Abstract
Adiponectin and resistin’s possible roles in weight regulation have received little attention. We tested the hypothesis that adipokine levels predict future weight gain in women in the Nurses’ Health Study. Among women who provided blood samples in 1990, we studied 1063 women who did not develop diabetes (‘healthy’) and 984 women who subsequently developed diabetes. Total and high molecular weight (HMW) adiponectin and resistin levels were measured using ELISA. Women who did not developed diabetes had a mean body mass index (BMI) of 26.3±6.0kg/m2 at baseline and gained 2.0±6.1kg over 4 years. Women who developed diabetes had a mean BMI of 30.1±5.4kg/m2 at baseline, and gained 2.4±7.1kg over 4 years. In women who did not developed diabetes, higher baseline levels of total and HMW adiponectin were associated with significantly greater weight gain after adjustment for age, BMI, physical activity, diet, and other covariates: women in the highest quintile of total adiponectin gained 3.18 kg compared to women in the lowest quintile who gained 0.80 kg (fully adjusted; p for trend<0.0001). Adiponectin was not significantly associated with weight gain in women who subsequently developed diabetes. Resistin levels were not associated with weight gain in either women who did or did not developed diabetes during the follow-up. We conclude that elevated adiponectin levels are associated with higher weight gain in healthy women, independently of confounding risk factors. High adiponectin production by adipocytes might be a sign of “healthy” adipose tissue with further capacity to store fat.
Introduction
Adipose tissue is now considered as a major endocrine organ and is the source of many proteins and hormones called adipokines. Among them, adiponectin is produced almost exclusively by adipocytes. Circulating levels are inversely correlated with obesity, insulin resistance, and risk of developing diabetes (1,2). Animal studies have raised the possibility that adiponectin has anti-inflammatory and insulin sensitizing proprieties, but very little attention has been directed toward possible weight-regulating functions. A few prospective human studies have tested the association between adiponectin levels and subsequent weight change (3,4,5), but were inconclusive. One of the explanations for the absence of significant association could be the confounding effect of existence of pre-disease states (especially ‘pre-diabetes’) in some of the individuals included in the populations that were studied.
In humans, resistin, another adipokine, is mainly produced by macrophages infiltrating adipose tissue in states of excess adiposity. Resistin levels are positively associated with obesity, and with insulin resistance in some studies (1), but the association may be explained by excess weight accumulation. One prospective study in Pima Indians showed that resistin was associated with future increases in percent body fat (6).
With this background in mind, we hypothesized that adiponectin (total and HMW) and resistin levels are associated with subsequent weight change in healthy women (who did not develop diabetes over a long follow up) participating in the Nurses’ Health Study, but not in women who subsequently developed diabetes.
Methods and Procedures
The Nurses’ Health Study was initially established in 1976 and enrolled 121 700 female US nurses who were 30 to 55 years old at baseline. Every two years, women were asked to fill health-related questionnaires. In 1989–1990, 32 826 participants provided blood samples. Women who provided a blood sample were similar to women who did not. In this analysis, we included participants who were free of diabetes, coronary heart disease, stroke, and cancer at the time of the blood sampling. Type 2 diabetes was identified using the biennially mailed questionnaire and was confirmed by a validated supplementary questionnaire about symptoms, diagnostic laboratory test results, and treatment for type 2 diabetes (7). Validation studies have shown a high validity of self-reported diabetes (98.4% of self-reported were confirmed by medical chart review) and low rates of false negatives (0.5%) (8). The total sample for the present analysis included 1063 apparently healthy women who did not develop diabetes and 984 women who developed diabetes over 12 years of prospective follow-up (1990–2002) in a nested type 2 diabetes case-control study. The study was approved by the institutional review board of Brigham and Women’s Hospital, Boston, MA.
Body weight was self-reported at each cycle, including at the time of blood sampling (1989–1990). Based on previous reports, we are confident that self-reported weight is highly accurate and reproducible in the NHS (9). Subsequent weight change from 1990 to 1994 was calculated using reported weight in 1994 minus reported weight in 1990. Recent weight change before baseline (blood samples) was calculated using reported weight in 1990 minus weight in 1988. Body mass index (BMI) was calculated as weight (in kg) divided by the squared height (in m2). Methods for assessment of family history of overweight (10), dietary intake (11), and physical activity (12) have been described previously. Details about cigarette smoking, menopausal status and use of hormonal replacement therapy (HRT) were reported in the 1988 questionnaire. The validity of self-reported body weight, dietary intake, and physical activity levels have been described previously (13). Resistin, total, and HMW adiponectin levels were measured by ELISA as described previously (2).
We present baseline characteristics using means and standard deviations (SD) or percentages for demographic and lifestyle data and adipokine levels using medians and interquartile ranges (IQR). We used Spearman correlations to assess univariate associations between adipokine levels and lifestyle characteristics (as continuous variables). We calculated the weight gain over 4 years for each quintile of total adiponectin levels measured at baseline; we first adjusted models for age and BMI, then for specific baseline variables (family history of overweight, smoking, post-menopausal status, and HRT). Finally, we further adjusted the model for recent weight change (1988–1990) and for lifestyle (diet score, physical activity, alcohol intake). We conducted the same analyses using quintile of HMW adiponectin or resistin levels at baseline. We stratified analyses by healthy women (who remained free of diabetes over follow-up), or in women who subsequently developed diabetes during follow-up. We considered 2-sided p-values <0.05 to be significant. All statistical analyses were conducted with SAS software 9.1 (SAS Institute, Cary, North Carolina).
Results
Baseline characteristics of women who did not develop diabetes and women who developed diabetes over follow-up are presented in Table 1. At baseline, both groups of women were in the middle-aged range (mean age 56 years old). Women who did not develop diabetes had a lower mean BMI at baseline (26.3 kg/m2) than the women who developed diabetes (30.1 kg/m2). The majority had a positive family history of overweight (71% and 79%). Over the 1990–1994 period, the average weight gain was 2.0 kg in ‘healthy’ women (who did not develop diabetes) and 2.4 kg in women who developed diabetes over the follow-up. Other details about demographic characteristics, lifestyle, and adipokine levels are also available in Table 1.
Table 1.
Baseline characteristics in 1989–1990 of women in the Nurses Health Study who did and did not develop diabetes over follow-up
| Characteristics | Women who did not develop diabetes a | Women who developed diabetes a | p-value | ||
|---|---|---|---|---|---|
| Mean (SD) or N,% or median [IQR] | Mean (SD) or N,% or median [IQR] | ||||
| n=1063 d | n=984 e | ||||
|
| |||||
| Age, y | 55.9 (7.0) | 56.1 (7.0) | 0.5 | ||
| Body-mass index, kg/m2 | 26.3 (6.0) | 30.1 (5.4) | <0.0001 | ||
| Weight gain 1990 to 1994 (kg) | 1.99 (6.06) | 2.40 (7.12) | 0.2 | ||
| Family history of overweight/obesity b | 685 | 70.9% | 726 | 79.1% | <0.0001 |
| Current smoking, % | 133 | 12.5% | 131 | 13.3% | 0.6 |
| Postmenopausal, % | 645 | 60.7% | 616 | 62.6% | 0.4 |
| Current postmenopausal hormone use, % | 393 | 37.3% | 281 | 29.0% | <0.0001 |
| Physical activity, metabolic equivalents/week | 15.5 (25.2) | 12.2 (15.0) | 0.0003 | ||
| Dietary intake | |||||
| Diet score c | 7.99 (2.69) | 8.01 (2.77) | 0.9 | ||
| Cereal fiber, g/day | 4.96 (2.60) | 4.54 (2.19) | <0.0001 | ||
| Glycemic load | 102.0 (17.9) | 102.1 (16.2) | 0.9 | ||
| Trans fatty acids, g/d | 2.54 (0.82) | 2.64 (0.82) | 0.005 | ||
| Polyunsaturated/saturated fatty acids ratio | 0.57 (0.18) | 0.56 (0.15) | 0.09 | ||
| Alcohol, g/day | 5.65 (9.12) | 3.43 (7.21) | <0.0001 | ||
| Total energy, kcal/day | 1769.8 (468.8) | 1827.2 (504.4) | 0.008 | ||
| Total adiponectin, μg/mL (median [IQR]) | 18.1 [10.0] | 11.4 [8.8] | <0.0001 | ||
| High-molecular weight adiponectin, μg/mL (median [IQR]) | 6.66 [5.61] | 3.58 [3.63] | <0.0001 | ||
| Resistin, ng/mL (median [IQR]) | 15.2 [9.8] | 16.4 [10.9] | <0.0001 | ||
diabetes incidence was assessed from 1990 until 2002
self-reported, at least one parent obese, defined by use of pictograms
Diet score = sum of Cereal fiber quintile, Trans fatty acids quintile, Polyunsaturated/saturated fatty acids ratio quintile, Glycemic load quintile (Trans fatty acids and Glycemic load scored in reverse, lowest quintile=4)
for women who did not develop diabetes: N=1054 for post-menopausal hormonal use and dietary intake data; n=1048 for physical activity data; n=966 for family history of overweight/obesity
for women who develop diabetes: N=968 for post-menopausal hormonal use; N=976 for dietary intake data; n=974 for physical activity data; n=918 for family history of overweight/obesity
Total and HMW adiponectin levels in women who did not develop diabetes
Greater weight gain was associated with higher total (Pearson r=0.10, p=0.001) and HMW adiponectin (r=0.09; p=0.006) levels at baseline in women who did not develop diabetes (Table 2). As expected, adiponectin levels at baseline were negatively associated with recent weight change (r= −0.08; p= 0.007), baseline BMI (r= −0.33; p<0.0001) and waist circumference (r= −0.32; p<0.0001). Higher levels of physical activity, better diet score, and alcohol intake were positively associated with higher total or HMW adiponectin levels in women who did not develop diabetes (Table 2).
Table 2.
Spearman correlations between characteristics and total adiponectin, HMW adiponectin, and resistin levels in women who did not develop diabetes and in women who developed diabetes in the NHS.
| Characteristics | Total adiponectin | HMW adiponectin | Resistin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women who did not develop diabetes | Women who developed diabetes | Women who did not develop diabetes | Women who developed diabetes | Women who did not develop diabetes | Women who developed diabetes | |||||||
| R | p-value | R | p-value | R | p-value | R | p-value | R | p-value | R | p-value | |
| Subsequent weight change from 1990–1994 (kg) | 0.10 | 0.001 | 0.04 | 0.23 | 0.09 | 0.006 | 0.07 | 0.04 | 0.03 | 0.29 | 0.01 | 0.66 |
| Recent weight change 1988–1990 (kg) | −0.08 | 0.007 | −0.01 | 0.65 | −0.05 | 0.13 | −0.02 | 0.49 | 0.01 | 0.64 | 0.05 | 0.14 |
| Age (years) | 0.15 | <.0001 | 0.19 | <.0001 | 0.14 | <.0001 | 0.18 | <.0001 | −0.08 | 0.01 | −0.09 | 0.01 |
| Body-mass index (kg/m2) | −0.33 | <.0001 | −0.20 | <.0001 | −0.35 | <.0001 | −0.23 | <.0001 | 0.22 | <.0001 | 0.13 | <.0001 |
| Waist circumference (cm) | −0.32 | <.0001 | −0.20 | <.0001 | −0.33 | <.0001 | −0.22 | <.0001 | 0.20 | <.0001 | 0.10 | 0.01 |
| Physical activity (metabolic equivalents/week) | 0.10 | 0.001 | 0.06 | 0.05 | 0.13 | <.0001 | 0.07 | 0.02 | −0.13 | <.0001 | −0.04 | 0.24 |
| Dietary intake | ||||||||||||
| Diet score a | 0.12 | <.0001 | 0.06 | 0.06 | 0.12 | <.0001 | 0.04 | 0.23 | −0.17 | <.0001 | −0.08 | 0.01 |
| Cereal fiber (g/day) | 0.09 | 0.004 | 0.02 | 0.55 | 0.08 | 0.01 | 0.02 | 0.63 | −0.12 | <.0001 | −0.06 | 0.05 |
| Glycemic load | −0.03 | 0.29 | −0.02 | 0.44 | −0.04 | 0.25 | −0.03 | 0.40 | 0.005 | 0.86 | −0.03 | 0.35 |
| Trans fatty acids (g/d) | −0.14 | <.0001 | −0.06 | 0.09 | −0.15 | <.0001 | −0.03 | 0.41 | 0.07 | 0.02 | 0.07 | 0.02 |
| Polyunsat/sat fatty acids ratio | 0.00003 | 0.99 | −0.01 | 0.83 | 0.003 | 0.90 | 0.001 | 0.98 | −0.15 | <.0001 | −0.06 | 0.08 |
| Alcohol (g/day) | 0.14 | <.0001 | 0.08 | 0.01 | 0.13 | <.0001 | 0.12 | 0.0002 | −0.07 | 0.03 | −0.04 | 0.19 |
| Total energy (kcal/day) | −0.01 | 0.77 | −0.05 | 0.15 | −0.02 | 0.60 | −0.04 | 0.22 | −0.003 | 0.92 | −0.02 | 0.50 |
Diet score = sum of Cereal fiber quintile, Trans fatty acids quintile, Polyunsaturated/saturated fatty acids ratio quintile, Glycemic load quintile (Trans fatty acids and Glycemic load scored in reverse, lowest quintile=4)
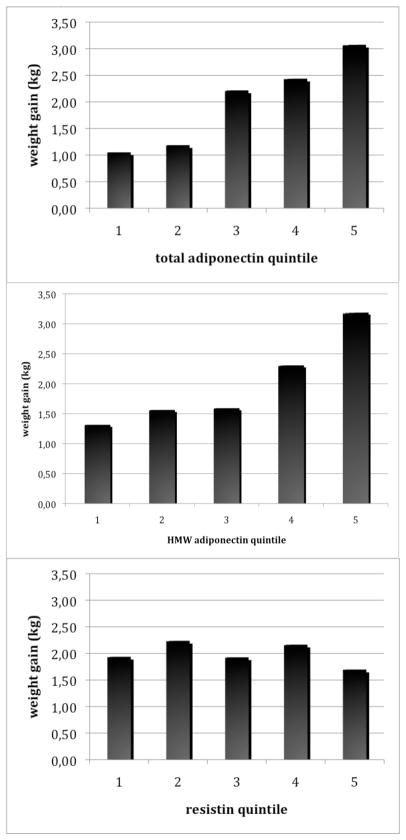
Weight gain increased per quintile of baseline adiponectin levels: women with adiponectin levels in the highest quintile gained 3.08 kg (95% CI=2.25–3.90) compared to women in the lowest quintile who gained 1.05 kg (95% CI=0.21–1.89) in age-BMI adjusted analyses (trend p-value =0.0001; Table 3). The trend across quintile was still highly significant after adjustment for family history of obesity, smoking, and post-menopausal status (p=0.0002) and seemed strengthened after further adjustment for recent weight change and lifestyle factors (p<0.0001; Table 3). Therefore, in the fully adjusted model, women who did not develop diabetes who had adiponectin levels in the highest quintile gained 3.18 kg compared with 0.80 kg for women in the lowest quintile. Multivariable models with HMW adiponectin resulted in similar patterns (Table 3). Mean age-BMI adjusted weight gain per quintile of total and HMW adiponectin in women who did not develop diabetes are illustrated in Figure 1.
Table 3.
Weight gain over 4 years per adipokines quintile at baseline in women who did not develop diabetes over follow-up, adjusted for confounding factors
| Quintile of adipokine concentration in women who remained free of diabetes | P for Trend | |||||
|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | ||
| Total adiponectin | 9.3 (0.05–11.9) | 14.2 (11.9–16.2) | 18.1 (16.2–20.2) | 21.7 (20.2–24.6) | 29.3 (24.7–42.1) | |
| Median (range) μg/mL | ||||||
| Weight gain (kg) crude | 1.31 (0.49–2.13) | 1.19 (0.37–2.00) | 2.23 (1.41–3.06) | 2.33 (1.51–3.16) | 2.91 (2.09–3.72) | 0.001 |
| Age-BMI adjusted | 1.05 (0.21–1.89) | 1.19 (0.38–2.00) | 2.22 (1.40–3.03) | 2.44 (1.61–3.26) | 3.08 (2.25–3.90) | 0.0001 |
| Multivariate model a | 0.96 (0.12–1.80) | 1.59 (0.78–2.40) | 2.32 (1.51–3.14) | 2.50 (1.69–3.31) | 3.13 (2.31–3.94) | 0.0002 |
| Multivariate model b plus lifestyle | 0.80 (−0.05–1.65) | 1.60 (0.80–2.41) | 2.29 (1.47–3.10) | 2.60 (1.78–3.41) | 3.18 (2.36–4.01) | <0.0001 |
| HMW adiponectin | 2.86 (0.24–4.01) | 4.80 (4.02–5.72) | 6.67 (5.73–7.87) | 9.26 (7.90–11.4) | 15.1 (11.5–43.7) | |
| Median (range) μg/mL | ||||||
| Weight gain (kg) crude | 1.46 (0.64–2.28) | 1.64 (0.82–2.46) | 1.67 (0.85–2.50) | 2.20 (1.38–3.02) | 3.00 (2.17–3.82) | 0.006 |
| Age-BMI adjusted | 1.32 (0.46–2.17) | 1.56 (0.75–2.38) | 1.59 (0.77–2.41) | 2.31 (1.49–3.12) | 3.19 (2.35–4.02) | 0.001 |
| Multivariate model a | 1.43 (0.58–2.28) | 1.88 (1.08–2.68) | 1.59 (0.77–2.41) | 2.42 (1.61–3.23) | 3.18 (2.35–4.02) | 0.004 |
| Multivariate model b plus lifestyle | 1.29 (0.44–2.15) | 1.91 (1.11–2.72) | 1.58 (0.76–2.40) | 2.40 (1.59–3.21) | 3.30 (2.46–4.13) | 0.002 |
| Resistin | 8.89 (2.25–10.7) | 12.1 (10.7–13.5) | 15.2 (13.6–16.9) | 19.4 (16.9–22.8) | 30.5 (23.0–131.6) | |
| Median (range) ng/mL | ||||||
| Weight gain (kg) crude | 1.89 (1.07–2.72) | 2.24 (1.41–3.06) | 2.00 (1.18–2.83) | 2.10 (1.28–2.93) | 1.73 (0.91–2.56) | 0.73 |
| Age-BMI adjusted | 1.94 (1.11–2.76) | 2.24 (1.42–3.06) | 1.93 (1.11–2.75) | 2.17 (1.34–2.99) | 1.70 (0.88–2.52) | 0.68 |
| Multivariate model a | 2.14 (1.32–2.96) | 2.23 (1.42–3.05) | 1.93 (1.11–2.75) | 2.22 (1.39–3.04) | 2.02 (1.20–2.83) | 0.84 |
| Multivariate model b plus lifestyle | 2.05 (1.22–2.87) | 2.33 (1.51–3.15) | 1.85 (1.04–2.67) | 2.21 (1.38–3.03) | 2.07 (1.25–2.88) | 0.95 |
adjusted for age, BMI and family history of overweight (1988), smoking (1988), menopausal status (and use of hormonal replacement; 1988)
multivariate model, further adjusted for weight change before, physical activity, diet score, alcohol
Figure 1. Weight gain per quintile of adipokine in women who did not develop diabetes over follow-up.
All analyses were adjusted for age and BMI. P-values for trend across quintile: total adiponectin p=0.0001; HMW adiponectin p=0.001; resistin p=0.68
Total and HMW adiponectin levels in women who developed diabetes
In contrast, weight gain in women who subsequently developed diabetes was not significantly associated with baseline total adiponectin (r= 0.04; p=0.23) and weakly associated with HMW adiponectin (r=0.07; p=0.04). Weight gain per adiponectin quintile (total and HMW) in women who subsequently developed diabetes is illustrated in Figure 2. Similarly, correlations between adiponectin levels and lifestyle characteristics were weaker or not significant in women who developed diabetes compared with correlations observed in women who did not develop diabetes (Table 2). Mean age-BMI adjusted weight gain per quintile of total and HMW adiponectin in women who developed diabetes are illustrated in Figure 2.
Figure 2. Weight gain per quintile of adipokine in women who developed diabetes over follow-up.
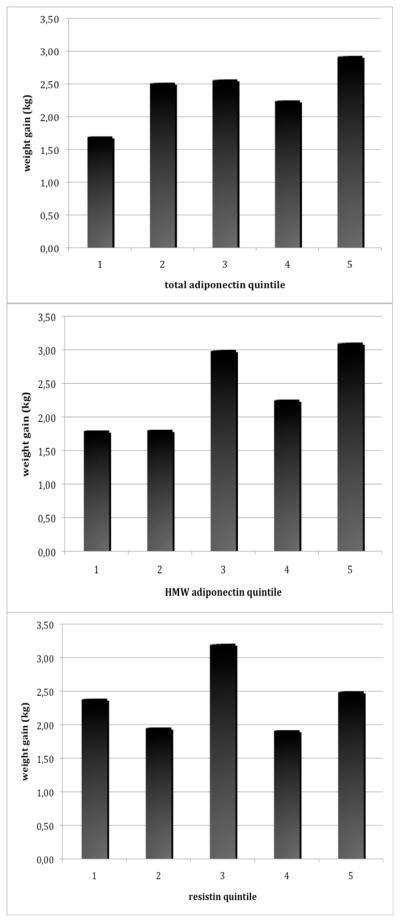
All analyses were adjusted for age and BMI. P-values for trend across quintile: total adiponectin p=0.18; HMW adiponectin p=0.06; resistin p=0.92
Resistin levels
Resistin was not associated with future weight change in women who did not develop diabetes (r=0.03; p=0.29) or in women who developed diabetes (r=0.01; p=0.66). In both groups, higher resistin levels were associated with higher BMI (and waist circumference) and worse diet score; resistin levels were negatively correlated with physical activity levels in women who did not develop diabetes only (Table 2). Inclusion of potential confounders in multivariable models did not reveal any significant association between resistin levels and future weight gain (table 3).
Discussion
We found that baseline elevated total or HMW adiponectin levels were associated with greater subsequent weight gain in women that we could consider as metabolically ‘healthy’: those who did not develop diabetes over a long follow up period. Associations were significant even after adjustment for characteristics known to influence weight change over time. In contrast, adiponectin levels were not associated with weight gain in women who subsequently developed diabetes. Resistin levels were not associated with weight gain in women who did or did not develop diabetes.
Previous epidemiologic studies investigating links between adiponectin levels and subsequent weight change have not seen significant associations (3,4,5). Multiple reasons could explain discrepancies between previous reports and our findings. Relatively small numbers and diverse ethnic backgrounds might explain part of the differences: Vorazova et al. (3) studied Pima Indians while Bennett et al. (5) studied Afro-Jamaicans. The Rancho Bernado Study (4) included a larger number of whites, but the study differed in that the population was elderly (mean age 75 at baseline) and most of the individuals showed spontaneous weight loss (and not weight gain) where concomitant diseases might have influenced the levels of adipokines and/or subsequent weight change. Indeed, the association could be confounded by presence of pre-disease states and be observable only in a ‘healthy’ population, as illustrated here in women who did not develop diabetes in the NHS. We have excluded women with diabetes, cardiovascular diseases, or cancer at baseline and have used data from women who did not develop diabetes over a long period of follow-up after blood sampling, so we are confident that our population is generally metabolically healthy and that the variation in weight represents “natural weight change” in free-living humans.
The positive association of elevated adiponectin levels with higher weight gain might be surprising to some degree, adiponectin being viewed as a ‘good’ adipokine, protecting against diabetes. We proposed that in ‘healthy’ individuals, higher adiponectin levels reflect ‘healthier’ adipocytes fully capable of their main function, that is, fat storage. Adipocytes need to be fully differentiated to produce adiponectin (14). In vitro studies have shown that as adipocytes age and accumulate intracellular triglycerides, they become hypertrophic and dysfunctional: they slow down triglyceride uptake, are less insulin-sensitive, and decrease adiponectin expression and secretion (15). This is concordant with adipose tissue biopsies from discordant twins demonstrating lower adiponectin mRNA in the heavier compared with the leaner twin (16). This is in line with elevated adiponectin levels in humans being a sign of healthy adipose tissue and its capacity to adapt to more fat accumulation.
In contrast, in women predisposed to develop diabetes, associations between adiponectin levels and weight gain were not as clear; this could be explained by metabolically dysfunctional adipose tissue appearing early in the pre-disease stage of diabetes. We know that individuals have different capacities to accumulate extra adipose tissue, and that for a similar level of adiposity we can observe very large variations in metabolic consequences (17). As a result, each individual may accumulate a certain amount of extra fat before adipose tissue becomes dysfunctional, meaning it has lower capacity to store energy efficiently, and more fat accumulates in peripheral sites, both hallmarks of insulin resistance states. In our analyses of women who subsequently developed diabetes, we can extrapolate that adiponectin production was altered by some degree of insulin resistance already present at the time of blood sampling, reflecting adipocyte dysfunction (and impaired capacity to store fat in the peripheral adipose tissue) well before the appearance of type 2 diabetes. In rhesus monkeys developing obesity and type 2 diabetes, it has been shown that adiponectin levels decrease very early in the weight gain process, in parallel with decrease insulin sensitivity, years before change in fasting glucose (18). We believe that a decrease in adiponectin level is an early sign of dysfunctional adipose tissue. In line with this hypothesis, our data show that recent previous weight gain was associated with lower adiponectin levels. It is also possible that adiponectin has local paracrine and/or endocrine effects to increase weight gain, but our data cannot directly assess this possibility.
We did not observe any association of resistin levels with subsequent weight change, as others have suggested (19). This is in contrast with the study conducted in Pima Indians that demonstrated that resistin levels were associated with future increases in percent body fat (6). As mentioned before, differences in ancestral background might have modified associations. In humans, resistin seems to be mainly produced by macrophages infiltrating the adipose tissue in states of excess weight; by consequence resistin cannot be viewed as a direct reflection of adipocyte function and serves as a negative control in our analyses. Interestingly, resistin levels were associated with lifestyle in the expected direction: high physical activity and better diet score were associated with lower resistin levels.
Our study has many strengths including a prospective design, a large sample insuring adequate power, validated questionnaires and inclusion of ‘healthy’ women to exclude confounding by pre-disease states. Our study also has limitations. Weights and heights were self-reported; however self-reported weights/heights in the NHS study were demonstrated to be valid (13). We had only a one-time measurement of adipokine levels; but one-time measurement of adipokines has been shown to produce reliable measures of exposure (20). The NHS is a cohort of mainly white, well-educated women, so our findings might not be generalizable to other populations.
In conclusion, elevated adiponectin levels are associated with greater subsequent weight gain in ‘healthy’ women, independently of baseline BMI, recent weight change, and lifestyle. Inter-individual variation in adaptation to fat accumulation is well known: some individuals develop adipose tissue dysfunction and insulin resistance while others are still “metabolically healthy” at a similar BMI. Healthy adipocytes produce high levels of adiponectin and so circulating levels of adiponectin could be a good sign of functionality of adipose tissue, including capacity to further accumulate triglycerides. Longitudinal serial measurement of adiponectin levels, peripheral insulin resistance and weight change will be necessary to test this hypothesis.
Acknowledgments
The data used is this report was taken from the Nurses’ Health Study, which is located in the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School. The National institutes of Health and the Intramural Research Program of the National Institute of Child Health and Human Development provided funding for this study (NIH grant DK58845). The funding source had no role in the collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication. JBM was supported by a Career Development Award from the American Diabetes Association and by NIDDK K24 DK080140. JBM currently has a research grant from GlaxoSmithKline, and has a consulting agreement with Interleukin Genetics, Inc. CSM is supported by NIH grants DK58785, DK79929, DK 081913, DK58845 and a discretionary grant from BIDMC.
Footnotes
Disclosure: The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2008;93:3165–3172. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vozarova B, Stefan N, Lindsay RS, et al. Low plasma adiponectin concentrations do not predict weight gain in humans. Diabetes. 2002;51:2964–2967. doi: 10.2337/diabetes.51.10.2964. [DOI] [PubMed] [Google Scholar]
- 4.Langenberg C, Bergstrom J, Laughlin GA, et al. Ghrelin, adiponectin, and leptin do not predict long-term changes in weight and body mass index in older adults: longitudinal analysis of the Rancho Bernardo cohort. Am J Epidemiol. 2005;162:1189–1197. doi: 10.1093/aje/kwi338. [DOI] [PubMed] [Google Scholar]
- 5.Bennett NR, Boyne MS, Cooper RS, et al. Impact of adiponectin and ghrelin on incident glucose intolerance and on weight change. Clin Endocrinol (Oxf) 2009;70:408–414. doi: 10.1111/j.1365-2265.2008.03344.x. [DOI] [PubMed] [Google Scholar]
- 6.Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, et al. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes. 2004;53:1279–1284. doi: 10.2337/diabetes.53.5.1279. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Meigs JB, Li TY, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 8.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 9.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 10.Field AE, Willett WC, Lissner L, et al. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity. 2007;15:967–976. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 11.Willett WC, Stampfer MJ. Implications of total energy intake for epidemiologic analysis. In: Willett WC, editor. Nutritional Epidemiology. Oxford University Press; Oxford: 1998. [Google Scholar]
- 12.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nature Reviews Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 14.Korner A, Wabitsch M, Seidel B, et al. Adiponectin expression in humans is dependent on differentiation of adipocytes and down-regulated by humoral serum components of high molecular weight. Biochemical & Biophysical Research Communications. 2005;337:540–550. doi: 10.1016/j.bbrc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 15.Yu YH, Zhu H. Chronological changes in metabolism and functions of cultured adipocytes: a hypothesis for cell aging in mature adipocytes. American Journal of Physiology - Endocrinology & Metabolism. 2004;286:E402–10. doi: 10.1152/ajpendo.00247.2003. [DOI] [PubMed] [Google Scholar]
- 16.Pietilainen KH, Kannisto K, Korsheninnikova E, et al. Acquired obesity increases CD68 and tumor necrosis factor-alpha and decreases adiponectin gene expression in adipose tissue: a study in monozygotic twins. Journal of Clinical Endocrinology & Metabolism. 2006;91:2776–2781. doi: 10.1210/jc.2005-2848. [DOI] [PubMed] [Google Scholar]
- 17.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. Journal of Clinical Endocrinology & Metabolism. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 18.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Chan JL, Yiannakouris N, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. Journal of Clinical Endocrinology & Metabolism. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RC, Ho GY, Xue X, et al. Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:1291–1293. doi: 10.1158/1055-9965.EPI-06-1089. [DOI] [PubMed] [Google Scholar]