Abstract
The detection sensitivity of silver nanoparticle (AgNP)-tagged goat immunoglobulin G (gIgG) microarrays was investigated by studying surface plasmon resonance (SPR) images captured in the visible wavelength range with the help of a Kretchmann-configured optical coupling set-up. The functionalization of anti-gIgG molecules on the AgNP surface was studied using transmission electron microscopy, photon correlation measurements and UV–visible absorption spectroscopy. A value of 1.3 × 107 M−1 was obtained for the antibody–antigen binding constant by monitoring the binding events at a particular resonance wavelength. The detection limit of this SPR imaging instrument is 6.66 nM of gIgG achieved through signal enhancement by a factor of larger than 4 owing to nanoparticle tagging with the antibody.
Keywords: plasmonics, antibody microarray, nanoparticle labelling
1. Introduction
The surface plasmon resonance imaging (SPRI) system offers high-fidelity, high-throughput capabilities of simultaneously monitoring multiple reactions owing to immobilization of biomolecules on a substrate at differently designated spots of micrometre sizes. The technique is a label-free, highly surface-sensitive detection mechanism of chemical and biological analytes in a real-time environment [1,2]. SPRI-based sensing systems have recently been employed for studying the hybridization mechanisms of different types of deoxyribonucleic acid (DNA) immobilized on discrete on-chip sites [3], weak protein–carbohydrate interactions for cellular recognition [4] and the kinetics of protein adsorption/desorption onto peptide microarrays [5,6]. SPRI detects the changes in refractive index in close proximity to the surface of a thin metal film as variations in light intensity reflected from the back of the film and thus requires no labelling [7].
In recent years, silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) have extensively been used as labels for biomolecules with a view to studying the DNA hybridization using the surface-enhanced resonance Raman scattering (SERRS) technique [8]. Highly reproducible SERRS activity was achieved with the intensity error lower than 15 per cent for AuNPs embedded with Raman active molecules, rhodamine 6G (R6G), when the AuNPs–R6G@Ag nanostructures were assembled on glass for 2 h with silver deposition based on silver enhancer solution for 2 min [9]. When protein membranes were labelled with 10 nm AuNPs in cells, high-sensitivity, stable three-dimensional imaging of individual nanoparticles was obtained using the aphotothermal interference contrast method [10]. The signal intensities were, however, low and prone to fluctuation. The active life was also limited owing to photobleaching if the distance for transfer of energy became longer than 10 nm. Noble metal nanoparticles exhibit tunable optical resonances in the visible range. Plasmon coupling was employed to continuously monitor separations between single pairs of AuNPs and AgNPs of up to 70 nm for longer than 50 min and the kinetics of single DNA hybridization events without any interference from photobleaching [11]. Antibodies were immobilized onto 300 nanospots on the sensing surface of the multiarray optical nanochip using their interaction with protein A and the concentrations of antigens were determined from the peak absorption intensity of the localized SPR spectra [12]. Metal nanoshells consisting of a spherical silica core nanoparticle surrounded by a thin gold shell were embedded into solid tumours for in vivo treatment for cancer. Because nanoshells are tunable to absorb near-infrared (NIR) light, their use was found to make thermal ablation treatment almost non-invasive, generating heat to the predefined tissue volume with minimum possible damage to intervening and surrounding normal tissue. Irreversible damage was observed to have occurred within 6 min on extracorporeal exposure of NIR (820 nm) light having intensity as low as 4 W cm−1 to a tumour spot with a 5 mm diameter. The temperature distribution was monitored by the phase-sensitive, fast-spoiled gradient-echo magnetic resonance, and the average temperature was found to be significantly higher than that of tissues treated without nanoshells [13].
In the present study, AgNPs have been employed to tag antibody for enhancing the detection capability. The functionalization of AgNPs with anti-goat immunoglobulin G (anti-gIgG) was characterized by transmission electron microscopy (TEM), photon correlation and UV–visible spectroscopies. The sensitivity of this AgNP-tagged anti-gIgG (AgNP–anti-gIgG) in sandwich immunoassays was examined in this investigation for the first time, by comparing reflected light intensity with untagged anti-gIgG at a fixed angle in our wavelength-scanning SPRI system. The easy, economical route to the synthesis of AgNPs has now been well established [14]. The use of AgNPs for the amplified SPRI immunoassay of gIgG provides a simple, cost-effective and sensitive sandwich immunoassay method compared with enzyme-linked immunosorbent assay [15].
2. Experimental
Materials such as gIgG, rabbit anti-gIgG, bovine serum albumin (BSA), 11-mercaptoundecanoic acid (MUA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma. The remaining chemicals such as ethanol, silver nitrate (AgNO3), sodium citrate, tetrahydrofuran (THF) and gold were obtained from the UK supplier of Sigma-Aldrich. All reagents were used as received. All stock solutions were prepared using deionized MilliQ water of resistivity 18 MΩ cm. AgNPs were synthesized by reducing 0.12 mM AgNO3 in 0.42 mM sodium citrate in 100 ml of water at a temperature near to the boiling point [16]. After cooling to room temperature in the dark, the reaction mixture was stabilized at pH 4.5 by adding 1 ml of THF to prevent further uncontrolled growth of nanoparticles of varying sizes [17]. AgNPs functionalized with anti-gIgG solution (AgNP–anti-gIgG) were prepared by constantly stirring the mixture of 1 ml of stock solution of AgNPs with 400 µl of 1 mg ml−1 anti-gIgG solution. The mixture was centrifuged three times at 9000 r.p.m. for 20 min. The precipitate was collected and dispersed in 5 ml of water. The solution was stirred for 15 min and used immediately.
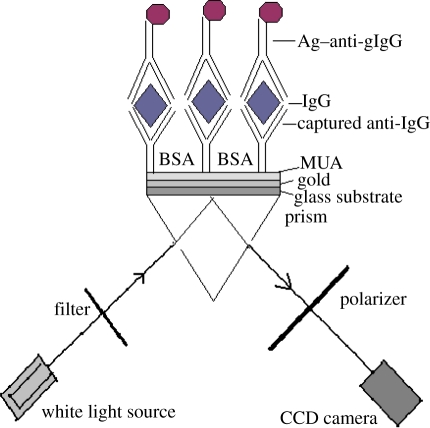
The nanoparticles and functionalized nanoparticles (with antibody in AgNP–anti-gIgG solution) were characterized by high-resolution TEM (JEOL JEM 2010), Zetasizer (Malvern Instruments, model ZEN 3600) and UV–visible spectroscopy (Shimadzu UV-VIS-3101PC). For the TEM examination of AgNPs and the AgNP–anti-gIgG complex, a drop of solution was applied onto a carbon-coated copper grid and observed under the accelerating voltage of 200 kV with different magnifications. The hydrodynamic diameter of the AgNPs was measured using photon correlation spectroscopy (PCS) on a Malvern Zetasizer 3600 instrument (Malvern Instruments, UK) before and after antibody functionalization. The instrument is equipped with an external red laser (wavelength of 633 nm) for illuminating the solution contained in a cell, and scattered light by the particles is detected in a non-invasive mode by placing an avalanche photodiode (quantum efficiency of greater than 50% at 633 nm) at 173° relative to the source. SPRI experiments were performed on the plasmon active gold films on the glass substrates and AgNP–anti-gIgG immobilization on the gold-coated glass substrate using the Kretschmann configuration for optical coupling of plasmons [18]. As shown in figure 1, the wavelength-scanning mechanism was similar to that proposed by Johansen et al. [19]. The p-polarized collimated beam from a white light source was incident on the right-angle prism surface at a fixed incident angle θ = 46°. The sample substrates were brought into optical contact with the prism (with an index of refraction np = 1.515) using an index-matching fluid. The total internal reflected light from the base of the prism was passed through a tunable filter capable of modulating the wavelength with an accuracy of 2.5 ± 0.5 nm. The images captured by a CCD camera in the dark in air were transferred to a personal computer for analysis. The resolving power and the chip area of the CCD are 4.65 µm and 5 mm × 3.5 mm, respectively. The mean greyscale intensity of each captured image at each wavelength was calculated using ImageJ 1.37t (National Institutes of Health, Bethesda, MD). The dark (0%) and bright (100%) detector response was set to 0 and 255 in greyscale intensity for calibration purposes.
Figure 1.
Schematic of surface plasmon resonance imaging (SPRI). Immunoassay of immunoglobulin G (IgG) based on silver nanoparticles (AgNPs). (Online version in colour.)
SPRI substrates were prepared by evaporating 47 nm thick gold (Au) films with a 1 nm chromium precursor layer on 2.5 × 3.5 cm2 BK7 microscope glass slides (Menzel-Glaser, Germany). Monolayers (SAM) of MUA were self-assembled on gold under mild conditions in order to control the surface charge density of probe biomolecules and surface wettability properties. The COOH-terminal functional groups of the MUA molecules provided the means for biomolecular probing through a chemical ‘linker’ and controlled the amount of the desired biomolecules. The chip was immersed in an ethanol solution of MUA (6.66 mM) overnight. After rinsing, the chip was activated with a mixed solution of EDC (200 mM) and NHS (50 mM) for 2 h, washed with deionized Millipore water and air dried. Antibody immobilization and sandwich immunoassay on the SAM-modified Au chips were performed by following the standard protocol [20]. Samples containing gIgG (6.66–660 nM), 178.5 mM anti-gIgG and BSA (20 mg ml−1) were prepared in 10 mM phosphate buffer saline at pH 7.4. All experiments were performed at room temperature. The assay was initiated when 0.5 ml of buffer containing anti-gIgG was incubated on the SAM-modified gold chip for 2 h in an incubator.
After extensive washing in buffer and water, the chip was dried in air. Using an x–y micro-spotting device, an array of spots about 350 µm in diameter of gIgG of varying concentration was produced on the immobilized captured antibody. After incubating in a humid chamber for 2 h and washing in buffer and water and air drying, the chip was spotted by AgNP–anti-gIgG solution exactly on the same position as previously spotted gIgG. The remaining area of the chip was covered by BSA solution for 1 h for reduction of non-specific adsorption. The chip incubation, washing and drying were then performed as before.
3. Results and discussion
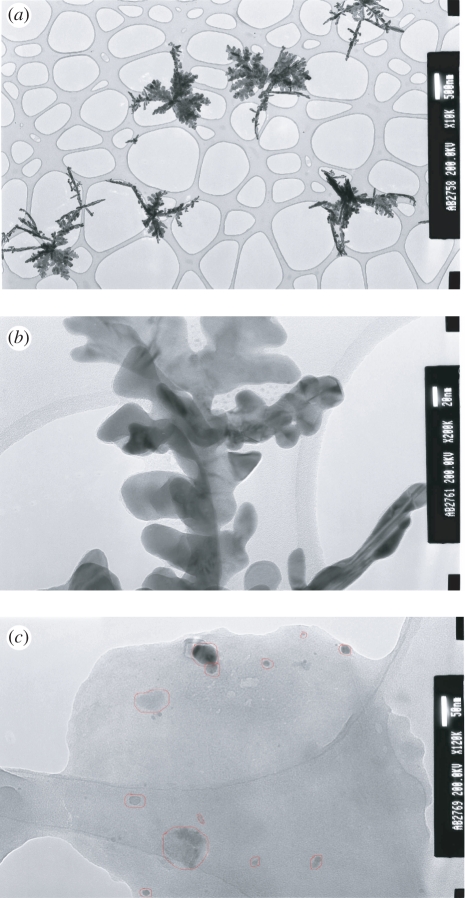
Transmission electron micrographs of AgNPs are displayed in figure 2. AgNPs synthesized by the citrate reduction method with the THF stabilizer were found to exhibit a significant variation in size and shape (figure 2a). The particle diameter was estimated to be in the range of 10–40 nm, and these values are lower than those reported earlier [16]. Long Ag nanorods were also visible in this electron micrograph and close observation revealed the existence of a few faceted nanoparticles. Excess silver in the solution is believed to favour aggregation, leading to the growth of the dendritic structure of Ag clusters, as shown in figure 2b. The growth of these tree-like structures of AgNPs can be explained in terms of the diffusion-limited aggregated model [21]. Figure 2c shows the TEM image of AgNPs functionalized with anti-gIgG in which non-uniform binding of AgNPs to the aggregated anti-gIgG surface can be clearly identified. The agglomerate became larger in size between 25 and 60 nm. It is to be noted that the IgG antibody is a Y-shaped structure with dimensions of 14 nm × 10 nm × 4 nm [22]. A value of approximately 0.2 was estimated for the ratio of conjugation between AgNPs and anti-gIgG in AgNP–anti-gIgG complexes.
Figure 2.
Transmission electron microscopy (TEM) images of (a,b) AgNPs and (c) AgNP-functionalized anti-gIgG (AgNP–anti-gIgG) using a 200-mesh TEM grid as the mask. Image resolutions are (a) 500 nm, (b) 20 nm and (c) 50 nm.
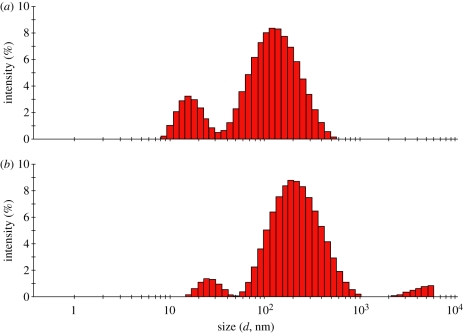
The results of PCS measurements are given in figure 3, which shows relative scattering intensity versus hydrodynamic particle size distribution (ISD) in the form of histograms for AgNPs before and after antibody functionalization. The hydrodynamic diameter refers to the size of a sphere that has the same average diffusion coefficient as the molecule being measured and depends upon surface structure, concentration and types of ions in the medium [23]. The ISD showed bi-modal characteristics in both cases. The antibody functionalization moved the position of the main peak mode from 120 to 190 nm while the intensity remained almost constant at 8 per cent. An increase from 16 to 25 nm was observed for the second peak mode with an accompanying decrease in the intensity from 3 to 1 per cent. The dependence of the intensity (I) of scattered light on the spherical particle diameter (d) obeys a power law in the form of I ∝ d6 according to the Rayleigh approximations [24]. The ratio of population of the large particles to that of the small particles was, therefore, estimated to be 5.94 × 10−6 before antibody functionalization, whereas this ratio increased to 4.15 × 10−5 after antibody functionalization. This implies that the small AgNPs grew in size in sufficiently large numbers as a result of the agglomeration with anti-gIgG, giving rise to a fivefold increase in the relative concentrations.
Figure 3.
Size distribution histograms of (a) AgNPs and (b) AgNP–anti-gIgG from the dynamic light scattering experiments. (Online version in colour.)
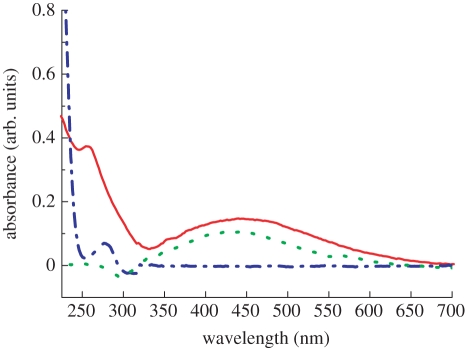
Figure 4 shows the UV–visible absorption spectrum of the AgNP–anti-gIgG complexes. The spectra for the AgNPs and pure anti-gIgG solution are included for comparison. The original concentration of AgNP–anti-gIgG solution was diluted by three times and an absorption spectrum of the resulting solution was studied. The colour of the AgNP solution changed from light grey to dark brown after functionalization with anti-gIgG. The position of the LSPR peak of AgNPs functionalized with anti-gIgG was red-shifted to 452 nm with respect to the peak of AgNPs at 430 nm. The amount of this red shift is nearly the same as that obtained for AgNPs individually modified with small 1-hexadecanethiol molecules [25]. However, the position of the AgNP absorption peak is not fixed but generally depends upon the distribution of the nanoparticles' sizes and shapes as a result of citrate reduction [26]. It has been recently reported that an increase in the silver to citrate ratio from 1 : 1.6 to 1 : 4.2 produced the variation in particle sizes between 36 and 66 nm, causing the absorption peak shift from 408 to 433 nm [27]. The absorption peaks for AgNP and AgNP–anti-gIgG complexes were both broadened owing to aggregation in the solution coupled with the change of inter particle distance [28]. The specific peak at 280 nm in pure anti-gIgG solution owing to aromatic amino acids (phenylalanine, tyrosine and tryptophan) was blue-shifted to 260 nm after bonding with AgNPs. UV–visible absorption, TEM and PCS measurements were repeated three times to ensure the formation of a stable AgNP–anti-gIgG complex.
Figure 4.
UV–visible absorption spectra of 12.46 µg ml−1 AgNPs (dotted line), 11.9 mM anti-gIgG (dashed-dotted line) and AgNP–anti-gIgG (solid line) solution (diluted three times). (Online version in colour.)
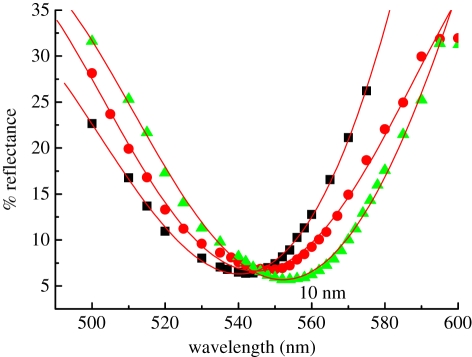
Figure 5 presents the SPR curves showing the variation in reflected intensity as a function of the incident wavelength corresponding to the fixed incident angle of 46°. These curves were obtained by the fourth-order polynomial fitting to experimental data. The dip in the reflectivity curve represents the resonance that occurs when the momentum of the incoming light is equal to the momentum of the plasmons. The dip is not sharp, implying that the surface plasmon wave was damped and the optical coupling became relatively stronger after immobilization. The SPRI resonance wavelength underwent a red shift from 542 to 546 nm when the Au surface was modified with the overlayer of MUA SAM. Further red shift of the resonance wavelength to 552 nm was observed owing to the anti-gIgG immobilization on the MUA-modified Au surface. This observation of red shifts is consistent with the results of absorption spectroscopy mentioned earlier. Using PC-based software with matrix formalism, values of 1.9 nm and 1.52 were obtained for the thickness and refractive index (at 542 nm) of the MUA SAM overlayer from the self-consistent solution of the Fresnel equations for reflectance close to resonance in a Kretschmann's four-layer geometry of glass/gold/MUA (or MUA + anti-gIG)/air. These values agree well with those reported from ellipsometric measurements [29]. A value of 60 nm for half-widths of all three SPRI curves indicates no appreciable change in the thickness owing to the immobilization [19]. The sensitivity Sn of an SPR sensor owing to the refractive indices is approximately taken to be proportional to the inverse of the cube of the refractive index of the MUA SAM [30]. The value of Sn is, therefore, of the order of 30 per cent.
Figure 5.
Comparison of SPRI reflectivity spectra of bare Au substrate (filled squares), modification with MUA (filled circles) and anti-goat immunoglobulin G (gIgG) (filled triangles) immobilization. The solid symbols show the experimental data, and the curves represent a fourth-order polynomial fit. (Online version in colour.)
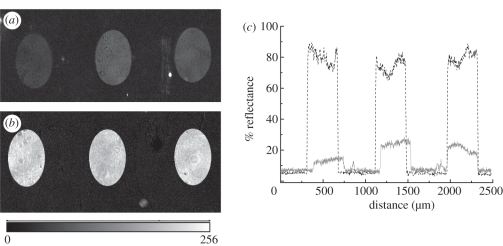
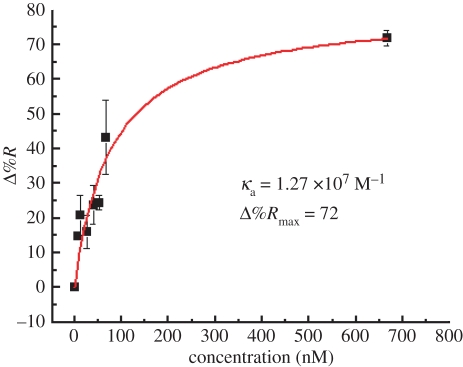
Figure 6a,b shows SPR images of 6.66 and 660 nM gIgG antigen spots on the sensor array on the immobilized anti-gIgG sensor surface. The angle of incidence was set at 46° and the incident wavelength was 552 nm. For the given surface coverage used here, the greyscale intensity in SPRI was taken to be linearly proportional to the materials deposited. These two different concentrations were chosen for the minimum and maximum reflectivity change owing to different mass depositions on the gold surface. It should be noted that the signal derived from the area between two spots can be used as a reference. The obtained image contrast of antigen spot compared with the base demonstrates the reduction in non-specific adsorption. The spatial distribution of percentage of reflectance (%R) of these images is calculated and shown in figure 6c. The SPR signal change was reproducible for the protein-adsorbed spots. The spatial resolution of these SPRI experiments was of the order of hundreds of micrometre, which provides enough resolution to resolve the spots. Since the changes in %R in the SPRI experiment were proportional to the amount of molecules adsorbed onto the surface, data from these measurements were used to quantitatively determine the concentration dependence of antibody–antigen binding on the surface. The percentage of reflectance change (Δ%R) was averaged and normalized over three spots with respect to the background anti-gIgG surface at the same wavelength. The nonlinear response between Δ%R and concentration is fitted to the following equation
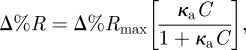 |
3.1 |
where κa is the affinity constant and C is the antigen concentration. Figure 7 shows the dose–response curve of the interaction of surface-immobilized anti-gIgG and gIgG where the concentration of gIgG is plotted against Δ%R. A value of 1.27 × 107 M−1 was obtained for the affinity constant, and this value is consistent with the general range of antibody–antigen interaction reported by McMahon & O'Kennedy [31].
Figure 6.
SPR image of the microarray goat immunoglobulin G (gIgG) spot: (a) 6.66 nM, (b) 660 nM at 552 nm (intensity in greyscale is shown at the bottom of the pictures) and (c) spatial distribution of reflectivity (%) of the gIgG spot in the microarray (solid and broken lines for 6.66 and 660 nM, respectively). (Online version in colour.)
Figure 7.
Response of Δ%R of immobilized anti-goat immunoglobulin G (gIgG) against the concentration of goat immunoglobulin G (gIgG) within the range of 6.66–660 nM. The reflectivity corresponds to that at 552 nm. (Online version in colour.)
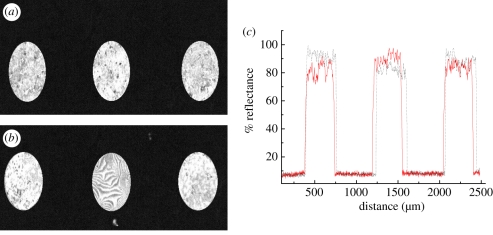
Optimization of signal enhancement of anti-gIgG after AgNP functionalization was performed with the help of SPRI. Figure 8 shows the post-binding SPR images of 6.66 and 660 nM gIgG spots after chip incubation in AgNP–anti-gIgG solution at 552 nm. The large-scale heterogeneity across the printing area was believed to be satisfactory for the AgNP-functionalized anti-gIgG surface. The excitation wavelength of 552 nm of the incident light was resonant with a fraction of SP resonances in irregularly shaped cluster composites of Ag films. This is attributed to the SPR of AgNPs in Ag-anti-IgG composite film, which is in agreement with the observed black spots in post-binding SPR images. The resonant coupling of incident light may also be associated with a strong scattering as a secondary process. The magnitude of the scattering efficiency and the relative contribution to the total extinction were shown to vary with particle size and shape, metal composition and surrounding medium [32,33]. Values of %R that were obtained from the spatial distribution of spots, as indicated in figure 8c, are not appreciably different. %R for concentrations of less than 6.66 nM of the AgNP–anti-IgG overlayer on the gold surface and low reflection required high-intensity illumination. The reflection for the overlayer with a concentration higher than 660 nM reached the pixel saturation value for the camera used [34].
Figure 8.
SPR images of the microarrayed AgNP–anti-goat immunoglobulin G (gIgG) spot of (a) 6.66 nM and (b) 660 nM at 552 nm and (c) the spatial distribution of the reflectivity (%) of the spots (solid and broken lines for 6.66 and 660 nM, respectively). (Online version in colour.)
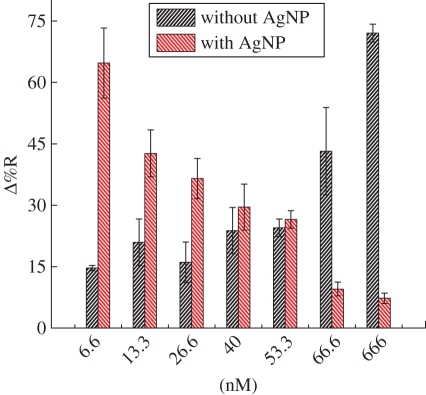
In order to overcome this instrument limitation, the dependence of the differential reflectance (Δ%R) of the AgNP–anti-IgG overlayer on the gold surface on concentrations of gIgG is presented in figure 9. Histograms of the overlayer without the AgNP tag are included for the sake of comparison. The signal factor owing to the tagging was estimated to be 4.33 for 6.66 nM gIgG. Signal enhancement for higher concentrations, such as 660 nM, was found to be relatively small (0.14). The low signal enhancement at the high gIgG concentration may be due to the formation of a denser layer of gIgG on the NP surface (steric hindrance effect), which reduced the rate of adsorption of the nanoparticle-tagged antibody. The reorientation and lateral diffusion of nanoparticle-tagged anti-gIgG was also expected to offer the available binding site as a favourable position [35].
Figure 9.
Change in percentage of reflectivity (Δ%R) for different concentrations of goat immunoglobulin G (gIgG) with and without AgNPs at an SPRI wavelength of 552 nm (average over three measurements). (Online version in colour.)
The minimum detection limit obtained in the present investigation compares very well with the published data for the human immunoglobulin G–antibody interactions that were studied using a similarly constructed SPRI system [36]. The SPRI detection limit of ovalbumin using electroaddressing of anti-ovalbumin IgG was found to be 2 nM [37]. A lower limit of 1 nM was achieved for detecting two clinically important low-molecular-weight proteins, beta(2)-microglobulin and cystatin C, using high-density multiplexed antibody arrays on gold surfaces in the SPRI system [38]. The range between 6.66 and 660 nM investigated in the present study is limited to the angle of incidence of 46°.
A control experiment was carried out with AgNP-functionalized BSA on the immobilized anti-gIgG-patterned chip. The Δ%R of the image was found to be within the limit of between 3 and 5 per cent. Non-specific binding was also performed in a separate measurement, by dropping 10 mM of anti-BSA solution on immobilized IgG spots for 30 min. The SPRI experiment was repeated after washing and drying the chip. However, no concentration-dependent response of Δ%R of the SPR images was observed, indicating the biological activity of the immobilized antibody.
4. Concluding remarks
The citrate reduction method with the THF stabilizer was employed to synthesize AgNPs with sizes ranging from 10 to 40 nm. The functionalization ratio between AgNPs with anti-gIgG in the stable AgNP–anti-gIgG complex was found to be approximately 1 : 5 from TEM analysis. The red shift of the absorption peaks owing to the functionalization of the anti-gIgG molecules on the AgNP surface is believed to have arisen from the increases in particle sizes. This interpretation is supported by the particle size distribution as measured by PCS. The binding of gIgG with an immobilized rabbit anti-gIgG surface was monitored by considering reflectivity changes in the percentage (Δ%R) of anti-gIgG at a fixed angle for the SPRI resonance wavelength. After nanoparticle functionalization with anti-gIgG, SPRI signal enhancement was found to be 4.33 times larger than that obtained without nanoparticle tagging. This result indicates the possibility of detection of low analyte concentrations in an early-stage tumour/cancer cell with the help of SPRI spectroscopy. The detection limit of the present investigation is in the nanomolar range, consistent with the published data. Differential spectroscopic SPR measurements using different incident angles have the potential to extend the detection range.
Acknowledgements
This work was supported by funding under the FP6 IIF Marie Curie Scheme from the European commission (proposal no. 22364) and EPSRC (DT/E010857/1). We are grateful to Prof. P. Vadgama for fruitful scientific discussions, and Rajdip and Madhurima Ray for useful suggestions.
References
- 1.Campbell C. T., Kim G. 2007. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 28, 2380–2392 10.1016/j.biomaterials.2007.01.047 (doi:10.1016/j.biomaterials.2007.01.047) [DOI] [PubMed] [Google Scholar]
- 2.Phillips K. S., Cheng Q. 2007. Recent advances in surface plasmon resonance based techniques for bioanalysis. Anal. Bioanal. Chem. 387, 1831–1840 10.1007/s00216-006-1052-7 (doi:10.1007/s00216-006-1052-7) [DOI] [PubMed] [Google Scholar]
- 3.Malic L., Veres T., Tabrizian M. 2009. Biochip functionalization using electrowetting-on-dielectric digital microfluidics for surface plasmon resonance imaging detection of DNA hybridization. Biosens. Bioelectron. 24, 2218–2224 10.1016/j.bios.2008.11.031 (doi:10.1016/j.bios.2008.11.031) [DOI] [PubMed] [Google Scholar]
- 4.Smith E. A., Thomas W. D., Kiessling L. L., Corn R. M. 2003. Surface plasmon resonance imaging studies of protein–carbohydrate interactions. J. Am. Chem. Soc. 125, 6140–6148 10.1021/ja034165u (doi:10.1021/ja034165u) [DOI] [PubMed] [Google Scholar]
- 5.Wegner G. J., Lee H. J., Corn R. M. 2002. Characterization and optimization of peptide arrays for the study of epitope–antibody interactions using surface plasmon resonance imaging. Anal. Chem. 74, 5161–5168 10.1021/ac025922u (doi:10.1021/ac025922u) [DOI] [PubMed] [Google Scholar]
- 6.Wegner G. J., Wark A. W., Lee H. J., Codner E., Saeki T., Fang S., Corn R. M. 2004. Real-time surface plasmon resonance imaging measurements for the multiplexed determination of protein adsorption/desorption kinetics and surface enzymatic reactions on peptide microarrays. Anal. Chem. 76, 5677–5684 10.1021/ac0494275 (doi:10.1021/ac0494275) [DOI] [PubMed] [Google Scholar]
- 7.Kanda V., Kariuki J. K., Harrison D. J., McDermott M. T. 2004. Label-free reading of microarray-based immunoassays with surface plasmon resonance imaging. Anal. Chem. 76, 7257–7262 10.1021/ac049318q (doi:10.1021/ac049318q) [DOI] [PubMed] [Google Scholar]
- 8.Graham D., et al. 2009. Functionalized nanoparticles for bioanalysis by SERRS. Biochem. Soc. Trans. 37, 697–701 10.1042/BST0370697 (doi:10.1042/BST0370697) [DOI] [PubMed] [Google Scholar]
- 9.Wang Y. L., Ren W., Dong S. J., Wang E. K. 2009. Characterization and optimization of AuNPs labeled by Raman reporters on glass based on silver enhancement. J. Raman Spectrosc. 40, 571–576 10.1002/jrs.2170 (doi:10.1002/jrs.2170) [DOI] [Google Scholar]
- 10.Cognet L., Tardin C., Boyer D., Choquet D., Tamarat P., Lounis B. 2003. Single metallic nanoparticle imaging for protein detection in cells. Proc. Natl Acad. Sci. USA 100, 11 350–11 355 10.1073/pnas.1534635100 (doi:10.1073/pnas.1534635100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnichsen C., Reinhard B. M., Liphardt J., Alivisatos A. P. 2005. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 23, 741–745 10.1038/nbt1100 (doi:10.1038/nbt1100) [DOI] [PubMed] [Google Scholar]
- 12.Endo T., Kerman K., Nagatani N., Hiepa H. M., Kim D. K., Yonezawa Y., Nakano K., Tamiya E. 2006. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core-shell structured nanoparticle layer nanochip. Anal. Chem. 78, 6465–6475 10.1021/ac0608321 (doi:10.1021/ac0608321) [DOI] [PubMed] [Google Scholar]
- 13.Hirsch L. R., Stafford R. J., Bankson J. A., Sershen S. R., Rivera B., Price R. E., Hazle J. D., Halas N. J., West J. L. 2003. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100, 13 549–13 554 10.1073/pnas.2232479100 (doi:10.1073/pnas.2232479100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolaymat T. M., El Badawy A. M., Genaidy A., Scheckel K. G., Luxton T. P., Suidan M. 2010. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 408, 999–1006 10.1016/j.scitotenv.2009.11.003 (doi:10.1016/j.scitotenv.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 15.Jin H. J., Zangar R. C. 2010. Antibody microarrays for high-throughput, multianalyte analysis. Cancer Biomark. 6, 281–290 [DOI] [PubMed] [Google Scholar]
- 16.Kamat P. V., Flumiani M., Hartland G. V. 1998. Picosecond dynamics of silver nanoclusters. Photoejection of electrons and fragmentation. J. Phys. Chem. B 102, 3123–3128 10.1021/jp980009b (doi:10.1021/jp980009b) [DOI] [Google Scholar]
- 17.Kempa T., Farrer R. A., Giersig M., Fourkas J. T. 2006. Photochemical synthesis and multiphoton luminescence of monodisperse silver nanocrystals. Plasmonics 1, 45–51 10.1007/s11468-006-9008-5 (doi:10.1007/s11468-006-9008-5) [DOI] [Google Scholar]
- 18.Kretschmann E., Raether H. Z. 1968. Radiative decay of non radiative surface plasmons excited by light. Natureforsch 23A, 2135–2136 [Google Scholar]
- 19.Johansen K., Arwin H., Lundström I., Liedberg B. 2000. Imaging surface plasmon resonance sensor based on multiple wavelengths: sensitivity considerations. Rev. Sci. Instrum. 71, 3530–3538 10.1063/1.1287631 (doi:10.1063/1.1287631) [DOI] [Google Scholar]
- 20.Gosling J. P. 1990. A decade of development in immunoassay methodology. Clin. Chem. 36, 1408–1427 [PubMed] [Google Scholar]
- 21.Kelly K. L., Coronado E., Zhao L., Schatz G. C. 2003. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B 107, 668–677 10.1021/jp026731y (doi:10.1021/jp026731y) [DOI] [Google Scholar]
- 22.Price C. P., Newman D. J. (eds) 1991. Principles and practice of immunoassay. London, UK: Macmillan [Google Scholar]
- 23.Kaszuba M., McKnight D., Connah M. T., McNeil-Watson F. K., Nobbmann U. 2008. Measuring sub-nanometre sizes using dynamic light scattering. J. Nanopart. Res. 10, 823–829 10.1007/s11051-007-9317-4 (doi:10.1007/s11051-007-9317-4) [DOI] [Google Scholar]
- 24.Tscharnuter W. 2000. Photon correlation spectroscopy in particle sizing. In Encyclopedia of analytical chemistry (ed. Meyers R. A.), pp. 5469–5485 Chichester, UK: John Wiley & Sons Ltd [Google Scholar]
- 25.McFarland A. D., Van Duyne R. P. 2003. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett. 3, 1057–1062 10.1021/nl034372s (doi:10.1021/nl034372s) [DOI] [Google Scholar]
- 26.Pillai Z. S., Kamat P. V. 2004. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 108, 945–951 10.1021/jp037018r (doi:10.1021/jp037018r) [DOI] [Google Scholar]
- 27.Li T., Albee B., Alemayehu M., Diaz R., Ingham L., Kamal S., Rodriguez M., Bishnoi S. W. 2010. Comparative toxicity study of Ag, Au, and Ag–Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 398, 689–700 10.1007/s00216-010-3915-1 (doi:10.1007/s00216-010-3915-1) [DOI] [PubMed] [Google Scholar]
- 28.Kreibig U., Vollmer M. 1995. Optical properties of metal clusters, vol. 25 Berlin, Germany: Springer-Verlag [Google Scholar]
- 29.Balasubramanian S., Revzin A., Sirnonian A. 2006. Electrochemical desorption of proteins from gold electrode surface. Electroanalysis 18, 1885–1892 10.1002/elan.200603627 (doi:10.1002/elan.200603627) [DOI] [Google Scholar]
- 30.Homola J. 1997. On the sensitivity of surface plasmon resonance sensors with spectral interrogation. Sensors Actuators B 41, 207–211 10.1016/S0925-4005(97)80297-3 (doi:10.1016/S0925-4005(97)80297-3) [DOI] [Google Scholar]
- 31.McMahon M. J., O'Kennedy R. 2001. The use of in vitro immunisation, as an adjunct to monoclonal antibody production, may result in the production of hybridomas secreting polyreactive antibodies. J. Immunol. Methods 258, 27–36 10.1016/S0022-1759(01)00481-1 (doi:10.1016/S0022-1759(01)00481-1) [DOI] [PubMed] [Google Scholar]
- 32.Lee K. S., El-Sayed M. A. 2006. Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 110, 19 220–19 225 10.1021/jp062536y (doi:10.1021/jp062536y) [DOI] [PubMed] [Google Scholar]
- 33.Van Dijk M. A., Lippitz M., Orrit M. 2005. Far-field optical microscopy of single metal nanoparticles. Acc. Chem. Res. 38, 594–601 10.1021/ar0401303 (doi:10.1021/ar0401303) [DOI] [PubMed] [Google Scholar]
- 34.Boecker D., Zybin A., Horvatic V., Grunwald C., Niemax K. 2007. Differential surface plasmon resonance imaging for high throughput bioanalyses. Anal. Chem. 79, 702–709 10.1021/ac061623j (doi:10.1021/ac061623j) [DOI] [PubMed] [Google Scholar]
- 35.Gupta S., Huda S., Kilpatrick P. K., Velev O. D. 2007. Characterization and optimization of gold nanoparticle-based silver-enhanced immunoassays. Anal. Chem. 79, 3810–3820 10.1021/ac062341m (doi:10.1021/ac062341m) [DOI] [PubMed] [Google Scholar]
- 36.Dong Y., Wilkop T., Xu D. K., Wang Z. Z., Cheng Q. 2008. Microchannel chips for the multiplexed analysis of human immunoglobulin G–antibody interactions by surface plasmon resonance imaging. Anal. Bioanal. Chem. 390, 1575–1583 10.1007/s00216-008-1849-7 (doi:10.1007/s00216-008-1849-7) [DOI] [PubMed] [Google Scholar]
- 37.Corgier B. P., Bellon S., Anger-Leroy M., Loic J., Blum L. J., Marquette C. A. 2009. Protein-diazonium adduct direct electrografting onto SPRI-biochip. Langmuir 25, 9619–9623 10.1021/la900762s (doi:10.1021/la900762s) [DOI] [PubMed] [Google Scholar]
- 38.Lee H. J., Nedelkov D., Corn R. M. 2006. Surface plasmon resonance imaging measurements of antibody arrays for the multiplexed detection of low molecular weight protein biomarkers. Anal. Chem. 78, 6504–6510 10.1021/ac060881d (doi:10.1021/ac060881d) [DOI] [PubMed] [Google Scholar]