Abstract
Functionalized diazo acetoacetates are prepared by an efficient Mukaiyama-Michael reaction between methyl 3-(trialkylsilanoxy)-2-diazo-3-butenoate and α,β-unsaturated enones. Vinyl ether and ketone derivatives are both accessible in good to excellent yield through this methodology. The mild Lewis acid zinc(II) triflate is the optimal catalyst, and it’s loading can be as low as 0.1 mol%. In addition, zinc triflate was also found to be a superior catalyst for the related Mukaiyama-aldol reaction.
Metal-catalyzed diazo decomposition of diazoacetoacetate derivatives are among the most well-known reactions for the construction of cyclopropane, cycloalkanone and β-lactam moieties.1,2 The advantages of diazoacetoacetate compounds compared to diazoacetates include their higher thermal and acid stability and greater reaction control during diazo decomposition.1 Traditionally these diazo compounds were prepared by multi-step synthesis involving diazo transfer as the final step.3,4 Alternative methods were developed which involve the base-mediated condensation of diazo compounds with various electrophiles.5,6 However these reactions generally require stoichiometric amounts of the reagents under relatively harsh reaction conditions. Although diazo compounds are known to lose their diazo functionality in the presence of Lewis acids,7 Calter and Wang independently found various methods for the Lewis acid mediated condensation of diazoacetoacetate compounds and electrophiles.8 Despite these advances, efficient syntheses of structurally complex diazo compounds under mild conditions remains a challenging problem.
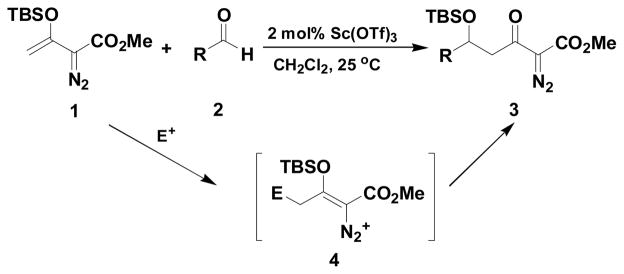
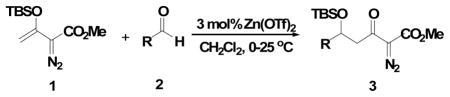
We recently published an efficient Mukaiyama-aldol reaction between methyl 3-(trialkylsilanoxy)-2-diazo-3-butenoate 19 and both aromatic and aliphatic aldehydes 2 (Scheme 1) catalyzed by scandium(III) triflate. 10 These reactions proceeded to form 3 in high yield without decomposition of the diazo group. Their success is due, at least in part, to the stability of the vinyldiazonium ion intermediate (4) that is formed by electrophilic addition to 1. However, the low Lewis acidity of scandium(III) triflate required relatively long reaction times and limted application to aldehydes with strong electron-withdrawing groups.
Scheme 1.
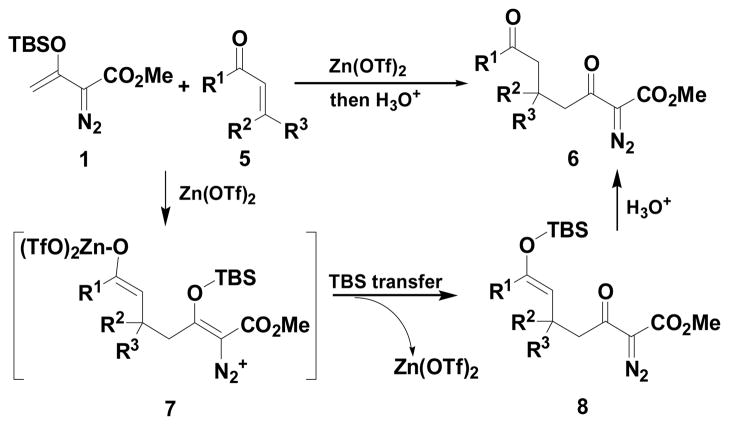
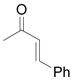
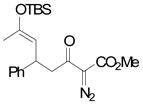
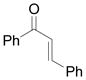
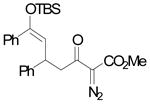
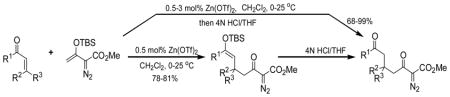
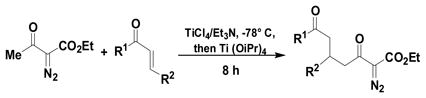
The Mukaiyama-Michael reaction that is the condensation between enolsilanes and α,β-unsaturated carbonyl compounds 11 is a suitable extension of the Mukaiyama-aldol reaction. Reactions mediated by stoichiometric amounts of strong Lewis acid catalysts have been reported,12 and the use of various lanthanides and other Lewis acids as catalysts have been extensively studied.13,14 In the only report of a Mukaiyama-Michael reaction to form diazozcetoacetates,8c TiCl4 is used in stoichiometric amounts to form the condensation product (eq 1); reactions occurred at −78°C, and 1,2-addition was competitive with 1,4-addition. In view of this, a Mukaiyama-Michael reaction between 1 and α,β-unsaturated carbonyl compounds using catalytic amounts of Lewis acid under milder conditions would be desirable. Herein we report that highly functionalized diazo compounds (6 and 8) can be prepared by an efficient Lewis acid-catalyzed Mukaiyama-Michael reaction between 1 and enones 5 (Scheme 2) under mild conditions with low catalyst loading of inexpensive zinc triflate. As in previous examples of Mukaiyama-Michael reactions with enolsilanes, silyl group transfer across seven atoms was considered to be a major challenge.
Scheme 2.
 |
(1) |
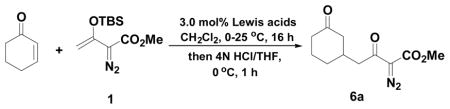
In catalyst screening with cyclohexenone we found that scandium(III) triflate, the previously preferred catalyst for Mukaiyama-aldol reactions of 1,10 was relatively ineffective for the Michael reaction with the same nucleophile. Thus, reaction of 1 with 1.1 equiv of cyclohexenone in the presence of 3.0 mol% Sc(OTf)3 gave the Mukaiyama-Michael reaction product (6a) in only 43% isolated yield after subsequent hydrolysis (entry 1, Table 1),. The low yield was determined to be caused by slow reaction as unreacted cyclohexenone and hydrolyzed silyl enol ether from 1 were also isolated. Lanthanium(III) triflate increased the yield slightly to 50%; however, the reaction was not significantly faster (entry 2). The use of boron trifluoride-etherate8b under the same conditions resulted in decomposition of 6a (entry 3).
Table 1.
Optimization of the Mukaiyama-Michael Reactiona
 | ||
|---|---|---|
| entry | Lewis acid | yield of 6a (%)b |
| 1 | Sc(OTf)3 | 43 |
| 2 | La(OTf)3 | 50 |
| 3 | BF3-OEt2 (100 mol%) | 0 |
| 4 | Cu(OTf)2 | 29 |
| 5 | AgOTf | 44 |
| 6 | Sn(OTf)2 | 48 |
| 7 | Zn(OTf)2 | 79 |
| 8c | Zn(OTf)2 | 96 |
Vinyldiazoacetate 1 (0.5 mmol) was added to a solution of enone 5a (0.55 mmol) and metal triflate (0.015 mol) in CH2Cl2 (2 mL). The solution was stirred at 0°C and then allowed to warm up to room temperature. After 16 hours, the crude reaction mixture was concentrated and hydrolyzed with 1:5 4N HCl/THF at 0°C. See Supporting Information for details.
Yield of isolated 6a following column chromatography.
1.5 equivalent of 1 was used under the same conditions.
In related studies of the Cu(II) catalyzed Mukaiyama-aldol reactions, silylation of the metal aldolate (silyl transfer) had been proposed to be the rate-limiting step.15a Furthermore, Ellis and Bosnich concluded that a Lewis acid having a strong alkoxide-Lewis acid bond will inhibit silyl transfer.15b Thus, we focused our attention on the use of less oxophilic Lewis acids. A screening of selected mild Lewis acids (Table 1, entries 4–7) revealed that the inexpensive zinc(II) triflate gave a significantly improved yield (79%) of Mukaiyama-Michael product 6a in the reaction between 1 and 1.1 equivalent of cyclohexenone (entry 7). The yield of 6a could be further improved to 96% with the use of 1.5 equiv 1 (entry 8).16,17 Other Lewis acids were found to be much less effective than Zn(OTf)2; with Cu(II) and Ag(I) triflate diazo decomposition occurs, resulting in a complex reaction mixture and low yield (entries 4, 5).1 Having established optimum conditions with Zn(OTf)2, efforts were undertaken to reduce the required amount of catalyst: a 96% yield of 6a was obtained with 0.5 mol % catalyst, and with only 0.1 mol % of Zn(OTf)2 the yield of 6a was 94%.
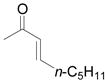
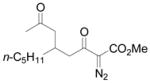
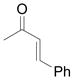
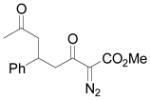
Using 3 mol% Zn(OTf)2 and the optimal conditions for production of 6 reactions of 1 with various α, β-unsaturated ketones were examined (Table 2). Cyclic enones 5a–5d and several acyclic enones 5h–5j all gave excellent yields of the desired product.18 β, β-Disubstituted enones such as 5e and 5g are sterically hindered and were previously reported to react sluggishly19 or give low yields of Mukaiyama-Michael product.14a However, although we found that its reaction with 1 was notably slower than with 5a, the Michael adduct 6e was still isolated in good yield with 3 mol% catalyst after 36 hours. Decreased reaction rate was also observed with 5g, but a good yield of 6g was obtained by increasing the concentration of reactants in the reaction solution. The reaction of methyl vinyl ketone 5f was facile; however, oligomerization was found to be the major side-reaction. The yield of 5f could be improved to 74% by using 2 equivalents of 1. Several of these reactions were performed in the presence of only 0.5 mol% zinc triflate catalyst and afforded comparable product yield (entries 1, 6, 7). In all cases, only 1,4-addition products were observed and isolated from the reaction mixture.
Table 2.
Zinc Triflate-Catalyzed Mukaiyama-Michael Reactions of 1 with Representative Enonesa
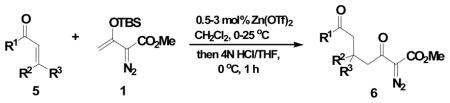 | ||||
|---|---|---|---|---|
| entry | enone 5 | prduct 6 | yieldb, % | |
| 1 | a |
|
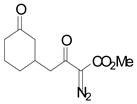
|
96 |
| 2 | b |
|
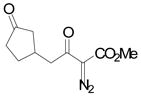
|
84 |
| 3 | c |
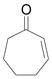
|
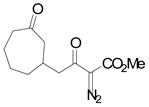
|
94 |
| 4c | d |
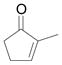
|
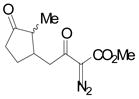
|
99 |
| 5d | e |
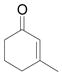
|
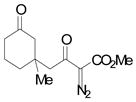
|
68 |
| 6e | f |
|
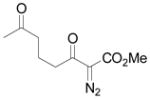
|
74 |
| 7d,f | g |
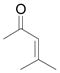
|
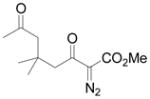
|
71 |
| 8 | h |
|
|
88 |
| 9 | i |
|
|
92 |
| 10 | j |
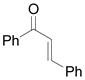
|
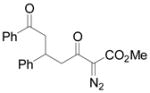
|
99 |
Vinyldiazoacetate 1 (0.75 mmol) was added to a solution of enone 5 (0.5 mmol) and zinc triflate (0.015 mmol) in CH2Cl2 (2 mL). The solution was stirred at 0°C and then allowed to warm to room temperature. After 16 hours, the crude reaction mixture was concentrated and then hydrolyzed with 1:5 4N HCl/THF at 0°C. See Supporting Information for details
Yield of isolated 6 following column chromatography.
The product was isolated as two diastereomers in 3:1 ratio.
Reaction time was 36 hours.
Two equivalents of 1 was used, 62% yield with 1.5 equiv. of 1; 71% yield with 0.5 mol % of catalyst.
Reaction was performed in 1 mL CH2Cl2, and the concentration of enone 5g was 0.5 M; 78% yield with 0.5 mol % catalyst.
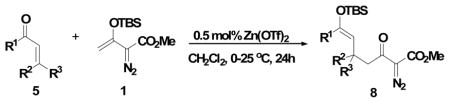
In performing these reactions we anticipated that silyl enol ether 8 would be the major product prior to hydrolysis, and indeed it was. However, the product mixture after 16 h in a reaction apparatus that was open to the atmosphere contained variable amounts of 6 and 8 that were marginally reproducible. However, when the reaction apparatus was closed to the atmosphere, only 8 was obtained. Zinc triflate is sufficiently hygroscopic to draw water into the reaction solution and cause the hydrolysis of 8. Reducing the amount of catalyst and performing the reaction in a closed apparatus resulted in good yields of 8, representative examples for which are given in Table 3.
Table 3.
Catalytic Mukaiyama-Michael Reactions of 1 with 0.5mol% catalyst loading and lead to product 8a
Vinyldiazoacetate 1 (0.5 mmol) was added to a solution of enone 5(0.6 mmol) and metal triflate (0.0025 mol) in CH2Cl2 (2 mL). The solution was stirred at 0°C and then allowed to warm up to room temperature and react for 24 hours.
Yield of isolated 5 following column chromatography.
The product was isolated as two diastereomers in 3:1 ratio favoring the E-isomer.
Recognizing the effectiveness of zinc triflate for the Mukaiyama-Michael reaction, we returned to the Mukaiyama-aldol reaction to examine its effectiveness for that transformation (Table 4). The isolated yields of both 3a and 3b were slightly higher with zinc triflate than with scandium triflate, and Zn(OTf)2 loading could be as low as 0.1 mol%. Cinnamaldehyde 2b undergoes exclusive 1,2-addition with 1 in the presence of zinc(II) triflate; no 1,4-addition product was observed (entry 2). Furthermore, zinc(II) triflate was found to catalyze the reactions of 4-nitrobenzaldehyde 2c and 2-nitrobenzaldehyde 2d, which had previously been found to be unreactive using scandium triflate (entries 3, 4).
Table 4.
Zinc(II) Triflate Catalyzed Mukaiyama-aldol Reactions of 1 with Representative Aldehydesa
In conclusion, an efficient Mukaiyama-Michael methodology involving reactions between a diazo-containing silyl enol ether and various α,β-unsaturated ketones has been developed using inexpensive, commercially available, zinc triflate. Reaction conditions are mild, product yields are high under low catalyst loading, and the catalyst is also effective in corresponding Mukiayama-aldol reactions. This methodology can be used for the efficient construction of highly substituted diazo-containing compounds and allow late-stage installation and manipulation of the diazo functionality.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support provided by the National Institutes of Health (GM46503) and the National Science Foundation.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. John Wiley & Sons; New York: 1998. [Google Scholar]; (b) Doyle MP. In: Comprehensive Organometallic Chemistry II. Hegedus LS, editor. 12 Chapter 5.2 Pergammon Press; New York: 1995. [Google Scholar]
- 2.Recent reviews: Merlic CA, Zechman AL. Synthesis. 2003:1137–1156.Davies HML, Antoulinakis EG. Org React. 2001;57:1–326.Hodgson DM, Pierard FYTM, Stupple PA. Chem Soc Rev. 2001;30:50–61.Doyle MP. In: In Catalytic Asymmetric Synthesis. 2. Ojima I, editor. John Wiley & Sons; New York: 2000. Doyle MP, Forbes DC. Chem Rev. 1998;98:911–935. doi: 10.1021/cr940066a.Khlebnikov AF, Novikov MS, Kostikov RR. Adv Heterocycl Chem. 1996;65:93–233.Padwa A, Austin DJ. Angew Chem, Int Ed Engl. 1994;33:1797–1815.
- 3.Review: Regitz M, Maas G. Diazo Compounds; Properties and Synthesis. Academic Press; Orlando, FL: 1986.
- 4.(a) Padwa A, Austin DJ, Price AT, Semones MA, Doyle MP, Protopopova MN, Winchester WR, Tran A. J Am Chem Soc. 1993;115:8669–8680. [Google Scholar]; (b) Doyle MP, Westrum LJ, Wolthuis WNE, See MM, Boone WP, Bagheri V, Pearson MM. J Am Chem Soc. 1993;115:958–964. [Google Scholar]; (c) Clemens RJ, Hyatt JA. J Org Chem. 1985;50:2431–2435. [Google Scholar]
- 5.Review: Zhao Y, Wang J. Synlett. 2005:2886–2892.
- 6.(a) Wenkert E, McPherson CA. J Am Chem Soc. 1972;94:8084–8090. [Google Scholar]; (b) Moody CJ, Morfitt CN. Synthesis. 1998:1039–1042. [Google Scholar]; (c) Kanemasa S, Araki T, Kanai T, Wada E. Tetrahedron Lett. 1999;40:5059–5062. [Google Scholar]; (d) Jiang N, Wang J. Tetrahedron Lett. 2002;43:1285–1287. [Google Scholar]; (e) Sa MM, Silveira GP, Bortoluzzi AJ, Padwa A. Tetrahedron. 2003;59:5441–5447. [Google Scholar]
- 7.Holmquist CR, Roskamp EJ. J Org Chem. 1989;54:3258–3260. [Google Scholar]
- 8.(a) Calter MA, Sugathapala PM, Zhu C. Tetrahedron Lett. 1997;38:3837–3840. [Google Scholar]; (b) Calter MA, Zhu C. J Org Chem. 1999;64:1415–1419. [Google Scholar]; (c) Deng G, Tian X, Qu Z, Wang J. Angew Chem, Int Ed. 2002;41:2773–2776. doi: 10.1002/1521-3773(20020802)41:15<2773::AID-ANIE2773>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Davies HML, Ahmed G, Churchill MR. J Am Chem Soc. 1996;118:10774–10780. [Google Scholar]
- 10.Doyle MP, Kundu K, Russell AE. Org Lett. 2005;7:5171–5174. doi: 10.1021/ol052003s. [DOI] [PubMed] [Google Scholar]
- 11.Narasaki K, Soai K, Mukaiyama T. Chem Lett. 1974:1223–1224. [Google Scholar]
- 12.For representative examples: Heathcock CH, Norman MH, Uehling DE. J Am Chem Soc. 1985;107:2797–2799.Sato T, Wakahara Y, Otera J, Nozai H, Fukuzumi S. J Am Chem Soc. 1991;113:4028–4030.Fujita Y, Fukuzumi S, Otera J. Tetrahedron Lett. 1997;38:2117–2120.Sankararaman S, Sudha R. J Org Chem. 1999;64:2155–2157. doi: 10.1021/jo982363b.Bellassoued M, Mouelhi S, Fromentin P, Gonzalez A. J Organometal Chem. 2005;690:2172–2179.
- 13.For representative examples of Mukaiyama-Michael reactions using catalytic amounts of Lewis acids: Matsuda I, Makino T, Hasegawa Y, Itoh K. Tetrahedron Lett. 2000;41:1409–1412.Miura K, Nakagawa T, Hosomi A. Synlett. 2003:2068–2070.Jaber N, Assie M, Fiaud J, Collin J. Tetrahedron. 2004;60:3075–3083.An DL, Peng Z, Orita A, Kurita A, Man-e S, Ohkubo K, Li X, Fukuzumi S, Otera J. Chem Eur J. 2006;12:1642–1647. doi: 10.1002/chem.200501091.Suga H, Takemoto H, Kakehi A. Heterocylces. 2007;71:361–371.Attanasi OA, Favi G, Filippone P, Lillini S, Mantellini F, Spinelli D, Stenta M. Adv Syn & Catal. 2007;349:907–915.
- 14.For representative examples of asymmetric Mukaiyama-Michael reactions using Lewis acid catalysts: Evans DA, Rovis T, Kozlowski M, Dowey CW, Tedrow JS. J Am Chem Soc. 2000;122:9134–9142.Evans DA, Scheidt KA, Johnston JN, Willis MC. J Am Chem Soc. 2001;123:4480–4491. doi: 10.1021/ja010302g.Desimoni G, Faita G, Filippone S, Mella M, Zampori M, Zema M. Tetrahedron. 2001;57:10203–10212.Sibi MP, Chen J. Org Lett. 2002;4:2933–2936. doi: 10.1021/ol026333d.Wang X, Adachi S, Iwai H, Takatsuki H, Fujita K, Kubo M, Oku A, Harada T. J Org Chem. 2003;68:10046–10057. doi: 10.1021/jo035379x.Suga H, Kitamura T, Kakehi A, Baba T. Chem Commum. 2004:1414–1415. doi: 10.1039/b402826k.Harada T, Adachi S, Wang X. Org Lett. 2004;6:4877–4879. doi: 10.1021/ol048071g.Ishihara K, Fushimi M. Org Lett. 2006;8:1921–1924. doi: 10.1021/ol060651l.Takenaka N, Abell JP, Yamamoto H. J Am Chem Soc. 2007;129:742–743. doi: 10.1021/ja0668320.
- 15.(a) Evans DA, Murry JA, Kozlowski MC. J Am Chem Soc. 1996;118:5814–5815. [Google Scholar]; (b) Ellis WW, Bosnich B. Chem Commun. 1998;193–194 [Google Scholar]
- 16.The use of other common solvents such as chloroform, toluene, THF and ether gave diminished yields of 6a.
- 17.In contrast to our report of Mukaiyama reaction (ref 10), the OTMS silyl enol ether analog of 1 gave la ower yield of 6 than did 1. The additional advantage of the OTBS silyl enol ether is its greater stability.
- 18.Other common Michael acceptors such as α, β-unsaturated esters, unsaturated nitriles and unsaturated nitro compounds were found to be unreactive under the standard reaction condition.
- 19.DuBay WJ, Grieco PA, Todd LJ. J Org Chem. 1994;59:6898–6899. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.