scores (r = −0.4, p<0.05) and a negative trend was found with the ADOS social scores (r = −0.26, p=0.1). The statistical significance of these correlations was assessed using a randomization test (see Experimental procedures).
Figure 4.
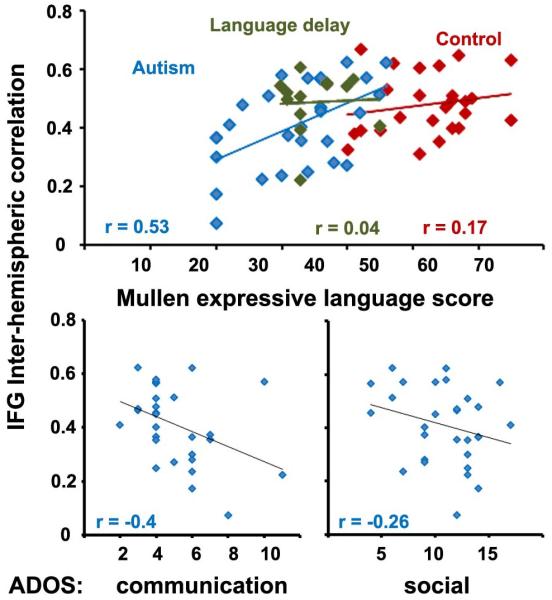
Inter-hemispheric correlation in IFG and verbal ability (top) or autism severity (bottom). Toddlers with autism (blue) showed a significant positive correlation between inter-hemispheric correlation value and their expressive language ability as measured by the Mullen test (top), while typically developing (red) and language delayed toddlers (green) did not. Toddlers with autism exhibited a significant negative correlation between inter-hemispheric correlation and the ADOS communications score (left) while exhibiting a negative trend with the ADOS social scores (right).
Control analyses
We performed several control analyses to rule out alternative interpretations of the results. First, the strength of inter-hemispheric synchrony in IFG did not depend on age in any group (Figure S4, panel A). Second, the spectral power of spontaneous fMRI activity was equivalent at all frequencies across all three groups (Figure S4, panel B). Weaker inter-hemispheric synchrony in IFG of toddlers with autism was, therefore, not a consequence of smaller/weaker spontaneous fluctuations, but rather a reflection of their disrupted temporal synchronization across the hemispheres. Third, the amount of time between sleep onset and the analyzed fMRI scan was equivalent across groups (p > 0.2 for all three between group comparisons, two tailed t-tests). This suggests that the toddlers of all three groups, on average, were in a similar state of sleep. Also note that the synchronization difference was specific to language areas rather than a general property of the whole cortex, which would be expected from a difference in arousal or vigilance. Furthermore, as mentioned above, the amplitude of spontaneous fMRI fluctuations was equivalent across the groups in all ROIs (Figure S4), indicating that there were no general differences in the amount of cortical activity exhibited by the three groups as may be expected in different sleep states.
Finally, we assessed whether there were any residual evoked responses evident in any of the analyzed ROIs despite having projected out the stimulus structure from each voxel. We estimated the fMRI responses in each ROI and each subject group for each of the four auditory stimulus types. Residual evoked responses, if present at all, were minimal and did not differ across the six ROIs or across the groups (Figure S5, panel A). Furthermore, the amplitude of any possible residual evoked responses was an order of magnitude smaller than the amplitude of spontaneous activity (Figure S5, panel B). This reassured us that the reported difference in synchronization between the groups was not driven by responses to the auditory stimuli, but, rather, by fluctuations in spontaneous activity.
Discussion
Our results suggest that reduced neural synchronization is a notable characteristic of autism, evident at very early stages of autism development. Compared with language-delayed and control toddlers, toddlers with autism exhibited significantly weaker inter-hemispheric synchronization in IFG and/or STG, two areas commonly associated with language processing (Figures 2 and 3). Furthermore, in the autism group, IFG synchronization strength was correlated with behavioral scores, scaling positively with language abilities and negatively with autism severity (Figure 4). Whether poor inter-hemispheric synchronization in putative language areas plays a causal role in generating autistic behavioral symptoms cannot be determined by this study. Nevertheless, the fact that poor synchronization was found in the language system of toddlers with autism and not in toddlers with language delay (both groups exhibited similarly low expressive language scores - Figure S6), suggests that reduced synchronization may reflect the existence of a specific pathophysiological mechanism that is unique to autism.
Poor synchronization as an early diagnostic tool
It is remarkable that quantifying the synchronization of spontaneous cortical activity during natural sleep holds such valuable information about the developmental state of a toddler. The majority of the toddlers with autism in our sample (72%) could be identified with high accuracy (84%) by the strength of inter-hemispheric correlation in putative language areas (Figures 3 and S2). These results were obtained when selecting a correlation threshold of 0.38. Raising the threshold would increase the number of identified toddlers with autism (higher sensitivity) at the expense of reduced accuracy (lower specificity). Regardless of the precise threshold chosen, these results suggest that quantifying spontaneous cortical activity during sleep may aid in the early diagnosis of autism and enable earlier intervention (Pierce et al., 2009; Zwaigenbaum et al., 2009). There are many clear advantages to this technique. Scanning during natural sleep does not require subject compliance, eliminating the possibility that group differences in brain activity arise from task differences or behavioral strategies. In fact, in toddlers it is practically the only way of avoiding incessant movement artifacts and random uncontrolled behaviors. Even more importantly, scanning during sleep permits the inclusion of individuals with severe autistic traits who are usually excluded from autism imaging studies. Note that this study is one of a handful of fMRI studies that include individuals with severe autism, a critical requirement for an early diagnostic tool and for thorough evaluation of hypotheses regarding autism neurophysiology.
Poor synchronization as a marker of common pathology
The disruption of synchronization during sleep may be generated by numerous pathophysiological mechanisms including abnormal anatomical connectivity, synaptic function, excitation-inhibition balance, local neural network structure/function, and so forth (Belmonte et al., 2004). The assumption is that these underlying pathophysiological mechanisms also disrupt cortical function during wakefulness, alter perception and behavior, and may generate autistic behavioral symptoms. While our study cannot pinpoint the underlying pathophysiological mechanism/s, the results do suggest that such mechanisms may exist in putative language areas at very early stages of autism development.
Our results are compatible with several recent reports of reduced resting-state synchronization in adolescents and adults with autism (Anderson et al., 2010; Cherkassky et al., 2006; Kennedy and Courchesne, 2008; Monk et al., 2009; Weng et al., 2010). Most importantly, one recent study has reported that adults and adolescents with autism exhibit significantly decreased inter-hemispheric synchronization in multiple cortical areas including a similar IFG area to the one described here (see Figure 3 in (Anderson et al., 2010). One speculative possibility is that reduced inter-hemispheric synchronization found during early autism development may persist and become even more widespread with age. Further studies exploring other aspects of cortical and sub-cortical synchronization are warranted for determining the spatial specificity of synchronization abnormalities throughout autism development.
Converging evidence from multiple fields of neurobiology, not just neuro-imaging, suggests that autism is a disorder of abnormal neural connectivity and synchronization (Levy et al., 2009). Genetic studies have reported abnormalities in genes associated with synaptic formation, maturation, and transmission in autism, which are expected to generate abnormally connected neural networks in individuals with autism (Geschwind and Levitt, 2007; Rubenstein and Merzenich, 2003). Electrophysiology studies in mouse models of autism have reported neural network abnormalities including excitation-inhibition imbalances (Gibson et al., 2008) and abnormal synaptic transmission (Etherton et al., 2009). Anatomical MRI studies have reported increased white matter volumes (Herbert et al., 2004) along with abnormal white matter myelination (Alexander et al., 2007; Ben Bashat et al., 2007). Finally, several fMRI studies in adults and adolescents with autism have reported abnormal synchronization across brain areas under active task conditions (Hasson et al., 2009; Jones et al., 2009) or spontaneously fluctuating during rest/sleep (Anderson et al., 2010; Cherkassky et al., 2006; Kennedy and Courchesne, 2008; Monk et al., 2009; Weng et al., 2010). The emerging hypothesis suggests that the formation of abnormal neural networks exhibiting irregular anatomical connections and/or irregular neural synchronization, leads to the development of autistic behavioral symptoms.
Our study supports this hypothesis in several novel ways. It presents evidence showing that synchronization is disrupted during early autism development (when toddlers are only beginning to manifest autistic behavioral symptoms) and that the extent of disruption is related to the severity of existing symptoms (Figure 4). With this in mind, it is tempting to speculate that early abnormal development marked by disrupted synchronization in key brain areas, such as those mediating language, may be at the core of autism pathophysiology.
Poor synchronization and cortical lateralization
Weak inter-hemispheric synchronization in language areas of toddlers with autism may be a signature of early “abnormal lateralization”. Responses to language seem to be lateralized in typically developing infants (Dehaene-Lambertz et al., 2002; Redcay et al., 2008), yet tend to exhibit reduced amplitudes and/or different lateralization in children with autism (Boddaert et al., 2004; Redcay and Courchesne, 2008). The significance of language lateralization for proper language development and maintenance is unknown (Hickok and Poeppel, 2007). Furthermore, the relationship between functional lateralization during language processing and inter-hemispheric synchronization during rest or sleep is also poorly understood. Spontaneous activity tends to correlate across areas that share a particular function (Fox and Raichle, 2007), suggesting that lateralized cortical systems such as language should exhibit less correlation across hemispheres than bilateral systems such as vision. Indeed, our results show weaker inter-hemispheric correlations in language areas as compared with visual areas across all groups (Figure 3). One might speculate that weaker inter-hemispheric synchronization in language areas of toddlers with autism suggests early “over lateralization” of language function.
Note that the directionality of lateralization to the left or right hemisphere cannot be determined using our data.
Uniqueness to autism
Delayed and impaired language capabilities are a defining hallmark of both autism and language delay diagnoses (DSM-IV-TR, 2000). While both groups exhibited equivalently reduced expressive language abilities in comparison to control toddlers, only those with autism exhibited the social abnormalities indicative of autism, as measured by the ADOS scale (Figure S6), suggesting that weak inter-hemispheric synchronization marks a pathological mechanism that is unique to autism. In the current study we did not include a group of toddlers with developmental delay who exhibit low IQ and lack the social symptoms of autism. It would be important to characterize inter-hemispheric synchronization in this additional group to determine whether the presented results are indeed unique to autism or not. In addition, it would be useful to perform longitudinal studies to determine the predictive value of poor synchronization by assessing the stability of individual autism diagnosis over time.
Final note
We would like to emphasize the importance of studying autism physiology specifically in infants and toddlers, at the developmental period where autistic symptoms and abnormal physiology begin to emerge (Courchesne et al., 2007). Studying early development is critical for understanding autism pathophysiology as it is manifested closer to “critical period” windows of development (Hensch, 2005). Such understanding may reveal novel intervention methods that could be applied prior to the closure of critical period windows before possibly irreversible cortical changes have occurred.
Experimental procedures
Subjects
Seventy-two toddlers participated in this study: twenty-nine with autism (mean age: 29 months, range: 12 to 46), thirteen with language delay (mean age: 19 months, range: 13 to 27), and thirty typically developing controls (mean age: 28 months, range: 13 to 46). All parents provided written informed consent and were paid for their participation. The UCSD human subject research protection program approved all experimental procedures. Toddlers were scanned late at night, during natural sleep, without the use of sedation.
Diagnosis
Toddlers were diagnosed by a clinical psychologist with over 10 years of experience in autism using the three initial modules of the Autism Diagnostic Observation Schedule; toddler, 1, or 2 and the Mullen scale for early learning (Mullen, 1995) (Figure S6). Autism diagnosis was based on clinical judgment and ADOS scores, with those meeting the criteria having a composite ADOS score larger than 10. In all toddlers, behavioral exams were performed within 3 months of the fMRI scan (typically they were performed within the same week). The diagnosis of toddlers with autism who were younger than 24 months at the time of the scan was confirmed at later ages (Table S2). Toddlers in the autism group did not include individuals with PDD-NOS or other less severe forms of autism. Toddlers were diagnosed with language delay if their expressive language score was below 40. On average, the expressive language scores were almost identical across autism and control groups, indicating a similar level of language difficulty/delay. However, only toddlers with autism exhibited the social and communication difficulties assessed by the ADOS test.
Data acquisition and preprocessing
Functional and anatomical data was acquired using a GE 1.5T Signa scanner located at the UCSD Radiology Imaging Laboratory in Sorrento Valley, California. Scanning was performed with a standard GE birdcage head coil used for RF transmit and receive. BOLD contrast was obtained using a T2* - sensitive echo planar imaging sequence (repetition time of 2000-2500 ms with 150-288 time-points in length depending on the precise protocol used, 31 slices, 3×3×3 mm voxels). Anatomical volumes were acquired with a T1-weighted SPGR pulse sequence (.94×.94×1.2 mm). Data was processed with the Brain Voyager software package (R. Goebel, Brain Innovation, Maastricht, The Netherlands). Preprocessing included 3D motion correction and temporal high-pass filtering with a cutoff frequency of 6 cycles per scan. In 18 cases (10 autism, 4 control, and 4 language delay), anecdotal head movements were found and the corresponding time-points were discarded. Functional images were aligned with the anatomical volume, and transformed to the Talairach coordinate system. Data was spatially smoothed using a Gaussian kernel with 8mm width at half height.
Auditory stimuli
Four different types of stimulus protocols were included in this study. All included blocks of auditory stimulation containing words, pseudo words, sentences, tones, or environmental sounds (e.g. train, phone, plane, and dog bark), which were 20-35 seconds in length and were interleaved with rest blocks of equal length. Any possible evoked responses to the stimulus were regressed out of the data as described below.
Regressing out stimulus structure and global mean
To ensure that the analyzed data contained only spontaneous cortical activity and no auditory evoked responses, we regressed out the relevant stimulus structure from each fMRI scan (Jones et al., 2009). This process included building a general linear model (GLM) of the expected hemodynamic responses to the auditory stimuli throughout the scan. We used linear regression to estimate the response amplitude (beta value) in every voxel to each stimulus condition and extracted the residual time course in each voxel. The analyses described throughout the manuscript were performed on these residuals. In a second step, we also regressed out the “global” (average) fMRI time-course across all gray matter voxels. We assumed that this average time-course reflected spontaneous “global” fluctuations due to arousal, heart rate, and respiration (Birn et al., 2006). This step was performed in an identical way to that described above except that here the “global” time course was used in place of the GLM with the resulting residuals describing the variability in each voxel that was not explained by the “global” time course. This analysis was performed separately for each subject.
Region of interest (ROI) definition
We defined six anatomical ROIs individually for each subject, manually selecting voxels along the following anatomical landmarks separately in each hemisphere: 1. Lateral occipital area – voxels surrounding the lateral occipital sulcus, 2. Anterior intraparietal sulcus – voxels surrounding the junction of anterior intraparietal sulcus and post-central sulcus, 3. Motor and somatosensory cortex – voxels surrounding the central sulcus around the “hand knob” landmark, 4. Superior temporal gyrus – voxels in the posterior part of the superior temporal gyrus (commonly referred to as “Wernicke’s area”), 5. Inferior frontal gyrus – voxels in the posterior part of the inferior frontal gyrus (commonly referred to as “Broca’s area”), 6. Lateral prefrontal cortex – voxels in the anterior part of the middle frontal gyrus. An example of ROI selection is described in Figure S1. Table S1 lists the average Talairach coordinates of each ROI in each group and Figure S1 shows a comparison of ROI sizes across the groups.
Seed correlation maps
Spontaneous fMRI activity was averaged across voxels of each left hemisphere ROI to compute six seed time-courses for each subject separately. The correlation between activity in each seed and the activity of every voxel in the cortex was then computed for each subject separately. Voxel-by-voxel correlation values were averaged across subjects of each group and displayed on the inflated brain of a representative subject (Figure 1). The average correlation values were thresholded at 0.3 with voxels exceeding this threshold displayed in distinct colors corresponding to each of the six seeds. A similar analysis was performed with the 7 toddlers exhibiting weakest IFG inter-hemispheric correlations (Figure S3).
Voxel-by-voxel interhemispheric correlation difference maps
To compare inter-hemispheric correlation strength across the groups, we first computed, separately for each subject, the correlation between the timecourses of each left-hemisphere voxel and its corresponding contralateral right-hemisphere voxel (determined by their Talairach X coordinate). This yielded a voxel-by-voxel measure of inter-hemispheric correlation for each subject, which was compared across groups using a random-effects analysis. Correlation values were normalized using the Fisher Transform and then two tailed t-tests were used to identify voxels with statistically significant between-group differences in correlation (Figure 2). Only voxel clusters exceeding 50mm3 are displayed in the statistical map, which was overlaid on the inflated anatomy of an exemplar subject.
ROI Correlation analysis
Spontaneous activity was averaged across voxels to compute a single timecourse for each ROI in each hemisphere. The correlation between timecourses of right and left ROIs was computed for each subject separately and then averaged across subjects of each group. We used both standard t-tests and randomization tests to assess the significance of differences in correlation values across the three groups (Figure 3). Randomization tests were carried out by generating a distribution of correlation differences for each pair of groups, according to the null hypothesis that there was no difference between groups, by randomly assigning individuals to either subject group (i.e., randomly shuffling subject identities). This randomization was repeated 10,000 times separately for each ROI to characterize ROI-specific randomized distributions. For the correlation difference between autism and either comparison group to be considered statistically significant, it had to fall above the 95th percentile of the relevant distribution (analogous to a one tailed t-test). Note that this statistical test does not assume that data are normally distributed and is, therefore, more conservative than a standard t-test. This was evident in that significance was always weaker when assessed with the former compared with the latter. The reported weaker inter-hemispheric correlations in autism (Figure 3) were significant using either statistical test.
The correlation between synchronization strength and behavioral measures (i.e. Mullen or ADOS scores, Figure 4) was computed for each ROI across individuals of each group separately. The statistical significance of these correlations was also determined using both randomization and t-test analyses. Here, the behavioral measures were shuffled across subjects to determine a distribution of correlation values expected by chance. For the real correlation to be considered significant, it had to exceed the 95th percentile of this random distribution. The reported significant relationships between synchronization strength and behavioral measures were significant when assessed with either statistical test.
Trigger average analysis
To determine whether there were any residual auditory evoked responses in the analyzed ROIs, we performed a “trigger average analysis”. Segments of data corresponding to the different blocks of stimulation were extracted, aligned to stimulus onset, and averaged. There were no visible BOLD increases at stimulus onset as would be expected from a stimulus evoked response in any of the ROIs or any of the groups (Figure S5).
Supplementary Material
Acknowledgments
Supported by NIMH Autism Center of Excellence grant P50-MH081755 (EC), NIMH R01-MH080134 (KP), NIMH R01-MH036840 (EC), NIH grant F31-MH080457 (ID), ISF and Bikura grants (RM), Pennsylvania Department of Health SAP grant 4100047862, NICHD/NIDCD PO1/U19, and Simons Foundation SFARI grant (MB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, Dubray MB, Lange N, Alexander AL, Abildskov T, Nielsen JA, Cariello AN, Cooperrider JR, et al. Decreased Interhemispheric Functional Connectivity in Autism. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson PC. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Belin P, Bourgeois M, Royer V, Barthelemy C, Mouren-Simeoni MC, Philippe A, Brunelle F, Samson Y, Zilbovicius M. Perception of complex sounds in autism: abnormal auditory cortical processing in children. Am J Psychiatry. 2004;161:2117–2120. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687, 1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- DSM-IV-TR . Diagnostic and statistical manual of mental disorders. American Psychiatric Press Inc.; Washington DC: 2000. [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Gelbard H, Vallines I, Harel M, Minshew N, Behrmann M. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2009;2:220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2009;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Paakko E, Oikarinen J, Vainionpaa V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med. 2000;44:373–378. doi: 10.1002/1522-2594(200009)44:3<373::aid-mrm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Flax JF, Guise KG, Sukul V, Benasich AA. Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Res. 2008;1223:42–49. doi: 10.1016/j.brainres.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol. 2010;23:124–130. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. American Guidance Services, Inc.; Circle Pines: 1995. [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld MY, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11:1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Glatt SJ, Liptak GS, McIntyre LL. The power and promise of identifying autism early: insights from the search for clinical and biological markers. Ann Clin Psychiatry. 2009;21:132–147. [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11:237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, Soddu A, Perlbarg V, Ledoux D, Brichant JF, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, Chawarska K, Constantino J, Dawson G, Dobkins K, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.