Abstract
Heparan sulfate proteoglycans are found at the cell surface and in the extracellular matrix, where they interact with a plethora of ligands. Over the last decade, new insights have emerged regarding the mechanism and biological significance of these interactions. Here, we discuss changing views on the specificity of protein–heparan sulfate binding and the activity of HSPGs as receptors and coreceptors. Although few in number, heparan sulfate proteoglycans have profound effects at the cellular, tissue, and organismal level.
Heparan sulfate proteoglycans at the cell surface and in the extracellular matrix act as receptors and coreceptors, with profound effects on growth factor action, cell adhesion, and tissue architecture.
Heparan sulfate proteoglycans (HSPGs) are glycoproteins, with the common characteristic of containing one or more covalently attached heparan sulfate (HS) chains, a type of glycosaminoglycan (GAG) (Esko et al. 2009). Cells elaborate a relatively small set of HSPGs (∼17) that fall into three groups according to their location: membrane HSPGs, such as syndecans and glycosylphosphatidylinositol-anchored proteoglycans (glypicans), the secreted extracellular matrix HSPGs (agrin, perlecan, type XVIII collagen), and the secretory vesicle proteoglycan, serglycin (Table 1). Much of the early work in the field concentrated on composition (size, chain number, and structure of the HS chains), biosynthesis, and binding properties of the chains. In 1985, the first somatic cell mutants altered in HSPG expression were identified (Esko et al. 1985), which allowed functional studies in the context of a cell culture model (Zhang et al. 2006). A decade later, the first HSPG mutants in a model organism (Drosophila melanogaster) were identified (Rogalski et al. 1993; Nakato et al. 1995; Häcker et al. 1997; Bellaiche et al. 1998; Lin et al. 1999), which was followed by identification of mutants in nematodes, tree frogs, zebrafish, and mice (Tables 2 and 3). HS is evolutionarily ancient and its composition has remained relatively constant from Hydra to humans (Yamada et al. 2007; Lawrence et al. 2008).
Table 1.
Heparan sulfate proteoglycans
| Class | Proteoglycan | Core mass (kDa)a | Chain type (number)b | Tissue | Human disease |
|---|---|---|---|---|---|
| Membrane-bound | Syndecan-1–syndecan-4 | 31–45 | HS (2–3) in Sdc2 and Sdc4; HS/CS (3–4 HS/1-2 CS) in Sdc1 and Sdc3 | Epithelial cells, fibroblasts | |
| Glypican-1–glypican-6 | 57–69 | HS (1–3) | Epithelial cells, fibroblasts | Simpson–Golabi–Behmel syndrome (overgrowth) (GPC3) (Pilia et al. 1996); omodysplasia (skeletal dysplasia) (GPC6) (Campos-Xavier et al. 2009) | |
| Betaglycan (part-time PG) | 110 | HS/CS (1–2) | Fibroblasts | ||
| Neuropilin-1 (part-time PG) | 130 | HS or CS (1) | Endothelial cells | ||
| CD44v3 | 37 | HS (1) | Lymphocytes | ||
| Secretory vesicles | Serglycin | 10–19 | Heparin/CS (10–15) | Mast cells, hematopoietic cells | |
| Extracellular matrix | Perlecan | 400 | HS (1–4) | Basement membranes | Schwartz–Jampel syndrome (skeletal dysplasia) (Nicole 2000; Arikawa-Hirasawa et al. 2001) |
| Agrin | 212 | HS (2–3) | Basement membranes | ||
| Collagen XVIII | 150 | HS (1–3) | Epithelial cells, basement membranes | Knobloch syndrome type I (Sertie et al. 2000) |
HS, heparan sulfate; CS, chondroitin sulfate; PG, proteoglycan.
aThe variation in core mass is because of species differences.
bThe number of chains is based on the number of putative attachment sites for chain initiation as well as data from the literature; the actual number of chains varies by method, tissue, and species.
Table 2.
Mutants altered in HSPG core proteins
| Gene | Proteoglycan | Phenotype (references) |
|---|---|---|
| Sdc1 | Syndecan-1 | Null allele: viable; increase in inflammation-mediated corneal angiogenesis (Gotte et al. 2002, 2005); corneal epithelial cells migrate more slowly, show reduced localization of α9 integrin during wound closure and fail to increase in proliferation after wounding (Stepp et al. 2002); enhanced leukocyte-endothelial interaction in the retina (Gotte et al. 2002, 2005); increase in medial and intimal smooth muscle cell replication and neointimal lesion after injury (Fukai et al. 2009); reduced cardiac fibrosis and dysfunction during angiotensin II–induced hypertension (Schellings et al. 2010); not required for follicle initiation and development (Richardson et al. 2009); accumulates plasma triglycerides, and shows prolonged circulation of injected human VLDL and intestinally derived chylomicrons (Stanford et al. 2009); juvenile mice resistant to carcinogen-induced tumorigenesis (McDermott et al. 2007); increased basal protein leakage and more susceptible to protein loss induced by combinations of IFN-γ, TNF-α, and increased venous pressure (Bode et al. 2008); exacerbates anti-GBM nephritis shifting Th1/Th2 balance toward a Th2 response (Rops et al. 2007); no role in hepatocyte infection by Plasmodium yoelii sporozoites (Bhanot 2002); normal larval development of Trichinella spiralis, but modestly reduced Th2 responses during infection (Beiting et al. 2006); less susceptible to Pseudomonas aeruginosa infection (Haynes et al. 2005); reduced P. aeruginosa infection rate and virulence (Park et al. 2001); protected from Staphylococcus aureus beta-toxin-induced lung injury (Hayashida et al. 2009a); exaggerated airway hyperresponsiveness, eosinophilia, and lung IL-4 responses to allergens (Xu et al. 2005); exaggerated CXC chemokines, neutrophilic inflammation, organ damage, and lethality in LPS endotoxemia (Hayashida et al. 2009b); prolonged recruitment of inflammatory cells in dextran sodium sulfate (DSS)-induced colitis and delayed type hypersensitivity (Masouleh et al. 2009; Floer et al. 2010). |
| Sdc2 | Syndecan-2 | No mutants reported. Sdc2 antisense impairs angiogenesis in human microvascular endothelial cells (Noguer et al. 2009); morpholinos inhibit cell migration and fibrillogenesis during embryogenesis in zebrafish (Arrington and Yost 2009). |
| Sdc3 | Syndecan-3 | Null allele: viable; altered feeding behavior (Strader et al. 2004); no phenotype in synovial endothelial cells (Patterson et al. 2005); enhanced long-term potentiation (LTP) in area CA1 (brain) and impaired performance in tasks assessing hippocampal function (Kaksonen et al. 2002); more sensitive to inhibition of food intake by the melanocortin agonist MTII (Reizes et al. 2003); perturbs laminar structure of the cerebral cortex as a result of impaired radial migration, and neural migration in the rostral migratory stream is impaired (Hienola et al. 2006); novel form of muscular dystrophy characterized by impaired locomotion, fibrosis, and hyperplasia of myonuclei and satellite cells (Cornelison et al. 2004). |
| Sdc4 | Syndecan-4 | Null allele: viable; enhanced fibrin deposition in degenerating fetal vessels in the placental labyrinth (Ishiguro et al. 2000); delayed angiogenesis in wound granulation tissue (Echtermeyer et al. 2001); defective subcellular localization of mTOR Complex2 and Akt activation in endothelial cells, affecting endothelial cell size, NOS, and arterial blood pressure (Partovian et al. 2008); decreased macrophage uptake of phospholipase A2-modified LDL (Boyanovsky et al. 2009); mesangial expansion, enhanced matrix collagens I and IV, fibronectin and focal segmental glomerulosclerosis in males, and induction of Sdc2 in glomeruli (Cevikbas et al. 2008); more susceptible to hepatic injury, and thrombin-cleaved form of osteopontin is significantly elevated after concanavalin-A injection (Kon et al. 2008); less damage in osteoarthritic cartilage in a surgically induced model of osteoarthritis (Echtermeyer et al. 2009); explanted satellite cells fail to reconstitute damaged muscle and are deficient in activation, proliferation, MyoD expression, myotube fusion, and differentiation (Cornelison et al. 2004); vibrissae are shorter and have a smaller diameter because of suboptimal response to fibroblast growth factors (Iwabuchi and Goetinck 2006); lower phosphorylation levels of focal adhesion kinase (Wilcox-Adelman et al. 2002); random migration of fibroblasts as a result of high delocalized Rac1 activity (Bass et al. 2007); defective RGD-independent cell attachment to transglutaminase-fibronectin matrices (Telci et al. 2008); impaired suppression of production of IL-1β by TGF-α (Ishiguro et al. 2002); decreased neutrophil recruitment and increased myofibroblast recruitment and interstitial fibrosis after bleomycin-treatment, no inhibition of fibrosis with recombinant CXCL10 protein (Jiang et al. 2010); hypersensitivity to LPS because of decreased TGF-β suppression of IL-1 production in monocytes and neutrophils (Ishiguro et al. 2001). |
| Gpc1 | Glypican-1 | Null allele: viable; reduced brain size (Jen et al. 2009). Athymic mutant mice show decreased tumor angiogenesis and metastasis (Aikawa et al. 2008). |
| Gpc2 | Glypican-2 | No mutants reported. |
| Gpc3 | Glypican-3 | Null allele: viable; resembles Simpson–Golabi–Behmel overgrowth syndrome, including somatic overgrowth, renal dysplasia, accessory spleens, polydactyly, and placentomegaly (Cano-Gauci et al. 1999; Chiao et al. 2002); defects in cardiac and coronary vascular development (Ng et al. 2009); alterations in Wnt signaling, in vivo inhibition of the noncanonical Wnt/JNK signaling, activation of canonical Wnt/β-catenin signaling (Song et al. 2005); increased Hedgehog signaling (Capurro et al. 2008); abnormal rates of proliferation and apoptosis in cortical and medullary collecting duct cells (Grisaru et al. 2001); delay in endochondral ossification, impairment in the development of the myelomonocytic lineage (Viviano et al. 2005). |
| Gpc4 | Glypican-4 | Zebrafish knypek controls cell polarity during convergent extension (Topczewski et al. 2001); craniofacial skeletal defects in adult fish (LeClair et al. 2009). |
| Gpc5 | Glypican-5 | No mutants reported. |
| Gpc6 | Glypican-6 | Impaired endochondral ossification and omodysplasia (Campos-Xavier et al. 2009). |
| Tgfbr3 | Betaglycan | Null allele: embryonic lethal; heart and liver defects (Stenvers et al. 2003); defect in seminiferous cord formation in E12.5–13.5 embryos (Sarraj et al. 2010). |
| Hspg2 | Perlecan | Null allele: embryonic lethal (E10–12); developmental angiogenesis altered in zebrafish (Zoeller et al. 2009); high incidence of malformations of the cardiac outflow tract, lack of well-defined spiral endocardial ridges (Costell et al. 2002); lower amounts of collagen IV and laminins in embryonic hearts, reduced function in infarcted hearts from heterozygous mice (Sasse et al. 2008); absence of acetylcholinesterase at the neuromuscular junctions (Arikawa-Hirasawa et al. 2002); cephalic and skeletal abnormalities (Arikawa-Hirasawa et al. 1999); cerebral ectopias, exencephaly (Girós et al. 2007); increased cross-sectional area of myosin heavy chain type IIb fibers in the tibialis anterior muscle (Xu et al. 2010b); diminished osteocyte canalicular pericellular area (Thompson et al. 2011). |
| Exon 3 deletion (Hspg23/3) viable: proteinuria after protein loading (Morita et al. 2005); monocyte/macrophage influx impaired in Hspg23/3Col18a1−/– mice in a model of renal ischemia/reperfusion (Celie et al. 2007). | ||
| Secreted as CSPG in some tissues (Danielson et al. 1992; Govindraj et al. 2002; Vogl-Willis and Edwards 2004; West et al. 2006), but relationship of CSPG isoform to phenotypes not established. | ||
| Prg1 | Serglycin | Null allele: viable; secretory granule defects in mast cells (Abrink et al. 2004); dense core formation is defective in mast cell granules (Henningsson et al. 2006); defective secretory granule maturation and granzyme B storage in cytotoxic T cells (Grujic et al. 2005); no effect on macrophages (Zernichow et al. 2006); platelets and megakaryocytes contain unusual scroll-like membranous inclusions (Woulfe et al. 2008); enlargement of multiple lymphoid organs, decrease in the proportion of CD4+ cells, more pronounced airway inflammatory response in older mice (Wernersson et al. 2009); increased virulence of Klebsiella pneumoniae (Niemann et al. 2007); defective regulation of antiviral CD8+ T-cell responses (Grujic et al. 2008). |
| Agrn | Agrin | Null allele: embryonic lethal; reduced number, size, and density of postsynaptic acetylcholine receptor aggregates in muscles; abnormal intramuscular nerve branching and presynaptic differentiation (Gautam et al. 1996,1999); smaller brains (Serpinskaya et al. 1999); abnormal development of interneuronal synapses (Gingras et al. 2007); increased resistance to excitotoxic injury (Hilgenberg et al. 2002); reduced number of cortical presynaptic and postsynaptic specializations (Ksiazek et al. 2007). |
| Floxed allele: Inactivation in podocytes does not affect glomerular charge selectivity or glomerular architecture (Harvey et al. 2007). | ||
| Col18a1 | Collagen XVIII | Null allele: viable; increased microvascular growth (Li and Olsen 2004); increased angiogenesis associated with atherosclerotic plaques (Moulton et al. 2004); delayed regression of blood vessels in the vitreous along the surface of the retina after birth and lack of or abnormal outgrowth of retinal vessels (Fukai et al. 2002); larger choroidal neovascularization lesions and increased vascular leakage (Marneros et al. 2007); accelerated healing and vascularization rate of excisional dorsal skin wounds (Seppinen et al. 2008); anomalous anastomoses of vasculature; disruption of the posterior iris pigment epithelial cell layer with release of melanin granules, severe thickening of the stromal iris basement membrane zone (Marneros and Olsen 2003); increase in the amount of retinal astrocytes (Hurskainen et al. 2005); more severe glomerular and tubulointerstitial injury in induced anti-GBM glomerulonephritis (Hamano et al. 2010); monocyte/macrophage influx impaired in Hspg23/3Col18a1−/– mice in a model of renal ischemia/reperfusion (Celie et al. 2007); mild chylomicronemia (Bishop et al. 2010). |
Table 3.
Mouse mutants altered in HS biosynthesis
| Gene | Enzyme | Phenotype |
|---|---|---|
| Xt1 | Xylosyltransferase-1 | No mutants reported. |
| Xt2 | Xylosyltransferase-2 | Null allele: viable; polycystic kidney and livers (Condac et al. 2007). |
| GalTI (β4GalT7) | Galactosyltransferase I | Human mutants: defective chondroitin substitution of decorin and biglycan in an Ehlers–Danlos patient (Gotte and Kresse 2005; Seidler et al. 2006). |
| GalTII (β3GalT6) | Galactosyltransferase II | No mutants reported. |
| Glcat1 | Glucuronyltransferase I | Null allele: embryonic lethal (4–8-cell stage) (Izumikawa et al. 2010). |
| Extl3 | N-acetylglucosaminyl transferase I | Floxed allele: Inactivation in islets decreases growth and insulin secretion (Takahashi et al. 2009). |
| Ext1/Ext2 | HS Copolymerase (N-acetylglucosaminyl-glucuronyltransferase) | Null allele: embryonic lethal (E6-7.5); lack of mesoderm differentiation (Lin et al. 2000; Stickens et al. 2005); heterozygotes develop rib growth plate exostoses (Stickens et al. 2005; Zak et al. 2011); unaltered vascular permeability in heterozygous mice (Xu et al. 2010a). |
| Floxed allele of Ext1: defective brain morphogenesis and midline axon guidance after nestin-Cre inactivation (Inatani et al. 2003); no effect on adaptive immune response in CD15Cre mice (Garner et al. 2008); altered T-cell and dendritic cell homing to lymph nodes in Tie2Cre mice (Bao et al. 2010); rib growth plate exostosis formation in Col2Cre mice (Jones et al. 2010; Matsumoto et al. 2010; Zak et al. 2011). | ||
| Ndst1 | N-acetylglucosaminyl N-deacetylase/N-sulfotransferase-1 | Null allele: Perinatal lethal; lung hypoplasia, defective forebrain, lens, and skull development (Fan et al. 2000; Ringvall et al. 2000; Grobe et al. 2005; Pan et al. 2006). |
| Floxed allele: decreased chemokine transcytosis and presentation and neutrophil infiltration in Tie2Cre mice (Wang et al. 2005); decreased allergen-induced airway hyperresponsiveness and inflammation because of reduction in recruitment of eosinophils, macrophages, neutrophils, and lymphocytes in Tie2Cre mice (Zuberi et al. 2009); decreased pathological angiogenesis in Tie2Cre mice (Fuster et al. 2007); decreased vascular VEGF-induced hyperpermeability (Xu et al. 2010a); decreased vascular smooth muscle cell proliferation, vessel size, and vascular remodeling after arterial injury in SM22αCre mice (Adhikari et al. 2010a); mild effect on T-cell response in Tie2Cre;Ndst2−/−mice (Garner et al. 2008); defective lacrimal gland development and Fgf10-Fgfr2b complex formation and signaling in LeCre mice (Pan et al. 2008); defective lobuloalveolar development in mammary gland (Crawford et al. 2010). | ||
| Ndst2 | N-acetylglucosaminyl N-deacetylase/N-sulfotransferase-2 | Null allele: viable; mast cell deficiency and defective storage of proteases (Forsberg et al. 1999; Humphries et al. 1999); compounding mutation with Ndst1 reduces l-selectin interactions (Wang et al. 2005). |
| Ndst3 | N-acetylglucosaminyl N-deacetylase/N-sulfotransferase-3 | Null allele: viable; floxed allele available (Pallerla et al. 2008). |
| Ndst4 | N-acetylglucosaminyl N-deacetylase/N-sulfotransferase-4 | No mutants reported. |
| Glce | Uronyl C5 epimerase | Null allele: perinatal lethal; renal agenesis (Li et al. 2003). |
| H2st | Uronyl 2-O-sulfotransferase | Null allele: perinatal lethal; renal agenesis; skeletal and ocular defects (Bullock et al. 1998; Merry et al. 2001); defective cerebral cortical development (McLaughlin et al. 2003); altered lacrimal gland development (Qu et al. 2011). |
| Floxed allele: altered lipoprotein clearance in AlbCre mice (Stanford et al. 2010); altered branching morphogenesis in the mammary gland (Garner et al. 2011). | ||
| H3st1 | Glucosaminyl 3-O-sulfotransferase 1 | Null allele: partially penetrant lethality; no alteration in coagulation (HajMohammadi et al. 2003); fertility defects because of impaired ovarian function and placenta development (Shworak et al. 2002; HajMohammadi et al. 2003). |
| H3st2 | Glucosaminyl 3-O-sulfotransferase 2 | Null allele; viable; no neuronal phenotype (Hasegawa and Wang 2008). |
| H3st3 | Glucosaminyl 3-O-sulfotransferase 3 | No mutants reported. |
| H3st4 | Glucosaminyl 3-O-sulfotransferase 4 | No mutants reported. |
| H3st5 | Glucosaminyl 3-O-sulfotransferase 5 | No mutants reported. |
| H3st6 | Glucosaminyl 3-O-sulfotransferase 6 | No mutants reported. |
| H6st1 | Glucosaminyl 6-O-sulfotransferase 1 | Null allele: embryonic lethal (Habuchi et al. 2007; Sugaya et al. 2008). |
| Gene trap allele: embryonic lethal; retinal axon guidance defects (Pratt et al. 2006). | ||
| Floxed allele: systemic inactivation embryonic lethal (Izvolsky et al. 2008); no change in plasma triglycerides in AlbCre mice (Stanford et al. 2010). | ||
| H6st2 | Glucosaminyl 6-O-sulfotransferase 2 | Null allele: viable (Sugaya et al. 2008); HS6ST-2, but not HS6ST-1, morphants in zebrafish show abnormalities in the branching morphogenesis of the caudal vein (Chen et al. 2005). |
| H6st3 | Glucosaminyl 6-O-sulfotransferase 3 | No mutants reported. |
| Hpa | Heparanase, transgene | Accelerated wound angiogenesis, enhanced delayed type hypersensitivity response (Zcharia et al. 2005; Edovitsky et al. 2006; Ilan et al. 2006); accumulation of intracellular crystals of protein Ym1 in macrophages (Waern et al. 2010); resistance to amyloid protein A amyloidosis (Li et al. 2005); age-related enlargement of lymphoid tissue and altered leukocyte composition (Wernersson et al. 2009). |
| Hpa | Heparanase | Null allele: viable; altered MMP-2 and MMP-14 expression (Zcharia et al. 2009). |
| Sulf1 | Endo-6-sulfatase 1 | Null allele: viable; esophageal defect (Ai et al. 2007; Ratzka et al. 2008); enhanced osteoarthritis, MMP-13, ADAMTS-5, and noggin elevated, col2a1 and aggrecan reduced in cartilage and chondrocytes (Otsuki et al. 2010). |
| Sulf2 | Endo-6-sulfatase 2 | Null allele: viable; behavioral defects (Lamanna et al. 2006); enhanced osteoarthritis, MMP-13, ADAMTS-5, and noggin elevated, col2a1 and aggrecan reduced in cartilage and chondrocytes (Otsuki et al. 2010). |
| Gene trap allele: Sulf2GT(pGT1TMpfs)155Ska, no phenotype (Lum et al. 2007). |
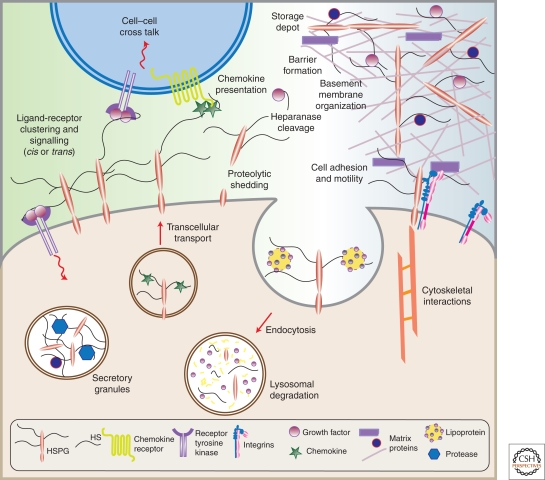
Figure 1 shows in pictorial form many of the systems in which HSPGs participate.
HSPGs are present in basement membranes (perlecan, agrin, and collagen XVIII), where they collaborate with other matrix components to define basement membrane structure and to provide a matrix for cell migration.
HSPGs are found in secretory vesicles, most notably serglycin, which plays a role in packaging granular contents, maintaining proteases in an active state, and regulating various biological activities after secretion such as coagulation, host defense, and wound repair.
HSPGs can bind cytokines, chemokines, growth factors, and morphogens, protecting them against proteolysis. These interactions provide a depot of regulatory factors that can be liberated by selective degradation of the HS chains. They also facilitate the formation of morphogen gradients essential for cell specification during development and chemokine gradients involved in leukocyte recruitment and homing.
HSPGs can act as receptors for proteases and protease inhibitors regulating their spatial distribution and activity.
Membrane proteoglycans cooperate with integrins and other cell adhesion receptors to facilitate cell-ECM attachment, cell–cell interactions, and cell motility.
Membrane HSPGs act as coreceptors for various tyrosine kinase-type growth factor receptors, lowering their activation threshold or changing the duration of signaling reactions.
Membrane HSPGs act as endocytic receptors for clearance of bound ligands, which is especially relevant in lipoprotein metabolism in the liver and perhaps in the formation of morphogen gradients during development.
Figure 1.
HSPGs have multiple activities in cells and tissues. (Adapted from Bishop et al. 2007; reprinted with permission from Nature Publishing Group © 2007.)
This article is divided into 10 subsections. The first three are written for investigators outside the field who may need some background information on the diversity of HSPGs and the interactions that occur with protein ligands. The subsequent sections describe seven systems that illustrate general principles or ideas that have undergone a significant shift over the last decade. Because of space limitations not all subjects can be considered or treated in appropriate depth and therefore the reader is referred to excellent recent review articles (Tkachenko et al. 2005; Bulow and Hobert 2006; Bishop et al. 2007; Lamanna et al. 2007; Bix and Iozzo 2008; Filmus et al. 2008; Ori et al. 2008; Rodgers et al. 2008; Sanderson and Yang 2008; Iozzo et al. 2009; Couchman 2010).
A BIRD'S-EYE VIEW OF STRUCTURE AND ASSEMBLY
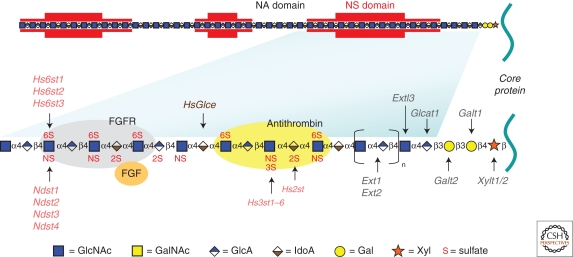
An idealized picture of a HSPG is shown in Figure 2. Each proteoglycan consists of a protein and one or more covalently attached HS chains. Comprehensive reviews have appeared on the assembly process and structural characterization of the chains, and therefore these subjects will not be discussed further here (Esko and Selleck 2002; Sugahara and Kitagawa 2002; Sasisekharan et al. 2006; Ori et al. 2008; Laremore et al. 2009). However, several features are important to consider in the context of their biological activities. (1) HSPGs are polyanionic and have unusual hydrodynamic volume because of the presence of long HS chains (40–300 sugar residues, ∼20–150 nm), sulfate groups, and uronic acids. Thus, different HSPGs often copurify by techniques that rely simply on the anionic characteristics of the chains or gel filtration. HS and other sulfated GAGs are amongst the most highly negatively charged biopolymers in nature and variation in the number and length of the chains gives rise to enormous polydispersity. (2) Some proteoglycans contain only one GAG chain (e.g., CD44v3 and betaglycan), whereas others have three to five chains (e.g., syndecan). Furthermore, the stoichiometry of GAG chain substitution can vary depending on source and growth conditions. “Part-time” proteoglycans can exist with or without a GAG chain (Table 1). (3) Some proteoglycans contain other types of glycans (e.g., asparagine-linked [N-linked] and serine/threonine-linked [O-linked] mucin-type chains). Some proteoglycans, such as syndecan-1, contain both HS and chondroitin/dermatan sulfate, another type of GAG. Other types of posttranslational modifications can occur (e.g., phosphorylation on cytoplasmic domains of trans-membrane proteoglycans). (4) The number and sulfation state of the chains can vary according to growth conditions and in response to growth factors. (5) The arrangement of negatively charged sulfate groups and the orientation of the carboxyl groups specify the location of ligand-binding sites. Furthermore, the sulfated residues are clustered in regions along the chain containing mixtures of iduronic acid and glucuronic acid (NS domains, Fig. 2) and are separated by nonsulfated domains rich in glucuronic acid (NA domains). (6) The pattern of sulfation, extent of uronic acid epimerization, and organization of the modified residues is generally thought to depend on the cell type in which HS is expressed rather than on the nature of the core protein (Kato et al. 1994). Thus, the overall composition of HS on different core proteins expressed by the same cell appears to be similar, but great variation occurs between cell types. This concept might be an oversimplification, as some variation has been suggested to occur in ligand-binding properties and composition dependent on the core protein (Shworak et al. 1993; Tveit et al. 2005).
Figure 2.
Heparan sulfate (HS) structure. HS biosynthesis initiates by the attachment of xylose to specific serine residues in HSPG core proteins followed by the formation of a linkage tetrasaccharide, glucuronic acid-galactose-galactose-xylose (GlcA-Gal-Gal-Xyl). Extl3 attaches the first N-acetyl-d-glucosamine (GlcNAc) residue and an enzyme complex composed of Ext1 and Ext2 alternately adds GlcA and GlcNAc to the nascent chain. The chains simultaneously undergo a series of processing reactions that begins by the removal of the acetyl groups from clusters of GlcNAc residues and substitution of the free amino groups with sulfate, catalyzed by one or more N-deacetylase-N-sulfotransferases (Ndst). The C5 epimerase (HsGlce) epimerizes d-glucuronic acids immediately adjacent to N-sulfoglucosamine units to l-iduronic acid (IdoA). A series of O-sulfotransferases can then add sulfate: uronyl 2-O-sulfotransferase (Hs2st) adds sulfate at C2 of the iduronic acids (and less frequently to glucuronic acids), 6-O-sulfotransferases (Hs6st1-3) add sulfate at C6 of the N-sulfoglucosamine units and less frequently to N-acetylglucosamine, and 3-O-sulfotransferases (Hs3st1, 2, 3a, 3b, 4, 5, 6) add sulfate at C3 of glucosamine units (N-sulfated or N-unsubstituted). As shown in the top of the figure by red shading, the modifications occur in clusters of variable length (N-sulfated or NS domains), which are interspersed by unmodified domains (N-acetylated or NA domains). The regions at the junction of these domains are sometimes called NA/NS domains (not shown) because the extent of processing is less. The modified domains make up binding sites for protein ligands as depicted for antithrombin, FGF and FGF receptor. The HS chains can be further modified once they arrive at the cell surface or in the ECM by two endosulfatases (Sulf1 and Sulf2), which remove specific sulfate groups located at C6 of glucosamine units, or by the action of extracellular heparanase or extracellular proteases (not shown). (Figure adapted from Bishop et al. 2007; reprinted with permission from Nature Publishing Group © 2007.)
GENERALIZATIONS ABOUT THE INTERACTION OF PROTEIN LIGANDS WITH HS PROTEOGLYCANS
HSPGs bind to many ligands, usually via the HS chains. In fact heparin, a highly sulfated form of HS, is often used as an “affinity” matrix for purifying proteins, and many of the growth factors in use today were purified by heparin affinity chromatography. Heparin-binding ligands include growth factors, cytokines, chemokines, enzymes, enzyme inhibitors, and extracellular matrix proteins. In other areas of glycobiology, glycan-binding proteins are referred to as “lectins,” a designation based on the presence of a carbohydrate recognition domain defined by characteristic protein folds or sequence motifs indicating their membership in an evolutionarily conserved gene family (Varki et al. 2009). In contrast, proteins that bind to HS appear to have evolved by convergent evolution; that is, they do not possess a specific fold or recognizable amino acid sequence pattern (Esko and Linhardt 2009). Heparin-binding sites often occur on the external surface of proteins or in shallow grooves lined with positively charged amino acids. Attempts have been made to define “consensus” sequences in heparin-binding proteins based on content and spacing of positively charged amino acid residues within linear sequences (Cardin and Weintraub 1989; Hileman et al. 1998; Capila and Linhardt 2002). However, the binding site for HS is often defined by positive residues contributed by noncontiguous segments of the protein. Although electrostatic interactions contribute much of the binding energy, hydrogen-bonding, van der Waal interactions, and hydrophobic effects also participate (Conrad 1998; Capila and Linhardt 2002).
The binding of a ligand to HS follows the same principles that underlie the interaction of other macromolecules, but the following considerations are important.
Dissociation constants for HS-dependent ligands range from millimolar to nanomolar values. Many growth factors bind with high affinity (e.g., fibroblast growth factors, FGFs), whereas many matrix proteins bind with low affinity (e.g., fibronectin). However, affinity does not dictate selectivity; some low affinity ligands achieve high avidity through dimerization (e.g., chemokines) or by clustering (e.g., fibronectin fibrils).
The HS chains can facilitate diffusion of ligands by allowing them to bind and slide or dissociate/reassociate through adjacent binding sites (mass action).
Binding can lead to a conformational change in the protein. The best-studied example is the allosteric effect of heparin on antithrombin.
HS can act as a template to approximate two proteins next to each other. Antithrombin inactivation of thrombin serves as the paradigm for this type of interaction, but other examples include the association of some growth factors with their receptor tyrosine kinases (e.g., FGF with FGF receptors).
In addition to the HS chains, the protein core of HSPGs can also bind ligands. For example, the Drosophila glypican ortholog, Dally, can directly interact with a number of morphogens, such as decapentaplegic (Dpp) and bone morphogenetic factor 4, in the absence of HS chains (Kirkpatrick et al. 2006). Expression of HS-deficient Dally can rescue several mutant phenotypes in Dally-deficient flies, indicating that a number of biologically relevant functions are mediated by the glypican protein core independently of HS. In mammals, the glypican-3 core protein interacts with hedgehog (Hh) independently of HS, and Gpc3-null embryos display increased Hh signaling, which might explain the overgrowth phenotype observed in patients lacking glypican-3 (Simpson–Golabi–Behmel syndrome) (Capurro et al. 2008). As discussed below, a peptide sequence in the core protein of syndecan-1 interacts with αVβ3 and αVβ5 integrins and modulates cell adhesion (Beauvais et al. 2004; McQuade et al. 2006). Finally, perlecan, collagen XVIII, and agrin are large proteins composed of multiple, functionally independent domains that can bind to other matrix components and growth factors (Iozzo et al. 2009).
ON THE SPECIFICITY OF BINDING
Although some ligands bind directly to the HSPG core proteins, the vast majority interact with sulfated domains within HS chains. Early studies of heparin and its interaction with antithrombin guided much of our thinking about the specificity of protein–HS interactions, but recent studies have broadened our view considerably. Heparin has high anticoagulant activity and higher-than-average overall sulfation (heparin contains aproximately 2.3 sulfate groups per disaccharide, whereas typical HS contains approximately 0.8 sulfate groups per disaccharide). The anticoagulant properties of heparin, however, do not depend on overall charge, but instead depend on a unique pentasaccharide with a specific arrangement of sulfate groups and uronic acid epimers, and requires a sulfate group positioned at C3 of the central glucosamine residue as shown in Figure 2 (Lindahl et al. 1980; Atha et al. 1985). This discovery suggested that the interaction of HS with other protein ligands might show similar selectivity.
Interestingly, 3-O-sulfated glucosamine residues are quite rare in HS, occurring about 1/20 disaccharides in heparin and less than 1/100 disaccharides in HS or not at all. Nevertheless, the 3-O-sulfotransferases comprise the largest family of HS sulfotransferases, with seven members (Fig. 2). Two of the enzymes can produce the antithrombin-binding sequence, whereas the others generate 3-O-sulfated sequences distinct in structure from the antithrombin-binding site, suggesting their purpose lies in the formation of binding sites for other ligands. One class of these sites interacts with glycoprotein gD of Herpes simplex virus-1 and appears to be required for infection (Shukla et al. 1999). Endogenous ligands include cyclophilin B (Vanpouille et al. 2007), FGF7 (Ye et al. 2001), and possibly the ectodomain of FGFR1 (McKeehan et al. 1999), but based on the large size of the 3-O-sulfotransferase family, other ligands undoubtedly exist.
Most ligands do not require 3-O-sulfation, but the study of heparin-antithrombin interaction set the stage conceptually for searching for specific arrangements of sulfated sugars to achieve selective binding. This technically challenging problem was initially approached by fractionating HS into pools that bound to the ligand or that did not, followed by compositional analysis or partial sequencing. Unfortunately, little variation in composition was noted, most likely because binding sites for most proteins represent only five to 12 sugars. To circumvent this problem, partially cleaved preparations were employed, eventually leading to the identification of minimally sized oligosaccharides that bound with reasonable affinity and that in some cases would initiate a biological response. Examples include, but are not limited to, FGF2 (Guimond et al. 1993; Maccarana et al. 1993), platelet-derived growth factor (Feyzi et al. 1997), platelet factor 4 (Maccarana and Lindahl 1993; Stringer and Gallagher 1997), MIP1α (Stringer et al. 2002, 2003), hepatocyte growth factor (scatter factor) (Lyon et al. 1994; Ashikari et al. 1995), vascular endothelial growth factor (Soker et al. 1994; Ono et al. 1999; Ashikari-Hada et al. 2005; Robinson et al. 2006), lipoprotein lipase (Parthasarathy et al. 1994; Spillmann et al. 2006), amyloid (Lindahl and Lindahl 1997), and L-selectin (Norgard-Sumnicht and Varki 1995; Wang et al. 2002). When FGF1 was studied in detail, it was noted that a range of HS octasaccharides that varied in the number as well as the positions of individual sulfate groups could bind with varying affinity (Kreuger et al. 2001). Furthermore, the formation of complexes between FGF1 and FGF receptors was promoted by a variety of saccharides of differing overall sulfate content. This apparent lack of specificity has a corollary in vivo; in Drosophila deletion of 2-O- or 6-O-sulfotransferases has no effect on FGF signaling and tracheal development, which depends on FGF (Kamimura et al. 2006). Inactivation of either enzyme results in elevated sulfation at other positions, suggesting a form of molecular compensation. Lack of both enzymes impairs FGF signaling and causes multiple patterning deficiencies, indicating that HS is essential but that the receptor ligand complex can accommodate differently sulfated oligosaccharides. It would appear that other ligand-receptor pairs might behave similarly, because many organs and tissues in mice bearing mutations in these sulfotransferases (or the epimerase that interconverts glucuronic acid to iduronic acid) develop normally (Table 3).
Nevertheless, specificity does exist. For example, mice lacking the 2-O-sulfotransferase suffer renal agenesis (Bullock et al. 1998), but other tissues develop normally. In the lacrimal gland, a decrease in overall sulfation of HS affects Fgf10-Fgfr2b signaling required for branching morphogenesis (Pan et al. 2008). Similar reduction in overall sulfation alters vasculogenesis (Jakobsson et al. 2006), tumor angiogenesis (Fuster et al. 2007), and vascular hyperpermeability (Xu et al. 2010a). Wnt signaling is specifically affected by removal of 6-O-sulfate groups on HS by a pair of cell surface endolytic-6-O-sulfatases (the Sulfs) that act at very restricted sites in the chain (Ai et al. 2003; Lamanna et al. 2008). Although the range of biological processes regulated in vivo by the Sulfs remains to be elucidated, growth factors including FGF, Wnt, and GDNF are clearly affected by their loss, resulting in developmental defects and early postnatal lethality (Ai et al. 2007; Holst et al. 2007). Thus, determining whether specific sequences mediate binding and biological action remains an active area of investigation.
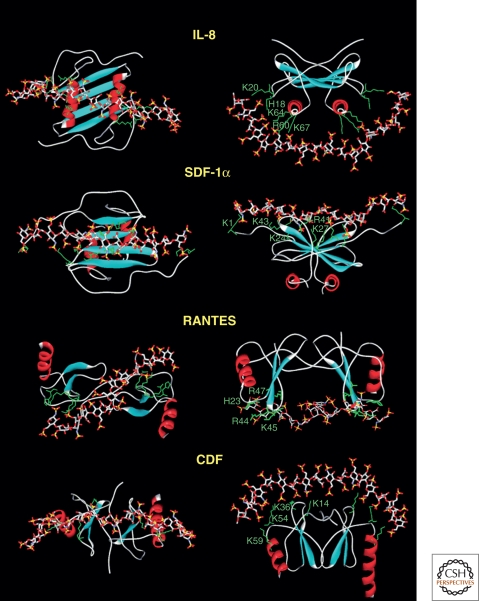
Chemokines are a subset of cytokines that interact with HS (Lortat-Jacob 2009). These small (8–10-kDa) secreted proteins signal through G protein–coupled receptors on cell surfaces to control cell migration in development, lymphocyte homing, inflammation, and wound repair. Binding of chemokines to HSPGs allows their local retention, protection from degradation (Sadir et al. 2004), activation by oligomerization (Proudfoot et al. 2003), and presentation to leukocytes (Handel et al. 2005). Interestingly, chemokines usually occur as dimers with positively charged domains oriented in different configurations (Fig. 3). Although no cocrystal structures are currently available, molecular docking suggests that an HS chain might fit along the dimer interface or can span the heparin-binding domains oriented on opposite sides of the dimer. In the latter case, the distance between the two binding sites in the dimer exceeds the length of a typical sulfated domain in HS (around four to eight sugars). Thus, the preferred organization of the bioactive segment of a chain might consist of two short sulfated domains (NS domains, Fig. 2) separated by a nonsulfated linker of a specific length (NA domain) (Lortat-Jacob et al. 2002). Examples of other ligands in which binding sites require extended or discontinuous domains include IFN-γ (Lortat-Jacob et al. 1995) and platelet factor 4 (Stringer and Gallagher 1997), and may include other paired systems, such as FGF/FGFR (Mulloy and Linhardt 2001) or VEGF/VEGFR/Nrp1 (Grunewald et al. 2010). Generating this arrangement would presumably require spatial control over the assembly process, but how this is achieved in vivo is not known (Lindahl and Li 2009).
Figure 3.
Docking of HS to chemokines. Molecular modeling was used to dock a fully sulfated heparin-like chain to several chemokines. The proteins are represented by ribbons except for the side chains of the basic amino acids directly involved in polysaccharide binding (green). The heparin molecule is represented by sticks. (Data from Lortat-Jacob et al. 2002.)
PROTEOGLYCANS AS CORECEPTORS
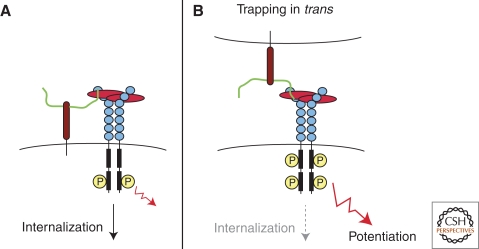
In 1991, Yayon et al. (1991) and Rapraeger et al. (1991) reported that cell surface HSPGs facilitate the formation and signaling of FGF2-FGF receptor complexes (Fig. 4A). As described above, exogenous heparin or HS can also potentiate the formation of complexes of FGF with FGF receptors. In this context, the HSPG or heparin is considered a “coreceptor,” because its function is to aid the formation of ligand-receptor complexes either through conformational change of ligand and/or receptor or by acting as a template to approximate ligand and receptor. In cells, activation usually occurs in cis by HSPGs expressed on the same cell as the signaling receptor (Fig. 4A). For example, altering HS sulfation selectively in endothelial cells using the Cre-lox system decreases pathological angiogenesis in vivo (Fuster et al. 2007). Similarly, altering HS in mammary epithelial cells has a striking impact on lobuloalveolar development, in spite of expression of HS in surrounding stromal cells (Crawford et al. 2010). Studies of lens development (Pan et al. 2006), branching morphogenesis in the lacrimal gland (Pan et al. 2008; Qu et al. 2011), axon guidance, and development of the central nervous system (Inatani et al. 2003; Yamaguchi et al. 2010) also suggest that HSPGs act as coreceptors in a cell-autonomous manner.
Figure 4.
Model for trans-activation of VEGF receptor by HSPGs. (A) Resident plasma membrane HSPGs can mediate VEGF interactions with its receptor in cis, inducing cell signaling and subsequent internalization of the complex. (B) HSPGs from an adjacent cell can also mediate VEGF interactions with its receptor in trans, delaying internalization of the signaling complex and enhancing VEGF response. (From Jakobsson et al. 2006; reprinted with permission from Elsevier © 2006.)
Nevertheless, the observation that exogenous heparin, HS or HSPGs can activate FGF signaling raised the possibility that HSPGs on one cell type might activate signaling in trans on adjacent cells. Kraemer and Yost provided the first in vivo evidence for trans-activation in their studies of left–right development in Xenopus. In this system ectodermal syndecan-2 transmits in a non-cell-autonomous fashion left–right information to migrating mesoderm via a growth factor signaling pathway (Kramer and Yost 2002). More recently, Jacobsson et al. analyzed VEGF signaling in embryoid bodies derived from mutant stem cell populations that were either deficient in HS biosynthesis or in VEGF receptor expression (Jakobsson et al. 2006). Although both mutant stem cell lines were unable to support VEGF signaling, generation of chimeras restored VEGF signaling and response. A model for how HSPGs might activate VEGF receptors in trans is shown in Figure 4B. As discussed below, germline stem cells are maintained in a stem cell niche by short-range trans-signaling mediated by glypicans expressed in niche cells acting on the stem cells (Hayashi et al. 2009). Trans-activation of receptors by HSPGs could potentially elicit stronger signaling responses by trapping the receptor at the cell surface in an activated state. Overall, the ability of HSPGs to trans-activate adjacent cells represents a novel type of cellular cross talk and may play an important role in regulating cellular differentiation and response during development.
Secreted HSPGs also can act in a non-cell-autonomous manner (e.g., by directly eliciting signaling responses in nearby cells). The HSPG agrin acts in this way to induce postsynaptic differentiation at the neuromuscular junction (Bezakova and Ruegg 2003). To carry out this function, agrin is secreted by motor neurons where it activates the receptor muscle-specific receptor tyrosine kinase (MuSK) on adjacent muscle cells. Signaling cascades induced by MuSK signaling result in cytoskeletal reorganization and subsequent aggregation of acetylcholine receptors on muscle cells, priming the neuromuscular junction for activation. Recent studies have shown that proteolytic processing of agrin at the neurological synapses by neurotrypsin releases an active carboxy-terminal fragment that subsequently induces the formation of dendritic filopodia on hippocampal neurons (Matsumoto-Miyai et al. 2009). Other proteoglycans can also undergo proteolytic processing to release bioactive domains that can act in an endocrine fashion, including collagen XVIII (Marneros and Olsen 2001) and perlecan (Bix and Iozzo 2008).
The coreceptor function of some proteoglycans, such as the trans-membrane syndecans, can be dynamically regulated by modulating their association with the cell surface through a process known as shedding (Bernfield et al. 1999; Manon-Jensen et al. 2010). Syndecan shedding is mediated by matrix metalloproteinases (MMP1, MMP7, MMP9, ADAM17) (Fitzgerald et al. 2000; Li et al. 2002; Endo et al. 2003; Ding et al. 2005; Brule et al. 2006; Pruessmeyer et al. 2010). Mechanistically, induced shedding of syndecan-1 appears to involve its cytoplasmic tail, which binds to Rab5, a small GTPase that regulates intracellular trafficking and signaling events (Hayashida et al. 2008). Rab5 may regulate shedding by inducing dissociation of syndecan-1 from β1 integrin (see below), thus exposing a normally cryptic cleavage site. Shedding has a major effect on the localization and signaling capacity of HS-bound ligands (Kato et al. 1998; Park et al. 2000b). For example, syndecan-1 shedding modulates chemokine-dependent inflammatory processes in models of tissue damage caused by noninfectious agents (Li et al. 2002; Xu et al. 2005b; Hayashida et al. 2009b). Syndecan-1 shed by implanted tumor cells can exert biologic effects distal to the primary tumor, driving formation of osteoclasts and bone destruction via bound heat-labile factors (Kelly et al. 2010). Some microorganisms can enhance host cell proteolytic shedding of syndecan-1 resulting in enhanced bacterial colonization (Park et al. 2000a, 2001). In all of these models, the activity of shed syndecan ectodomains depends on the HS chains, suggesting their activity depends on ligands bound to the chains.
PROTEOGLYCANS AS ENDOCYTIC RECEPTORS
Although often overlooked, membrane HSPGs also act as endocytic receptors, and undergo constitutive as well as ligand-induced endocytosis (Williams and Fuki 1997; Belting 2003). Although the precise mechanism of endocytosis is unclear, it appears to occur independently of clathrin, caveolin, and dynamin, but in a lipid-raft-dependent manner involving vesicles of unusual composition (Zimmermann et al. 2005; Wittrup et al. 2010). Ligands bound to the HS chains “piggy back” into the cell through this route. The endocytic activity of HSPGs plays a significant physiological role in lipid metabolism. Recent genetic evidence showed that mice lacking syndecan-1 accumulate both liver-derived and dietary triglycerides in the form of remnant lipoprotein particles (Stanford et al. 2009). Altering the structure of HS by selective inactivation of sulfotransferases in hepatocytes led to the same phenotype and showed that the HS chains on syndecan-1 represent the binding site for the lipoproteins (MacArthur et al. 2007; Stanford et al. 2010).
Other membrane HSPGs, such as syndecan-2 and syndecan-4 and glypicans, also can mediate uptake of ligands in cultured cells (Fuki et al. 2000). Furthermore, uptake can be induced by FGF2 or by antibody-induced clustering (Fuki et al. 1997; Tkachenko et al. 2004; Zimmermann et al. 2005). Although recycling of internalized HSPGs has been observed (Fransson et al. 1995; Zimmermann et al. 2005), most HSPGs end up in lysosomes, where they undergo degradation by lysosomal proteases and exolytic glycosidases and sulfatases. Bound ligands also are degraded, which provides a mechanism for delivering nutrients to cells or for removal of bioactive factors from the environment. The formation of tissue gradients of morphogens (discussed below) may depend in part on continuous clearance of the ligands through an endocytic mechanism (Lander et al. 2002; Marois et al. 2006; Ren et al. 2009).
Viruses and other pathogens can exploit HSPGs to transit from the extracellular environment to the inside of cells. The membrane-penetrating peptide, HIV-tat, is released from HIV-infected cells and then enters surrounding cells using HSPGs (Green and Loewenstein 1988; Frankel and Pabo 1988). Based in part on HIV-tat, a number of cell-penetrating-peptides have been generated, typically rich in arginine or lysine residues that will facilitate interaction with HSPGs (Poon and Gariepy 2007). In addition, synthetic positively charged transporters, such as guanidinylated aminoglycosides have been prepared. Conjugation of these carriers to drugs, toxins, enzymes, oligonucleotides, as well as quantum dots can mediate delivery of cargo into the cells via HSPGs (Elson-Schwab et al. 2007; Sarrazin et al. 2010).
HSPGs can also mediate transcellular transport. Wang et al. showed HSPG-mediated chemokine transport across endothelial cells and its dependence on the sulfation state of the HS chains (Wang et al. 2005). As discussed below, cell surface HSPGs are also engaged in cell adhesion, which is dependent on the interaction of HS chains with ECM proteins such as fibronectin. Whether cell attachment and endocytosis are mutually exclusive or perhaps mediated through different membrane proteoglycans is unclear.
PROTEOGLYCANS AS ADHESION RECEPTORS
HSPGs play several roles in cell adhesion and in the determination of cell shape. These processes depend on binding of cell surface HS to “heparin-binding” domains present in matrix proteins, such as fibronectin, laminins, vitronectin, thrombospondin, and some fibrillar collagens (Bernfield et al. 1999). Syndecan-4 provides an interesting example of a mechanical and functional link between the ECM and the actin cytoskeleton. Syndecan-4 is widely expressed during development and in most adult tissues and is a central component of focal adhesions (Oh and Couchman 2004). Fibroblasts lacking syndecan-4 have an altered actin cytoskeleton and multiple HS chains are required to cluster syndecan-4 on the plasma membrane (Gopal et al. 2010). Consistent with this idea, overexpression of syndecan-4 in CHO cells results in increased focal adhesion formation, organization of cytoskeletal stress fibers, and decreased cell motility (Longley et al. 1999). The activity of syndecan-4 depends on multimerization, which occurs via recruitment of PIP2, activation of PKC-α, and downstream signaling through the RhoA pathway (Oh et al. 1997a,b,c). Interestingly, multimerization of syndecan-4 is prevented by phosphorylation of its cytoplasmic tail by PKC-δ in response to FGF2 signaling (Murakami et al. 2002). FGF2 is a mitogen and during proliferation, cells need to detach to undergo cytokinesis. Thus, syndecan-4 has a central role in coordinating cytoskeletal changes that take place during adhesion and cell proliferation.
As described in other articles (e.g., Schwartz 2010; Campbell and Humphries 2011; Geiger and Yamada 2011; Watt and Fujiwara 2011), integrins mediate various interactions between cells and ECM components. Integrins can recognize short peptide sequences (e.g., RGD) present in many ECM proteins, and binding leads to activation, intracellular signaling via kinases and other enzymes, and focal adhesion formation (Cox et al. 2006). When fibroblasts attach and spread in response to fibronectin fragments containing the RGD site, the formation of focal adhesions and stress fibers can often take place only if the heparin-binding domain of the fibronectin is also present (Saoncella et al. 1999; Woods et al. 2000; Morgan et al. 2007). The HS chains of syndecan-4 bind to fibronectin, which together with integrin, induce formation of focal adhesions and stress fibers (Fig. 5).
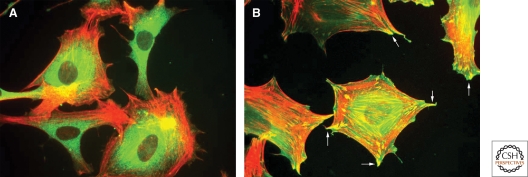
Figure 5.
The role of syndecan-4 in focal adhesion. (A) Fibroblasts attach and spread through α5β1 integrin on coverslips coated with the integrin-binding domain of fibronectin but they do not form focal adhesions. (B) Focal adhesions (arrows) form only after engagement of syndecan-4 HS chains after the addition of the heparin-binding domain (HepII) from fibronectin. (Data for image from Okina et al. 2009.)
Syndecan-1 (and syndecan-4) also can regulate activation of αvβ3 and αvβ5 integrin by way of interaction of the extracellular domain of the proteoglycan with the β-integrin subunit (Beauvais et al. 2004, 2009; McQuade et al. 2006). The engagement of these receptors occurs outside the cell via a defined peptide segment in syndecan-1, but the activation mechanism is cytoplasmic and occurs via a talin-dependent, inside-out signaling pathway that requires syndecan-1 clustering. The HS chains of syndecan-1 are required, presumably by facilitating syndecan-1 clustering along aggregated ECM components.
HSPGs also can facilitate cell–cell adhesion. During the inflammatory response, endothelial HS interacts with L-selectin on passing leukocytes to aid in the initial tethering of leukocytes to the lumenal surface of the endothelium (Wang et al. 2005; Celie et al. 2009). This interaction may depend on the presence of an unusual N-unsubstituted glucosamine unit in HS (Norgard-Sumnicht and Varki 1995) perhaps in combination with fully sulfated domains (Smits et al. 2010). Interestingly, heparinoids administered intravenously to mice dramatically reduce leukocyte infiltration in response to inflammation (Wang et al. 2002) by disruption of endogenous HS-selectin (Wang et al. 2005) or sialyl Lewisa/x-selectin interactions (Koenig et al. 1998; Stevenson et al. 2007). After passing the endothelial cell layer, leucocytes encounter the vascular basement membrane. Leukocyte migration through this barrier is considered to involve local degradation by matrix metalloproteinases and secreted heparanase, which may be necessary for dissolution of HSPG in the basement membrane (Vreys and David 2007; Li and Vlodavsky 2009).
PROTEOGLYCANS REGULATE GROWTH FACTOR BINDING TO ECM AND CELL MIGRATION
The ECM provides a structural network for mediating and regulating cellular movement (e.g., during development and wound repair). One of the ways the ECM regulates cell migration is to directly bind growth factors, such as platelet-derived growth factor (PDGF), providing directional and stimulatory cues for moving cells (Smith et al. 2009). Interestingly, the association of PDGF with ECM appears to be dependent on HS, but does not involve direct binding to HS (Symes et al. 2010). Similarly, HS-dependent interactions between fibronectin and VEGF have been reported (Mitsi et al. 2006). The mechanism by which HS regulates the binding of growth factors to fibronectin appears to stem from its ability to induce the transition of fibronectin from a globular form to a more stable extended form, revealing growth factor–binding sites (Mitsi et al. 2008). This activity depends on the size and composition of the chains, as shown by studies in which only heparin chains longer than 22 saccharides and with sulfation at the 6-O- and N-positions of glucosamine units retained the ability to modify fibronectin structure and allow VEGF binding (Mitsi et al. 2006).
Tissue-specific expression of different proteoglycans during zebrafish embryogenesis also has been shown to play a role in determining the structure and function of the extracellular matrix. Syndecan-2 expression in the extraembryonic yolk syncytial layer induces fibronectin and laminin matrix assembly throughout the embryo and directs primordial cell migration (Arrington and Yost 2009). Interestingly, overexpression of syndecan-2 in the embryo does not rescue embryonic defects resulting from yolk syncytial layer deficiency, suggesting that proteoglycans in specific cell types can act in a unique manner because of either positional or structural differences. Other studies have also shown that the loss of specific proteoglycans such as syndecan-4 in Xenopus laevis can have adverse effects on neural crest cell migration (Matthews et al. 2008) and convergent extension movements (Munoz et al. 2006). These model systems provide a powerful empirical approach for determining the participation of HSPGs in matrix deposition and cell migration.
PROTEOGLYCANS AND BARRIER ACTIVITY
HSPGs were long thought to be a filtration barrier for charged macromolecules in the kidney, but recent studies cast doubt on this idea. The basement membrane of the kidney filtration structure, the glomerulus, contains HSPGs such as agrin, perlecan, and collagen XVIII, and HS accounts for much of the negative charge in the glomerular basement membrane (GBM) (Miner 1999; Raats et al. 2000). Early studies in which HS was removed by perfusion of rat kidneys with heparinase suggested that HS was essential for filtration of large charged proteins such as ferritin and albumin (Kanwar et al. 1980). Correlations of reduced HS levels in the GBM, increased heparanase expression and proteinuria were also made in patients with various kidney diseases such as diabetic nephropathy, further suggesting a putative barrier function for GBM HS (Makino et al. 1992; Tamsma et al. 1994; van den Hoven et al. 2006; Wijnhoven et al. 2006). However, confusion arose when subsequent in vivo studies failed to substantiate that removal of GBM HS with heparinase can result in acute proteinuria, and in fact enzyme digestion actually prevented proteinuria induced by removal of sialic acids (Wijnhoven et al. 2007a,b). Furthermore, tissue-specific deletion of the major GBM HSPGs in mice does not cause proteinuria (Rossi et al. 2003; Harvey et al. 2007; Goldberg et al. 2009), nor does complete ablation of HS biosynthesis in mouse podocytes until 8 months of age when proximal tubule abnormalities become prevalent (Chen et al. 2008). Taken together, it appears that the actual function of HS in the glomerulus is associated with its control of podocyte behavior and not as an ultrafiltration barrier.
Although HSPGs do not appear to play a substantial role in permselectivity in the kidney, cell surface HSPGs of the syndecan family have been shown to play a major role in maintaining the barrier integrity of the intestinal epithelium (Bode et al. 2006, 2008). The loss of syndecan-1 or its GAG chains from the intestinal epithelium has been shown to correlate with an efflux of plasma proteins into the intestinal lumen, causing a potentially lethal condition known as protein-losing enteropathy (PLE) (Murch et al. 1993, 1996; Westphal et al. 2000). The relationship between loss of syndecan-1 and PLE appears to be because of the ability of syndecan-1 to down-regulate inflammatory cytokines, such as IFN-γ and TNF-α, which work together to disrupt interepithelial integrity. Thus, it is thought that loss of syndecan-1 or its HS chains in the intestine exposes the epithelium to cytokine insult, resulting in the disruption of cell–cell interactions and PLE (Bode et al. 2008). Syndecan-1 may play a direct role in sealing the gaps between intestinal epithelial cells, acting as a physical barrier to prevent protein leakage (Bode et al. 2008). Importantly, the administration of nonanticoagulant heparin to syndecan-1-deficient mice as well as to one patient with PLE has proven effective at correcting protein leakage (Bode et al. 2008; Liem et al. 2008), suggesting that patients suffering from barrier dysfunction diseases associated with HSPG deficiency could be treated similarly.
MORPHOGEN AND CHEMOKINE GRADIENTS
Morphogens are signaling molecules that are expressed in restricted regions of tissue and can form gradients that specify cellular differentiation and patterning during development. Studies of morphogen diffusion in the Drosophila wing disk have shown that some morphogens, such as wingless (Wg), hedgehog (Hh) and Dpp, require HS for effective diffusion and will not cross cellular regions deficient in HS (Jackson et al. 1997; The et al. 1999; Tsuda et al. 1999; Baeg et al. 2001; Bornemann et al. 2004). Similar studies in the Drosophila wing disc revealed that glypicans are essential for morphogen diffusion (Belenkaya et al. 2004; Han et al. 2005; Yan and Lin 2009). These findings suggest a model of morphogen mobility known as restricted diffusion, where morphogens are transferred from one HSPG to the next at the cell surface, moving from regions of high concentration to regions of low concentration along a path that is defined by the interacting ligand (Yan and Lin 2009). Although this model might describe the mechanism by which HSPGs regulate short-range morphogen gradients, other modes of morphogen transmission are thought to exist. For example, in studies of morphogen diffusion in the Drosophila wing disc, Eaton and colleagues have described exocellular vesicles (argosomes) and lipoprotein particles (Lipophorin) that can mediate the transmission of morphogens over long distances (Greco et al. 2001; Eugster et al. 2007). Interestingly, the packaging of morphogens, such as Wg into argosomes, is HS-dependent and membrane-associated glypicans can recruit Lipophorin containing lipid-modified forms of Hh and Wg to disc tissue. Lander has discussed in detail the complexity of factors that can affect the shape of morphogen gradients and other ways that HSPGs participate in this process (Lander et al. 2002; Lander 2007).
Glypicans may play an important role in morphogen gradient formation because of their mode of attachment to the cell surface. Unlike other proteoglycans, glypicans are bound to the cell membrane via a GPI anchor, allowing diffusion to occur in the outer leaflet of the plasma membrane and affiliation with specific membrane structures such as lipid rafts (Taylor et al. 2009; Gutierrez and Brandan 2010). The ability of glypicans to localize into lipid rafts may allow them to associate more directly with a number of morphogens, such as Hh and Wg, which are themselves lipidated (Rietveld et al. 1999; Zhai et al. 2004). It is also interesting to note that the GPI anchor of glypicans can be cleaved from the cell surface by the hydrolase Notum (Kirkpatrick et al. 2004; Kreuger et al. 2004), a process that may impact the ability of these proteoglycans to regulate morphogen gradients. In support of this idea, overexpression of a secreted form of glypican that lacks a GPI anchor dramatically expands the range of the Hh activity in the Drosophila wing disc (Takeo et al. 2005). The reason for this expansion is unclear, but may be caused by a stabilizing effect of this secreted proteoglycan on Hh as it diffuses from its source. Alternatively, secreted glypican may interfere with Hh posttranslational processing events, such as cholesterol modification. HSPGs can modulate morphogen mobility by promoting their association with modifier enzymes such as ADAMs (a disintegrin and metalloproteinases) and transglutaminases (Dierker et al. 2009a,b). Whether the ability of HSPGs to mediate restricted diffusion and morphogen modification represents distinct mechanisms for the control of gradients is currently unclear.
One should also keep in mind that many other factors diffuse through the ECM en route to their final destinations and therefore would encounter HSPGs on the surfaces of cells, in the interstitial matrix or in a basement membrane. For example, lipoprotein lipase is expressed by adipocytes and skeletal and cardiac myocytes, but its site of action is on the lumenal side of the capillary endothelium in resident blood vessels. A recent study has shown that deletion of collagen XVIII results in chylomicronemia caused by decreased presentation of the lipase in the vasculature (Bishop et al. 2010). Because deficiency of collagen XVIII causes thickening of basement membranes (Utriainen et al. 2004), the decreased presentation of the lipase might be caused by delayed diffusion through the basement membrane underlying capillaries in tissues involved in lipolysis. Plasma lipids are normal in perlecan mutants lacking the HS attachment sites (Tran-Lundmark et al. 2008; Bishop et al. 2010), indicating specificity might exist in the interaction of the lipase with HSPGs in the matrix.
STEM CELL NICHE
The generation, maintenance and repair of different tissues during development is regulated by stem cell populations that reside in defined cellular microenvironments known as stem cell niches. These niches are essential for determining the ability of stem cells to retain a self-perpetuating pluripotent state or to differentiate into committed tissue specific progenitors (Nurcombe and Cool 2007). Interestingly, many of the signaling molecules involved in stem cell maintenance, such as Wnts and FGFs, are regulated by HSPGs (Sato et al. 2004; Xu et al. 2005a,c). Furthermore, embryonic stem cells change the structure of their HS as they differentiate into specific lineages (Johnson et al. 2007; Baldwin et al. 2008).
To directly address the role of HSPGs in stem cell differentiation, mouse embryonic stem cells with mutations in HS biosynthesis have been studied. Embryonic stem cells that lack HS because of Ext1 gene deficiency are incapable of differentiation on removal of leukemia inhibitory factor, apparently caused by a defective response to FGF (Kraushaar et al. 2010). These findings were corroborated by studies in embryonic stem cells lacking Ndst1/2, which also cannot differentiate in response to FGF because of reduced sulfation (Lanner et al. 2010). In addition, mouse embryonic stem cells deficient in Ndst1/2 were found to be unable to respond to VEGF, preventing their differentiation into blood capillary structures (Jakobsson et al. 2006). Taken together, these studies substantiate the importance of HS in stem cell differentiation at least ex vivo.
To address how HSPGs might regulate stem cells in their native cellular environments, Nakato and colleagues examined whether glypican participated in the maintenance of stem cells in the Drosophila germline stem cell niche. Interestingly, this function appears to be related to the ability of glypicans to restrict the localization and activity of the morphogen Dpp to the outer boundary of the niche (Hayashi et al. 2009). Stem cells directly adjacent to this Dpp-rich pocket were shown to be activated in trans by this morphogen and remained pluripotent. Daughter cells that were not able to physically associate with this region remained resistant to Dpp signaling and subsequently underwent differentiation. These findings will likely have important implications for stem-cell-based treatments of disease and for the design of synthetic matrices for stem-cell-based tissue engineering.
CONCLUDING REMARKS
The purpose of this article was to provide an overview of HSPGs and their biological roles in the ECM. As described above, HSPGs bind many ligands, modulate numerous cellular activities, and aid in tissue architecture and physiology. The examples selected for presentation represent only a subset of activities associated with HSPGs. However, it is striking that so many essential activities appear to be regulated by such a small family of macromolecules. Understanding how cells regulate the expression and composition of HSPGs to achieve these diverse activities in a coordinated fashion is a major biological problem to solve. The problem may be as complex as unraveling the genetic code, given the enormous complexity of heparan sulfate.
ACKNOWLEDGMENTS
The authors acknowledge grants GM33063 and HL57345 (to J.D.E) and F32DK085905 (to W.C.L) from the National Institutes of Health and a grant from Fondation pour la Recherche Medicale (to S.S.).
Footnotes
Editors: Richard Hynes and Kenneth Yamada
Additional Perspectives on Extracellular Matrix Biology available at www.cshperspectives.org
REFERENCES
- Abrink M, Grujic M, Pejler G 2004. Serglycin is essential for maturation of mast cell secretory granule. J Biol Chem 279: 40897–40905 [DOI] [PubMed] [Google Scholar]
- Adhikari N, Basi DL, Townsend D, Rusch M, Mariash A, Mullegama S, Watson A, Larson J, Tan S, Lerman B, et al. 2010. Heparan sulfate Ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. J Mol Cell Cardiol 49: 287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP Jr 2003. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol 162: 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai X, Kitazawa T, Do AT, Kusche-Gullberg M, Labosky PA, Emerson CP Jr 2007. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development 134: 3327–3338 [DOI] [PubMed] [Google Scholar]
- Aikawa T, Whipple CA, Lopez ME, Gunn J, Young A, Lander AD, Korc M 2008. Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J Clin Invest 118: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y 1999. Perlecan is essential for cartilage and cephalic development. Nat Genet 23: 354–358 [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, Yamada Y 2001. Dyssegmental dysplasia, Silverman–Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet 27: 431–434 [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y 2002. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nature Neurosci 5: 119–123 [DOI] [PubMed] [Google Scholar]
- Arrington CB, Yost HJ 2009. Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development 136: 3143–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari S, Habuchi H, Kimata K 1995. Characterization of heparan sulfate oligosaccharides that bind to hepatocyte growth factor. J Biol Chem 270: 29586–29593 [DOI] [PubMed] [Google Scholar]
- Ashikari-Hada S, Habuchi H, Kariya Y, Kimata K 2005. Heparin regulates vascular endothelial growth factor 165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J Biol Chem 280: 31508–31515 [DOI] [PubMed] [Google Scholar]
- Atha DH, Lormeau JC, Petitou M, Rosenberg RD, Choay J 1985. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry 24: 6723–6729 [DOI] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N 2001. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128: 87–94 [DOI] [PubMed] [Google Scholar]
- Baldwin RJ, ten Dam GB, van Kuppevelt TH, Lacaud G, Gallagher JT, Kouskoff V, Merry CL 2008. A developmentally regulated heparan sulfate epitope defines a subpopulation with increased blood potential during mesodermal differentiation. Stem Cells 26: 3108–3118 [DOI] [PubMed] [Google Scholar]
- Bao X, Moseman EA, Saito H, Petryanik B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, et al. 2010. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33: 817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ 2007. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol 177: 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, Burbach BJ, Rapraeger AC 2004. The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J Cell Biol 167: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC 2009. Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med 206: 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Park PW, Appleton JA 2006. Synthesis of syndecan-1 by skeletal muscle cells is an early response to infection with Trichinella spiralis but is not essential for nurse cell development. Infect Immun 74: 1941–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X 2004. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell 119: 231–244 [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, The I, Perrimon N 1998. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 394: 85–88 [DOI] [PubMed] [Google Scholar]
- Belting M 2003. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem Sci 28: 145–151 [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M 1999. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777 [DOI] [PubMed] [Google Scholar]
- Bezakova G, Ruegg MA 2003. New insights into the roles of agrin. Nat Rev Mol Cell Biol 4: 295–308 [DOI] [PubMed] [Google Scholar]
- Bhanot P 2002. Plasmodium yoelii sporozoites infect syndecan-1 deficient mice. Molec Biochem Parasitol 123: 143–144 [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446: 1030–1037 [DOI] [PubMed] [Google Scholar]
- Bishop JR, Passos-Bueno MR, Fong L, Stanford KI, Gonzales JC, Yeh E, Young SG, Bensadoun A, Witztum JL, Esko JD, et al. 2010. Deletion of the basement membrane heparan sulfate proteoglycan type XVIII collagen causes hypertriglyceridemia in mice and humans. PLoS ONE 5: e13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RV 2008. Novel interactions of perlecan: Unraveling perlecan's role in angiogenesis. Microsc Res Tech 71: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L, Murch S, Freeze HH 2006. Heparan sulfate plays a central role in a dynamic in vitro model of protein-losing enteropathy. J Biol Chem 281: 7809–7815 [DOI] [PubMed] [Google Scholar]
- Bode L, Salvestrini C, Park PW, Li JP, Esko JD, Yamaguchi Y, Murch S, Freeze HH 2008. Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. J Clin Invest 118: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R 2004. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development 131: 1927–1938 [DOI] [PubMed] [Google Scholar]
- Boyanovsky BB, Shridas P, Simons M, van der Westhuyzen DR, Webb NR 2009. Syndecan-4 mediates macrophage uptake of group V secretory phospholipase A2-modified LDL. J Lipid Res 50: 641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, Gattegno L 2006. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology 16: 488–501 [DOI] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA 1998. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev 12: 1894–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow HE, Hobert O 2006. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol 22: 375–407 [DOI] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ 2011. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3: a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Xavier AB, Martinet D, Bateman J, Belluoccio D, Rowley L, Tan TY, Baxova A, Gustavson KH, Borochowitz ZU, Innes AM, et al. 2009. Mutations in the heparan-sulfate proteoglycan glypican 6 (GPC6) impair endochondral ossification and cause recessive omodysplasia. Am J Hum Genet 84: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, et al. 1999. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson–Golabi–Behmel syndrome. J Cell Biol 146: 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capila I, Linhardt RJ 2002. Heparin-protein interactions. Angew Chem Int Edit 41: 391–412 [DOI] [PubMed] [Google Scholar]
- Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J 2008. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell 14: 700–711 [DOI] [PubMed] [Google Scholar]
- Cardin AD, Weintraub HJ 1989. Molecular modeling of protein–glycosaminoglycan interactions. Arteriosclerosis 9: 21–32 [DOI] [PubMed] [Google Scholar]
- Celie JW, Rutjes NW, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, Drager AM, Zweegman S, Kessler FL, Beelen RH, et al. 2007. Subendothelial heparan sulfate proteoglycans become major l-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol 170: 1865–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celie JW, Beelen RH, van den Born J 2009. Heparan sulfate proteoglycans in extravasation: assisting leukocyte guidance. Front Biosci 14: 4932–4949 [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Schaefer L, Uhlig P, Robenek H, Theilmeier G, Echtermeyer F, Bruckner P 2008. Unilateral nephrectomy leads to up-regulation of syndecan-2- and TGF-β-mediated glomerulosclerosis in syndecan-4 deficient male mice. Matrix Biol 27: 42–52 [DOI] [PubMed] [Google Scholar]
- Chen E, Stringer SE, Rusch MA, Selleck SB, Ekker SC 2005. A unique role for 6-O sulfation modification in zebrafish vascular development. Dev Biol 284: 364–376 [DOI] [PubMed] [Google Scholar]
- Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ 2008. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int 74: 289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlessinger D, Pilia G, Efstratiadis A 2002. Overgrowth of a mouse model of the Simpson–Golabi–Behmel syndrome is independent of IGF signaling. Dev Biol 243: 185–206 [DOI] [PubMed] [Google Scholar]
- Condac E, Silasi-Mansat R, Kosanke S, Schoeb T, Towner R, Lupu F, Cummings RD, Hinsdale ME 2007. Polycystic disease caused by deficiency in xylosyltransferase 2, an initiating enzyme of glycosaminoglycan biosynthesis. Proc Natl Acad Sci 104: 9416–9421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad HE 1998. Heparin-binding proteins Academic Press, San Diego [Google Scholar]
- Cornelison DDW, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB 2004. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev 18: 2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M, Carmona R, Gustafsson E, González-Iriarte M, Fässler R, Muñoz-Chápuli R 2002. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ Res 91: 158–164 [DOI] [PubMed] [Google Scholar]
- Couchman JR 2010. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26: 89–114 [DOI] [PubMed] [Google Scholar]
- Cox BD, Natarajan M, Stettner MR, Gladson CL 2006. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem 99: 35–52 [DOI] [PubMed] [Google Scholar]
- Crawford BE, Garner OB, Bishop JR, Zhang DY, Bush KT, Nigam SK, Esko JD 2010. Loss of the heparan sulfate sulfotransferase, Ndst1, in mammary epithelial cells selectively blocks lobuloalveolar development in mice. PLoS ONE 5: e10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson KG, Martinez-Hernandez A, Hassell JR, Iozzo RV 1992. Establishment of a cell line from the EHS tumor: Biosynthesis of basement membrane constituents and characterization of a hybrid proteoglycan containing heparan and chondroitin sulfate chains. Matrix 11: 22–35 [DOI] [PubMed] [Google Scholar]
- Dierker T, Dreier R, Migone M, Hamer S, Grobe K 2009a. Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked hedgehog oligomers. J Biol Chem 284: 32562–32571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker T, Dreier R, Petersen A, Bordych C, Grobe K 2009b. Heparan sulfate–modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem 284: 8013–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Lopez-Burks M, Sanchez-Duran JA, Korc M, Lander AD 2005. Growth factor–induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J Cell Biol 171: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P 2001. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest 107: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, et al. 2009. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med 15: 1072–1076 [DOI] [PubMed] [Google Scholar]
- Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, Elkin M 2006. Role of endothelial heparanase in delayed-type hypersensitivity. Blood 107: 3609–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson-Schwab L, Garner OB, Schuksz M, Crawford BE, Esko JD, Tor Y 2007. Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem 282: 13585–13591 [DOI] [PubMed] [Google Scholar]
- Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H 2003. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem 278: 40764–40770 [DOI] [PubMed] [Google Scholar]
- Esko JD, Kimata K, Lindahl U 2009. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of glycobiology (ed. Varki A, et al. ), pp. 229–248 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Esko JD, Linhardt RJ 2009. Proteins that bind sulfated glycosaminoglycans. in Essentials of glycobiology (ed. Varki A, et al. ), pp. 501–512 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Esko JD, Selleck SB 2002. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435–471 [DOI] [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci 82: 3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster C, Panakova D, Mahmoud A, Eaton S 2007. Lipoprotein–heparan sulfate interactions in the Hh pathway. Dev Cell 13: 57–71 [DOI] [PubMed] [Google Scholar]
- Fan G, Xiao L, Cheng L, Wang X, Sun B, Hu G 2000. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett 467: 7–11 [DOI] [PubMed] [Google Scholar]
- Feyzi E, Lustig F, Fager G, Spillmann D, Lindahl U, Salmivirta M 1997. Characterization of heparin and heparan sulfate domains binding to the long splice variant of platelet-derived growth factor A chain. J Biol Chem 272: 5518–5524 [DOI] [PubMed] [Google Scholar]
- Filmus J, Capurro M, Rast J 2008. Glypicans. Genome Biol 9: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang ZH, Park PW, Murphy G, Bernfield M 2000. Shedding of syndecan-1 and-4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol 148: 811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floer M, Gotte M, Wild MK, Heidemann J, Gassar ES, Domschke W, Kiesel L, Luegering A, Kucharzik T 2010. Enoxaparin improves the course of dextran sodium sulfate-induced colitis in syndecan-1-deficient mice. Am J Pathol 176: 146–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellén L 1999. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400: 773–776 [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55: 1189–1193 [DOI] [PubMed] [Google Scholar]
- Fransson LÅ, Edgren G, Havsmark B, Schmidtchen A 1995. Recycling of a glycosylphosphatidylinositol-anchored heparan sulphate proteoglycan (glypican) in skin fibroblasts. Glycobiology 5: 407–415 [DOI] [PubMed] [Google Scholar]
- Fuki IV, Meyer ME, Williams KJ 2000. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem J 351: 607–612 [PMC free article] [PubMed] [Google Scholar]
- Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemelä M, Ilves M, Li E, Pihlajaniemi T, et al. 2002. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J 21: 1535–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai N, Kenagy RD, Chen L, Gao L, Daum G, Clowes AW 2009. Syndecan-1: An inhibitor of arterial smooth muscle cell growth and intimal hyperplasia. Arterioscler Thromb Vasc Biol 29: 1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki IV, Kuhn KM, Lomazov IR, Rothman VL, Tuszynski GP, Iozzo RV, Swenson TL, Fisher EA, Williams KJ 1997. The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J Clin Invest 100: 1611–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster MM, Wang L, Castagnola J, Sikora L, Reddi K, Lee PHA, Radek K, Schuksz M, Bishop JR, Gallo RL, et al. 2007. Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J Cell Biol 177: 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner OB, Yamaguchi Y, Esko JD, Videm V 2008. Small changes in lymphocyte development and activation in mice through tissue-specific alteration of heparan sulphate. Immunology 125: 420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner OB, Bush KT, Nigam KB, Yamaguchi Y, Xu D, Esko JD, Nigam SK 2011. Stage-dependent regulation of mammary ductal branching by heparan sulfate and HGF-cMet signaling. Dev Biol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR 1996. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85: 525–535 [DOI] [PubMed] [Google Scholar]
- Gautam M, DeChiara TM, Glass DJ, Yancopoulos GD, Sanes JR 1999. Distinct phenotypes of mutant mice lacking agrin, MuSK, or rapsyn. Brain Res Dev Brain Res 114: 171–178 [DOI] [PubMed] [Google Scholar]
- Geiger B, Yamada KM 2011. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3: a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras J, Rassadi S, Cooper E, Ferns M 2007. Synaptic transmission is impaired at neuronal autonomic synapses in agrin-null mice. Dev Neurobiol 67: 521–534 [DOI] [PubMed] [Google Scholar]
- Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M 2007. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev Biol 7: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH 2009. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant 24: 2044–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Bober A, Whiteford JR, Multhaupt HA, Yoneda A, Couchman JR 2010. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J Biol Chem 285: 14247–14258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, Kresse H 2005. Defective glycosaminoglycan substitution of decorin in a patient with progeroid syndrome is a direct consequence of two point mutations in the galactosyltransferase I (β4GalT-7) gene. Biochem Genet 43: 65–77 [DOI] [PubMed] [Google Scholar]
- Gotte M, Joussen AM, Klein C, Andre P, Wagner DD, Hinkes MT, Kirchhof B, Adamis AP, Bernfield M 2002. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest Ophthalmol Vis Sci 43: 1135–1141 [PubMed] [Google Scholar]
- Gotte M, Bernfield M, Joussen AM 2005. Increased leukocyte–endothelial interactions in syndecan-1-deficient mice involve heparan sulfate–dependent and –independent steps. Curr Eye Res 30: 417–422 [DOI] [PubMed] [Google Scholar]
- Govindraj P, West L, Koob TJ, Neame P, Doege K, Hassell JR 2002. Isolation and identification of the major heparan sulfate proteoglycans in the developing bovine rib growth plate. J Biol Chem 277: 19461–19469 [DOI] [PubMed] [Google Scholar]
- Greco V, Hannus M, Eaton S 2001. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106: 633–645 [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM 1988. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55: 1179–1188 [DOI] [PubMed] [Google Scholar]
- Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND 2001. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol 231: 31–46 [DOI] [PubMed] [Google Scholar]
- Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD 2005. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development 132: 3777–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grujic M, Braga T, Lukinius A, Eloranta ML, Knight SD, Pejler G, Abrink M 2005. Serglycin-deficient cytotoxic T lymphocytes display defective secretory granule maturation and granzyme B storage. J Biol Chem 280: 33411–33418 [DOI] [PubMed] [Google Scholar]
- Grujic M, Christensen JP, Sørensen MR, Abrink M, Pejler G, Thomsen AR 2008. Delayed contraction of the CD8+ T cell response toward lymphocytic choriomeningitis virus infection in mice lacking serglycin. J Immunol 181: 1043–1051 [DOI] [PubMed] [Google Scholar]
- Grunewald FS, Prota AE, Giese A, Ballmer-Hofer K 2010. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta 1804: 567–580 [DOI] [PubMed] [Google Scholar]
- Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC 1993. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem 268: 23906–23914 [PubMed] [Google Scholar]
- Gutierrez J, Brandan E 2010. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol 30: 1634–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K 2007. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem 282: 15578–15588 [DOI] [PubMed] [Google Scholar]
- Häcker U, Lin XH, Perrimon N 1997. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development 124: 3565–3573 [DOI] [PubMed] [Google Scholar]
- HajMohammadi S, Enjyoji K, Princivalle M, Christi P, Lech M, Beeler D, Rayburn H, Schwartz JJ, Barzegar S, De Agostini AI, et al. 2003. Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. J Clin Invest 111: 989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano Y, Okude T, Shirai R, Sato I, Kimura R, Ogawa M, Ueda Y, Yokosuka O, Kalluri R, Ueda S 2010. Lack of collagen XVIII/endostatin exacerbates immune-mediated glomerulonephritis. J Am Soc Nephrol 21: 1445–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X 2005. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 132: 667–679 [DOI] [PubMed] [Google Scholar]
- Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE 2005. Regulation of protein function by glycosaminoglycans–As exemplified by chemokines. Annu Rev Biochem 74: 385–410 [DOI] [PubMed] [Google Scholar]
- Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH 2007. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Wang F 2008. Visualizing mechanosensory endings of TrkC-expressing neurons in HS3ST-2-hPLAP mice. J Comp Neurol 511: 543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, Nakato H 2009. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol 187: 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Stahl PD, Park PW 2008. Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. J Biol Chem 283: 35435–35444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida A, Bartlett AH, Foster TJ, Park PW 2009a. Staphylococcus aureus β-toxin induces lung injury through syndecan-1. Am J Pathol 174: 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Parks WC, Park PW 2009b. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood 114: 3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes A, Ruda F, Oliver J, Hamood AN, Griswold JA, Park PW, Rumbaugh KP 2005. Syndecan 1 shedding contributes to Pseudomonas aeruginosa sepsis. Infect Immun 73: 7914–7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsson F, Hergeth S, Cortelius R, Abrink M, Pejler G 2006. A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components. FEBS J 273: 4901–4912 [DOI] [PubMed] [Google Scholar]
- Hienola A, Tumova S, Kulesskiy E, Rauvala H 2006. N-syndecan deficiency impairs neural migration in brain. J Cell Biol 174: 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman RE, Fromm JR, Weiler JM, Linhardt RJ 1998. Glycosaminoglycan–protein interactions: Definition of consensus sites in glycosaminoglycan binding proteins. BioEssays 20: 156–167 [DOI] [PubMed] [Google Scholar]
- Hilgenberg LGW, Ho KD, Lee D, O'Dowd DK, Smith MA 2002. Agrin regulates neuronal responses to excitatory neurotransmitters in vitro and in vivo. Molec Cell Neurosci 19: 97–110 [DOI] [PubMed] [Google Scholar]
- Holst CR, Bou-Reslan H, Gore BB, Wong K, Grant D, Chalasani S, Carano RA, Frantz GD, Tessier-Lavigne M, Bolon B, et al. 2007. Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS ONE 2: e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang CF, Sharpe AH, Stevens RL 1999. Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400: 769–772 [DOI] [PubMed] [Google Scholar]
- Hurskainen M, Eklund L, Hägg PO, Fruttiger M, Sormunen R, Ilves M, Pihlajaniemi T 2005. Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J 19: 1564–1566 [DOI] [PubMed] [Google Scholar]
- Ilan N, Elkin M, Vlodavsky I 2006. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol 38: 2018–2039 [DOI] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y 2003. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science 302: 1044–1046 [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Zoeller JJ, Nystrom A 2009. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol Cells 27: 503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Nakamura E, Ito M, Nagasaka T, Kobayashi H, Kusugami K, Saito H, et al. 2000. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Dev Dyn 219: 539–544 [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, et al. 2001. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem 276: 47483–47488 [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kojima T, Muramatsu T 2002. Syndecan-4 as a molecule involved in defense mechanisms. Glycoconj J 19: 315–318 [DOI] [PubMed] [Google Scholar]
- Iwabuchi T, Goetinck PF 2006. Syndecan-4 dependent FGF stimulation of mouse vibrissae growth. Mech Dev 123: 831–841 [DOI] [PubMed] [Google Scholar]
- Izumikawa T, Kanagawa N, Watamoto Y, Okada M, Saeki M, Sakano M, Sugahara K, Sugihara K, Asano M, Kitagawa H 2010. Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I-deficient mice. J Biol Chem 285: 12190–12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izvolsky KI, Lu J, Martin G, Albrecht KH, Cardoso WV 2008. Systemic inactivation of Hs6st1 in mice is associated with late postnatal mortality without major defects in organogenesis. Genesis 46: 8–18 [DOI] [PubMed] [Google Scholar]
- Jackson SM, Nakato H, Sugiura M, Jannuzi A, Oakes R, Kaluza V, Golden C, Selleck SB 1997. dally, a Drosophila glypican, controls cellular responses to the TGF-β-related morphogen, Dpp. Development 124: 4113–4120 [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellen L, Claesson-Welsh L 2006. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell 10: 625–634 [DOI] [PubMed] [Google Scholar]
- Jen YH, Musacchio M, Lander AD 2009. Glypican-1 controls brain size through regulation of fibroblast growth factor signaling in early neurogenesis. Neural Dev 4: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. 2010. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 120: 2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CE, Crawford BE, Stavridis M, Ten Dam G, Wat AL, Rushton G, Ward CM, Wilson V, van Kuppevelt TH, Esko JD, et al. 2007. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells 25: 1913–1923 [DOI] [PubMed] [Google Scholar]
- Jones KB, Piombo V, Searby C, Kurriger G, Yang B, Grabellus F, Roughley PJ, Morcuende JA, Buckwalter JA, Capecchi MR, et al. 2010. A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes. Proc Natl Acad Sci 107: 2054–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Pavlov I, Võikar V, Lauri SE, Hienola A, Riekki R, Lakso M, Taira T, Rauvala H 2002. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Molec Cell Neurosci 21: 158–172 [DOI] [PubMed] [Google Scholar]
- Kamimura K, Koyama T, Habuchi H, Ueda R, Masu M, Kimata K, Nakato H 2006. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J Cell Biol 174: 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS, Linker A, Farquhar MG 1980. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol 86: 688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Wang H, Bernfield M, Gallagher JT, Turnbull JE 1994. Cell surface syndecan-1 on distinct cell types differs in fine structure and ligand binding of its heparan sulfate chains. J Biol Chem 269: 18881–18890 [PubMed] [Google Scholar]
- Kato M, Wang H, Kainulainen V, Fitzgerald ML, Ledbetter S, Ornitz DM, Bernfield M 1998. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med 4: 691–697 [DOI] [PubMed] [Google Scholar]
- Kelly T, Suva LJ, Nicks KM, MacLeod V, Sanderson RD 2010. Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis. J Bone Miner Res 25: 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick CA, Dimitroff BD, Rawson JM, Selleck SB 2004. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell 7: 513–523 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Knox SM, Staatz WD, Fox B, Lercher DM, Selleck SB 2006. The function of a Drosophila glypican does not depend entirely on heparan sulfate modification. Dev Biol 300: 570–582 [DOI] [PubMed] [Google Scholar]
- Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A 1998. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest 101: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon S, Ikesue M, Kimura C, Aoki M, Nakayama Y, Saito Y, Kurotaki D, Diao H, Matsui Y, Segawa T, et al. 2008. Syndecan-4 protects against osteopontin-mediated acute hepatic injury by masking functional domains of osteopontin. J Exp Med 205: 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ 2002. Ectodermal syndecan-2 mediates left–right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell 2: 115–124 [DOI] [PubMed] [Google Scholar]
- Kraushaar DC, Yamaguchi Y, Wang L 2010. Heparan sulfate is required for embryonic stem cells to exit from self-renewal. J Biol Chem 285: 5907–5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Salmivirta M, Sturiale L, Giménez-Gallego G, Lindahl U 2001. Sequence analysis of heparan sulfate epitopes with graded affinities for fibroblast growth factors 1 and 2. J Biol Chem 276: 30744–30752 [DOI] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM 2004. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell 7: 503–512 [DOI] [PubMed] [Google Scholar]
- Ksiazek I, Burkhardt C, Lin S, Seddik R, Maj M, Bezakova G, Jucker M, Arber S, Caroni P, Sanes JR, et al. 2007. Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci 27: 7183–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna WC, Baldwin RJ, Padva M, Kalus I, Ten Dam G, van Kuppevelt TH, Gallagher JT, von Figura K, Dierks T, Merry CL 2006. Heparan sulfate 6-O-endosulfatases: discrete in vivo activities and functional co-operativity. Biochem J 400: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna WC, Kalus I, Padva M, Baldwin RJ, Merry CL, Dierks T 2007. The heparanome–The enigma of encoding and decoding heparan sulfate sulfation. J Biotechnol 129: 290–307 [DOI] [PubMed] [Google Scholar]
- Lamanna WC, Frese MA, Balleininger M, Dierks T 2008. Sulf loss influences N-, 2-O-, and 6-O-sulfation of multiple heparan sulfate proteoglycans and modulates fibroblast growth factor signaling. J Biol Chem 283: 27724–27735 [DOI] [PubMed] [Google Scholar]
- Lander AD 2007. Morpheus unbound: Reimagining the morphogen gradient. Cell 128: 245–256 [DOI] [PubMed] [Google Scholar]
- Lander AD, Nie Q, Wan FY 2002. Do morphogen gradients arise by diffusion? Dev Cell 2: 785–796 [DOI] [PubMed] [Google Scholar]
- Lanner F, Lee KL, Sohl M, Holmborn K, Yang H, Wilbertz J, Poellinger L, Rossant J, Farnebo F 2010. Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. Stem Cells 28: 191–200 [DOI] [PubMed] [Google Scholar]
- Laremore TN, Zhang F, Dordick JS, Liu J, Linhardt RJ 2009. Recent progress and applications in glycosaminoglycan and heparin research. Curr Opin Chem Biol 13: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD 2008. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem 283: 33674–33684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClair EE, Mui SR, Huang A, Topczewska JM, Topczewski J 2009. Craniofacial skeletal defects of adult zebrafish Glypican 4 (knypek) mutants. Dev Dyn 238: 2550–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Olsen BR 2004. Increased angiogenic response in aortic explants of collagen XVIII/endostatin-null mice. Am J Pathol 165: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JP, Vlodavsky I 2009. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost 102: 823–828 [DOI] [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC 2002. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111: 635–646 [DOI] [PubMed] [Google Scholar]
- Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U 2003. Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. J Biol Chem 278: 28363–28366 [DOI] [PubMed] [Google Scholar]
- Li JP, Galvis ML, Gong F, Zhang X, Zcharia E, Metzger S, Vlodavsky I, Kisilevsky R, Lindahl U 2005. In vivo fragmentation of heparan sulfate by heparanase overexpression renders mice resistant to amyloid protein A amyloidosis. Proc Natl Acad Sci 102: 6473–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem YS, Bode L, Freeze HH, Leebeek FW, Zandbergen AA, Paul Wilson J 2008. Using heparin therapy to reverse protein-losing enteropathy in a patient with CDG-Ib. Nat Clin Pract Gastroenterol Hepatol 5: 220–224 [DOI] [PubMed] [Google Scholar]
- Lin XH, Buff EM, Perrimon N, Michelson AM 1999. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development 126: 3715–3723 [DOI] [PubMed] [Google Scholar]
- Lin X, Wei G, Shi ZZ, Dryer L, Esko JD, Wells DE, Matzuk MM 2000. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol 224: 299–311 [DOI] [PubMed] [Google Scholar]
- Lindahl U, Li JP 2009. Interactions between heparan sulfate and proteins—Design and functional implications. Int Rev Cell Mol Biol 276: 105–159 [DOI] [PubMed] [Google Scholar]
- Lindahl B, Lindahl U 1997. Amyloid-specific heparan sulfate from human liver and spleen. J Biol Chem 272: 26091–26094 [DOI] [PubMed] [Google Scholar]
- Lindahl U, Backstrom G, Thunberg L, Leder IG 1980. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci 77: 6551–6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley RL, Woods A, Fleetwood A, Cowling GJ, Gallagher JT, Couchman JR 1999. Control of morphology, cytoskeleton and migration by syndecan-4. J Cell Sci 112: 3421–3431 [DOI] [PubMed] [Google Scholar]
- Lortat-Jacob H 2009. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol 19: 543–548 [DOI] [PubMed] [Google Scholar]
- Lortat-Jacob H, Turnbull JE, Grimaud JA 1995. Molecular organization of the interferon γ-binding domain in heparan sulphate. Biochem J 310: 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortat-Jacob H, Grosdidier A, Imberty A 2002. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci 99: 1229–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum DH, Tan J, Rosen SD, Werb Z 2007. Gene trap disruption of the mouse heparan sulfate 6-O-endosulfatase gene, Sulf2. Mol Cell Biol 27: 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M, Deakin JA, Mizuno K, Nakamura T, Gallagher JT 1994. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J Biol Chem 269: 11216–11223 [PubMed] [Google Scholar]
- MacArthur JM, Bishop JR, Wang L, Stanford KI, Bensadoun A, Witztum JL, Esko JD 2007. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest 117: 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarana M, Lindahl U 1993. Mode of interaction between platelet factor 4 and heparin. Glycobiology 3: 271–277 [DOI] [PubMed] [Google Scholar]
- Maccarana M, Casu B, Lindahl U 1993. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem 268: 23898–23905 [PubMed] [Google Scholar]
- Makino H, Ikeda S, Haramoto T, Ota Z 1992. Heparan sulfate proteoglycans are lost in patients with diabetic nephropathy. Nephron 61: 415–421 [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y, Couchman JR 2010. Proteoglycans in health and disease: The multiple roles of syndecan shedding. Febs J 277: 3876–3889 [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR 2001. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol 20: 337–345 [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR 2003. Age-dependent iris abnormalities in collagen XVIII/endostatin deficient mice with similarities to human pigment dispersion syndrome. Investig Ophthal Visual Sci 44: 2367–2372 [DOI] [PubMed] [Google Scholar]
- Marneros AG, She H, Zambarakji H, Hashizume H, Connolly EJ, Kim I, Gragoudas ES, Miller JW, Olsen BR 2007. Endogenous endostatin inhibits choroidal neovascularization. FASEB J 21: 3809–3818 [DOI] [PubMed] [Google Scholar]
- Marois E, Mahmoud A, Eaton S 2006. The endocytic pathway and formation of the Wingless morphogen gradient. Development 133: 307–317 [DOI] [PubMed] [Google Scholar]
- Masouleh BK, Ten Dam GB, Wild MK, Seelige R, van der Vlag J, Rops AL, Echtermeyer FG, Vestweber D, van Kuppevelt TH, Kiesel L, et al. 2009. Role of the heparan sulfate proteoglycan syndecan-1 (CD138) in delayed-type hypersensitivity. J Immunol 182: 4985–4993 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Irie F, Mackem S, Yamaguchi Y 2010. A mouse model of chondrocyte-specific somatic mutation reveals a role for Ext1 loss of heterozygosity in multiple hereditary exostoses. Proc Natl Acad Sci 107: 10932–10937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Luscher D, Hettwer S, Wolfel J, Ladner AP, Ster J, Gerber U, et al. 2009. Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent agrin cleavage. Cell 136: 1161–1171 [DOI] [PubMed] [Google Scholar]
- Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R 2008. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 135: 1771–1780 [DOI] [PubMed] [Google Scholar]
- McDermott SP, Ranheim EA, Leatherberry VS, Khwaja SS, Klos KS, Alexander CM 2007. Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene 26: 1407–1416 [DOI] [PubMed] [Google Scholar]
- McKeehan WL, Wu XC, Kan M 1999. Requirement for anticoagulant heparan sulfate in the fibroblast growth factor receptor complex. J Biol Chem 274: 21511–21514 [DOI] [PubMed] [Google Scholar]
- McLaughlin D, Karlsson F, Tian N, Pratt T, Bullock SL, Wilson VA, Price DJ, Mason JO 2003. Specific modification of heparan sulphate is required for normal cerebral cortical development. Mech Dev 120: 1481–1488 [DOI] [PubMed] [Google Scholar]
- McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC 2006. Syndecan-1 regulates αvβ5 integrin activity in B82L fibroblasts. J Cell Sci 119: 2445–2456 [DOI] [PubMed] [Google Scholar]
- Merry CLR, Bullock SL, Swan DC, Backen AC, Lyon M, Beddington RSP, Wilson VA, Gallagher JT 2001. The molecular phenotype of heparan sulfate in the Hs2st−/− mutant mouse. J Biol Chem 276: 35429–35434 [DOI] [PubMed] [Google Scholar]
- Miner JH 1999. Renal basement membrane components. Kidney Int 56: 2016–2024 [DOI] [PubMed] [Google Scholar]
- Mitsi M, Hong Z, Costello CE, Nugent MA 2006. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry 45: 10319–10328 [DOI] [PubMed] [Google Scholar]
- Mitsi M, Forsten-Williams K, Gopalakrishnan M, Nugent MA 2008. A catalytic role of heparin within the extracellular matrix. J Biol Chem 283: 34796–34807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD 2007. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol 8: 957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Yoshimura A, Inui K, Ideura T, Watanabe H, Wang L, Soininen R, Tryggvason K 2005. Heparan sulfate of perlecan is involved in glomerular filtration. J Am Soc Nephrol 16: 1703–1710 [DOI] [PubMed] [Google Scholar]
- Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X 2004. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation 110: 1330–1336 [DOI] [PubMed] [Google Scholar]
- Mulloy B, Linhardt RJ 2001. Order out of complexity–Protein structures that interact with heparin. Curr Opin Struct Biol 11: 623–628 [DOI] [PubMed] [Google Scholar]
- Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J 2006. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol 8: 492–500 [DOI] [PubMed] [Google Scholar]
- Murakami M, Horowitz A, Tang S, Ware JA, Simons M 2002. Protein kinase C (PKC) δ regulates PKCα activity in a syndecan-4-dependent manner. J Biol Chem 277: 20367–20371 [DOI] [PubMed] [Google Scholar]
- Murch SH, MacDonald TT, Walker-Smith JA, Levin M, Lionetti P, Klein NJ 1993. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet 341: 711–714 [DOI] [PubMed] [Google Scholar]
- Murch SH, Winyard PJD, Koletzko S, Wehner B, Cheema HA, Risdon RA, Phillips AD, Meadows N, Klein NJ, Walker-Smith JA 1996. Congenital enterocyte heparan sulphate deficiency with massive albumin loss, secretory diarrhoea, and malnutrition. Lancet 347: 1299–1301 [DOI] [PubMed] [Google Scholar]
- Nakato H, Futch TA, Selleck SB 1995. The division abnormally delayed (dally) gene: A putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development 121: 3687–3702 [DOI] [PubMed] [Google Scholar]
- Ng A, Wong M, Viviano B, Erlich JM, Alba G, Pflederer C, Jay PY, Saunders S 2009. Loss of glypican-3 function causes growth factor-dependent defects in cardiac and coronary vascular development. Dev Biol 335: 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole S 2000. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz–Jampel syndrome (chondrodystrophic myotonia). Nature Genetics 26: 480–483 [DOI] [PubMed] [Google Scholar]
- Niemann CU, Abrink M, Pejler G, Fischer RL, Christensen EI, Knight SD, Borregaard N 2007. Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 109: 4478–4486 [DOI] [PubMed] [Google Scholar]
- Noguer O, Villena J, Lorita J, Vilaró S, Reina M 2009. Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Exp Cell Res 315: 795–808 [DOI] [PubMed] [Google Scholar]
- Norgard-Sumnicht KE, Varki A 1995. Endothelial heparan sulfate proteoglycans that bind to l-selectin have glucosamine residues with unsubstituted amino groups. J Biol Chem 270: 12012–12024 [DOI] [PubMed] [Google Scholar]
- Nurcombe V, Cool SM 2007. Heparan sulfate control of proliferation and differentiation in the stem cell niche. Crit Rev Eukaryot Gene Expr 17: 159–171 [DOI] [PubMed] [Google Scholar]
- Oh ES, Couchman JR 2004. Syndecans-2 and -4; close cousins, but not identical twins. Mol Cells 17: 181–187 [PubMed] [Google Scholar]
- Oh ES, Couchman JR, Woods A 1997a. Serine phosphorylation of syndecan-2 proteoglycan cytoplasmic domain. Arch Biochem Biophys 344: 67–74 [DOI] [PubMed] [Google Scholar]
- Oh ES, Woods A, Couchman JR 1997b. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J Biol Chem 272: 11805–11811 [DOI] [PubMed] [Google Scholar]
- Oh ES, Woods A, Couchman JR 1997c. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J Biol Chem 272: 8133–8136 [DOI] [PubMed] [Google Scholar]
- Okina E, Manon-Jensen T, Whiteford JR, Couchman JR 2009. Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand J Med Sci Sports 19: 479–489 [DOI] [PubMed] [Google Scholar]
- Ono K, Hattori H, Takeshita S, Kurita A, Ishihara M 1999. Structural features in heparin that interact with VEGF165 and modulate its biological activity. Glycobiology 9: 705–711 [DOI] [PubMed] [Google Scholar]
- Ori A, Wilkinson MC, Fernig DG 2008. The heparanome and regulation of cell function: Structures, functions and challenges. Front Biosci 13: 4309–4338 [DOI] [PubMed] [Google Scholar]
- Otsuki S, Hanson SR, Miyaki S, Grogan SP, Kinoshita M, Asahara H, Wong CH, Lotz MK 2010. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc Natl Acad Sci 107: 10202–10207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallerla SR, Lawrence R, Lewejohann L, Pan Y, Fischer T, Schlomann U, Zhang X, Esko JD, Grobe K 2008. Altered heparan sulfate structure in mice with deleted NDST3 gene function. J Biol Chem 283: 16885–16894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X 2006. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133: 4933–4944 [DOI] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X 2008. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 135: 301–310 [DOI] [PubMed] [Google Scholar]
- Park PW, Pier GB, Preston MJ, Goldberger O, Fitzgerald ML, Bernfield M 2000a. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J Biol Chem 275: 3057–3064 [DOI] [PubMed] [Google Scholar]
- Park PW, Reizes O, Bernfield M 2000b. Cell surface heparan sulfate proteoglycans: Selective regulators of ligand-receptor encounters. J Biol Chem 275: 29923–29926 [DOI] [PubMed] [Google Scholar]
- Park PW, Pier GB, Hinkes MT, Bernfield M 2001. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature 411: 98–102 [DOI] [PubMed] [Google Scholar]
- Parthasarathy N, Goldberg IJ, Sivaram P, Mulloy B, Flory DM, Wagner WD 1994. Oligosaccharide sequences of endothelial cell surface heparan sulfate proteoglycan with affinity for lipoprotein lipase. J Biol Chem 269: 22391–22396 [PubMed] [Google Scholar]
- Partovian C, Ju R, Zhuang ZW, Martin KA, Simons M 2008. Syndecan-4 regulates subcellular localization of mTOR complex2 and Akt activation in a PKCα-dependent manner in endothelial cells. Molec Cell 32: 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AM, Gardner L, Shaw J, David G, Loreau E, Aguilar L, Ashton BA, Middleton J 2005. Induction of a CXCL8 binding site on endothelial syndecan-3 in rheumatoid synovium. Arthritis Rheum 52: 2331–2342 [DOI] [PubMed] [Google Scholar]
- Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D 1996. Mutations in GPC3, a glypican gene, cause the Simpson–Golabi–Behmel overgrowth syndrome [see comments]. Nat Genet 12: 241–247 [DOI] [PubMed] [Google Scholar]
- Poon GM, Gariepy J 2007. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem Soc Trans 35: 788–793 [DOI] [PubMed] [Google Scholar]
- Pratt T, Conway CD, Tian NM, Price DJ, Mason JO 2006. Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. J Neurosci 26: 6911–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot AEI, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TNC, Kosco-Vilbois MH 2003. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci 100: 1885–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, et al. 2010. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem 285: 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Carbe C, Tao C, Powers A, Lawrence R van Kuppevelt TH, Cardoso WV, Grobe K, Esko JD, Zhang X 2011. Lacrimal gland development and Fgf10-Fgfr2b signaling are controlled by 2-0- and 6-0-sulfated heparan sulfate. J Bioi Chem 286: 14435–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raats CJI, Van den Born J, Berden JHM 2000. Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int 57: 385–400 [DOI] [PubMed] [Google Scholar]
- Rapraeger AC, Krufka A, Olwin BB 1991. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 252: 1705–1708 [DOI] [PubMed] [Google Scholar]
- Ratzka A, Kalus I, Moser M, Dierks T, Mundlos S, Vortkamp A 2008. Redundant function of the heparan sulfate 6-O-endosulfatases Sulf1 and Sulf2 during skeletal development. Dev Dyn 237: 339–353 [DOI] [PubMed] [Google Scholar]
- Reizes O, Benoit SC, Strader AD, Clegg DJ, Akunuru S, Seeley RJ 2003. Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Ann NY Acad Sci 994: 66–73 [DOI] [PubMed] [Google Scholar]
- Ren Y, Kirkpatrick CA, Rawson JM, Sun M, Selleck SB 2009. Cell type-specific requirements for heparan sulfate biosynthesis at the Drosophila neuromuscular junction: Effects on synapse function, membrane trafficking, and mitochondrial localization. J Neurosci 29: 8539–8550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GD, Fantauzzo KA, Bazzi H, Määttä A, Jahoda CAB 2009. Dynamic expression of Syndecan-1 during hair follicle morphogenesis. Gene Express Patterns 9: 454–460 [DOI] [PubMed] [Google Scholar]
- Rietveld A, Neutz S, Simons K, Eaton S 1999. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J Biol Chem 274: 12049–12054 [DOI] [PubMed] [Google Scholar]
- Ringvall M, Ledin J, Holmborn K, Van Kuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellén L, Forsberg E 2000. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem 275: 25926–25930 [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Mulloy B, Gallagher JT, Stringer SE 2006. VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J Biol Chem 281: 1731–1740 [DOI] [PubMed] [Google Scholar]
- Rodgers KD, San Antonio JD, Jacenko O 2008. Heparan sulfate proteoglycans: A GAGgle of skeletal-hematopoietic regulators. Dev Dyn 237: 2622–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski TM, Williams BD, Mullen GP, Moerman DG 1993. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes 7: 1471–1484 [DOI] [PubMed] [Google Scholar]
- Rops AL, Götte M, Baselmans MH, van den Hoven MJ, Steenbergen EJ, Lensen JF, Wijnhoven TJ, Cevikbas F, van den Heuvel LP, van Kuppevelt TH, et al. 2007. Syndecan-1 deficiency aggravates anti-glomerular basement membrane nephritis. Kidney Int 72: 1204–1215 [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R 2003. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J 22: 236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadir R, Imberty A, Baleux F, Lortat-Jacob H 2004. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem 279: 43854–43860 [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y 2008. Syndecan-1: A dynamic regulator of the myeloma microenvironment. Clin Exp Metastasis 25: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF 1999. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci 96: 2805–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraj MA, Escalona RM, Umbers A, Chua HK, Small C, Griswold M, Loveland K, Findlay JK, Stenvers KL 2010. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (betaglycan) knockout mice. Biol Reprod 82: 153–162 [DOI] [PubMed] [Google Scholar]
- Sarrazin S, Wilson B, Sly WS, Tor Y, Esko JD 2010. Guanidinylated neomycin mediates heparan sulfate–dependent transport of active enzymes to lysosomes. Mol Ther 18: 1268–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasisekharan R, Raman R, Prabhakar V 2006. Glycomics approach to structure–function relationships of glycosaminoglycans. Annu Rev Biomed Eng 8: 181–231 [DOI] [PubMed] [Google Scholar]
- Sasse P, Malan D, Fleischmann M, Roell W, Gustafsson E, Bostani T, Fan Y, Kolbe T, Breitbach M, Addicks K, et al. 2008. Perlecan is critical for heart stability. Cardiovasc Res 80: 435–444 [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH 2004. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10: 55–63 [DOI] [PubMed] [Google Scholar]
- Schellings MWM, Vanhoutte D, van Almen GC, Swinnen M, Leenders JJG, Kubben N, van Leeuwen REW, Hofstra L, Heymans S, Pinto YM 2010. Syndecan-1 amplifies angiotensin II–induced cardiac fibrosis. Hypertension 55: 249–256 [DOI] [PubMed] [Google Scholar]
- Schwartz MA 2010. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol 2: a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler DG, Faiyaz-Ul-Haque M, Hansen U, Yip GW, Zaidi SH, Teebi AS, Kiesel L, Gotte M 2006. Defective glycosylation of decorin and biglycan, altered collagen structure, and abnormal phenotype of the skin fibroblasts of an Ehlers–Danlos syndrome patient carrying the novel Arg270Cys substitution in galactosyltransferase I (β4GalT-7). J Mol Med 84: 583–594 [DOI] [PubMed] [Google Scholar]
- Seppinen L, Sormunen R, Soini Y, Elamaa H, Heljasvaara R, Pihlajaniemi T 2008. Lack of collagen XVIII accelerates cutaneous wound healing, while overexpression of its endostatin domain leads to delayed healing. Matrix Biol 27: 535–546 [DOI] [PubMed] [Google Scholar]
- Serpinskaya AS, Feng G, Sanes JR, Craig AM 1999. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev Biol 205: 65–78 [DOI] [PubMed] [Google Scholar]
- Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR 2000. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum Mol Genet 9: 2051–2058 [DOI] [PubMed] [Google Scholar]
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai XM, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99: 13–22 [DOI] [PubMed] [Google Scholar]
- Shworak NW, Kojima T, Rosenberg RD 1993. Isolation and characterization of ryudocan and syndecan heparan sulfate proteoglycans, core proteins, and cDNAs from a rat endothelial cell line. Haemostasis 23: 161–176 [DOI] [PubMed] [Google Scholar]
- Shworak NW, HajMohammadi S, DeAgostini AI, Rosenberg RD 2002. Mice deficient in heparan sulfate 3-O-sulfotransferase-1: Normal hemostasis with unexpected perinatal phenotypes. Glycoconj J 19: 355–361 [DOI] [PubMed] [Google Scholar]
- Smith EM, Mitsi M, Nugent MA, Symes K 2009. PDGF-A interactions with fibronectin reveal a critical role for heparan sulfate in directed cell migration during Xenopus gastrulation. Proc Natl Acad Sci 106: 21683–21688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits NC, Kurup S, Rops AL, Ten Dam GB, Massuger LF, Hafmans T, Turnbull JE, Spillmann D, Li JP, Kennel SJ, et al. 2010. The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. J Biol Chem 285: 41143–41151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Goldstaub D, Svahn CM, Vlodavsky I, Levi B-Z, Neufeld G 1994. Variations in the size and sulfation of heparin modulate the effect of heparin on the binding of VEGF165 to its receptors. Biochem Biophys Res Commun 203: 1339–1347 [DOI] [PubMed] [Google Scholar]
- Song HH, Shi W, Xiang Y-Y, Filmus J 2005. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem 280: 2116–2125 [DOI] [PubMed] [Google Scholar]
- Spillmann D, Lookene A, Olivecrona G 2006. Isolation and characterization of low sulfated heparan sulfate sequences with affinity for lipoprotein lipase. J Biol Chem 281: 23405–23413 [DOI] [PubMed] [Google Scholar]
- Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum J.L, Esko JD 2009. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest 119: 3236–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Wang L, Castagnola J, Song D, Bishop JR, Brown JR, Lawrence R, Bai X, Habuchi H, Tanaka M, et al. 2010. Heparan sulfate 2-O-sulfotransferase is required for triglyceride-rich lipoprotein clearance. J Biol Chem 285: 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ 2003. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor–deficient embryos. Mol Cell Biol 23: 4371–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, Iglesia DDS, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, et al. 2002. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci 115: 4517–4531 [DOI] [PubMed] [Google Scholar]
- Stevenson JL, Varki A, Borsig L 2007. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb Res 120: S107–S111 [DOI] [PubMed] [Google Scholar]
- Stickens D, Zak BM, Rougier N, Esko JD, Werb Z 2005. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132: 5055–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ 2004. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J Clin Invest 114: 1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer SE, Gallagher JT 1997. Specific binding of the chemokine platelet factor 4 to heparan sulfate. J Biol Chem 272: 20508–20514 [DOI] [PubMed] [Google Scholar]
- Stringer SE, Forster MJ, Mulloy B, Bishop CR, Graham GJ, Gallagher JT 2002. Characterization of the binding site on heparan sulfate for macrophage inflammatory protein 1α. Blood 100: 1543–1550 [PubMed] [Google Scholar]
- Stringer SE, Nelson MS, Gupta P 2003. Identification of an MIP-1α-binding heparan sulfate oligosaccharide that supports long-term in vitro maintenance of human LTC-ICs. Blood 101: 2243–2245 [DOI] [PubMed] [Google Scholar]
- Sugahara K, Kitagawa H 2002. Heparin and heparan sulfate biosynthesis. IUBMB Life 54: 163–175 [DOI] [PubMed] [Google Scholar]
- Sugaya N, Habuchi H, Nagai N, Ashikari-Hada S, Kimata K 2008. 6-O-sulfation of heparan sulfate differentially regulates various fibroblast growth factor-dependent signalings in culture. J Biol Chem 283: 10366–10376 [DOI] [PubMed] [Google Scholar]
- Symes K, Smith EM, Mitsi M, Nugent MA 2010. Sweet cues: How heparan sulfate modification of fibronectin enables growth factor guided migration of embryonic cells. Cell Adh Migr 4: 507–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, Ikeda T, Sugihara K, Asano M, Yoshikawa T, et al. 2009. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun 383: 113–118 [DOI] [PubMed] [Google Scholar]
- Takeo S, Akiyama T, Firkus C, Aigaki T, Nakato H 2005. Expression of a secreted form of Dally, a Drosophila glypican, induces overgrowth phenotype by affecting action range of Hedgehog. Dev Biol 284: 204–218 [DOI] [PubMed] [Google Scholar]
- Tamsma JT, van den Born J, Bruijn JA, Assmann KJ, Weening JJ, Berden JH, Wieslander J, Schrama E, Hermans J, Veerkamp JH, et al. 1994. Expression of glomerular extracellular matrix components in human diabetic nephropathy: Decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 37: 313–320 [DOI] [PubMed] [Google Scholar]
- Taylor DR, Whitehouse IJ, Hooper NM 2009. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog 5: e1000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telci D, Wang Z, Li X, Verderio EAM, Humphries MJ, Baccarini M, Basaga H, Griffin M 2008. Fibronectin-tissue transglutaminase matrix rescues RGD-impaired cell adhesion through syndecan-4 and β1 integrin co-signaling. J Biol Chem 283: 20937–20947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The I, Bellaiche Y, Perrimon N 1999. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol Cell 4: 633–639 [DOI] [PubMed] [Google Scholar]
- Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB, Wang L, Duncan RL, Farach-Carson MC 2011. Perlecan/Hspg2 deficiency alters the pericellular space of the lacuno-canalicular system surrounding osteocytic processes in cortical bone. J Bone Mineral Res 26: 618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko E, Lutgens E, Stan RV, Simons M 2004. Fibroblast growth factor 2 endocytosis in endothelial cells proceed via syndecan-4-dependent activation of Rac1 and a Cdc42-dependent macropinocytic pathway. J Cell Sci 117: 3189–3199 [DOI] [PubMed] [Google Scholar]
- Tkachenko E, Rhodes JM, Simons M 2005. Syndecans: New kids on the signaling block. Circ Res 96: 488–500 [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Armores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L 2001. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell 1: 251–264 [DOI] [PubMed] [Google Scholar]
- Tran-Lundmark K, Tran PK, Paulsson-Berne G, Friden V, Soininen R, Tryggvason K, Wight TN, Kinsella MG, Boren J, et al. 2008. Heparan sulfate in perlecan promotes mouse atherosclerosis: Roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res 103: 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, et al. 1999. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature 400: 276–280 [DOI] [PubMed] [Google Scholar]
- Tveit H, Dick G, Skibeli V, Prydz K 2005. A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin–Darby canine kidney cells. J Biol Chem 280: 29596–29603 [DOI] [PubMed] [Google Scholar]
- Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T 2004. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet 13: 2089–2099 [DOI] [PubMed] [Google Scholar]
- van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, et al. 2006. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int 70: 2100–2108 [DOI] [PubMed] [Google Scholar]
- Vanpouille C, Deligny A, Delehedde M, Denys A, Melchior A, Lienard X, Lyon M, Mazurier J, Fernig DG, Allain F 2007. The heparin/heparan sulfate sequence that interacts with cyclophilin B contains a 3-O-sulfated N-unsubstituted glucosamine residue. J Biol Chem 282: 24416–24429 [DOI] [PubMed] [Google Scholar]
- Varki A, Etzler ME, Cummings RD, Esko JD 2009. Discovery and classification of glycan-binding proteins. In Essentials of glycobiology (ed. Varki A, et al. ), pp. 375–386 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Viviano BL, Silverstein L, Pflederer C, Paine-Saunders S, Mills K, Saunders S 2005. Altered hematopoiesis in glypican-3-deficient mice results in decreased osteoclast differentiation and a delay in endochondral ossification. Dev Biol 282: 152–162 [DOI] [PubMed] [Google Scholar]
- Vogl-Willis CA, Edwards IJ 2004. High glucose-induced alterations in subendothelial matrix perlecan leads to increased monocyte binding. Arterioscler Thromb Vasc Biol 24: 858–863 [DOI] [PubMed] [Google Scholar]
- Vreys V, David G 2007. Mammalian heparanase: What is the message? J Cell Mol Med 11: 427–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waern I, Jia J, Pejler G, Zcharia E, Vlodavsky I, Li JP, Wernersson S 2010. Accumulation of Ym1 and formation of intracellular crystalline bodies in alveolar macrophages lacking heparanase. Mol Immunol 47: 1467–1475 [DOI] [PubMed] [Google Scholar]
- Wang LC, Brown JR, Varki A, Esko JD 2002. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of l- and p-selectins. J Clin Invest 110: 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fuster M, Sriramarao P, Esko JD 2005. Endothelial heparan sulfate deficiency impairs l-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol 6: 902–910 [DOI] [PubMed] [Google Scholar]
- Watt FM, Fujiwara H 2011. Cell–extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol 3: a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S, Braga T, Sawesi O, Waern I, Nilsson KE, Pejler G, Abrink M 2009. Age-related enlargement of lymphoid tissue and altered leukocyte composition in serglycin-deficient mice. J Leuk Biol 85: 401–408 [DOI] [PubMed] [Google Scholar]
- West L, Govindraj P, Koob TJ, Hassell JR 2006. Changes in perlecan during chondrocyte differentiation in the fetal bovine rib growth plate. J Orthop Res 24: 1317–1326 [DOI] [PubMed] [Google Scholar]
- Westphal V, Murch S, Kim S, Srikrishna G, Winchester B, Day R, Freeze HH 2000. Reduced heparan sulfate accumulation in enterocytes contributes to protein-losing enteropathy in a congenital disorder of glycosylation. Am J Pathol 157: 1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnhoven TJ, Lensen JF, Rops AL, van der Vlag J, Kolset SO, Bangstad HJ, Pfeffer P, van den Hoven MJ, Berden JH, van den Heuvel LP, et al. 2006. Aberrant heparan sulfate profile in the human diabetic kidney offers new clues for therapeutic glycomimetics. Am J Kidney Dis 48: 250–261 [DOI] [PubMed] [Google Scholar]
- Wijnhoven TJ, Lensen JF, Wismans RG, Lamrani M, Monnens LA, Wevers RA, Rops AL, van der Vlag J, Berden JH, van den Heuvel LP, et al. 2007a. In vivo degradation of heparan sulfates in the glomerular basement membrane does not result in proteinuria. J Am Soc Nephrol 18: 823–832 [DOI] [PubMed] [Google Scholar]
- Wijnhoven TJ, Lensen JF, Wismans RG, Lefeber DJ, Rops AL, van der Vlag J, Berden JH, van den Heuvel LP, van Kuppevelt TH 2007b. Removal of heparan sulfate from the glomerular basement membrane blocks protein passage. J Am Soc Nephrol 18: 3119–3127 [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman SA, Denhez F, Goetinck PF 2002. Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem 277: 32970–32977 [DOI] [PubMed] [Google Scholar]
- Williams KJ, Fuki IV 1997. Cell-surface heparan sulfate proteoglycans: Dynamic molecules mediating ligand catabolism. Curr Opin Lipidol 8: 253–262 [DOI] [PubMed] [Google Scholar]
- Wittrup A, Zhang SH, Svensson KJ, Kucharzewska P, Johansson MC, Morgelin M, Belting M 2010. Magnetic nanoparticle-based isolation of endocytic vesicles reveals a role of the heat shock protein GRP75 in macromolecular delivery. Proc Natl Acad Sci 107: 13342–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Longley RL, Tumova S, Couchman JR 2000. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys 374: 66–72 [DOI] [PubMed] [Google Scholar]
- Woulfe DS, Lilliendahl JK, August S, Rauova L, Kowalska MA, Abrink M, Pejler G, White JG, Schick BP 2008. Serglycin proteoglycan deletion induces defects in platelet aggregation and thrombus formation in mice. Blood 111: 3458–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Fuster MM, Lawrence R, Esko JD 2010a. Heparan sulfate regulates VEGF165 And VEGF121-mediated vascular hyperpermeability. J Biol Chem 286: 734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Ichikawa N, Kosaki K, Yamada Y, Sasaki T, Sakai LY, Kurosawa H, Hattori N, Arikawa-Hirasawa E 2010b. Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol 29: 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O'Sullivan C, Delavan-Boorsma K, Mok M, Bronstein A, Carpenter MK 2005a. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 23: 315–323 [DOI] [PubMed] [Google Scholar]
- Xu J, Park PW, Kheradmand F, Corry DB 2005b. Endogenous attenuation of allergic lung inflammation by syndecan-1. J Immunol 174: 5758–5765 [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA 2005c. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2: 185–190 [DOI] [PubMed] [Google Scholar]
- Yamada S, Morimoto H, Fujisawa T, Sugahara K 2007. Glycosaminoglycans in Hydra magnipapillata (Hydrozoa, Cnidaria): Demonstration of chondroitin in the developing nematocyst, sting organelle, and structural characterization of glycosaminoglycans. Glycobiology 17: 886–894 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Inatani M, Matsumoto Y, Ogawa J, Irie F 2010. Roles of heparan sulfate in mammalian brain development current views based on the findings from Ext1 conditional knockout studies. Prog Mol Biol Transl Sci 93: 133–152 [DOI] [PubMed] [Google Scholar]
- Yan D, Lin X 2009. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol 1: a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM 1991. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64: 841–848 [DOI] [PubMed] [Google Scholar]
- Ye S, Luo YD, Lu WQ, Jones RB, Linhardt RJ, Capila I, Toida T, Kan M, Pelletier H, McKeehan WL 2001. Structural basis for interaction of FGF-1, FGF-2, and FGF-7 with different heparan sulfate motifs. Biochemistry 40: 14429–14439 [DOI] [PubMed] [Google Scholar]
- Zak SM, Schuksz M, Koyama E, Mundy C Wells DE, Yamaguchi Y, Pacifici M, Esko JD 2011. Compound heterozygous loss of Ext1 and Ext2 is sufficient for formation of multiple exostoses in mouse ribs and long bones. Bone 48: 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, Metzger S, Sarid R, Naggi A, Casu B, Ilan N, et al. 2005. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. FASEB J 19: 211–221 [DOI] [PubMed] [Google Scholar]
- Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP 2009. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS ONE 4: e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernichow L, Abrink M, Hallgren J, Grujic M, Pejler G, Kolset SO 2006. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-α secretion in response to lipopolysaccharide. J Biol Chem 281: 26792–26801 [DOI] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S 2004. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem 279: 33220–33227 [DOI] [PubMed] [Google Scholar]
- Zhang L, Lawrence R, Frazier BA, Esko JD 2006. CHO glycosylation mutants: Proteoglycans. Methods Enzymol 416: 205–221 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N'Kuli F, Courtoy PJ, David G 2005. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell 9: 377–388 [DOI] [PubMed] [Google Scholar]
- Zoeller JJ, Whitelock JM, Iozzo RV 2009. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol 28: 284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi RI, Ge XN, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM, Frenzel EM, Fuster MM, Esko JD, Rao SP, et al. 2009. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol 183: 3971–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]