Abstract
Fibronectin (FN) is a multidomain protein with the ability to bind simultaneously to cell surface receptors, collagen, proteoglycans, and other FN molecules. Many of these domains and interactions are also involved in the assembly of FN dimers into a multimeric fibrillar matrix. When, where, and how FN binds to its various partners must be controlled and coordinated during fibrillogenesis. Steps in the process of FN fibrillogenesis including FN self-association, receptor activities, and intracellular pathways have been under intense investigation for years. In this review, the domain organization of FN including the extra domains and variable region that are controlled by alternative splicing are described. We discuss how FN–FN and cell–FN interactions play essential roles in the initiation and progression of matrix assembly using complementary results from cell culture and embryonic model systems that have enhanced our understanding of this process.
Formation of fibrillar fibronectin matrices involves interactions with cell surface integrins that induce fibronectin conformational changes required for self-association. Cell culture and embryonic model systems have provided key insights into the mechanisms that initiate and regulate the fibronectin assembly process.
As a ubiquitous component of the extracellular matrix (ECM), fibronectin (FN) provides essential connections to cells through integrins and other receptors and regulates cell adhesion, migration, and differentiation. FN is secreted as a large dimeric glycoprotein with subunits that range in size from 230 kDa to 270 kDa (Mosher 1989; Hynes 1990). Variation in subunit size depends primarily on alternative splicing. FN was first isolated from blood more than 60 years ago (Edsall 1978), and this form is called plasma FN. The other major form, called cellular FN, is abundant in the fibrillar matrices of most tissues. Although FN is probably best known for promoting attachment of cells to surfaces, this multidomain protein has many interesting structural features and functional roles beyond cell adhesion.
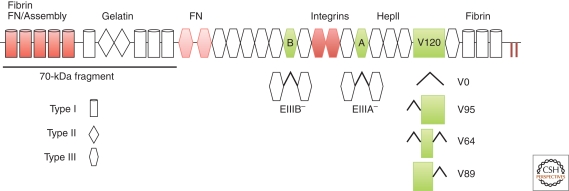
FN is composed of three different types of modules termed type I, II, and III repeats (Fig. 1) (Petersen et al. 1983; Hynes 1990). These repeats have distinct structures. Although the conformations of type I and type II repeats are maintained by pairs of intramodule disulfide bonds, the type III repeat is a 7-stranded β-barrel structure that lacks disulfide bonds (Main et al. 1992; Leahy et al. 1996, 1992) and, therefore, can undergo conformational changes. FN type III repeats are widely distributed among animal, bacterial, and plant proteins and are found in both extracellular and intracellular proteins (Bork and Doolittle 1992; Tsyguelnaia and Doolittle 1998).
Figure 1.
FN domain organization and isoforms. Each FN monomer has a modular structure consisting of 12 type I repeats (cylinders), 2 type II repeats (diamonds), and 15 constitutive type III repeats (hexagons). Two additional type III repeats (EIIIA and EIIIB, green) are included or omitted by alternative splicing. The third region of alternative splicing, the V region (green box), is included (V120), excluded (V0), or partially included (V95, V64, V89). Sets of modules comprise domains for binding to other extracellular molecules as indicated. Domains required for fibrillogenesis are in red: the assembly domain (repeats I1-5) binds FN, III9-10 contains the RGD and synergy sequences for integrin binding, and the carboxy-terminal cysteines form the disulfide-bonded FN dimer (‖). The III1-2 domain (light red) has two FN binding sites that are important for fibrillogenesis. The amino-terminal 70-kDa fragment contains assembly and gelatin-binding domains and is routinely used in FN binding and matrix assembly studies.
Sets of adjacent modules form binding domains for a variety of proteins and carbohydrates (Fig. 1). ECM proteins, including FN, bind to cells via integrin receptors, αβ heterodimers with two transmembrane subunits (Hynes 2002). FN-binding integrins have specificity for one of the two cell-binding sites within FN, either the RGD-dependent cell-binding domain in III10 (Pierschbacher and Ruoslahti 1984) or the CS1 segment of the alternatively spliced V region (IIICS) (Wayner et al. 1989; Guan and Hynes 1990). Some integrins require a synergy sequence in repeat III9 for maximal interactions with FN (Aota et al. 1994; Bowditch et al. 1994). Another family of cell surface receptors is the syndecans, single-chain transmembrane proteoglycans (Couchman 2010). Syndecans use their glycosaminoglycan (GAG) chains to interact with FN at its carboxy-terminal heparin-binding (HepII) domain (Fig. 1) (Saunders and Bernfield 1988; Woods et al. 2000), which binds to heparin, heparan sulfate, and chondroitin sulfate GAGs (Hynes 1990; Barkalow and Schwarzbauer 1994). Syndecan binding to the HepII domain enhances integrin-mediated cell spreading and intracellular signaling, suggesting that syndecans act as coreceptors with integrins in cell–FN binding (Woods and Couchman 1998; Morgan et al. 2007).
A major site for FN self-association is within the amino-terminal assembly domain spanning the first five type I repeats (I1-5) (Fig. 1) (McKeown-Longo and Mosher 1985; McDonald et al. 1987; Schwarzbauer 1991b; Sottile et al. 1991). This domain plays an essential role in FN fibrillogenesis. As a major blood protein, FN interacts with fibrin during blood coagulation, also using the I1-5 domain (Mosher 1989; Hynes 1990). As fibrin polymerizes, factor XIII transglutaminase covalently cross-links glutamine residues near the amino terminus of FN to fibrin α chains (Mosher 1975; Corbett et al. 1997). The amino-terminal domain has multiple binding partners in addition to FN and fibrin; these include heparin, S. aureus, and other bacteria, thrombospondin-1, and tenascin-C (Hynes 1990; Ingham et al. 2004; Schwarz-Linek et al. 2006). Adjacent to this domain is the gelatin/collagen-binding domain composed of type I and type II modules (Ingham et al. 1988). This domain also binds to tissue transglutaminase (Radek et al. 1993) and fibrillin-1 (Sabatier et al. 2009). Within the 15 type III repeats reside several FN binding sites that interact with the amino-terminal assembly domain as well as three sites of alternative splicing that generate multiple isoforms. At the carboxyl terminus is a pair of cysteine residues that form the FN dimer through antiparallel disulfide bonds (Hynes 1990). This dimerization may be facilitated by disulfide isomerase activity located in the last set of type I repeats (Langenbach and Sottile 1999).
The diverse set of binding domains provides FN with the ability to interact simultaneously with other FN molecules, other ECM components (e.g., collagens and proteoglycans), cell surface receptors, and extracellular enzymes (Pankov and Yamada 2002; Fogelgren et al. 2005; Hynes 2009; Singh et al. 2010). Multitasking by FN probably underlies its essential role during embryogenesis (George et al. 1993). Furthermore, FN's interactions can be modulated by exposure or sequestration of its binding sites within matrix fibrils, through the presence of ECM proteins that bind to FN, or through variation in structure by alternative splicing.
DERIVATION AND FUNCTIONS OF ALTERNATIVELY SPLICED FN ISOFORMS
The primary gene transcript of FN is alternatively spliced to generate multiple mRNAs, each encoding a distinct FN subunit. Three sites of alternative splicing lie amongst the type III repeats: extra type III domains EIIIB/ED-B (between III7 and III8) and EIIIA/ED-A (between III11 and III12), and the variable (V) region/IIICS (between III14 and III15) (Hynes 1990; Schwarzbauer 1991a). EIIIA and EIIIB are single type III modules coded for by single exons that are either included or skipped during splicing (Fig. 1). Neither EIIIA nor EIIIB are included in plasma FN, whereas cellular FN monomers can have neither, one, or both of these extra domains. EIIIA and EIIIB modules are highly conserved having identical sequences in virtually all mammals, from humans to the giant panda (JE Schwarzbauer, unpubl.). This conservation contrasts with other type III repeats; for example, the RGD-containing III10 module is identical among primates but diverges in other mammals.
Splicing at the V region occurs by subdivision of a large exon that encodes the V region plus the first half of the type III15 module (Schwarzbauer et al. 1983). Alternative 3′ splice sites are used to generate mRNAs encoding three splice variants: V0 which lacks the entire V region and V95 or V120 which include 95 or 120 amino acids of the V region, respectively. V0, V95, and V120 are common in mammals but humans have two additional splice variants (V89 and V64) arising from usage of a unique 5′ splice site located 267 bases into the exon (Fig. 1) (Mosher 1989; Hynes 1990). Chickens and frogs differ from mammals and have only two splice variants at the V region (Norton 1987; DeSimone et al. 1992). In addition, dog cartilage contains a novel splice variant that lacks the V region, III15 and I10 (MacLeod et al. 1996). Most plasma FN molecules are heterodimers composed of one V0 and one V+ subunit (Hynes 1990). Cellular FN, on the other hand, has very few V0 subunits; most subunits are V+, containing part or all of the V region.
The exciting finding of FN alternative splicing raised the idea that each of these regions could carry out a unique function. All three sites are positioned to have an effect on cell adhesion. EIIIA and EIIIB flank the RGD/synergy integrin-binding region, whereas EIIIA and the V region reside on either side of the HepII domain (Fig. 1). In fact, the V region has a direct role in cell adhesion by binding to α4 integrins through a site in the first 25 amino acids (called CS1) (Wayner et al. 1989; Guan and Hynes 1990; Nojima et al. 1990) and through a secondary CS5 site located 65 residues carboxy-terminal to CS1 (Mould et al. 1991). The V region also has effects on the activities of the HepII domain in FN fibrillogenesis (Santas et al. 2002), and it has heparin-binding activity (Mostafavi-Pour et al. 2001) which may allow the V region to modulate syndecan binding to FN. EIIIA also appears to participate in cell adhesion because it binds to α4 and α9 integrins (Liao et al. 2002) and its presence in dimeric FN enhances HT1080 cell attachment (Manabe et al. 1997). Cell binding to EIIIB has not been reported.
The V region has functions other than adhesion. The V region controls FN dimer secretion such that any V0-V0 dimers that form are retained in the ER and degraded intracellularly (Schwarzbauer et al. 1989). V0-V+ FN dimers are more efficiently incorporated into fibrin clots than are dimers with two V regions (Wilson and Schwarzbauer 1992), which may explain, at least partly, the unique subunit composition of plasma FN. V+ FN is widely expressed and deposited into the ECM in essentially all tissues (ffrench-Constant and Hynes 1989; Oyama et al. 1989). Interestingly, however, V0-containing dimers are commonly found in tissues because a significant fraction of the FN in tissues is derived from plasma (Moretti et al. 2007). Some tissue-specific and disease-associated changes in V region variants have been reported (Oyama et al. 1993; Kumazaki et al. 1999; Schofield and Humphries 1999; Trefzer et al. 2006). Monoclonal antibodies have been generated that show differential reactivity with FN in tumors and developing tissues compared to FN in normal adult tissues. Two of these so-called “oncofetal” epitopes are affected by alternative splicing. One site is recognized only when a certain Thr residue in the V region is O-glycosylated (Matsuura et al. 1988). The other site is a cryptic region that becomes exposed with inclusion of EIIIB (Ventura et al. 2010).
In contrast to the prevalence of V+ FN in most tissues, EIIIA and EIIIB levels are low in adult tissues (Oyama et al. 1989; Pagani et al. 1991), but are up-regulated during development (ffrench-Constant and Hynes 1989; Pagani et al. 1991; Peters et al. 2002), with injury (ffrench-Constant et al. 1989; Kilian et al. 2008), in tumors (Schwarzbauer et al. 1987; Koukoulis et al. 1993; Castellani et al. 1994; Kaczmarek et al. 1994; Astrof and Hynes 2009), and in other diseases (Van Vliet et al. 2001; Kilian et al. 2008). Taken together, the changes in the inclusion of EIIIA and EIIIB suggest that these modules have activities that affect tissue organization and cell–ECM interactions such as might occur in tissue remodeling.
Specific functional roles for EIIIA and EIIIB have not been clearly defined from cell culture experiments. Therefore, generation of null mutations in mice provides a critical test of their requirements. Mice lacking either EIIIA or EIIIB are viable and fertile showing that each individual domain is dispensable for embryogenesis (Fukuda et al. 2002; Muro et al. 2003; Tan et al. 2004). Furthermore, neither is required for neovascularization in mice (Astrof et al. 2004). EIIIA-null mice are reported to have defects in wound repair (Muro et al. 2003) and are less prone to develop atherosclerotic plaques (Tan et al. 2004). Proliferation and FN matrix assembly by cells cultured from EIIIB-null mice were reduced (Fukuda et al. 2002). In contrast to the relatively mild effects in single null mice, double knock out of both EIIIA and EIIIB resulted in embryonic lethality with multiple vascular defects, some of which were specific to the genetic background of the mouse strain (Astrof et al. 2007). Although the defects in mice lacking both EIIIA and EIIIB are severe, mechanistic insights into specific functions for each splice variant in vivo remain to be uncovered.
Alternative splicing appears to contribute to protein solubility and stability. Plasma FN tends to be more soluble than cellular FN at physiological pH (Hynes 1990) and also contains many more V0-V+ dimers suggesting that the presence or absence of the V region may affect partitioning of FN between tissue matrices and body fluids. Inclusion of EIIIB makes FN more sensitive to proteolysis (Zardi et al. 1987) suggesting a role in protein stability that might allow more rapid turnover of FN at sites where EIIIB+ FN levels are high, such as during tissue remodeling where production of extracellular proteases is up-regulated. Thus, circumstantial evidence suggests that, along with any specific functions, alternative splicing might have a general effect on the properties of FN dimers and fibrils by modulating protein solubility and sensitivity to proteolysis.
FN FIBRILLOGENESIS: THE STEP-WISE CONVERSION OF FN DIMERS TO FIBRILS
Multimeric FN fibrils are prevalent in most vertebrate tissues and are the major functional form of FN (Fig. 2). Assembly of FN fibrils is a cell-mediated process that depends on binding of FN dimers to integrins (Ali and Hynes 1977; McDonald et al. 1987; Wu et al. 1993). These receptors promote FN–FN interactions outside the cell and concomitantly link to the actin cytoskeleton via their cytoplasmic domains. Thus, they coordinate FN fibril architecture with cell shape and intracellular signaling (Wierzbicka-Patynowski and Schwarzbauer 2003; Singh et al. 2010). We have a reasonable understanding of the steps involved in initiation of fibril assembly, but detailed information about the interactions and mechanisms as well as the events that promote fibril maturation into detergent-insoluble matrix remains to be elucidated.
Figure 2.
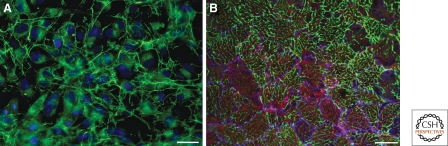
FN matrices in culture and in vivo. (A) Indirect immunofluorescence image shows a typical FN matrix assembled by cells in monolayer culture. Dexamethasone-treated HT1080 fibrosarcoma cells (Brenner et al. 2000) were fixed and permeabilized, and FN was visualized by staining with anti-FN antibodies and fluorescein-tagged secondary antibodies. Fibrils (green) extend around and between the cells whose nuclei are visualized by staining DNA with DAPI (blue). Intracellular staining adjacent to the nuclei is most likely FN that is trafficking through the secretory pathway. The organization of fibrils assembled by fibroblast-like cells (A) differs from the fibril arrangements formed by blastocoel roof cells (B) which grow in a multilayered sheet. (B) Indirect immunofluorescence image of the blastocoel roof from a mid-late gastrula stage Xenopus laevis embryo shows native assembly state of the FN matrix at this stage. This is an en face view of the innermost layer of cells in the multicell layered blastocoel roof. These cells face the fluid-filled blastocoel and assemble FN fibrils on their free surfaces. FN fibrils (green) extend across surfaces of cells outlined by C-cadherin staining (blue). Cortical assembly of actin (red) is required for the formation of FN fibrils from pericellular FN that initially assembles at cell–cell boundaries. Note the apparent incomplete progression, or delay, of FN fibril assembly by some cells in the layer. These are cells that have entered the layer as gastrulation proceeds and as a consequence of radial intercalation. Once resident in this layer these cells begin to assemble matrix on their newly “free” surfaces. (Scale bars, 25 µm.)
The basic model for FN assembly that has emerged over the past 20-plus years depends on processes that promote cell binding, FN–FN interactions, and conversion into stable, deoxycholate (DOC) detergent-insoluble matrix fibrils (McKeown-Longo and Mosher 1983; Mao and Schwarzbauer 2005; Singh et al. 2010). FN is secreted as a soluble, covalent dimer (Mosher 1989; Hynes 1990), and the dimer structure is essential for fibrillogenesis (Schwarzbauer 1991b). The dimer has a compact conformation that depends, in part, on long-range interactions between type III modules III2-3 and III12-14 (Johnson et al. 1999). This conformation prevents fibril formation in solution; fibrillogenesis is dependent on FN binding to cells (McDonald 1988). Integrin α5β1 is the primary receptor for binding to soluble FN (Huveneers et al. 2008) and recognizes the RGD and synergy sites in the III9-10 modules (Pierschbacher and Ruoslahti 1984; Aota et al. 1994). FN binding to α5β1 induces receptor clustering which brings together bound FN dimers. Initially, the integrin-bound FN is in a compact conformation but as assembly progresses, it undergoes conformational changes to become more extended (Baneyx et al. 2001). The FN conformations and interactions within fibrils impart these structures with significant elasticity (Ohashi et al. 1999; Baneyx et al. 2002).
FN–FN Interactions during Fibrillogenesis
The amino-terminal assembly domain has an essential role in fibrillogenesis as shown by deletion, mutagenesis, and antibody-blocking studies (McDonald et al. 1987; Schwarzbauer 1991b; Sottile et al. 1991). This domain consists of repeats I1-5 within the amino-terminal 70-kDa fragment (Fig. 1) and binds to multiple sites within FN. Two major binding sites for the assembly domain are within the first two type III repeats, one site in native III2 and a second cryptic site that is exposed in denatured III1 (Aguirre et al. 1994; Hocking et al. 1994). The III1-2 domain has a unique structure compared to other pairs of type III modules in that it has an extra-long linker that may facilitate folding to regulate FN interactions (Leahy et al. 1996; Vakonakis et al. 2007). Results from a variety of approaches support the conclusion that conformational changes in the III1-2 domain are involved in FN binding to this region. Mechanical stretching of FN enhances binding of 70 kDa (Zhong et al. 1998). Changes in the fluorescence resonance energy transfer (FRET) signal from a CFP-III1-2-YFP FRET sensor protein were detected on binding of 70 kDa (Karuri et al. 2009). Complementary mutations that eliminate a salt-bridge interaction between III1 and III2 increase binding of 70 kDa (Karuri et al. 2009). A key role for III1-2 in fibril formation is shown by blockade of assembly by antibodies to III1-2 (Chernousov et al. 1987, 1991), and by deletion of III1-2 from recombinant FN (Sechler et al. 2001). Furthermore, anastellin, a FN fragment containing part of III1, binds to FN at multiple sites in III1-3 (Ohashi and Erickson 2005) and induces formation of fibrous FN aggregates that are DOC-insoluble (Morla et al. 1994). These results lead to the conclusion that III1-2 plays a critical role in FN assembly and may be essential for conversion of fibrils into a DOC-insoluble form (Morla and Ruoslahti 1992; Sechler et al. 2001).
The 70-kDa fragment binds to other sites in FN including the HepII domain and a cryptic site in III4-5 (Bultmann et al. 1998; Maqueda et al. 2007). Current evidence suggests that these interactions are not essential for fibrillogenesis, so what might be the roles of these sites? One possibility is that FN–FN interactions at multiple sites might be needed to shift the assembly reaction toward fibrillogenesis by reducing the rate of dissociation of interacting FN dimers. Another possibility is that fibril strength and stability may be enhanced by interactions along the length of the FN subunits, and this could reduce fibril breakage in response to the application of force (Engler et al. 2009). If fibrillogenesis works, in part, like a zipper, then a third possibility is that multiple interacting sites may act like zipper teeth with the alignment of FN molecules determined by the initial interaction. Finally, it is possible that the cryptic sites may become exposed only after FN is within a fibril which might position them to initiate fibril branching (Singh et al. 2010). All of these potential mechanisms for fibril formation and function remain to be tested.
FN Receptor Requirements and Intracellular Connections
FN binding to integrins promotes formation of adhesion complexes where integrin cytoplasmic tails make connections with the actin cytoskeleton (Dubash et al. 2009). The continuity of interactions from FN through integrins to actin stress fibers is critical for matrix assembly. Stress fibers, which are contractile actin-myosin filaments, generate tension at sites of contact between integrins and FN. α5β1 integrin engagement with the cytoskeleton allows these receptors to translocate away from these contact sites along actin filaments (Pankov et al. 2000) and, in the process, to pull on bound FN molecules and promote fibrillogenesis (Pankov et al. 2000; Ohashi et al. 2002). Treatments that stimulate cell contractility are known to induce conformational changes that expose FN binding sites (Zhong et al. 1998) and to enhance incorporation of FN into fibrils (Zhang et al. 1994). Although α5β1 integrin is primarily responsible for FN matrix assembly, other integrins can form FN fibrils but appropriate stimulation, such as with Mn2+ or with activating anti-integrin antibodies, is usually required in vitro. These other integrins include α4β1 (Sechler et al. 2000), αvβ1 (Zhang et al. 1993; Yang and Hynes 1996), αvβ3 (Wennerberg et al. 1996; Wu et al. 1996; Takahashi et al. 2007), and αIIbβ3 (Olorundare et al. 2001). Syndecan-1 and syndecan-2 also participate in FN assembly (Klass et al. 2000; Galante and Schwarzbauer 2007; Stepp et al. 2010).
Focal adhesion kinase (FAK) is activated by integrin binding to FN. Its activation is essential for matrix assembly in vitro, and matrix is significantly diminished in FAK-null embryos (Ilic et al. 2004). Many other signaling molecules are activated by integrin binding to FN, but inhibition or knockdown of these molecules, including Src, paxillin, PI-3-kinase, and protein kinase C, delays but does not eliminate fibrillogenesis (Somers and Mosher 1993; Wierzbicka-Patynowski and Schwarzbauer 2002; Wierzbicka-Patynowski et al. 2007). There appear to be parallel or overlapping sets of signaling pathways that can support matrix assembly and, if one pathway is blocked, other downstream molecules can be recruited to take their place.
One key molecule in regulating assembly is Rho GTPase. Activation of Rho GTPase and downstream Rho kinase stimulates cell contractility (Yoneda et al. 2005; Dubash et al. 2009) and plays a critical role in fibrillogenesis (Zhang et al. 1994; Zhong et al. 1998; Yoneda et al. 2007). Modulation of Rho activity affects the incorporation of FN into matrix fibrils and changes matrix reactivity with certain antibodies (Zhang et al. 1994; Zhong et al. 1998; Smith et al. 2007). Importantly, Rho action exposes binding sites required for FN–FN interactions (Zhong et al 1998). In fact, contractility promotes integrin engagement of FN's synergy site and thus increases α5β1–FN bond strength (Friedland et al. 2009); this effect on bond strength could explain why the synergy site is required for matrix assembly (Nagai et al. 1991; Sechler et al. 1997). Fibrils within a matrix are under significant tension and relax to as little as one-quarter of their original length when tension is removed, for example, when one end of a fibril is released from its attachment site (Ohashi et al. 1999). Thus, cell contractility and tension are needed to initiate fibrillogenesis and to maintain fibril architecture in established matrices.
Fibrillogenesis and Conversion to DOC Insolubility
Initiation of assembly depends on FN binding to integrins and subsequent conformational changes in bound FN. These events lead to interactions between FN dimers to form multimers and short fibrils that are soluble in DOC detergent (Choi and Hynes 1979; McKeown-Longo and Mosher 1983). FN dimers associate end-to-end (Dzamba and Peters 1991), and continued deposition then serves to lengthen and thicken the fibrils. With time, FN fibrils are irreversibly converted from DOC-soluble into DOC-insoluble matrix (Choi and Hynes 1979; McKeown-Longo and Mosher 1983). Fibril insolubility appears to depend on strong noncovalent, protein–protein interactions (Chen and Mosher 1996; Ohashi and Erickson 2009) and is likely to involve β-strand exchange interactions as have been observed in the formation of amyloid-like fibrils in a solution of partially denatured III9 modules (Litvinovich et al. 1998). Insoluble FN matrix may form through a dock-and-lock mechanism (Esler et al. 2000) in which FN dimers first associate reversibly with fibrils and then undergo conformational changes to become irreversibly incorporated into fibrils. Recombinant FN with III1-2 deleted (FNΔIII1-2) was severely deficient in conversion into DOC-insoluble material (Sechler et al. 2001) which suggests that this domain may participate in forming strong protein interactions. However, strong interactions involving other domains are possible because a recombinant FN that lacks a larger region (FNΔIII1-7) forms DOC-insoluble matrix at a more rapid rate than wild type FN (Sechler et al. 1996).
FN fibrillogenesis and formation of DOC-insoluble matrix influence the onset and progression of certain diseases. For example, mutations in FN have been linked to the hereditary kidney disease glomerulopathy with fibronectin deposits (GFND) (Castelletti et al. 2008) characterized by granular deposits of FN aggregates (Strøm et al. 1995). Two of the mutations associated with this disease are in FN repeat III13 and cause a reduction in heparin-binding activity. These mutations may also affect syndecan binding to the HepII domain. Because syndecan-2 is required for FN matrix assembly (Klass et al. 2000; Galante and Schwarzbauer 2007), this disease may progress through loss of syndecan–FN interactions resulting in disorganized fibrils. Clearly, much more research is needed about how FN matrix becomes insoluble and how dysregulation of this step contributes to diseases and scarring.
FN FIBRILLOGENESIS: DEVELOPMENTAL MECHANISMS AND CONSEQUENCES OF ASSEMBLY
FN was a primary early focus of analyses of ECM functions in embryogenesis and it has been implicated in many fundamental developmental processes (Dzamba et al. 2001; Astrof and Hynes 2009; Rozario and DeSimone 2010). Unlike many other ECM proteins including the collagens and laminins, FN appears to be a chordate innovation. FN-like genes have yet to be identified for any protostome (Hynes and Zhao 2000; Rubin et al. 2000) or nonchordate deuterostome (Whittaker et al. 2006; Tucker and Chiquet-Ehrismann 2009). Thus, it has been suggested that FN and other proteins such as von Willebrand factor and the tenascins may have first arisen along with the blood vasculature and/or neural crest of the vertebrates (Hynes and Zhao 2000; Whittaker et al. 2006). Whether or not the origins of these tissues are linked to the emergence of the FN protein, it is clear that FN is an indispensable component of vertebrate ECMs involved in a wide range of developmental events.
FN is expressed early in vertebrate embryogenesis, and initial studies of both avian (Critchley et al. 1979; Duband and Thiery 1982; Sanders 1982) and amphibian embryos (Boucaut and Darribere 1983a,b; Lee et al. 1984) revealed that FN is assembled prior to the initiation of gastrulation movements. During gastrulation, an elaborate network of FN is detected predominantly at the basal surfaces of the endoderm and ectoderm in the chick (Krotoski et al. 1986) and along the blastocoel roof in amphibians (Lee et al. 1984) consistent with the role of the FN matrix as a migratory substrate for the mesoderm. The importance of FN to mesoderm migration was subsequently confirmed using blocking antibodies and RGD-containing peptides in both systems (Boucaut et al. 1984a,b; Brown and Sanders 1991; Winklbauer et al. 1996). FN and other ECM proteins including laminins and tenascin have also been suggested to provide guidance cues for many other cells and tissues including primordial germ cells in frogs (Heasman et al. 1981) and mice (ffrench-Constant et al. 1991), and avian neural crest (Duband and Thiery 1982; Rovasio et al. 1983; Boucaut et al. 1984). Targeted disruption of the FN gene in mice is embryonic lethal, and these embryos display severe defects in mesoderm development affecting notochord and somite formation, and normal development of the heart and vasculature (George et al. 1993; Georges-Labouesse et al. 1996). Morpholino knockdowns of FN in zebrafish are also characterized by failures of mesoderm migration and somite boundary formation (Jülich et al. 2005; Latimer and Jessen 2010). In the zebrafish natter mutant, which lacks one of the two FN genes expressed in this species, defects in heart tube formation are observed and attributable to failures in organization of the myocardial epithelium but not myocardial cell migration per se, even though initiation of cell movement does appear to require deposition of FN specifically at the midline (Trinh and Stainier 2004). Clearly, these and other studies of FN and its many roles in embryonic development point to the importance not only of proper regulation of FN expression but also the localized assembly of FN-containing ECM at the right “times and places” to promote normal morphogenesis and differentiation.
Importance of FN Fibrillogenesis and Assembly States
How ECM is assembled at cell and tissue surfaces has become an increasingly important question in large part because of a growing realization that the three-dimensional (3D) organization of the ECM can have distinct instructive properties in terms of cellular responses. FN, along with other ECM glycoproteins, collagens, and proteoglycans, are assembled into complex 3D microenvironments that provide structural support to cells and tissues, and restrict the diffusion of growth factors and other soluble signaling molecules to direct or influence cell behavior, proliferation and growth, gene expression, and cell fate specification (Rozario and DeSimone 2010). The physical and mechanical properties of the ECM and corresponding mechanisms of rigidity-sensing used by cells in contact with it also play important roles in these processes (McBeath et al. 2004; Paszek et al. 2005; Engler et al. 2006). During embryogenesis, matrix assembly is initiated and assembled matrices are subsequently remodeled as development proceeds. This raises the interesting possibility that progressive changes in assembly state can serve to regulate cell behaviors, possibly by acting as a developmental “checkpoint” for subsequent morphogenetic and/or cell-fate decisions.
Regulated FN deposition is essential for cleft formation during epithelial branching morphogenesis with both localized synthesis and transient assembly of FN observed at sites of forming clefts (Sakai et al. 2003). Inhibition of FN assembly or knock-down of FN synthesis blocks cleft formation and branching, whereas the addition of exogenous FN promotes these processes. Interestingly, the assembly of FN at sites of cleft formation was associated with a reduction in cadherin-dependent cell–cell adhesions suggesting a possible mechanistic link or adhesive “crosstalk” critical for branching morphogenesis to proceed (Sakai et al. 2003). Onodera et al. (2010) recently identified the Btbd7 gene as a key regulator of branching in this system. Btbd7 is expressed by cells in the emerging clefts in response to local FN accumulation. Btbd7 induces Snail2 and suppresses E-cadherin expression thereby promoting morphogenetic changes required for cleft formation.
Methods used to block or disrupt the normal deposition and assembly of FN fibrils in embryos have included injection of RGD peptides, antibodies directed against β1 integrins (Darribere et al. 1990) or FN (Marsden and DeSimone 2001), and the expression of integrin β1 dominant-negative constructs (Marsden and DeSimone 2003). However, these manipulations effectively block initial FN binding to cell surfaces. Thus, any contributions of intermediate states of assembly to a given developmental process are not readily resolvable using such reagents. Darribere and Schwarzbauer (2000) injected recombinant forms of FN into amphibian blastula-stage embryos that coassembled with endogenous FN and formed disorganized chimeric matrices. Gastrulation movements were perturbed in these embryos highlighting the importance of ECM architecture in directing normal cell behaviors. Nakatsuji and Johnson (Nakatsuji et al. 1982; Nakatsuji and Johnson 1984) have proposed that the physical orientation of assembled FN fibrils along the blastocoel roof is critical for directed cell migration of the mesendoderm; however, it remains unclear whether contact guidance is an intrinsic property of the fibrillar network laid down in vivo. Nevertheless, there is ample evidence that FN fibrils are critical for normal mesendodermal protrusive behaviors (Winklbauer and Keller 1996; Winklbauer et al. 1996; Nagel and Winklbauer 1999) possibly through a chemotactic mechanism involving the sequestration of PDGF by assembled fibrils (Nagel et al. 2004). Although artificial substrates conditioned with the fibrillar matrix of the blastocoel roof clearly support the directional migration of mesendodermal cells and explants in vitro (Nakatsuji and Johnson 1983, 1984; Shi et al. 1987; Winklbauer et al. 1992), mesendoderm will still move directionally toward the animal pole in the presence of nonfibrillar FN if the normal circular geometry of the tissue is maintained (Davidson et al. 2002; Rozario et al. 2009).
More recently, a 70-kDa amino-terminal fragment of FN known to block FN fibril assembly (Fig. 1) (McKeown-Longo and Mosher 1985; McDonald et al. 1987) was used to prevent the nascent pericellular FN matrix that first accumulates along the blastocoel roofs of blastula and early gastrula-stage Xenopus embryos (Fig. 2) from forming fibrils (Rozario et al. 2009). FN fibrillogenesis normally proceeds in this system coincident in space and time (Davidson et al. 2004, 2008) with the three major morphogenetic movements that drive gastrulation (radial intercalation and epiboly, mediolateral intercalation and convergent extension, and mesendoderm migration), each of which has been shown to require FN (Winklbauer and Keller 1996; Marsden and DeSimone 2001, 2003; Davidson et al. 2002, 2006). Inhibition of FN fibril assembly but not FN binding to cell surfaces has no apparent effect on axis elongation indicating that convergent extension is largely unaffected in the absence of FN fibrils. In contrast, radial intercalation of blastocoel roof cells is perturbed when FN fibrillogenesis is blocked, resulting in a failure of epibolic spreading and thinning of the blastocoel roof (Rozario et al 2009). Randomization of the normally parallel alignment of mitotic spindles in dividing roof cells also occurs in the absence of FN fibrils and this likely contributes to the failure of these cells to intercalate properly; polarized cell division was shown previously to be required for normal morphogenetic movements in Xenopus gastrulae (Marsden and DeSimone 2001) and in other systems (Wei and Mikawa 2000; Gong et al. 2004). Finally, the velocity of mesendoderm cell migration along the blastocoel roof is increased in embryos lacking fibrils (Rozario et al 2009) suggesting that the fibrillar FN substrate normally slows the migration of these cells, possibly because of differences in adhesive strength (Palecek et al. 1997) or integrin clustering (Guo et al. 2002). In normal embryos, the migrating mesendoderm remodels and reduces the apparent density of fibrillar FNs assembled by the blastocoel-roof cells as they pass over it (Davidson et al. 2004).
Together, these observations provide compelling support for the hypothesis that at least some developmental events are regulated by the physical assembly state and 3D architecture of FN-rich ECMs. Thus, how FN assembly and remodeling are spatio-temporally controlled in vivo becomes critical to our understanding of morphogenetic mechanisms and possibly the regulation of gene expression, cell fate, and proliferation. It remains to be established to what extent cellular responses to progressive changes in FN assembly state in embryos reflect actual differences in FN conformation (e.g., exposure of cryptic binding sites) and density (e.g., haptotaxis, number of cell-binding sites), and/or the overall mechanical properties (e.g., stiffness, 3D organization) and composition of the assembled matrix (e.g., binding of other matrix molecules, growth factors and cytokines).
Unlike the situation with 2D or even 3D cell-culture models, FN matrix assembly in embryos frequently proceeds coincident with highly dynamic cell movements and tissue rearrangements. During primitive streak formation in the chick, dramatic displacements of FN and fibrillin are observed in register with epiblastic cell movements (Czirok et al. 2006; Zamir et al. 2006). Similar correlations between FN assembly, remodeling, and cell movements are also apparent in frog embryos undergoing convergence and extension movements (Davidson et al. 2008). These observations highlight the importance of considering not only the biomechanical context in which fibril assembly initiates but also the likely functional roles of a FN matrix that translocates along with cells and tissues during morphogenesis. These studies underscore our limited understanding of dynamic cell–ECM interactions particularly as they relate to forces generated by the cells and tissues that drive morphogenesis in vivo.
How Is FN Matrix Assembly Initiated in Embryos?
As we have discussed, much is now known about mechanisms of FN matrix assembly based on studies of mammalian cells in culture (Singh et al. 2010). A key feature of current models of this process involves the generation of cell tension through integrins on stiff substrates (Halliday and Tomasek 1995; Zhang et al. 1997; Zhong et al. 1998; Pankov et al. 2000) but how FN assembly initiates in vivo on the free surfaces of multicell-layered tissues is less clear. The initial assembly of FN in Xenopus embryos serves as a useful example of this problem. A pericellular nonfibrillar FN matrix first begins to assemble at the free surfaces of cells in the multilayered blastocoel roof that face the blastocoel cavity, which is a fluid-filled space rich in soluble FN (Lee et al. 1984). FN “puncta” assembled at cell surfaces give way to an elaborate fibrillar matrix that crosses cell boundaries, and becomes thicker over time (Fig. 2). Assembly and remodeling are surprisingly dynamic and complex with individual fibrils forming and breaking connections with one another, undergoing rapid recoil and displaying considerable movement relative to the cells on which they form (Davidson et al. 2008). Although the question of how FN assembly is initiated in this system has been of interest to developmental biologists for many years, no obvious differences in FN or integrin α5β1 expression have been reported that could account for the tight spatio-temporal regulation of assembly; all cells in the embryo express the integrin and FN is secreted into the blastocoel at concentrations sufficient to allow assembly well before assembly begins (Lee et al. 1984; Joos et al. 1995; Winklbauer 1998). In contrast, some of the first clues to emerge suggested that cell–cell adhesion and tissue tension may instead play a critical role in regulating FN assembly (Winklbauer 1998; Dzamba et al. 2009).
During the period in which FN assembly begins, blastocoel-roof cells change shape from round to polygonal (Dzamba et al. 2009), indicating that cell–cell adhesion and tension have increased (Lecuit and Lenne 2007). Moreover, experimentally applied mechanical deformations designed to increase or decrease tension in the blastocoel roof promoted or blocked FN fibril assembly, respectively (Dzamba et al. 2009). Overexpression of cadherins in blastocoel-roof cells also resulted in a precocious change in cell shape and early initiation of FN fibril assembly, whereas inhibition of cadherin adhesion blocked fibril formation (Dzamba et al. 2009). C-cadherin mediated adhesion is normally greater in the blastocoel roof than in other parts of the embryo at these stages (Reintsch and Hausen 2001) and, whereas changes in C-cadherin adhesion are known to occur during gastrulation, this does not appear to be the result of alterations in C-cadherin levels at the cell surface (Brieher and Gumbiner 1994). So, how might cadherin adhesion normally be regulated in cells of the blastocoel roof, and can regional changes in cell adhesion regulate FN matrix assembly, an integrin-dependent process?
To address these issues, Dzamba et al. (2009) began by investigating the role of the noncanonical Wnt/PCP (planar cell polarity) signaling pathway in FN matrix assembly. Inhibition of Wnt/PCP signaling with a dominant-negative Wnt11 construct prevented FN fibril assembly, which could in turn be rescued by expressing constitutively active forms of Rac or Rho. Surprisingly, reduced Wnt/PCP signaling had little effect on the ability of these cells to apply traction stresses to artificial FN substrates whereas average traction stresses were reduced on C-cadherin-FC substrates under these same conditions (Dzamba et al. 2009). This result is consistent, however, with previous studies in the zebrafish in which disruptions in Wnt/PCP signaling were correlated with reduced cadherin adhesion and tissue cohesivity (Ulrich et al. 2005). Based on these data, a model for FN fibril assembly in tissues was proposed in which cadherin-dependent changes in cell–cell adhesion result in a reorganization of the cortical actin cytoskeleton (Dzamba et al. 2009). A Rac- and Pak-dependent contractile event is then required for translocation of integrins with bound FN from sites of cell–cell contact where the pericellular matrix first forms. Thus, nascent cell–cell adherens-like junctions in this early embryonic tissue perform an analogous function to focal adhesions in vitro by generating the tension on integrins necessary to expose cryptic sites within FN that are required for self-association and fibril formation. It will be of considerable interest in the future to establish whether a similar mechanism(s) is widely used by other tissues in vivo to regulate FN matrix assembly.
Both FN (Marsden and DeSimone 2001; Davidson et al. 2006; Latimer and Jessen 2010) and the Wnt/PCP pathway (Heisenberg et al. 2000; Wallingford et al. 2000) are required for convergent extension movements in Xenopus and the zebrafish. FN alone is insufficient to rescue convergent extension defects in the absence of Wnt/PCP signals (Davidson et al. 2006). These observations suggest that one important function of the Wnt/PCP pathway in embryogenesis is the regulation of FN matrix assembly, which in turn is required for subsequent morphogenetic movements. However, whereas FN is clearly required for the protrusive activity that drives convergence and extension movements (Davidson et al. 2006), Wnt/PCP signals are essential for directed cell polarity. Other mechanisms have also been reported to contribute to matrix assembly in embryos. For example, syndecan-1 and -2 promote FN matrix assembly at gastrulation in Xenopus (Kramer and Yost 2002). A connection to syndecans may extend beyond just matrix assembly because it has also been reported that syndecan-4 regulates Wnt/PCP signaling and is required for normal convergence and extension movements. In this instance, FN is proposed to act in concert with syndecan-4 to activate Wnt/PCP signaling (Muñoz et al. 2006). Other cell-signaling pathways have been implicated in the regulation of matrix assembly in development. For example, FAK and Ena/Vasp are required for FN assembly at intersomitic boundaries in Xenopus (Kragtorp and Miller 2006). In addition, Jülich et al. (2009) reported that Eph/Ephrin signaling is required for integrin α5β1 clustering at emergent somite borders in the zebrafish resulting in localized assembly of the FN matrix. The requirement for fibronectin and integrin α5β1 in somitogenesis is conserved among multiple vertebrate species including frog (Kragtorp and Miller 2007) and mouse (Yang et al. 1993; Yang and Hynes 1996). The overall conclusion from these studies is that FN matrix assembly is a complex process that is tightly regulated in vivo and throughout embryogenesis. A key challenge remains the identification of mechanisms of cell adhesion, signaling, and force generation that combine to establish the proper spatio-temporal control of FN deposition.
CONCLUSIONS AND PERSPECTIVES
The integration of work from many labs has provided mechanistic insights into how soluble dimeric FN is converted into insoluble multimeric fibrils and a rudimentary understanding of the functions of multiple alternatively spliced FN isoforms. Matrix assembly requires FN, integrins, and molecules that connect integrins to the cytoskeleton. On the FN side of the plasma membrane, direct interactions with receptors combined with temporally and spatially regulated exposure of FN self-association sites orchestrate fibril formation. At the membrane, integrin clustering provides tethering sites to initiate fibrillogenesis. Inside the cell, integrin connections to the cytoskeleton align actin filaments with extracellular fibrils. Information is exchanged between inside and out through activation of specific signaling molecules (e.g., FAK and Rho), through integrin translocation, which applies tension and may induce essential conformational changes, and through crosstalk with cadherins at cell–cell contacts in intact tissues.
This sketch of the assembly process covers the major events but many mechanistic questions remain to be solved. For example, our understanding of how FN dimers are organized within a multimeric fibril is limited. Are there specific FN domains that interact to initiate fibrillogenesis or is initiation a stochastic process? Is the organization of molecules in a fibril dependent on a specific spatiotemporal process that links tension, integrin translocation, and FN conformational changes? Are FN molecules oriented within fibrils to present certain binding sites on the fibril surface? During fibril growth, are FN dimers added at the ends, laterally, or both? To address these and other questions will require new or enhanced microscopic and biochemical methods for dissecting multicomponent complexes in situ.
Innumerable studies using cultured cells to define and dissect specific steps in FN fibrillogenesis have laid the groundwork. However, stationary cells in culture may represent a specialized case of assembly because, in vivo, matrix assembly often occurs during periods of cell movement and morphogenesis. Moreover, studies in vivo and with 3D substrates in vitro show that the cellular microenvironment affects matrix assembly. A deeper understanding of the mechanisms of matrix assembly, the generation of tension, the potential roles for other ECM proteins, and fibril contributions to matrix stiffness and cell traction must take into account dynamic cellular behaviors and the biomechanics involved in promoting (or limiting) assembly.
ACKNOWLEDGMENTS
The authors acknowledge support from the NIH (CA44627 and GM59383 to J.E.S.; HD26402 and GM094793 to D.W.D.). We thank Cara Carraher and Bette Dzamba for critical reading of the manuscript. We also thank Bette Dzamba for contributing the immunofluorescence image of Xenopus blastocoel roof cells and Dan Slone for immunofluorescence of HT1080 cell FN matrix.
Footnotes
Editors: Richard O. Hynes and Kenneth M. Yamada
Additional Perspectives on Extracellular Matrix Biology available at www.cshperspectives.org
REFERENCES
- Aguirre KM, McCormick RJ, Schwarzbauer JE 1994. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem 269: 27863–27868 [PubMed] [Google Scholar]
- Ali IU, Hynes RO 1977. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta 471: 16–24 [DOI] [PubMed] [Google Scholar]
- Aota S, Nomizu M, Yamada K 1994. The short amino acid sequence pro-his-ser-arg-asn in human fibronectin enhances cell adhesive function. J Biol Chem 269: 24756–24761 [PubMed] [Google Scholar]
- Astrof S, Hynes RO 2009. Fibronectins in vascular morphogenesis. Angiogenesis 12: 165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Crowley D, Hynes RO 2007. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol 311: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Crowley D, George EL, Fukuda T, Sekiguchi K, Hanahan D, Hynes RO 2004. Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis. Mol Cell Biol 24: 8662–8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V 2001. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci 98: 14464–14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V 2002. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci 99: 5139–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkalow FJ, Schwarzbauer JE 1994. Interactions between fibronectin and chondroitin sulfate are modulated by molecular context. J Biol Chem 269: 3957–3962 [PubMed] [Google Scholar]
- Bork P, Doolittle RF 1992. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci 89: 8990–8994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T 1983a. Fibronectin in early amphibian embryos. Migrating mesodermal cells contact fibronectin established prior to gastrulation. Cell Tissue Res 234: 135–145 [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T 1983b. Presence of fibronectin during early embryogenesis in amphibian Pleurodeles waltlii. Cell Differ 12: 77–83 [DOI] [PubMed] [Google Scholar]
- Boucaut J-C, Darribere T, Poole TJ, Aoyama H, Yamada KM, Thiery JP 1984a. Biologically active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol 99: 1822–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut JC, Darribère T, Boulekbache H, Thiery JP 1984b. Prevention of gastrulation but not neurulation by antibodies to fibronectin in amphibian embryos. Nature 307: 364–367 [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribère T, Poole TJ, Aoyama H, Yamada KM, Thiery JP 1984c. Biologically active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol 99: 1822–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch RD, Hariharan M, Tominna EF, Smith JW, Yamada KM, Getzoff E, Ginsberg M 1994. Identification of a novel integrin binding site in fibronectin: Differential utilization by β3 integrins. J Biol Chem 269: 10856–10863 [PubMed] [Google Scholar]
- Brenner KA, Corbett SA, Schwarzbauer JE 2000. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene 19: 3156–3163 [DOI] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM 1994. Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J Cell Biol 126: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Sanders EJ 1991. Interactions between mesoderm cells and the extracellular matrix following gastrulation in the chick embryo. J Cell Sci 99: 431–441 [DOI] [PubMed] [Google Scholar]
- Bultmann H, Santas AJ, Pesciotta Peters DM 1998. Fibronectin fibrillogenesis involves the heparin II binding domain of fibronectin. J Biol Chem 273: 2601–2609 [DOI] [PubMed] [Google Scholar]
- Castelletti F, Donadelli R, Banteria F, Hildebrandt F, Zipfel PF, Bresin E, Otto E, Skerka C, Renieri A, Todeschini M, et al. 2008. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci 105: 2538–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, Zardi L 1994. The fibronectin isoform containing the ED-B oncofetal domain: A marker of angiogenesis. Int J Cancer 59: 612–618 [DOI] [PubMed] [Google Scholar]
- Chen H, Mosher DF 1996. Formation of sodium dodecyl sulfate–stable fibronectin multimers. J Biol Chem 271: 9084–9089 [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Fogerty FJ, Koteliansky VE, Mosher DF 1991. Role of the I-9 and III-1 modules of fibronectin in the formation of an extracellular fibronectin matrix. J Biol Chem 266: 10851–10858 [PubMed] [Google Scholar]
- Chernousov MA, Faerman AI, Frid MG, Printseva OY, Koteliansky VE 1987. Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly. FEBS Lett 217: 124–128 [DOI] [PubMed] [Google Scholar]
- Choi MG, Hynes RO 1979. Biosynthesis and processing of fibronectin in NIL.8 hamster cells. J Biol Chem 254: 12050–12055 [PubMed] [Google Scholar]
- Corbett SA, Lee L, Wilson CL, Schwarzbauer JE 1997. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem 272: 24999–5005 [DOI] [PubMed] [Google Scholar]
- Couchman JR 2010. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26: 89–114 [DOI] [PubMed] [Google Scholar]
- Critchley DR, England MA, Wakely J, Hynes RO 1979. Distribution of fibronectin in the ectoderm of gastrulating chick embryos. Nature 280: 498–500 [DOI] [PubMed] [Google Scholar]
- Czirok A, Zamir EA, Filla MB, Little CD, Rongish BJ 2006. Extracellular matrix macroassembly dynamics in early vertebrate embryos. Curr Top Dev Biol 73: 237–258 [DOI] [PubMed] [Google Scholar]
- Darribere T, Schwarzbauer JE 2000. Fibronectin matrix composition and organization can regulate cell migration during amphibian development. Mech Dev 92: 239–250 [DOI] [PubMed] [Google Scholar]
- Darribere T, Guida K, Larjava H, Johnson KE, Yamada KM, Thiery JP, Boucaut JC 1990. In vivo analysis of integrin b1 subunit function in fibronectin matrix assembly. J Cell Biol 110: 1813–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Keller R, DeSimone DW 2004. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn 231: 888–895 [DOI] [PubMed] [Google Scholar]
- Davidson L, Dzamba B, Keller R, Desimone D 2008. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn 237: 2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW 2002. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: Combined roles for integrin α5β1, fibronectin, and tissue geometry. Dev Biol 242: 109–129 [DOI] [PubMed] [Google Scholar]
- Davidson LA, Marsden M, Keller R, DeSimone DW 2006. Integrin α5β1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol 16: 833–844 [DOI] [PubMed] [Google Scholar]
- DeSimone DW, Norton PA, Hynes RO 1992. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev Biol 149: 357–369 [DOI] [PubMed] [Google Scholar]
- Duband JL, Thiery JP 1982. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Dev Biol 93: 308–323 [DOI] [PubMed] [Google Scholar]
- Dubash AD, Menold MM, Samson T, Boulter E, García-Mata R, Doughman R, Burridge K 2009. Chapter 1. Focal adhesions: New angles on an old structure. Int Rev Cell Mol Biol 277: 1–65 [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Peters DM 1991. Arrangement of cellular fibronectin in noncollagenous fibrils in human fibroblast cultures. J Cell Sci 100: 605–612 [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Bolton MA, DeSimone DW 2001. The integrin family of cell adhesion molecules. In Cell adhesion (ed. Beckerle MC), pp. 100–154 Oxford University Press, Oxford [Google Scholar]
- Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW 2009. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell 16: 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall JT 1978. Some early history of cold-insoluble globulin. Ann N Y Acad Sci 312: 1–10 [DOI] [PubMed] [Google Scholar]
- Engler AJ, Chan M, Boettiger D, Schwarzbauer JE 2009. A novel mode of cell detachment from fibrillar fibronectin matrix under shear. J Cell Sci 122: 1647–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689 [DOI] [PubMed] [Google Scholar]
- Esler WP, Stimson ER, Jennings JM, Vinters HV, Ghilardi JR, Lee JP, Mantyh PW, Maggio JE 2000. Alzheimer's disease amyloid propagation by a template-dependent dock-lock mechanism. Biochemistry 39: 6288–6295 [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Hynes RO 1989. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development 106: 375–388 [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Hollingsworth A, Heasman J, Wylie CC 1991. Response to fibronectin of mouse primordial germ cells before, during and after migration. Development 113: 1365–1373 [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Van De Water L, Dvorak HF, Hynes RO 1989. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 109: 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelgren B, Polgár N, Szauter KM, Ujfaludi Z, Laczkó R, Fong KS, Csiszar K 2005. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem 280: 24690–24697 [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D 2009. Mechanically activated integrin switch controls α5β1 function. Science 323: 642–644 [DOI] [PubMed] [Google Scholar]
- Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, Sato M, Kuriyama K, Yasui N, Sekiguchi K 2002. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res 62: 5603–5610 [PubMed] [Google Scholar]
- Galante LL, Schwarzbauer JE 2007. Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. J Cell Biol 179: 999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119: 1079–1091 [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse EN, George EL, Rayburn H, Hynes RO 1996. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn 207: 145–156 [DOI] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE 2004. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430: 689–693 [DOI] [PubMed] [Google Scholar]
- Guan J-L, Hynes RO 1990. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor a4b1. Cell 60: 53–61 [DOI] [PubMed] [Google Scholar]
- Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M 2002. Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res 62: 6837–6845 [PubMed] [Google Scholar]
- Halliday NL, Tomasek JJ 1995. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res 217: 109–117 [DOI] [PubMed] [Google Scholar]
- Heasman J, Hynes RO, Swan AP, Thomas V, Wylie CC 1981. Primordial germ cells of Xenopus embryos: The role of fibronectin in their adhesion during migration. Cell 27: 437–447 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405: 76–81 [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ 1994. Fibronectin's III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem 269: 19183–19191 [PubMed] [Google Scholar]
- Huveneers S, Truong H, Fässler R, Sonnenberg A, Danen EH 2008. Binding of soluble fibronectin to integrin α5β1—Link to focal adhesion redistribution and contractile shape. J Cell Sci 121: 2452–2462 [DOI] [PubMed] [Google Scholar]
- Hynes RO 1990. Fibronectins. Springer-Verlag, New York [Google Scholar]
- Hynes RO 2002. Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Hynes RO 2009. The extracellular matrix: Not just pretty fibrils. Science 326: 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Zhao Q 2000. The evolution of cell adhesion. J Cell Biol 150: F89–96 [DOI] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, Han Q, Kim JB, Howerton K, Baumbusch C, Ogiwara N, et al. 2004. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci 117: 177–187 [DOI] [PubMed] [Google Scholar]
- Ingham KC, Brew SA, Erickson HP 2004. Localization of a cryptic binding site for tenascin on fibronectin. J Biol Chem 279: 28132–28135 [DOI] [PubMed] [Google Scholar]
- Ingham KC, Brew SA, Isaacs BS 1988. Interaction of fibronectin and its gelatin-binding domains with fluorescent-labeled chains of type I collagen. J Biol Chem 263: 4624–4628 [PubMed] [Google Scholar]
- Johnson KJ, Sage H, Briscoe G, Erickson HP 1999. The compact conformation of fibronectin is determined by intramolecular ionic interactions. J Biol Chem 274: 15473–15479 [DOI] [PubMed] [Google Scholar]
- Joos TO, Whittaker CA, Meng F, DeSimone DW, Gnau V, Hausen P 1995. Integrin α 5 during early development of Xenopus laevis. Mech Dev 50: 187–199 [DOI] [PubMed] [Google Scholar]
- Jülich D, Geisler R, Holley SA, Consortium TS 2005. Integrinα5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev Cell 8: 575–586 [DOI] [PubMed] [Google Scholar]
- Jülich D, Mould AP, Koper E, Holley SA 2009. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development 136: 2913–2921 [DOI] [PubMed] [Google Scholar]
- Kaczmarek J, Castellani P, Nicolo G, Spina B, Allemanni G, Zardi L 1994. Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer 59: 11–16 [DOI] [PubMed] [Google Scholar]
- Karuri NW, Lin Z, Rye HS, Schwarzbauer JE 2009. Probing the conformation of the fibronectin III1-2 domain by fluorescence resonance energy transfer. J Biol Chem 284: 3445–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian O, Dahse R, Alt V, Zardi L, Hentschel J, Schnettler R, Kosmehl H 2008. mRNA expression and protein distribution of fibronectin splice variants and high-molecular weight tenascin-C in different phases of human fracture healing. Calcif Tissue Int 83: 101–111 [DOI] [PubMed] [Google Scholar]
- Klass CM, Couchman JR, Woods A 2000. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J Cell Sci 113: 493–506 [DOI] [PubMed] [Google Scholar]
- Koukoulis GK, Howeedy MK, Korhen M, Virtanen I, Gould VE 1993. Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic, and neoplastic breast. J Submicrosc Cytol Pathol 25: 285–295 [PubMed] [Google Scholar]
- Kragtorp KA, Miller JR 2006. Regulation of somitogenesis by Ena/VASP proteins and FAK during Xenopus development. Development 133: 685–695 [DOI] [PubMed] [Google Scholar]
- Kragtorp KA, Miller JR 2007. Integrin α5 is required for somite rotation and boundary formation in Xenopus. Dev Dyn 236: 2713–2720 [DOI] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ 2002. Ectodermal syndecan-2 mediates left–right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell 2: 115–124 [DOI] [PubMed] [Google Scholar]
- Krotoski DM, Domingo C, Bronner-Fraser M 1986. Distribution of a putative cell surface receptor for fibronectin and laminin in the avian embryo. J Cell Biol 103: 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki T, Mitsui Y, Hamada K, Sumida H, Nishiyama M 1999. Detection of alternative splicing of fibronectin mRNA in a single cell. J Cell Sci 112: 1449–1453 [DOI] [PubMed] [Google Scholar]
- Langenbach KJ, Sottile J 1999. Identification of protein-disulfide isomerase activity in fibronectin. J Biol Chem 274: 7032–7038 [DOI] [PubMed] [Google Scholar]
- Latimer A, Jessen JR 2010. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol 29: 89–96 [DOI] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP 1996. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell 84: 155–164 [DOI] [PubMed] [Google Scholar]
- Leahy DJ, Hendrickson WA, Aukhil I, Erickson HP 1992. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science 258: 987–991 [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne P-F 2007. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 8: 633–644 [DOI] [PubMed] [Google Scholar]
- Lee G, Hynes R, Kirschner M 1984. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell 36: 729–740 [DOI] [PubMed] [Google Scholar]
- Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L 2002. The EIIIA segment of fibronectin is a ligand for integrins α9β1 and α4β1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem 277: 14467–14474 [DOI] [PubMed] [Google Scholar]
- Litvinovich SV, Brew SA, Aota S, Akiyama SK, Haudenschild C, Ingham KC 1998. Formation of amyloid-like fibrils by self-association of a partially unfolded fibronectin type III module. J Mol Biol 280: 245–258 [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Burton-Wurster N, Gu DN, Lust G 1996. Fibronectin mRNA splice variant in articular cartilage lacks bases encoding the V, III-15, and I-10 protein segments. J Biol Chem 271: 18954–18960 [DOI] [PubMed] [Google Scholar]
- Main AL, Harvey TS, Baron M, Boyd J, Campbell ID 1992. The three-dimensional structure of the tenth type III module of fibronectin: An insight into RGD-mediated interactions. Cell 71: 671–678 [DOI] [PubMed] [Google Scholar]
- Manabe R, Oh-e N, Maeda T, Fukuda T, Sekiguchi K 1997. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J Cell Biol 139: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE 2005. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24: 389–399 [DOI] [PubMed] [Google Scholar]
- Maqueda A, Moyano JV, Hernández Del Cerro M, Peters DM, Garcia-Pardo A 2007. The heparin III-binding domain of fibronectin (III4-5 repeats) binds to fibronectin and inhibits fibronectin matrix assembly. Matrix Biol 26: 642–651 [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW 2001. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: Novel roles for integrin and fibronectin. Development 128: 3635–3647 [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW 2003. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol 13: 1182–1191 [DOI] [PubMed] [Google Scholar]
- Matsuura H, Takio K, Titani K, Greene T, Levery SB, Salyan ME, Hakomori S 1988. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J Biol Chem 263: 3314–3322 [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483–495 [DOI] [PubMed] [Google Scholar]
- McDonald JA 1988. Extracellular matrix assembly. Annu Rev Cell Biol 4: 183–207 [DOI] [PubMed] [Google Scholar]
- McDonald JA, Quade BJ, Broekelman TJ, LaChance R, Forsman K, Hasegawa E, Akiyama S 1987. Fibronectin's cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J Biol Chem 262: 2957–2967 [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF 1983. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol 97: 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF 1985. Interaction of the 70,000-mol. wt. amino terminal fragment of fibronectin with matrix-assembly receptor of fibroblasts. J Cell Biol 100: 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti FA, Chauhan AK, Iaconcig A, Porro F, Baralle FE, Muro AF 2007. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. J Biol Chem 282: 28057–28062 [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD 2007. Synergistic control of cell adhesion by integrins and syndecans. Nature Rev Mol Cell Biol 8: 957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A, Ruoslahti E 1992. A fibronectin self-assembly site involved in fibronectin matrix assembly: Reconstruction in a synthetic peptide. J Cell Biol 118: 421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A, Zhang Z, Ruoslahtl E 1994. Superfibronectin is a functionally distinct form of fibronectin. Nature 367: 193–196 [DOI] [PubMed] [Google Scholar]
- Mosher DF 1975. Cross-linking of cold-insoluble globulin by fibrin stabilizing factor. J Biol Chem 260: 6614–6621 [PubMed] [Google Scholar]
- Mosher DF, ed. 1989. Fibronectin. Academic Press, New York [Google Scholar]
- Mostafavi-Pour Z, Askari JA, Whittard JD, Humphries MJ 2001. Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: Roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants. Matrix Biol 20: 63–73 [DOI] [PubMed] [Google Scholar]
- Mould AP, Komoriya A, Yamada KM, Humphries MJ 1991. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin α4β1: Inhibition of α4β1 function by RGD peptide homologues. J Biol Chem 266: 3579–3585 [PubMed] [Google Scholar]
- Muñoz R, Moreno M, Oliva C, Orbenes C, Larraín J 2006. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol 8: 492–500 [DOI] [PubMed] [Google Scholar]
- Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE 2003. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol 162: 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamakawa N, Aota S, Yamada SS, Akiyama SK, Olden K, Yamada KM 1991. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol 114: 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Winklbauer R 1999. Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development 126: 1975–1984 [DOI] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, Winklbauer R 2004. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development 131: 2727–2736 [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Johnson KE 1983. Conditioning of a culture substratum by the ectodermal layer promotes attachment and oriented locomotion by amphibian gastrula mesodermal cells. J Cell Sci 59: 43–60 [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Johnson KE 1984. Experimental manipulation of a contact guidance system in amphibian gastrulation by mechanical tension. Nature 307: 453–5 [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Gould AC, Johnson KE 1982. Movement and guidance of migrating mesodermal cells in Ambystoma maculatum gastrulae. J Cell Sci 56: 207–222 [DOI] [PubMed] [Google Scholar]
- Nojima Y, Humphries MJ, Mould AP, Komoriya A, Yamada KM, Schlossman SF, Morimoto C 1990. VLA-4 mediates CD3-dependent CD4+ T cell activation via the CS1 alternatively spliced domain of fibronectin. J Exp Med 172: 1185–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton PA, Hynes RO 1987. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol 7: 4297–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Erickson HP 2005. Domain unfolding plays a role in superfibronectin formation. J Biol Chem 280: 39143–39151 [DOI] [PubMed] [Google Scholar]
- Ohashi T, Erickson HP 2009. Revisiting the mystery of fibronectin multimers: The fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol 28: 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP 1999. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc Natl Acad Sci 96: 2153–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP 2002. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci 115: 1221–1229 [DOI] [PubMed] [Google Scholar]
- Olorundare OE, Peyruchaud O, Albrecht RM, Mosher DF 2001. Assembly of a fibronectin matrix by adherent platelets stimulated by lysophosphatidic acid and other agonists. Blood 98: 117–124 [DOI] [PubMed] [Google Scholar]
- Onodera T, Sakai T, Hsu JC-f, Matsumoto K, Chiorini JA, Yamada KM 2010. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science 329: 562–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama F, Hirohashi S, Sakamoto M, Titani K, Sekiguchi K 1993. Coordinate oncodevelopmental modulation of alternative splicing of fibronectin pre-messenger RNA at ED-A, ED-B, and CS1 regions in human liver tumors. Cancer Res 53: 2005–2011 [PubMed] [Google Scholar]
- Oyama F, Murata Y, Suganuma N, Kimura T, Titani K, Sekiguchi K 1989. Patterns of alternative splicing of fibronectin pre-mRNA in human adult and fetal tissues. Biochemistry 28: 1428–1434 [DOI] [PubMed] [Google Scholar]
- Pagani F, Zagato L, Vergani C, Casari G, Sidoli A, Baralle FE 1991. Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J Cell Biol 113: 1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF 1997. Integrin-ligand binding properties govern cell-substratum adhesiveness. Nature 385: 537–540 [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM 2002. Fibronectin at a glance. J Cell Sci 115: 3861–3863 [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Hahn C, Yamada KM 2000. Integrin dynamics and matrix assembly: Tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol 148: 1075–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254 [DOI] [PubMed] [Google Scholar]
- Peters J, Sechrist J, Luetolf S, Loredo G, Bronner-Fraser M 2002. Spatial expression of the alternatively spliced EIIIB and EIIIA segments of fibronectin in the early chicken embryo. Cell Commun Adhes 9: 221–238 [DOI] [PubMed] [Google Scholar]
- Petersen TE, Thogersen HC, Skorstengaard K, Vibe-Pedersen K, Sahl P, Sottrup-Jensen L, Magnusson S 1983. Partial primary structure of bovine plasma fibronectin: Three types of internal homology. Proc Natl Acad Sci 80: 137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E 1984. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309: 30–33 [DOI] [PubMed] [Google Scholar]
- Radek JT, Jeong J-M, Prasanna Murthy SN, Ingham K, Lorand L 1993. Affinity of human erythrocyte transglutaminase for a 42-kDa gelatin-binding fragment of human plasma fibronectin. Proc Natl Acad Sci 90: 3152–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reintsch WE, Hausen P 2001. Dorsoventral differences in cell–cell interactions modulate the motile behaviour of cells from the Xenopus gastrula. Dev Biol 240: 387–403 [DOI] [PubMed] [Google Scholar]
- Rovasio RA, Delouvee A, Yamada KM, Timpl R, Thiery JP 1983. Neural crest cell migration: Requirements for exogenous fibronectin and high cell density. J Cell Biol 96: 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW 2010. The extracellular matrix in development and morphogenesis: A dynamic view. Dev Biol 341: 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T, Dzamba B, Weber G, Davidson L, Desimone D 2009. The physical state of fibronectin matrix differentially regulates morphogenetic movements in vivo. Dev Biol 327: 386–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, et al. 2000. Comparative genomics of the eukaryotes. Science 287: 2204–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, et al. 2009. Fibrillin assembly requires fibronectin. Mol Biol Cell 20: 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM 2003. Fibronectin requirement in branching morphogenesis. Nature 423: 876–881 [DOI] [PubMed] [Google Scholar]
- Sanders EJ 1982. Ultrastructural immunocytochemical localization of fibronectin in the early chick embryo. J Embryol Exp Morphol 71: 155–170 [PubMed] [Google Scholar]
- Santas AJ, Peterson JA, Halbleib JL, Craig SE, Humphries MJ, Peters DM 2002. Alternative splicing of the IIICS domain in fibronectin governs the role of the heparin II domain in fibrillogenesis and cell spreading. J Biol Chem 277: 13650–13658 [DOI] [PubMed] [Google Scholar]
- Saunders S, Bernfield M 1988. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol 106: 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield KP, Humphries MJ 1999. Identification of fibronectin IIICS variants in human bone marrow stroma. Blood 93: 410–411 [PubMed] [Google Scholar]
- Schwarz-Linek U, Höök M, Potts JR 2006. Fibronectin-binding proteins of gram-positive cocci. Microbes Infect 8: 2291–2298 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE 1991a. Fibronectin: From gene to protein. Curr Opin Cell Biol 3: 786–791 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE 1991b. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol 113: 1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, Spencer CS, Wilson CL 1989. Selective secretion of alternatively spliced fibronectin variants. J Cell Biol 109: 3445–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, Patel RS, Fonda D, Hynes RO 1987. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J 6: 2573–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, Tamkun JW, Lemischka IR, Hynes RO 1983. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell 35: 421–431 [DOI] [PubMed] [Google Scholar]
- Sechler JL, Corbett SA, Schwarzbauer JE 1997. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol Biol Cell 8: 2563–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Cumiskey AM, Gazzola DM, Schwarzbauer JE 2000. A novel RGD-independent fibronectin assembly pathway initiated by α4β1 integrin binding to the alternatively spliced V region. J Cell Sci 113: 1491–1498 [DOI] [PubMed] [Google Scholar]
- Sechler JL, Rao H, Cumiskey AM, Vega-Colon I, Smith MS, et al. 2001. A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J Cell Biol 154: 1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Takada Y, Schwarzbauer JE 1996. Altered rate of fibronectin matrix assembly by deletion of the first type III repeats. J Cell Biol 134: 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi DL, Delarue M, Darribere T, Riou JF, Boucaut J-C 1987. Experimental analysis of the extension of the dorsal marginal zone in Pleurodeles waltl gastrulae. Development 100: 147–161 [DOI] [PubMed] [Google Scholar]
- Singh P, Carraher C, Schwarzbauer JE 2010. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26: 397–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V 2007. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 5: e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers CE, Mosher DF 1993. Protein kinase C modulation of fibronectin matrix assembly. J Biol Chem 268: 22277–22280 [PubMed] [Google Scholar]
- Sottile J, Schwarzbauer JE, Selegue J, Mosher DF 1991. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J Biol Chem 266: 12840–12843 [PubMed] [Google Scholar]
- Stepp MA, Daley WP, Bernstein AM, Pal-Ghosh S, Tadvalkar G, Shashurin A, Palsen S, Jurjus RA, Larsen M 2010. Syndecan-1 regulates cell migration and fibronectin fibril assembly. Exp Cell Res 316: 2322–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm EH, Banfi G, Krapf R, Abt AB, Mazzucco G, Monga G, Gloor F, Neuweiler J, Riess R, Stosiek P, et al. 1995. Glomerulopathy associated with predominant fibronectin deposits: A newly recognized hereditary disease. Kidney Int 48: 163–170 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, et al. 2007. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol 178: 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL 2004. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104: 11–18 [DOI] [PubMed] [Google Scholar]
- Trefzer U, Chen Y, Herberth G, Hofmann MA, Kiecker F, Guo Y, Sterry W 2006. The monoclonal antibody SM5-1 recognizes a fibronectin variant whcih is widely expressed in melanoma. BMC Cancer 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh LA, Stainier DYR 2004. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell 6: 371–382 [DOI] [PubMed] [Google Scholar]
- Tsyguelnaia I, Doolittle RF 1998. Presence of a fibronectin type III domain in a plant protein. J Mol Evol 46: 612–614 [DOI] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R 2009. Evidence for the evolution of tenascin and fibronectin early in the chordate lineage. Int J Biochem Cell Biol 41: 424–434 [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schötz E-M, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 9: 555–564 [DOI] [PubMed] [Google Scholar]
- Vakonakis I, Staunton D, Rooney LM, Campbell ID 2007. Interdomain association in fibronectin: Insight into cryptic sites and fibrillogenesis. EMBO J 26: 2575–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet A, Baelde HJ, Vleming LJ, de Heer E, Bruijn JA 2001. Distribution of fibronectin isoforms in human renal disease. J Pathol 193: 256–262 [DOI] [PubMed] [Google Scholar]
- Ventura E, Sassi F, Parodi A, Balza E, Borsi L, Castellani P, Carnemolla B, Zardi L 2010. Alternative splicing of the angiogenesis associated extra-domain B of fibronectin regulates the accessibility of the B-C loop of the type III repeat 8. PLoS One 5: e9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbächer U, Fraser SE, Harland RM 2000. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81–85 [DOI] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG 1989. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol 109: 1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Mikawa T 2000. Formation of the avian primitive streak from spatially restricted blastoderm: Evidence for polarized cell division in the elongating streak. Development 127: 87–96 [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R 1996. β1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol 132: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, Bergeron K-F, Whittle J, Brandhorst BP, Burke RD, Hynes RO 2006. The echinoderm adhesome. Dev Biol 300: 252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE 2002. Regulatory role for Src and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J Biol Chem 277: 19703–19708 [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE 2003. The ins and outs of fibronectin matrix assembly. J Cell Sci 116: 3269–3276 [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Mao Y, Schwarzbauer JE 2007. Continuous requirement for pp60-Src and phospho-paxillin during fibronectin matrix assembly by transformed cells. J Cell Physiol 210: 750–756 [DOI] [PubMed] [Google Scholar]
- Wilson CL, Schwarzbauer JE 1992. The alternatively spliced V region contributes to the differential incorporation of plasma and cellular fibronectins into fibrin clots. J Cell Biol 119: 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R 1998. Conditions for fibronectin fibril formation in the early Xenopus embryo. Dev Dyn 212: 335–345 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Keller RE 1996. Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev Biol 177: 413–426 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M, Selchow A, Wacker S 1996. Mesoderm migration in the Xenopus gastrula. Int J Dev Biol 40: 305–311 [PubMed] [Google Scholar]
- Winklbauer R, Selchow A, Nagel M, Angres B 1992. Cell interaction and its role in mesoderm cell migration during Xenopus gastrulation. Dev Dyn 195: 290–302 [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR 1998. Syndecans: Synergistic activators of cell adhesion. Trends Cell Biol 8: 189–194 [DOI] [PubMed] [Google Scholar]
- Woods A, Longley RL, Tumova S, Couchman JR 2000. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys 374: 66–72 [DOI] [PubMed] [Google Scholar]
- Wu C, Bauer JS, Juliano RL, McDonald JA 1993. The α5β1 integrin fibronectin receptor, but not the α5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem 268: 21883–21888 [PubMed] [Google Scholar]
- Wu C, Hughes PE, Ginsberg MH, McDonald JA 1996. Identification of a new biological function for the integrin αvβ3: Initiation of fibronectin matrix assembly. Cell Adhes Commun 4: 149–158 [DOI] [PubMed] [Google Scholar]
- Yang JT, Hynes RO 1996. Fibronectin receptor functions in embryonic cells deficient in α5β1 integrin can be replaced by αv integrins. Mol Biol Cell 7: 1737–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO 1993. Embryonic mesodermal defects in α5 integrin-deficient mice. Development 119: 1093–1105 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Multhaupt HAB, Couchman JR 2005. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Ushakov D, Multhaupt HA, Couchman JR 2007. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell 18: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir EA, Czirók A, Cui C, Little CD, Rongish BJ 2006. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc Natl Acad Sci 103: 19806–19811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardi L, Carnemolla B, Siri A, Petersen TE, Paolella G, Sebastio G, Baralle FE 1987. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J 6: 2337–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF 1994. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol 127: 1447–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF 1997. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell 8: 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E 1993. The avb1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 122: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141: 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]