Abstract
Finite-element analysis was used to investigate the extent of bias in the ichnological fossil record attributable to body mass. Virtual tracks were simulated for four dinosaur taxa of different sizes (Struthiomimus, Tyrannosaurus, Brachiosaurus and Edmontosaurus), in a range of substrate conditions. Outlines of autopodia were generated based upon osteology and published soft-tissue reconstructions. Loads were applied vertically to the feet equivalent to the weight of the animal, and distributed accordingly to fore- and hindlimbs where relevant. Ideal, semi-infinite elastic–plastic substrates displayed a ‘Goldilocks’ quality where only a narrow range of loads could produce tracks, given that small animals failed to indent the substrate, and larger animals would be unable to traverse the area without becoming mired. If a firm subsurface layer is assumed, a more complete assemblage is possible, though there is a strong bias towards larger, heavier animals. The depths of fossil tracks within an assemblage may indicate thicknesses of mechanically distinct substrate layers at the time of track formation, even when the lithified strata appear compositionally homogeneous. This work increases the effectiveness of using vertebrate tracks as palaeoenvironmental indicators in terms of inferring substrate conditions at the time of track formation. Additionally, simulated undertracks are examined, and it is shown that complex deformation beneath the foot may not be indicative of limb kinematics as has been previously interpreted, but instead ridges and undulations at the base of a track may be a function of sediment displacement vectors and pedal morphology.
Keywords: footprint, finite-element analysis, trackway, computer modelling, dinosaur
1. Introduction
The sample we have of the body fossil record is notoriously incomplete [1–4] and may be fundamentally biased by environmental and taxon-specific factors that potentially hamper our interpretation of ecological and evolutionary dynamics through deep time [5–8]. Interdependent environmental and taxon-specific biases are equally likely to affect the ichnological, or trace fossil, record. The potential for systematic bias towards ichnofossils produced by larger animals has previously been recognized in the field of vertebrate palaeoichnology, and particularly in the dinosaur track record [9]. Sites preserving only the tracks of very large saurischian dinosaurs (i.e. track lengths greater than 0.5 m) are generally recognized as size-biased assemblages and presumed to represent sedimentary conditions in which only animals above a certain threshold of body mass were capable of producing recognizable tracks [9]. However, beyond this supposition, the influence of body size on the recorded diversity of vertebrate ichnofossil assemblages is poorly understood both qualitatively and quantitatively. It is therefore imperative that the process of track formation and the variables associated with environment and animal biology are investigated.
The relationship between the size of an animal and the load applied to the substrate is not straightforward. Given that pressure is a measure of force over area, the resultant pressure exerted on the sediment surface is a function not only of the animal's mass (as weight), but also the geometry of the autopodia. Quadrupedal animals benefit from more feet in contact with the ground, further reducing the load on the substrate beneath any single foot, as compared with a similar-sized biped. In addition to size, foot morphology also plays an important role in determining the magnitude of deformation expressed as the depth of a track. Differing shapes present different paths for sediment movement, resulting in variable distributions of force that affect the extent to which any given foot may indent a substrate [10,11]. These considerations are not trivial if data on fossil track occurrences and abundances are to be used in ‘higher level’ [12] palaeobiological and palaeoecological inferences. Allen [13] noted over a decade ago that a widespread understanding of track formation lagged behind knowledge of anatomical aspects and distributions of fossil tracks, and despite a number of rigorous experimental studies in the intervening years [14–20], this still remains the case.
Among vertebrates, the Dinosauria represent a useful model system for studying track formation; their high taxonomic diversity and long evolutionary history yield an array of disparate foot morphologies and a huge range in body mass with which to test for possible biological factors underpinning preservational bias. The group contains small and large obligate bipeds, quadrupeds and supposed intermediate locomotor strategists (e.g. facultative bipedalism [21–23]) that may have exerted different underfoot pressures according to foot geometry and body shape (i.e. mass distribution). Coupled with a vast quantity of research describing dinosaur tracks spanning more than a century and a half [24–26], dinosaurs provide the ideal basis on which to further our understanding of fossil track formation, and the size-related biases associated therewith.
In this paper, information on foot anatomy and mass distribution from osteological evidence and soft tissue reconstructions are integrated with geotechnical theory and computer simulation to explore the potential for size bias in the vertebrate track record. In addition, features related to underfoot pressure and foot morphology were examined in surface and subsurface planes (true tracks and undertracks). Previous work on track formation using computer simulation has explored independently the effects of substrate consistency [27], foot anatomy [11] and force [28]. This paper aims to present a combined study in which the quantifiable variables of track formation are considered as a whole system, in the hope of elucidating aspects of preservational bias inherent in the fossil track record.
2. Methods
The following experiments used parallel finite-element analysis (FEA) software developed by authors Margetts and Falkingham, using the freely available ParaFEM libraries (www.parafem.org.uk) to model track formation [11,27–29]. A number of dinosaur tracks were simulated over a range of substrates in order to explore bias in their formation resulting from substrate- or taxon-specific factors.
2.1. The virtual foot
Four dinosaurs (Struthiomimus, Edmontosaurus, Tyrannosaurus and Brachiosaurus) were chosen to create a varied virtual track assemblage on a cohesive substrate, representing a range of body masses, and including obligate bipeds, an obligate quadruped and a facultative biped (table 1). These particular taxa were also chosen because they all have published data on body mass and centre of mass (CM) position [30,31], and represent a wide range in size, mass and autopodial morphology. The taxa were not selected in order to create some geotemporally correct track assemblage.
Table 1.
Mass, weight, foot metrics and pressures used to represent various dinosaur taxa used in this study. Data for Struthiomimus, Edmontosaurus and Tyrannosaurus from Bates et al. [30], and data for Brachiosaurus from Henderson [31].
| trackmaker | mass (kg) | force (kN) | foot length (m) | foot surface area (m2) | pressure (kN m−2) |
|---|---|---|---|---|---|
| Struthiomimus | 423 | 4.15 | 0.336 | 0.026 | 161.21 |
| Edmontosaurus (biped) | 813 | 7.98 | 0.29 | 0.052 | 151.92 |
| Edmontosaurus Quadruped manus | 813 | 2.55 | 0.12 | 0.011 | 241.39 |
| Edmontosaurus Quadruped pes | 813 | 5.42 | 0.29 | 0.052 | 103.31 |
| Tyrannosaurus | 7654 | 75.09 | 0.72 | 0.234 | 320.26 |
| Brachiosaurus manus | 25 922 | 95.11 | 0.6 | 0.144 | 662.29 |
| Brachiosaurus pes | 25 922 | 159.19 | 0.87 | 0.401 | 396.58 |
| Edmontosaurusa | 813 | 7.98 | n.a. | 0.063 | 126.46 |
| Brachiosaurusa | 25 922 | 254.29 | n.a. | 0.545 | 466.59 |
aEdmontosaurus and Brachiosaurus are also shown with pressure values from manus and pes combined.
A track is formed through the interaction of three factors; force, foot anatomy and substrate [32,33]. Force applied and foot anatomy are both dependent upon the track maker. To apply a reasonable force, the body mass for each dinosaur was taken from the literature ([30,31]; see table 1). The reader is directed to Bates et al. [30] for a comprehensive discussion on the confidence of CM and body mass reconstructions. Animals spend a very small proportion of their time moving at anything more than a walking speed, and it would therefore be expected that most tracks are made by walking animals. Indeed, this is corroborated by the numbers of trackways showing walking, rather than running gaits [34,35]. Stride length is positively correlated with speed [36,37], meaning that at low speeds, the hip joint and CM will move a shorter distance horizontally from the contact between the foot and the ground, resulting in a smaller angle of ground reaction force (GRF) [37]. As such, for the purposes of this paper, a purely vertical component to the applied force was assumed. Force distributed through feet in contact with the ground was taken as the weight of the animal, calculated as mass × gravity (9.81 m s−2). An animal of 100 kg would therefore exert a vertical force upon the ground of 981 N.
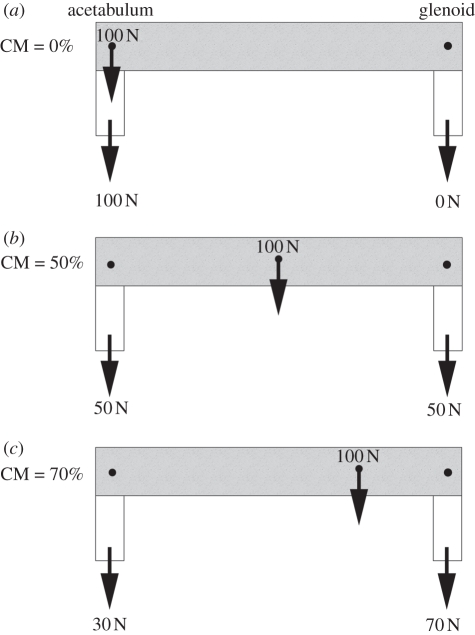
For a biped, maximum force is transmitted through a single foot when the opposite foot is raised, so the pressure applied in this case was equal to the weight of the animal divided by the surface area of a single foot. This is to approximate the peak force at any one time during limb support. In the case of quadrupedalism, CM plays a role in determining how much of the animal's weight is distributed to the fore- and hindlimbs, after which the two sets of limbs can be treated separately as bipeds [37]. This is a simplification of the loads experienced by the autopodia of a quadruped during locomotion, but provides reasonable input values for the purposes of this paper. CM estimates for Edmontosaurus and Brachiosaurus were taken from the literature (see [30] for Edmontosaurus CM and [31] for Brachiosaurus) and used to apportion force between fore- and hindlimbs. The amount of the animal's weight given to each pair of limbs was equal to the relative position of the CM between the pelvic and pectoral girdles, i.e. a CM 60 per cent of the way from the pectoral girdle to the pelvic girdle would imply a weight distribution of 60 per cent to the hindlimbs, and 40 per cent to the forelimbs [31] (figure 1). Treating the animal as two linked bipeds with appropriate weights was sufficient for the purposes of these experiments (see [37] for walking models of quadrupeds represented as two bipeds in tandem). While this may be a very simplified solution that ignores the effects of complex gaits, walking velocities and limb kinematics of dinosaurs are unknown and employing the loading regime outlined above avoids incorporating additional unfounded assumptions into the simulations. Consideration is given to the effects of duty factor and locomotion later in the discussion.
Figure 1.
Loads beneath fore- and hindlimbs as determined by CM position. A CM position of 50% gleno-acetabular position applies equal load to both fore- and hindlimbs. As the CM is positioned more anterior or more posterior, more load is applied to the fore- or hindlimbs, respectively.
Hadrosauridae have been interpreted as primarily bipedal with facultative quadrupedalism at either low [21,23] or high [38] speeds, based on anatomical features in the forelimbs suggestive of either mode of locomotion, and trackway evidence also supporting both gait reconstructions [35,39,40]. Edmontosaurus tracks were therefore simulated as being made by both a bipedal animal and a quadrupedal animal.
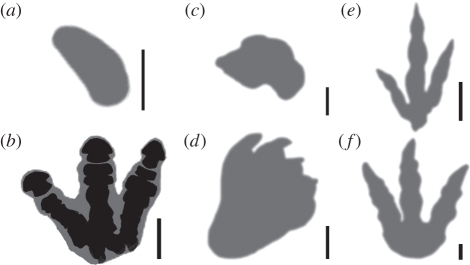
The indenters, or ‘virtual feet’, were created by producing outlines around ventral views of reconstructed skeletal autopodia (figure 2). Skeletal geometry was scaled to the same size as the specimens used by Bates et al. [30] and Henderson [31] so as to remain consistent with mass estimates. The outlines were then increased in size to account for soft tissue. The outline of the Edmontosaurus manus does not follow the osteology as closely as the other indenters, instead being based on the exceptionally preserved hadrosaur body fossil MRF 03 (though scaled to the specimen used by [30]), as figured in Sellers et al. [38, fig. 5], where the manus soft tissue takes a ‘mitten’-like form over the skeleton. This is supported by hadrosaur manus tracks illustrated by Lockley & Wright [39], and those described as ‘crescent shaped’ by Currie [41]. The indenters representing the manus and pes of Brachiosaurus were generated as in Falkingham et al. [28] from reconstructions by Wright [42]. These outlines defined the nodes and elements that would be loaded on the FE substrate volume (figure 2). For each animal, a volume of substrate was created for each foot to be indented into. Only one pes needed to be indented for each bipedal condition, and only one manus and one pes for the Brachiosaurus and quadrupedal Edmontosaurus.
Figure 2.
Foot outlines used to create indenters. (a) Edmontosaurus manus, (b) pes, (c) Brachiosaurus manus, (d) pes (adapted from [42]), (e) Struthiomimus and (f) Tyrannosaurus. Scale bars, 0.1 m. Edmontosaurus pes shows how foot outline was derived based on osteology.
2.2. The virtual substrate
An elastic-perfectly plastic von Mises model was applied in order to model a cohesive clay-like substrate. The mechanical properties of the substrate were defined by the undrained shear strength (Cu), Young's modulus (E) and Poisson's ratio (v). These parameters relate, respectively, to
— The strength of the substrate, that is, how much stress is needed before failure of the sediment (permanent deformation). Essentially a measure of cohesion between grains, shear strength is most strongly affected by water content [10,43]. Typical values of Cu in sediments located on the tidal banks of the Bahia Blanca Estuary, Argentina, were shown to range between 50 and 150 kN m−2 in the surface 1 m [44]. Values of Cu according to the British Standards for Geotechnical Engineering are summarized along with field testing methods in table 2.
— The stiffness of the substrate—how much deformation is recoverable through elastic behaviour before (and after) plastic deformation takes place. The value of Young's modulus is typically 1000× the value of Cu in cohesive substrates [45].
— The compressibility of the substrate. In an entirely incompressible substrate, v = 0.5. Such a substrate will not change in volume when deformed, resulting in expansion equal to compression along an axis perpendicular to that of the primary stress [10]. An incompressible substrate could be considered to be a fully saturated sediment, in which void space air has been completely replaced by water (note: though water is technically compressible to some extent, at the magnitude of forces dealt with here, it can safely be considered incompressible). Typical values for saturated clay or mud would be 0.4–0.5 [46].
Table 2.
Undrained strength classification of clays according to BS 8004:1986, along with simple field tests (from [10]).
| stiffness state | undrained strength (kN m−2) | test |
|---|---|---|
| hard | >300 | can be scratched by thumb nail |
| very stiff | 150–300 | can be indented by thumb nail |
| stiff | 75–150 | can be indented slightly by thumb |
| firm | 40–75 | thumb makes impression easily |
| soft | 20–40 | finger pushed in up to 10 mm |
| very soft | <20 | finger easily pushed in up to 25 mm |
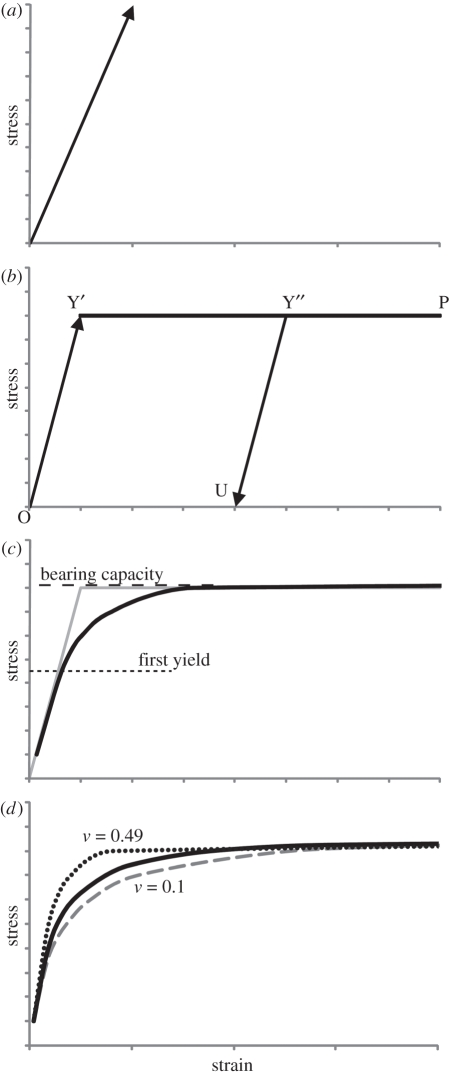
Many palaeontological FEA studies concerning stress within bone use elastic models, in which there is a linear relationship between stress and strain (figure 3a), determined by E. The introduction of a failure criterion (Cu), however, produces an elastic-perfectly plastic model, whereby initial loading deforms the material in a recoverable elastic manner (line O–Y′ in figure 3b) until the load is sufficient to plastically deform the substrate. Further loading equals or exceeds the bearing capacity and results in failure, where the substrate can no longer support the load (line Y′–P in figure 3b). When the load is removed, recovery occurs along a line parallel to the original elastic deformation (line Y″–U in figure 3b). On the scale of individual elements, this relationship is clear and well defined, but over an entire mesh, where some elements may be in a plastic state and others in an elastic one, the relationship becomes less defined, with a curved portion where plastic deformation occurs (figure 3c).
Figure 3.
(a) Elastic stress–strain relationship, (b) elastic-perfectly plastic stress–strain relationship. Initial elastic deformation occurs along line O–Y′ until stress exceeds the strength of the substrate, at which point failure occurs and deformation takes place along line Y′–P. If loading is halted at Y″, and then removed, elastic recovery occurs along line Y″–U, parallel to initial deformation O–Y′. (c) The effects of an elastic-perfectly plastic model distributed over a substrate volume, in which parts are in plastic failure, and others are in the elastic region. (d) The effects of Poisson's ratio on the overall form of the stress–strain relationship within a substrate; dotted line, v = 0.49; solid line, v = 0.3; dashed line, v = 0.1.
A soft clay-like substrate was generated in the FEA simulations using 20-node hexahedral elements. The 20-node element is required in this case because of the nature of the deformation; indenting into soft substrate causes a large gradient of deformation from negative vertical displacement beneath the edge of the indenter, to positive vertical displacement adjacent to the indenter. Eight-node elements lack the numerical flexibility to deal with such a gradient, and so by increasing the number of nodes defining the element, a more accurate solution can be found. The volume of substrate modelled was equal to four times the foot length in all dimensions in order to avoid boundary effects [13].
2.3. The process of indenting
While the foot of an animal can move at joints and the soft tissue is deformable to an extent, as a whole, the foot can be considered rigid compared with the non-rigid substrate. In order to create a rigid loaded area, rigid-body interface elements were generated above the area on the mesh that would be loaded [47], essentially creating a solid meshed foot on the surface of the virtual substrate. The weight of the animal was then applied to the interface elements to generate a uniform load over the foot. The ‘foot’ was loaded vertically as a static analysis (i.e. independent of loading rate), and then removed vertically in order to allow the substrate to recover the elastic part of the deformation, as would be the case in the formation of a real track. The effects of loading rate are considered later in §4.
For each indenter, substrates were generated with a high Cu, and this was incrementally lowered until the substrate could no longer support the load (i.e. bearing capacity was exceeded). In all cases, E was equal to 1000× Cu, and v = 0.4. Maximum depth of indentation beneath the virtual foot was recorded in each experiment, as this value is a fair indication of the degree to which a track is observable. Additionally, surface tracks and undertracks were visualized and qualitatively observed.
3. Results
If the body mass and total foot surface area of the models are logged, it can be shown that foot surface area is proportional to mass0.7 (see electronic supplementary material, S1). This is close to the relationship predicted by isometric scaling, where surface area is proportional to mass2/3 [48,49], and suggests that as animals increase in size, the pressure exerted on the substrate (discounting the effects of locomotion) increases at a proportionally greater rate. The small sample size used in this study means that any difference in this relationship between quadrupeds and bipeds cannot be observed, nor can the effects of allometric scaling be explored.
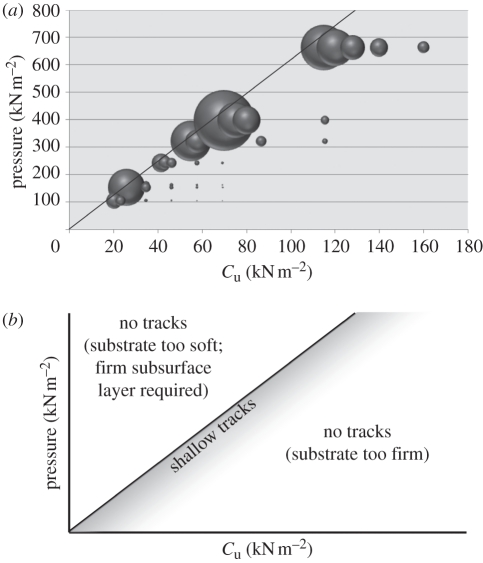
In homogeneous substrates, there is a very narrow range of Cu values for any given pressure that allow the formation of observable surface tracks (figure 4). If Cu is higher than this value, indenters fail to deform the substrate to an appreciable degree, attaining maximum track depths of less than a millimetre. Given that many of the autopodia used were tens of centimetres in length, such deformation cannot realistically be considered to be an observable track. Lower values of Cu than this narrow range cannot support the applied load, and the substrate fails. The maximum load a substrate can support beneath an indenter can be approximated by calculating the bearing capacity beneath a circular indenter under the specified load using the following equation [50]:
| 3.1 |
where S is a shape factor equal to 1 + 0.2 × (breadth/length).
Figure 4.
(a) Bubble plot of maximum track depth for a given load on varying substrates. Size of bubbles qualitatively represent maximum depth of track. Line shown denotes the predicted minimum Cu required to support any given load applied to a perfectly circular indenter. (b) Diagrammatic of results. Tracks are only formed to any significant depth at values approximately equal to those defined by the line, or to the left of the line only if a firmer underlying layer is present. Below and to the right of the line, loads are insufficient to produce tracks of significant depth.
Using this equation, it can be seen that a substrate for which Cu = 100 kN m−2 will fail when the load on a circular indenter (S = 1.2) reaches 616.8 kN m−2. The approximated failure point for circular indenters at any given load is plotted as a line in figure 4. This prediction is not the true value of bearing capacity for any specific track, however, owing to variations in foot morphology. However, as can be seen from figure 4, this approximation is sufficiently close as to highlight the relationship.
4. Discussion
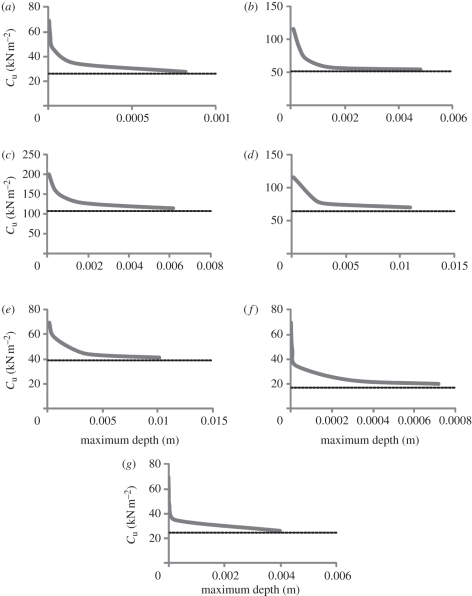
The range of Cu in which tracks of significant depth can be generated is very small regardless of foot morphology or load (figure 5). This limits tracks made in truly homogeneous substrates to an extremely narrow range of pressures and subsequently producer sizes and foot morphology. Below the minimum value of Cu, a track will be formed providing there is a firmer substrate layer beneath. If there is no firmer subsurface layer, the substrate cannot support the load, and the animal in question will be unable to traverse the area without becoming mired.
Figure 5.
Graphs showing maximum track depth and substrate Cu for each indenter. The horizontal dashed line depicts theoretical bearing capacity for a circular indenter applying the same pressure. (a) Struthiomimus, (b) Tyrannosaurus, (c) Brachiosaurus manus, (d) Brachiosaurus pes, (e) Edmontosaurus manus (quadrupedal loading), (f) Edmontosaurus pes (quadrupedal loading), and (g) Edmontosaurus pes (bipedal loading). Each graph shows there is a very narrow range in which tracks are generated, but the substrate is still able to support the load.
A key observation is that in simulating track formation in a homogeneous semi-infinite elastic-perfectly plastic substrate, generation of tracks to any significant depth is difficult to achieve—many tracks were so shallow that for real tracks of a similar depth, it would be unreasonable to expect discovery in the field. This is because the load required to plastically deform the substrate and the maximum load that the substrate can support are very close, implying that a very specific pressure is required to generate a track in a homogeneous substrate. There is therefore a ‘Goldilocks’ quality to homogeneous substrates regarding possible track formation. A faunal assemblage represented by tracks at a given tracksite will be strongly biased towards the largest animals the substrate can support, resulting in a very low diversity of recorded body sizes. Taxa exerting more pressure beneath their feet than the substrate can support will avoid the area or become mired, while animals producing less pressure than is required to create a track will not leave observable impressions.
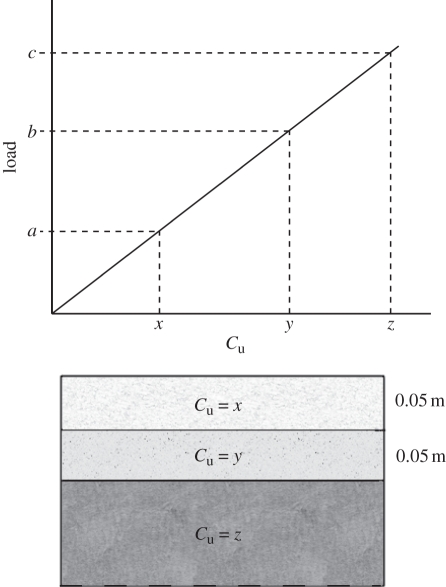
More commonly, substrates are polyphasic, with heterogeneous mechanical properties varying vertically and laterally. If a substrate is underlain by a firmer layer (e.g. compacted sediment or rock), then tracks will be formed if the surface layer fails. If we consider the scenario of a series of substrate layers becoming progressively firmer with depth (figure 6), it is observed that any animal creating sufficient load as to deform the uppermost substrate will generate a track. Refining this stratification such that Cu increases gradually with depth results in the intuitive case that heavier animals generate deeper tracks. It can be seen from figure 4b that this being the case, there is a much larger range of possible track-bearing substrates for animals exerting a greater pressure (i.e. by being larger or moving faster). Given the shallow nature of the tracks modelled in homogeneous substrates, it becomes apparent that most real tracks must therefore be formed in mechanically heterogeneous substrates, or in relatively shallow homogeneous substrates underlain by rock.
Figure 6.
Hypothetical scenario in which three substrate layers are considered, where Cu increases with depth. Animals producing loads that cause the surface layer to fail, but not the subsequent layer (a < load < b) will create tracks of 0.05 m maximum depth. Animals producing loads sufficient to deform layer two, but insufficient to deform layer three (a < b < load < c) will generate tracks to 0.1 m depth. Animals producing loads above the bearing capacity of layer three (load > c) will be unable to traverse the substrate, while animals producing less pressure than is required to deform the surface layer (load < a) will not generate tracks.
If a substrate is stratified with mechanically distinct layers, then the depths and surface areas of present tracks can be used to infer the depth and mechanical properties of these layers at the time of track formation. A track-bearing surface on which small and medium tracks are impressed to a similar depth, yet on which tracks made by larger animals appear deeper, will indicate a mechanically homogeneous surface layer as deep as the small and medium tracks (after accounting for subsequent weathering/erosion). Such consideration of tracks as palaeo-penetrometers may prove useful in interpretations of palaeoenvironment, at least in so far as determining substrate conditions at the time of track formation.
Examining the biases inherent in track formation as a consequence of animal size permits discussion of genuine and artificial signals regarding diversity as interpreted from tracksites. A track site limited to large producers, e.g. a purely sauropod track assemblage, is likely to be a preservational artefact, or indistinguishable from such. Smaller animals may have been abundant at such a site, but unable to produce tracks in the substrate. Allen [51] noted in his discussion of the Flandrian deposits of the inner Bristol Channel and Severn Estuary that the fauna represented by tracks lacked records of smaller mammals such as foxes and dogs, arguing this was a preservational rather than an ecological issue. The results presented here support this hypothesis, and may be applicable to other fossil tracksites dominated by large fauna such as Fumanya, Spain [52,53], or the tracksites at Paluxy River, Glen Rose, TX, USA [54]. In the case of the Glen Rose tracks, the site is dominated by large sauropod and medium to large theropod tracks. One trackway has been interpreted as showing the interaction of a theropod and sauropod [55], implying that the trackways were contemporary. Given the considerable depth of both the theropod and sauropod tracks, and the lack of tracks from smaller animals, the trackways appear to be consistent with the Goldilocks effect. Both the theropod and sauropod exceeded the bearing capacity of the surface mud, and indented deep tracks until supported by firmer substrate layers beneath the surface. However, the depth of the soft surface mud may have been too great for smaller animals to safely traverse while leaving tracks, resulting in the formation and subsequent preservation only of the largest animals present. A similar case can be made for the sauropod trackways at Fumanya, where deep sauropod tracks dominate. Shallow theropod tracks were previously reported from the site, but have since been subjected to weathering and are no longer present [52]. In this case, only larger animals were able to produce tracks, resulting in an impoverished track assemblage, whose low diversity has been exacerbated further by weathering and the complete removal of the shallower tracks left by smaller animals.
Given that there is a strong bias towards greater underfoot pressures, the preservation potential of track assemblages representing mixed age groups (herd behaviour) is greatly reduced; there will be a strong bias towards preserving only the largest members of the group. If the adults within a group are particularly large, as in the case of sauropods for instance, the range of substrates traversable by the group will be constrained by the minimum substrate strength that can support the largest animals. As such, tracks from smaller individuals become far less likely to form and subsequently preserve, because the substrates over which the animals move may not be soft enough to record the passage of smaller, juvenile forms. This reduction in preservation potential of mixed-age herds is supported by the fossil record; Myers & Fiorillo [56] noted that of 13 sauropod trackway associations indicating gregarious behaviour, only three sites contained tracks from multiple age groups.
The presence on a single track-bearing surface of both small and large true tracks (rather than transmitted or undertracks), indented to approximately the same depth (evidently halted by a firmer subsurface layer), is likely to be more indicative of true diversity in the area at the time of track formation (providing effects of time averaging can be removed). Presence of small, shallow tracks and large, deep tracks may not be indicative of true diversity, however, if the large deep tracks are particularly deep. In such a scenario, it is possible that medium-sized animals produce too great a pressure underfoot to be supported by the soft surface layer, but sink too far before reaching a supportive layer as to be able to traverse the area. This highlights the importance of considering not just the tracks present at a tracksite, but their total three-dimensional morphology, including foot anatomy and track depth, in order to make interpretations about faunal diversity.
The Goldilocks effect described in this study is mitigated when a substrate is exposed for a period of time during which the mechanical properties alter, such as when a substrate is drying out. Changes in mechanical properties will undoubtedly be the rule, rather than the exception, but the rate at which these changes occur will determine the overall applicability of the Goldilocks effect to the fossilized tracksite. In cases where the substrate dries out over a relatively prolonged period, the Goldilocks effect will be applicable over short time spans, but will not be evident over the recording life of the substrate, or in the preserved track surface. The presence of sedimentary features such as drying cracks or displacement rims that are unique to some trackways and not others may shed some light on the preservational context, and as to whether the Goldilocks effect noted here applies to a given track assemblage.
The experiments carried out in this study have used body mass to apply a force through the autopodia in a number of taxa. Loading in this way assumes a direct relationship between body mass and force, and was carried out as a rate-independent (i.e. static) analysis as the focus of the work was to explore bias relating to size, not necessarily locomotor mode. This was done to avoid incorporating unfounded assumptions into the simulations, given that the habitual gaits of dinosaurs are unknown. Nevertheless, consideration must be given to the effects of locomotion, duty factor and limb kinetics and kinematics. As an animal begins to move, the GRF gains a horizontal (forward–backward) component in order to move the animal forwards [57]. This force vector may also incorporate a lateral component depending on the animal's gait. As speed increases, the magnitude of the GRF also increases. In terms of pressure applied, the pressure beneath an animal's foot will increase as speed increases. As an animal increases in speed, the minimum Cu required to support the load also increases, such that a substrate that previously would be incapable of recording an animal standing or moving slowly may fail beneath the foot of a running animal.
As an animal traverses a substrate, the rate of loading is intrinsically linked to the speed and duty factor of the animal, with loading rate increasing as duty factor decreases. An increased loading rate results in more resistance from the substrate, and the result is that deformation occurs to a lesser extent (see electronic supplementary material, S2). However, at higher speeds, an animal exerts a greater force upon the substrate, as noted above. As such, although the loading rate increases with speed, the effects on substrate displacement will be mitigated by the increased load applied by the foot. It is important to note that the analyses carried out in this study were static, and thus did not account for the effects of rate-dependent loading and thixotropy. Exploring this complex interplay between loading magnitude and loading rate is beyond the scope of this study, and is impossible without comprehensive locomotor reconstructions of the animals in question. Instead, the Goldilocks effect can be considered to be the base mechanic around which other confounding factors such as limb dynamics, locomotion and substrate thixotropy have an effect. To provide some insight into these complex issues, and their relationship to the Goldilocks effect, the results from a series of simple dynamic simulations are provided and discussed in electronic supplementary material, S2.
Given the static vertical loading conditions employed in this study, the relationship between size and track-forming potential could be predicted to a reasonable degree with simple mechanics and geotechnical theory, as evidenced by the close correlation between equation (3.1) and the results (figure 4a). However, FEA provides benefits over simple mechanics. Simulating track formation allows for differing foot morphologies to be tested, which was shown to be a potentially important factor by Falkingham et al. [11]. FEA also allows us to explore the full three-dimensional volume of simulated tracks. The importance of understanding the relationship between foot morphology, three-dimensional deformation and undertrack production will be demonstrated by the discussion of features observed in the models.
5. Discussion of individual track features
The simulations undertaken for this study present an opportunity to investigate track features at the original track surface, and in subsurface undertracks. Specific features related to autopodia morphology and undertrack depth are discussed here. In order to generate deeper tracks and associated undertracks, failure was allowed to occur, but was halted when maximum depth reached 0.05 m, approximating a firm subsurface layer.
The values of mass, foot morphology and CM position used for the Edmontosaurus produce differing pressures between manus and pes (151.93 kN m−2 and 103.31 kN m−2, respectively). These differing pressures imply that substrates of different Cu are required to support the loads, which in turn creates a range of substrates (Cu = 20–40 kN m−2), where the manus causes the substrate to fail, but the pes does not. In such substrates, if underlain by a firmer layer, only the manus will generate tracks. This is the same mechanism as described in detail by Falkingham et al. [28] for sauropod manus-only trackways. It is interesting to note that the resulting pressure beneath the pes when bipedal locomotion is assumed is less than for the manus in a quadrupedal mode of locomotion. Depending on the validity of the mass, CM and foot outline input parameters used here, this may support the hypothesis that differing modes of locomotion may have been used for traversing different substrates. The values employed in this study would suggest quadrupedalism to be potentially more advantageous on firmer substrates, where the manus will not sink, while a bipedal mode of locomotion would allow traversal of softer substrates. However, this suggestion is based only on underfoot pressures, and further factors such as stability will ultimately determine gait. It may therefore be unwise to infer otherwise unknown locomotor styles from trackways in which the substrate conditions at the time of track formation cannot be constrained. The reader is directed to Wilson et al. [58] for discussion of a trackway in which an ornithopod trackmaker transitions between bipedal and quadrupedal gaits as substrate changes.
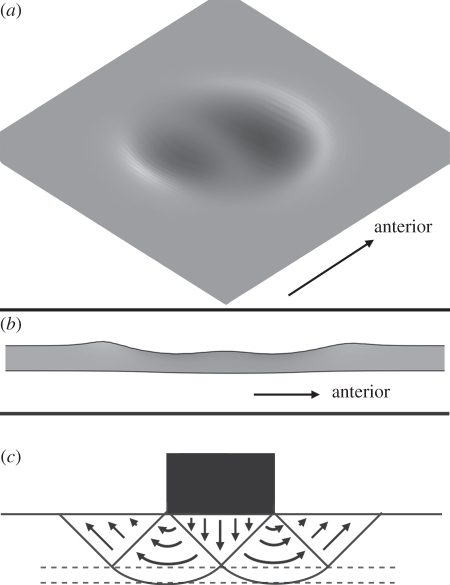
As described above for the Edmontosaurus track simulations, and as described by Falkingham et al. [28], there are a range of substrates in which only the manus, and not the pes, of Brachiosaurus produce enough pressure to deform the substrate. When the subsurface undertracks generated by manus and pes are visualized, important features can be observed. The bowl-like form of the Brachiosaurus pes (figure 7) is reminiscent of a number of reported sauropod tracks (e.g. [59,60]). The simulations here indicate that such a bowl-like form is potentially characteristic of undertracks. This is consistent with the assumption that the plantar surface of the foot was approximately flat, given that the shape of foot required to form bowl-like tracks would prove unstable on firm ground.
Figure 7.
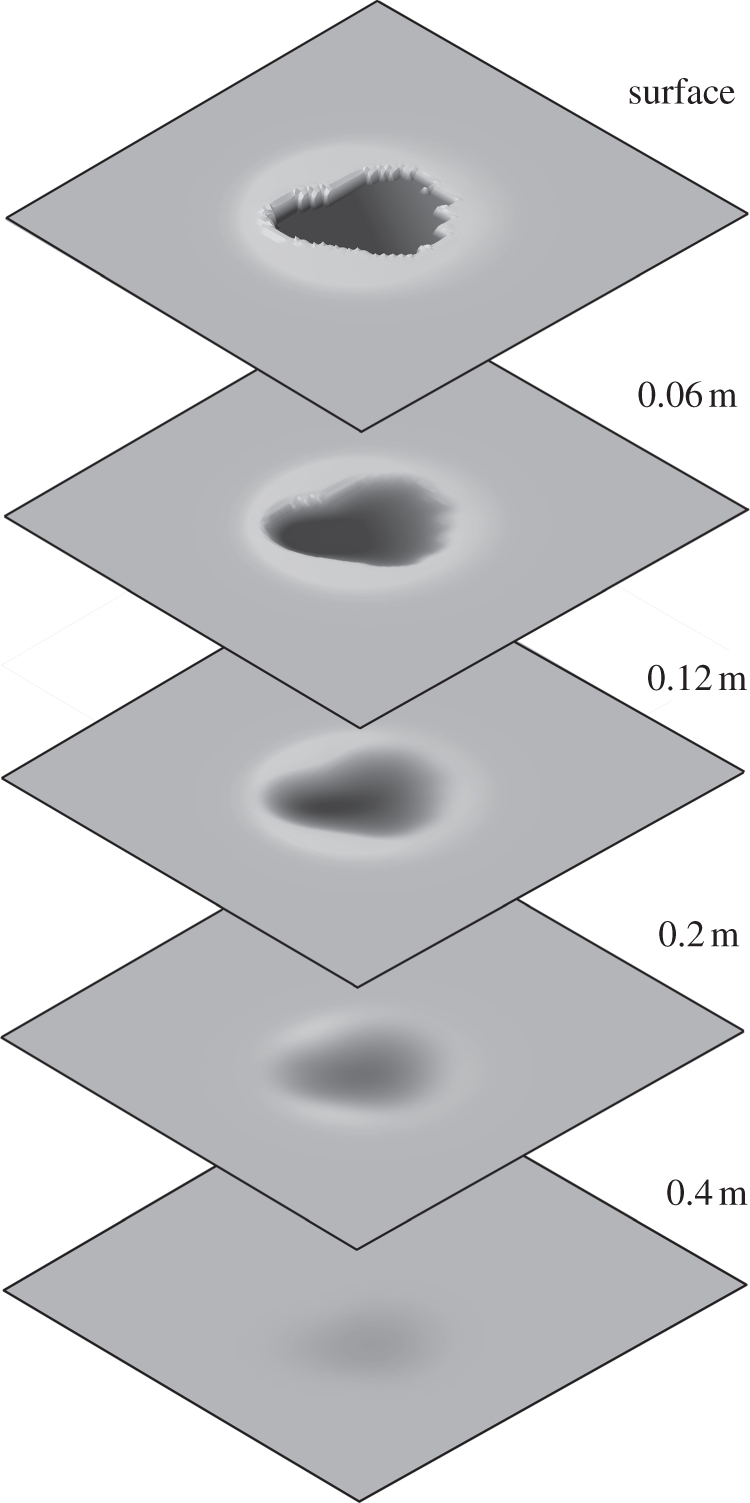
Series of undertracks as generated by the Brachiosaurus pes, seen in isometric view. Note the bowl-like form of successive undertracks, as compared with the flat interior and distinct outlines of the uppermost tracks. Darker shading represents deeper parts of the track.
When the Brachiosaurus manus track is observed as a subsurface undertrack at approximately 0.2 m depth, a ridge running transversely across the track can be seen (figure 8a,b). This ridge appears superficially similar to the undulating track surface hypothesized to result from three-phase movement of the foot [16,61]. However, with full control of all input variables, it is known that in this case the loading was carried out in an entirely vertical manner, evenly distributed through a flat foot, and so the ridge cannot be a function of limb kinematics or foot anatomy. Instead, this ridge is produced through the displacement of sediment according to Prandtl theory [10]. As substrate is deformed by a load, it is pushed down and out from beneath the indenter (figure 8c). The base of the actively deforming zone of substrate undulates against the rigid, non-moving zone [13]. A cross section through this area results in a subsurface track containing a ridge of non-deformed substrate. This effect is seen in the Brachiosaurus manus track because of the round shape of the indenter. This ridge is very subtle, and its absence from the other simulated tracks implies that its formation is closely linked with indenter morphology. Dynamic limb motion consisting of more complex, non-vertical loading will deform this ridge accordingly (e.g. see theropod track in [16]); however, fossil track evidence indicates that, as is the case here, sauropods placed the manus vertically, at least when traversing soft substrates [62]. Further study is required to fully understand this phenomenon and to avoid erroneous interpretation of tracks with undulating bases. Note that some previously described ridges in sauropod tracks (e.g. [63]) appear more defined and morphologically different to those outlined here, and we do not suggest this mode of ridge formation for those cases.
Figure 8.
(a) Isometric and (b) cross-section views of sauropod manus undertrack at a depth of 0.2 m (track generated in substrate of Cu = 110 kN m−2 and halted when track depth reached 0.05 m). Note the transverse ridge running medio-laterally through the track, appearing similar to the three-phase track described by Manning ([16, fig. 6a]; [64, fig. 12.7]). Darker shading represents deeper parts of the track. (c) Theoretical displacement beneath a strip load in a cohesive substrate. If a track is exposed in a layer corresponding to that marked, tracks may appear to contain an internal ridge running across the widest part of the track ((c) modified from [13]).
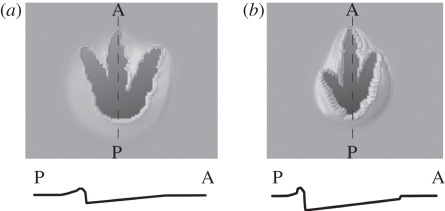
Both theropod tracks (Struthiomimus and Tyrannosaurus) indented to a considerably greater degree at the posterior of the virtual foot (figure 9). The pes of the Edmontosaurus also exhibited this feature, albeit to a lesser extent. This effect is a function of the shapes of the indenters as seen in Falkingham et al. [11]. The appearance of a deeper posterior track portion under uniform loading of a flat indenter has important consequences for interpretations of limb kinematics from fossil tracks. Commonly, the morphology of real tridactyl tracks is deeper beneath the distal areas of the digits as a result of the increased pressure as the animal kicks off, but in the simulated case there is no such loading regime. The development of a deeper track beneath a larger, more compact part of the foot would mean that the ‘two-phase’ interaction of the foot (weight bearing and toe off) described by Thulborn & Wade [61] could potentially produce a track with the appearance of a ‘three-phase’ foot–substrate interaction (which precedes the above phases with touch-down), where the heel and toes are deeper than the centre portion of the track [16,64]. It has been proposed that the two-phase and three-phase modes of locomotion represent knee-based and hip-based retraction of the limb, respectively ([64] and references therein), and that the associated pressures across the foot differ accordingly. However, if vertical loading, as has been used here, produces a deeper ‘heel’ in tridactyl tracks without a ‘heel-down’ kinematic phase, then attempting to differentiate between the locomotor modes of theropod dinosaurs and birds from fossil tracks may be considerably more difficult than has previously been assumed without ongoing experimental studies.
Figure 9.
(a) Tyrannosaurus and (b) Struthiomimus tracks viewed in plan and cross-section though digit III. Vertical dispalcement is greater at the posterior of the track owing to the compact nature of the indenter in this area. Darker shading represents deeper parts of the track.
The resultant tracks from these simulations are relevant for studies of tracks where interpretations of locomotion have been made based on the ‘pitch’ of the track [57,65]. Such interpretations must consider as an alternative, or at least confounding factor, varying shear strength (as a function of water content) throughout the total substrate layer at the time of track formation, resulting in track pitch altering systematically along a trackway. Alternatively, variations in track ‘pitch’ may be influenced by grain size or compositional differences, given that sand responds in the opposite manner to mud [11], allowing greater deformation beneath digits.
6. Conclusions
The simulation of tracks from a series of dinosaur taxa ranging in size from 400 to 25 000 kg shows a linear relationship between body mass and substrate shear strength required to produce observable tracks. The point of failure for a given track and subsequently the shear strength of the substrate at the time of track formation can be approximated by calculating the bearing capacity required for a circular indenter of equal size and load. Variations around this approximation are due to the effects of foot shape.
Tracks of significant depth are not possible in homogeneous, cohesive substrates without the presence of a firmer subsurface layer, because failure of the substrate will result in the animal being unable to traverse the area. A homogeneous cohesive substrate will only record tracks from the largest animals that substrate can support without failing. There is, however, a strong bias towards tracks made by larger animals if there is a firmer substrate beneath a softer layer. This Goldilocks effect means that for a homogeneous substrate, loading conditions (that is, the animal size, locomotion and foot morphology) must be ‘just right’ in order for the animal to be able to traverse the area but still form tracks. This has wide-ranging implications for interpretations of palaeodiversity and palaeoecology based on vertebrate track assemblages preserved in lithified muds and silts.
Presence of small and large tracks indented to the same depth on a single track-bearing surface (assuming time-averaging/transmitted tracks can be accounted for) offer the highest possibility of presenting a true representation of faunal diversity in the area at the time of track formation. Caution is strongly advised in making any interpretations of faunal diversity or population dynamics from track assemblages where all tracks have been produced by similar-sized producers. Such assemblages most likely represent a strongly biased preservation, or an ‘instantaneous’ event. Substrates which have dried out over relatively long time periods will provide a fuller record of faunal diversity in the area, but will be subject to biases and other erroneous data associated with time-averaging.
Specific features regarding track and undertrack formation have been noted for this range of indenters based on dinosaur taxa. Bowl-like sauropod pedal impressions may be indicative of being undertracks, potentially of significant depth. Internal ridges may form in tracks due to the vectors of displacement beneath a uniform load (as in the Brachiosaurus manus), or as a result of autopodia morphology causing non-uniform displacement under uniform loading (as in the tridactyl tracks). That these features can be formed independent of limb kinematics is of great importance, and highlights the need for further experimental work to clarify the specifics of their formation.
The approach used here, of computer simulation using FEA, has allowed the generation of tracks and associated undertracks for a range of animal sizes that would be difficult to replicate using physical modelling. Employing computational methods has also catered for constancy in input variables between experiments, and has provided the ability to easily and systematically manipulate those variables. We recognize that this study makes a number of assumptions and simplifications in terms of loading, and expect subsequent research to build on the methods used here to produce more complex models. Many of the conclusions and observations recorded here are related to the mechanics of substrates under load, and we hope that this will encourage further research into the effects of complex limb kinematics and kinetics on track formation, in light of the confounding geotechnical effects described here.
Acknowledgements
P.L.F. and K.T.B. were funded by the Natural Environment Research Council (NER/S/A/2006/14033 and NER/S/A/2006/14101, respectively). FEA simulations were run on the HPCx supercomputing service, using Engineering and Physical Sciences Research Council grant EP/F055595/1, awarded to L.M. We would also like to thank James Jepson for commenting on an early draft of the manuscript, and also Jeff Wilson and one anonymous reviewer for their constructive, helpful comments.
References
- 1.Benton M. J., Storrs G. W. 1994. Testing the quality of the fossil record: Paleontological knowledge is improving. Geology 22, 111–114 (doi:10.1130/0091-7613(1994)022<0111:ttqotf>2.3.co;2) [DOI] [Google Scholar]
- 2.Darwin C. R. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 502 pp. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- 3.Foote M., Sepkoski J. J. 1999. Absolute measures of the completeness of the fossil record. Nature 398, 415–417 10.1038/18872 (doi:10.1038/18872) [DOI] [PubMed] [Google Scholar]
- 4.Maxwell W. D., Benton M. J. 1990. Historical tests of the absolute completeness of the fossil record of tetrapods. Paleobiology 16, 322–335 [Google Scholar]
- 5.Benson R. B. J., Butler R. J., Lindgren J., Smith A. S. 2010. Mesozoic marine tetrapod diversity: mass extinctions and temporal heterogeneity in geological megabiases affecting vertebrates. Proc. R. Soc. B 277, 829–834 10.1098/rspb.2009.1845 (doi:10.1098/rspb.2009.1845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foote M. 1997. Estimating taxonomic durations and preservation probability. Paleobiology 23, 278–300 [Google Scholar]
- 7.Mannion P. D., Upchurch P., Carrano M. T., Barrett P. M. 2010. Testing the effect of the rock record on diversity: a multidisciplinary approach to elucidating the generic richness of sauropodomorph dinosaurs through time. Biol. Rev. (doi:10.1111/j.1469–185X.2010.00139.x) [DOI] [PubMed] [Google Scholar]
- 8.Smith A. B., McGowan A. J. 2007. The shape of the phanerozoic marine palaeodiversity curve: how much can be predicted from the sedimentary rock record of western Europe? Palaeontology 50, 765–774 10.1111/j.1475-4983.2007.00693.x (doi:10.1111/j.1475-4983.2007.00693.x) [DOI] [Google Scholar]
- 9.Lockley M. G., Conrad K. 1989. The palaeoenvironmental context, preservation, and palaeoecological significance of dinosaur tracksites in the western USA. In Dinosaur tracks and traces (eds Gillette D. D., Lockley M. G.), pp. 121–134 Cambridge, UK: Cambridge University Press [Google Scholar]
- 10.Craig R. F. 2004. Craig's soil mechanics, 447 pp. Abingdon, UK: Spon Press [Google Scholar]
- 11.Falkingham P. L., Margetts L., Manning P. L. 2010. Fossil vertebrate tracks as palaeopenetrometers: confounding effects of foot morphology. Palaios 25, 356–360 10.2110/palo.2009.p09-164r (doi:10.2110/palo.2009.p09-164r) [DOI] [Google Scholar]
- 12.Witmer L. M. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional morphology in vertebrate paleontology (ed. Thomason J. J.), pp. 19–33 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Allen J. R. L. 1997. Subfossil mammalian tracks (Flandrian) in the Severn Estuary, SW Britain: mechanics of formation, preservation and distribution. Phil. Trans. R. Soc. Lond. B 352, 481–518 10.1098/rstb.1997.0035 (doi:10.1098/rstb.1997.0035) [DOI] [Google Scholar]
- 14.Gatesy S. M., Middleton K. M., Jenkins F. A., Shubin N. H. 1999. Three-dimensional preservation of foot movements in Triassic theropod dinosaurs. Nature 399, 141–144 10.1038/20167 (doi:10.1038/20167) [DOI] [Google Scholar]
- 15.Manning P. L. 1999. Dinosaur track formation, preservation and interpretation: fossil and laboratory simulated dinosaur track studies. PhD thesis, University of Sheffield (England), UK [Google Scholar]
- 16.Manning P. L. 2004. A new approach to the analysis and interpretation of tracks: examples from the dinosauria. In The application of ichnology to palaeoenvironmental and stratigraphic analysis (ed. McIlroy D.), pp. 93–123 London, UK: Special Publications, Geological Society [Google Scholar]
- 17.Milàn J. 2006. Variations in the morphology of emu (Dromaius novaehollandiae) tracks reflecting differences in walking pattern and substrate consistency: ichnotaxonomic implications. Palaeontology 49, 405–420 10.1111/j.1475-4983.2006.00543.x (doi:10.1111/j.1475-4983.2006.00543.x) [DOI] [Google Scholar]
- 18.Milàn J., Bromley R. G. 2006. True tracks, undertracks and eroded tracks, experimental work with tetrapod tracks in laboratory and field. Palaeogeogr. Palaeoclimatol. Palaeoecol. 231, 253–264 10.1016/j.palaeo.2004.12.022 (doi:10.1016/j.palaeo.2004.12.022) [DOI] [Google Scholar]
- 19.Milàn J., Bromley R. G. 2008. The impact of sediment consistency on track and undertrack morphology: experiments with Emu tracks in layered cement. Ichnos 15, 19–27 10.1080/10420940600864712 (doi:10.1080/10420940600864712) [DOI] [Google Scholar]
- 20.Milàn J., Clemmensen L. B., Bonde N. 2004. Vertical sections through dinosaur tracks (Late Triassic lake deposits, East Greenland)—undertracks and other subsurface deformation structures revealed. Lethaia 37, 285–296 10.1080/00241160410002036 (doi:10.1080/00241160410002036) [DOI] [Google Scholar]
- 21.Galton P. M. 1970. The posture of hadrosaurian dinosaurs. J. Paleontol. 44, 464–473 [Google Scholar]
- 22.Galton P. M., Upchurch P. 2004. Prosauropoda. In The dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 232–258, 2nd edn. Berkeley, CA: University of California Press [Google Scholar]
- 23.Weishampel D. B., Horner J. R. 1992. Hadrosauridae. In The Dinosauria (eds Weishampel D. B., Dodson P., Osmólska), pp. 534–561 London, UK: University of California Press [Google Scholar]
- 24.Hitchcock E. 1858. Ichnology of New England. A report on the sandstone of the Connecticut Valley, especially its fossil footmarks, 220 pp. Boston, MA: W. White: (Reprinted by New York, NY: Arno Press 1974.) [Google Scholar]
- 25.Lockley M. G. 1987. Dinosaur tracks symposium signals a renaissance in vertebrate ichnology. Paleobiology 13, 246–252 [Google Scholar]
- 26.Sarjeant W. A. S. 1974. A history and bibliography of the study of fossil vertebrate footprints in the British isles. Palaeogeogr. Palaeoclimatol. Palaeoecol. 16, 265–378 10.1016/0031-0182(74)90024-8 (doi:10.1016/0031-0182(74)90024-8) [DOI] [Google Scholar]
- 27.Falkingham P. L., Margetts L., Smith I. M., Manning P. L. 2009. Reinterpretation of palmate and semi-palmate (webbed) fossil tracks; insights from finite element modelling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 271, 69–76 10.1016/j.palaeo.2008.09.011 (doi:10.1016/j.palaeo.2008.09.011) [DOI] [Google Scholar]
- 28.Falkingham P. L., Bates K. T., Margetts L., Manning P. L. 2010. Simulating sauropod manus-only trackway formation using finite-element analysis. Biol. Lett. 10.1098/rsbl.2010.0403 (doi:10.1098/rsbl.2010.0403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margetts L., Smith I. M., Leng J., Manning P. L. 2006. Parallel three-dimensional finite element analysis of dinosaur trackway formation. In Numerical methods in geotechnical engineering (ed. Schweiger H. F.), pp. 743–749 London, UK: Taylor & Francis [Google Scholar]
- 30.Bates K. T., Manning P. L., Hodgetts D., Sellers W. I. 2009. Estimating body mass properties of dinosaurs using laser imaging and 3D computer modelling. PLoS ONE 4, e4532. 10.1371/journal.pone.0004532 (doi:10.1371/journal.pone.0004532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson D. M. 2006. Burly gaits: centers of mass, stability, and the trackways of sauropod dinosaurs. J. Vertebrate Paleontol. 26, 907–921 10.1671/0272-4634(2006o26[907:BGCOMS]2.0.CO;2 (doi:10.1671/0272-4634(2006o26[907:BGCOMS]2.0.CO;2) [DOI] [Google Scholar]
- 32.Minter N. J., Braddy S. J., Davies R. B. 2007. Between a rock and a hard place: arthropod trackways and ichnotaxonomy. Lethaia 40, 365–375 10.1111/j.1502-3931.2007.00035.x (doi:10.1111/j.1502-3931.2007.00035.x) [DOI] [Google Scholar]
- 33.Padian K., Olsen P. E. 1984. Footprints of the komodo monitor and the trackways of fossil reptiles. Copeia 1984, 662–671 10.2307/1445147 (doi:10.2307/1445147) [DOI] [Google Scholar]
- 34.Alexander R. M. 1989. Dynamics of dinosaurs & other extinct giants, 167 pp. Chichester, UK: Columbia University Press [Google Scholar]
- 35.Thulborn R. A. 1990. Dinosaur tracks, 410 pp. London, UK: Chapman & Hall [Google Scholar]
- 36.Alexander R. M. 1976. Estimates of speeds of dinosaurs. Nature 261, 129–130 10.1038/261129a0 (doi:10.1038/261129a0) [DOI] [Google Scholar]
- 37.Alexander R. M. 1977. Mechanics and scaling of terrestrial locomotion. In Scale effects in animal locomotion (ed. Pedley T. J.), pp. 93–110 London, UK: Academic Press [Google Scholar]
- 38.Sellers W. I., Manning P. L., Lyson T., Stevens K., Margetts L. 2009. Virtual palaeontology: gait reconstruction of extinct vertebrates using high performance computing. Palaeontol. Electron. 12/11A, 26 [Google Scholar]
- 39.Lockley M. G., Wright J. L. 2001. Trackways of large quadrupedal ornithopods from the cretaceous: a review. In Mesozoic vertebrate life (eds Tanke D. H., Carpenter K.), pp. 428–442 Bloomington, IN: Indiana University press [Google Scholar]
- 40.Moratalla J. J., Sanz J. L., Jiménez R., Lockley M. G. 1992. A quadrupedal ornitjopod trackway from the lower Cretaceous of La Rioja (Spain): inferences on gait and hand structure. J. Vertebrate Paleontol. 12, 150–157 10.1080/02724634.1992.10011445 (doi:10.1080/02724634.1992.10011445) [DOI] [Google Scholar]
- 41.Currie P. J. 1983. Hadrosaur trackways from the Lower Cretaceous of Canada. Acta Palaeontol. Pol. 28, 63–73 [Google Scholar]
- 42.Wright J. 2005. Sauropod tracks and their importance in the study of the functional morphology and paleoecology of sauropods. In The sauropods: evolution and paleobiology (eds Curry Rogers K. A., Wilson J. A.), pp. 252–284 London, UK: University of California Press, Ltd [Google Scholar]
- 43.Marty D., Meyer C., Billion-Bruyst P. 2006. Sauropod trackway patterns expression of special behaviour related to substrate consistency? An example from the Late Jurassic of northwestern Switzerland. Hantkeniana 5, 38–41 [Google Scholar]
- 44.Ginsberg S. S., Perillo G. M. E. 1990. Channel bank recession in the Bahia Blanca Estuary, Argentina. J. Coast. Res. 6, 999–1009 [Google Scholar]
- 45.Leach R. 1994. Engineering properties of wetland soils. WRP technical note SG-RS-1.2 WRP technical note SG-RS-1.2, pp. 1–7 U.S. Army Engineer Research and Development Center, Vicksburg, MS, USA [Google Scholar]
- 46.Newcomb D. E., Birgisson B. 1999. Measuring in situ mechanical properties of pavement subgrade soils, 73 pp. Washington, DC: Transportation Research Board [Google Scholar]
- 47.Potts D. M., Zdravković L. 1999. Finite element analysis in geotechincal engineering: theory, 440 pp. London, UK: Thomas Telford [Google Scholar]
- 48.Alexander R. M. 2003. Principles of animal locomotion, 371 pp. Oxford, UK: Princeton University Press [Google Scholar]
- 49.Bennett M. B. 1999. Foot areas, ground reaction forces and pressures beneath the feet of kangaroos, wallabies and rat-kangaroos (Marsupialia: Macropodoidea). J. Zool. 247, 365–369 10.1111/j.1469-7998.1999.tb00999.x (doi:10.1111/j.1469-7998.1999.tb00999.x) [DOI] [Google Scholar]
- 50.Skempton A. W. 1951. The bearing capacity of clays. Proc. Build. Res. Congr. 1, 180–189 [Google Scholar]
- 51.Allen J. R. L. 1989. Fossil vertebrate tracks and indenter mechanics. J. Geol. Soc. 146, 600–602 10.1144/gsjgs.146.4.0600 (doi:10.1144/gsjgs.146.4.0600) [DOI] [Google Scholar]
- 52.Bates K. T., Rarity F., Manning P. L., Hodgetts D., Vila B., Oms O., Galobart À., Gawthorpe R. 2008. High-resolution LiDAR and photogrammetric survey of the Fumanya dinosaur tracksites (Catalonia): implications for the conservation and interpretation of geological heritage sites. J. Geol. Soc. Lond. 165, 115–127 10.1144/0016-76492007-033 (doi:10.1144/0016-76492007-033) [DOI] [Google Scholar]
- 53.Vila B., Oms O., Galobart À. 2005. Manus-only titansaurid trackway from Fumanya (Maastrichtian, Pyrenees): further evidence for underprint origin. Lethaia 38, 211–218 10.1080/00241160510013312 (doi:10.1080/00241160510013312) [DOI] [Google Scholar]
- 54.Farlow J. O., et al. 2010. Dinosaur tracksites of the paluxy river (glen rose formation, lower cretaceous), dinosaur valley state park, somervell county, texas. Geol. Soc. Am. Abstr. Programs 42, 94 [Google Scholar]
- 55.Thomas D. A., Farlow J. O. 1997. Tracking a dinosaur attack. Sci. Am. 277, 74–79 10.1038/scientificamerican1297-74 (doi:10.1038/scientificamerican1297-74) [DOI] [Google Scholar]
- 56.Myers T. S., Fiorillo A. R. 2009. Evidence for gregarious behavior and age segregation in sauropod dinosaurs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 274, 96–104 10.1016/j.palaeo.2009.01.002 (doi:10.1016/j.palaeo.2009.01.002) [DOI] [Google Scholar]
- 57.Mossman D. J., Brüning R., Powell H. P. 2003. Anatomy of a Jurassic theropod trackway from Ardley, Oxfordshire, UK. Ichnos 10, 195–207 10.1080/10420940390257941 (doi:10.1080/10420940390257941) [DOI] [Google Scholar]
- 58.Wilson J. A., Marsicano C. A., Smith R. M. H. 2009. Dynamic locomotor capabilities revealed by early dinosaur trackmakers from Southern Africa. PLoS ONE 4, e7331. 10.1371/journal.pone.0007331 (doi:10.1371/journal.pone.0007331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ensom P. C. 2002. Vertebrate trace fossils in the Purbeck limestone group of southern England. Spec. Pap. Palaeontol. 68, 203–220 [Google Scholar]
- 60.Lee Y. N., Huh M. 2002. Manus-only sauropod tracks in the Uhangri formation (upper Cretaceous), Korea and their paleobiological implications. J. Paleontol. 76, 558–564 (doi:10.1666/0022-3360(2002)076<0558:MOSTIT>2.0.CO;2) [DOI] [Google Scholar]
- 61.Thulborn R. A., Wade M. 1989. A footprint as a history of movement. In Dinosaur tracks and traces (eds Gillette D. D., Lockley M. G.), pp. 51–56 Cambridge, UK: Cambridge University Press [Google Scholar]
- 62.Milàn J., Christiansen P., Mateus O. 2005. A three-dimensionally preserved sauropod manus impression from the Upper Jurassic of Portugal: implications for sauropod manus shape and locomotor mechanics. Kaupia 14, 47–52 [Google Scholar]
- 63.Hwang K.-G., Lockley M. G., Huh M., Paik I. S. 2008. A reinterpretation of dinosaur footprints with internal ridges from the Upper Cretaceous Uhangri formation, Korea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 258, 59–70 10.1016/j.palaeo.2007.10.029 (doi:10.1016/j.palaeo.2007.10.029) [DOI] [Google Scholar]
- 64.Manning P. L. 2008. T. rex speed trap. In T. rex symposium volume (eds Carpenter K., Larson P. L.), pp. 205–231 Bloomington, IN: Indiana University Press [Google Scholar]
- 65.Day J. J., Norman D. B., Upchurch P., Powell H. P. 2002. Biomechanics—dinosaur locomotion from a new trackway. Nature 415, 494–495 10.1038/415494a (doi:10.1038/415494a) [DOI] [PubMed] [Google Scholar]