Abstract
Over the last three decades, advances in biochemical pathology and human genetics have illuminated one of the most enigmatic subjects in biomedicine—neurodegeneration. Eponymic diseases of the nervous system such as Alzheimer's, Parkinson's, and Huntington's diseases that were long characterized by mechanistic ignorance have yielded striking progress in our understanding of their molecular underpinnings. A central theme in these and related disorders is the concept that certain normally soluble neuronal proteins can misfold and aggregate into oligomers and amyloid fibrils which can confer profound cytotoxicity. Perhaps the foremost example, both in terms of its societal impact and how far knowledge has moved toward the clinic, is that of Alzheimer's disease (AD). Here, we will review the classical protein lesions of the disorder that have provided a road map to etiology and pathogenesis. We will discuss how elucidating the genotype-to-phenotype relationships of familial forms of Alzheimer's disease has highlighted the importance of the misfolding and altered proteostasis of two otherwise soluble proteins, amyloid β-protein and tau, suggesting mechanism-based therapeutic targets that have led to clinical trials.
Misfolded amyloid β and tau proteins cause the neurodegeneration seen in Alzheimer's. Small compounds that target amyloid β production and antibodies that prevent aggregate formation could be effective therapies.
Among the human disorders marked by protein misfolding and aggregation, Alzheimer's disease looms large. This enormously common degeneration of limbic and association cortices and related subcortical nuclei slowly robs its victims of their most human qualities: memory, reasoning, abstraction, and language. The disease has no doubt existed for millennia but was often confused with other syndromes that also presented as “senile dementia,” that is, progressive cognitive decline after middle age. The description of the clinico-pathological syndrome by the Bavarian psychiatrist, Alois Alzheimer, in 1906 established a neuropathological phenotype that has enabled considerable diagnostic specificity, although until recently only at the end of the patient’s life. The microscopic lesions that Alzheimer called attention to—senile (amyloid) plaques and neurofibrillary tangles—have also provided a crucial starting point for approaching molecular pathogenesis. Indeed, the principal reasons that substantial progress toward deciphering the disease has accrued are its high prevalence and the robustness of its histological signature.
Not surprisingly, the study of Alzheimer’s disease has had its share of controversy. Given the cytological and biochemical complexity of the disorder, it has been difficult to come to agreement about the temporal sequence of events that leads to the dementia and which steps are most amenable to intervention. However, in recent years, a considerable consensus has developed that certain molecular events in the brain occur years or even decades prior to the first symptoms, and a rough outline of the pathogenic cascade has emerged. Although our understanding is certainly incomplete, advances in the field have led to the design of mechanism-based therapeutics that are now undergoing the painstaking process of clinical evaluation.
PROTEIN CHEMICAL NATURE OF THE DIAGNOSTIC BRAIN LESIONS
Progress in elucidating the biology of AD first arose from the compositional analyses of amyloid plaques and neurofibrillary tangles in the mid-1980s. Attempts to isolate the subunit proteins of these lesions were met with some skepticism, as it was argued that the plaques and tangles might be end-stage lesions that would provide little useful information about etiology and early pathogenesis. It has become increasingly apparent that this concern was ill-founded.
The amyloid deposits found in meningo-cerebral blood vessels and neuritic plaques in AD are composed of extracellular fibrils of the amyloid β-proteins (Aβ) (Glenner and Wong 1984a; Masters et al. 1985). Although these deposits contain skeins of insoluble amyloid fibrils (8–10 nm in diameter), these are intermixed with a poorly defined array of nonfibrillar (“amorphous”) forms of the peptide. Once it was established by protein sequencing of isolated amyloid deposits that Aβ was the subunit protein of both vascular amyloid (Glenner and Wong 1984a) and plaque cores (Masters et al. 1985), immunohistochemistry with antibodies to Aβ revealed innumerable plaque-like deposits in AD brain tissue that appeared to lack the surrounding dystrophic neurites and altered microglia and astrocytes which are features of the neuritic plaques. Such lesions, referred to as “diffuse” or “preamyloid” plaques, represent Aβ deposits that are mostly in a nonfibrillar, apparently granular form in the neuropil (Tagliavini et al. 1988; Yamaguchi et al. 1988). Antibodies that selectively recognize the carboxyl termini of various Aβ peptides have shown that diffuse (nonneuritic) deposits are largely composed of the highly amyloidogenic 42-residue form (Aβ42) (Iwatsubo et al. 1994), which has two extra hydrophobic amino acids (Ala and Ile) at its carboxyl terminus compared to the more abundantly generated Aβ40 peptide. Aβ deposits do not occur simply in these two extreme forms (diffuse and neuritic) but rather as a continuum in which complex mixtures of fibrillar, granular, and even soluble (nonparticulate) forms of the peptide are associated with highly varying degrees of surrounding glial and neuritic alteration. The extent of microvascular Aβ deposition in AD brains usually correlates poorly with the amount of Aβ plaques, and its importance in contributing to the dementia remains a subject of research (Verbeek et al. 2000).
In regions of the Alzheimer brain that are not strongly implicated in the clinical syndrome (e.g., cerebellum and thalamus), most Aβ deposits are of the diffuse type and thus accompanied by relatively little peri-plaque gliosis and neuritic dystrophy. A frequently voiced concern of the “amyloid (or Aβ) hypothesis” of AD is that plaques can be found in the cortex of apparently healthy aged subjects (who were usually not tested for subtle cognitive dysfunction before death). However, these are primarily diffuse plaques that appear to be less bioactive (i.e., they lack significant surrounding neuritic and glial cytopathology). A rough analogy has been drawn to many cholesterol-rich fatty streaks in the coronary arteries of individuals who have not yet experienced clinically noticeable cardiovascular events.
Neurofibrillary tangles are generally intraneuronal cytoplasmic bundles of paired, helically wound ∼10-nm filaments (PHFs), often interspersed with straight ∼10-nm filaments (Geser et al. 2008). Neurofibrillary tangles usually occur in large numbers in AD brains, particularly in entorhinal cortex, hippocampal formation, amygdala, association cortices of the frontal, temporal, and parietal lobes, and certain subcortical nuclei that project to these regions. The subunit protein of the PHF is the microtubule-associated protein, tau. PHFs are not limited to the tangles found in the cell bodies of neurons but also occur in many of the dystrophic neurites present within and outside of the amyloid plaques. The tau present in PHF comprises hyperphosphorylated, relatively insoluble forms of this normally highly soluble cytosolic protein (Grundke-Iqbal et al. 1986; Kosik et al. 1986; Nukina and Ihara 1986). The tau aggregates in the tangles are often complexed with ubiquitin, a feature they share with numerous other intraneuronal protein inclusions in etiologically diverse disorders such as Parkinson’s disease and Lewy body dementia. If this ubiquitination represents an attempt to remove the tau filaments by way of degradation in the proteasome, it appears to be largely unsuccessful.
The two classical proteinaceous lesions of AD can occur independently in humans. Tangles composed of tau aggregates that are biochemically similar (though usually not identical) to those in AD have been described in a dozen or more neurodegenerative diseases in which one generally finds no Aβ deposits and neuritic plaques. Conversely, Aβ deposits (mostly of the diffuse type) can be seen in aged “normal” cortex in the virtual absence of tangles. There are some cases of AD itself that are “tangle-poor,” that is, very few neurofibrillary tangles are found in the neocortex despite abundant Aβ plaques (Terry et al. 1987). In many such cases, one finds an alternate form of neuronal inclusion, the Lewy body, composed principally of fibrils of α-synuclein (Hansen et al. 1993). The fact that neurofibrillary tangles composed of altered, aggregated forms of tau protein occur in disorders (e.g., subacute sclerosing panencephalitis, Kuf’s disease, and progressive supranuclear palsy) in the absence of Aβ deposition suggests that tangles can arise in the course of a variety of etiologically distinct neuronal insults.
Aβ IS GENERATED BY REGULATED PROTEOLYSIS OF A LARGE PRECURSOR PROTEIN
The purification and partial sequencing of the Aβ protein from meningovascular amyloid deposits in AD (Glenner and Wong 1984b) and Down’s syndrome (Glenner and Wong 1984b) enabled the subsequent cloning of the gene encoding the β-amyloid precursor protein (APP) (Kang et al. 1987). Aβ is derived from APP by sequential proteolytic cleavages by enzymes generally referred to as β-secretase and γ-secretase. APP comprises a group of ubiquitously expressed polypeptides whose heterogeneity arises from both alternative exon splicing and posttranslational modifications (e.g., N- and O-linked glycosylation, phosphorylation, and sulfation) (Weidemann et al. 1989). Deletion of the APP gene in mice results in neither early mortality nor appreciable early morbidity, although cerebral gliosis, changes in locomotor and cognitive behaviors and other deficits develop with age (Zheng et al. 1995; Ring et al. 2007), and knockdown of APP in rat embryos impairs radial glial-guided migration of immature neurons during cortical development (Young-Pearse et al. 2007). The lack of a lethal phenotype from APP deletion presumably results from mammals expressing two closely homologous Type 1 glycoproteins, the amyloid precursor-like proteins (APLPs) (Wasco et al. 1992; Slunt et al. 1994).
The recognition that the last 12–14 residues of Aβ derived from the transmembrane region of APP gave rise to a conundrum: How could Aβ be found as a free peptide in the extracellular amyloid deposits? It was generally assumed that neurons would need to undergo an initial injury to their membranes to allow the unknown protease that creates the carboxyl terminus of Aβ (γ-secretase) to access the intramembrane region of APP. In contrast, it was discovered in 1992 that Aβ is constitutively secreted by healthy cells throughout life and normally circulates in the cerebrospinal fluid (CSF) and plasma of humans and lower mammals (Haass et al. 1992; Seubert et al. 1992; Shoji et al. 1992). APP holoproteins undergo alternative processing events (Fig. 1).
Figure 1.
Schematic diagrams of the β-amyloid precursor protein (APP) and its principal metabolic derivatives. The upper diagram depicts the largest of the known APP alternate splice forms, comprising 770 amino acids. Regions of interest are indicated at their correct relative positions. A 17-residue signal peptide occurs at the amino terminus (box with vertical lines). Two alternatively spliced exons of 56 and 19 amino acids are inserted at residue 289; the first contains a serine protease inhibitor domain of the Kunitz type (KPI). A single membrane-spanning domain (TM) at amino acids 700–723 is indicated by the vertical dotted lines. The amyloid β-protein (Aβ) fragment includes 28 residues just outside the membrane plus the first 12–14 residues of the transmembrane domain. In the middle diagram, the arrow indicates the site (after residue 687; same site as the white dot in the Aβ region of APP in the upper diagram) of a constitutive proteolytic cleavage made by protease(s) designated α-secretase that enables secretion of the large, soluble ectodomain of APP (APPs- α) into the medium and retention of the 83 residue carboxy-terminal fragment in the membrane. The C83 fragment can undergo cleavage by protease called γ-secretase at residue 711 or residue 713 to release the p3 peptides. The lower diagram depicts the alternative proteolytic cleavage after residue 671 by enzyme called β-secretase that results in the secretion of the slightly truncated APPs- β molecule and the retention of a 99-residue carboxy-terminal fragment. The C99 fragment can also undergo heterogenous cleavages by γ-secretase to release the Aβ peptides.
The most common scission occurs between residues 16 and 17 of the Aβ region (12 residues amino-terminal to the transmembrane domain) and is affected by one of the α-secretases, which are members of the ADAM family of membrane-anchored metalloproteases (Sisodia 1992; Buxbaum et al. 1998; Kuhn et al. 2010). The soluble ectodomain region (APPs-α) is released into vesicle lumens and from the cell surface, leaving behind a membrane-retained carboxy terminal fragment (CTF) of 83 amino acids (C83) (Fig. 1). Some APP holoproteins that do not undergo α-secretase cleavage may instead be cut by β-secretase, particularly in neurons, releasing a truncated form of APPs (APPs-β) from the cell (Seubert et al. 1993) and leaving a 99-residue CTF (C99) in the membrane (Fig. 1). C99 can subsequently be cleaved by the unusual proteolytic activity referred to as γ-secretase to release Aβ into vesicle lumens and extracellular fluid. C83 can also undergo cleavage by γ-secretase to generate a peptide (p3) comprising the latter two-thirds of Aβ. In most cells, a much smaller portion of total cellular APP undergoes cleavage by β- than by α-secretase. The β-secretase that generates the principal amino terminus of Aβ (aspartate-1) is a single-transmembrane aspartyl protease (BACE) with its active site in its ectodomain (Sinha et al. 1999; Vassar et al. 1999; Yan et al. 1999).
Several functions have been attributed to the APP ectodomain, including the inhibition of certain serine proteases (in the case of those splice forms with a Kunitz protease inhibitor motif), enhancement of cell–cell and cell–substrate adhesion, neuritotrophic and other growth-promoting effects, and neuroprotective properties (Anliker and Muller 2006; Zheng and Koo 2006). The hydrophilic APP intracellular domain (AICD) that is released into the cytoplasm by the intramembrane proteolysis of C83 or C99 (Fig. 1) could have a signaling function (analogous to another α- and γ-secretase substrate, Notch), but putative activities of this type have not been widely confirmed (Hebert et al. 2006). No evidence has emerged that a fundamental function of APP is lost in AD patients. Instead, AD-causing APP mutations are all clustered near the secretase processing sites and seem to act by a gain-of-toxic-function mechanism, namely the increased production of the neurotoxic Aβ fragment.
THE GENETICS OF ALZHEIMER’S DISEASE
Estimates of the proportion of AD cases that are genetically based have varied widely from as low as 10% to as high as 40% or 50%, and some investigators believe that, in the fullness of time, virtually all cases will be shown to have some genetic determinants. The discovery that the ε4 allele of apolipoprotein E is a normal polymorphism that greatly increases the risk of AD (Strittmatter et al. 1993) underscores that most genetic factors predisposing to AD do not occur in a simple medelian pattern and are thus difficult to identify in genetic epidemiological studies. There are four widely confirmed genes in which mutations or polymorphisms result in AD, and numerous additional candidate genes (e.g., clusterin [Apo J]; complement receptor 1) are in various stages of confirmation. Missense mutations in APP account for a tiny fraction (far less than 0.01%) of all Alzheimer’s cases, but they appear to be informative about the pathogenic mechanisms of AD in general. Inheritance of one or two ε4 alleles of ApoE is by far the most prevalent genetic factor predisposing to AD (Saunders et al. 1993; Strittmatter et al. 1993), accounting for upward of 30% of cases. Nevertheless, some humans homozygous for ApoE4 still show no AD symptoms in their ninth decade. The third and fourth genes implicated in familial forms of AD are presenilin 1 (PS1) and presenilin 2 (PS2); dominantly inherited missense mutations result in aggressive disease, with clinical onset between ages ∼40 and ∼65 (Levy-Lahad et al. 1995; Rogaev et al. 1995; Sherrington et al. 1995).
Despite the prominence of tau accumulation in neurofibrillary tangles and dystrophic neurites, the tau gene has not been found to be mutant in familial AD. Instead, mutations in tau cause a form of frontotemporal dementia (Hong et al. 1998; Hutton et al. 1998; Spillantini et al. 1998). The disorder, which generally occurs without amyloid deposits, is characterized by widespread tangle formation associated with specific biochemical alterations in the microtubule-binding properties of the mutant tau (Lee et al. 2001). The discovery of tau mutations in this distinct form of dementia has shown that severe neurofibrillary tangle formation does not lead to secondary accumulation of Aβ. Both APP and presenilin mutations in AD and tau mutations in frontotemporal dementia support the view that the profound alteration of wild-type tau in AD can follow Aβ accumulation, but not vice versa.
GENOTYPE-TO-PHENOTYPE RELATIONSHIPS IN FAMILIAL AD
Cultured cells and transgenic mice have been used to model the effects of each of the four genes that have been unequivocally implicated in familial AD (Table 1). In all cases, inherited alterations in the gene products have been linked to increases in the production and/or deposition of Aβ. This work provides the strongest support for the hypothesis that cerebral Aβ accumulation can be causative of AD. The APP gene can cause AD in two distinct ways: via overexpression because of increased dosage of the wild-type gene (e.g., in trisomy 21 [Down’s syndrome] and in patients with APP microduplications on chromosome 21q) or else via missense mutations that increase the amyloidogenic cleavages of APP by either β- or γ-secretase. In Down’s syndrome, a lifelong increase in APP expression and the resultant overproduction of both Aβ40 and Aβ42 peptides (Rumble et al. 1989; Tokuda et al. 1997) is responsible for the appearance of many diffuse plaques as early as age 12. Down’s subjects usually develop diffuse plaques composed solely of Aβ42 in their teens and early 20s, with subsequent accrual of Aβ40 peptides onto these plaques and the gradual appearance of surrounding microgliosis, astrocytosis, neuritic dystrophy, and neurofibrillary tangles beginning in their 20s or 30s (Mann et al. 1995; Lemere et al. 1996a).
Table 1.
Genetic factors predisposing to AD: relationships to the β-amyloid phenotype
| Chromosome | Gene defect | Phenotype |
|---|---|---|
| 21 | βAPP mutations | Increased productions of all Aβ peptides or Aβ42 peptides |
| 19 | ApoE4 polymorphism | Increased density of Aβ40 plaques and vascular deposits |
| 14 | Presenilin 1 mutations | Increased production of Aβ42 peptides |
| 1 | Presenilin 2 mutations | Increased production of Aβ42 peptides |
The more than 20 known APP missense mutations that cause AD are clustered at the β-secretase cleavage site, just after the γ-secretase site, or else in the middle of the Aβ region (Fig. 2). A double missense mutation immediately amino-terminal to Aβ allows increased cleavage by β-secretase. Mutations occurring after the γ-secretase cleavage site enhance the relative production of the longer and more self-aggregating Aβ42 peptide. Mutations within Aβ (Levy et al. 1990; Hendriks et al. 1992) generally enhance the aggregation of the peptide. Importantly, no AD-causing mutations in the APP polypeptide away from the Aβ region have been documented.
Figure 2.
βAPP mutations genetically linked to familial Alzheimer’s disease or related disorders. The sequence within APP that contains the Aβ and transmembrane region is expanded and shown by the single-letter amino acid code. The underlined residues represent the Aβ1-42 peptide. The vertical broken lines indicate the location of the transmembrane domain. The bold letters below the sequence indicate the currently known missense mutations identified in certain patients with familial Alzheimer’s disease or hereditary cerebral hemorrhage with amyloidosis. The family with the AG92G mutation can experience cerebral hemorrhages from amyloid angiopathy and/or Alzheimer’s disease as the phenotypes. Three-digit numbers refer to the residue number according to the βAPP770 isoform.
The disease-promoting mechanism of inheriting one or two ApoE4 alleles is not settled. Considerable evidence suggests that this isoform leads to decreased clearance and/or enhanced aggregation of Aβ in the brain (Rebeck et al. 1993; Schmechel et al. 1993; Ma et al. 1994; Evans et al. 1995). A decrease in Aβ deposits occurs when APP transgenic mice are crossed with mice lacking ApoE (Bales et al. 1997). When these offspring are then crossed with mice expressing human ApoE3 or ApoE4, both resultant genotypes show more Aβ deposits than the mice lacking ApoE, but the ApoE4-expressing mice show significantly more than those with ApoE3 (Holtzman et al. 2000). Other possible mechanisms of the effect of ApoE4 in promoting the AD phenotype have been proposed (Mahley and Huang 2009). However, the observation in humans that not only parenchymal amyloid deposits but also those in meningeal vessels (and sometimes just the latter) accumulate as a function of rising ApoE4 gene dosage (Greenberg et al. 1995) strongly suggests that ApoE4 serves to increase tissue Aβ deposition.
When presenilin 1 and 2 were cloned in 1995, the mechanism by which missense mutations in these polytopic proteins produced AD was open and not necessarily expected to involve a change in Aβ economy. However, it soon became known that PS 1 or 2 missense mutations increase the ratio of Aβ42 to Aβ40 in the patients’ plasma and cultured fibroblast media (Scheuner et al. 1996). Modeling of these mutations in cultured cells and mice confirmed this result (Borchelt et al. 1996; Citron et al. 1997). Crossing mice transgenic for mutant APP with mice expressing mutant PS1 leads to an accelerated AD-like phenotype, with Aβ42 plaques occurring as early as 3 mo of age (Holcomb et al. 1998). Moreover, the ability of PS mutations to selectively enhance Aβ42 deposition has been documented directly in patients’ brains (Lemere et al. 1996b; Mann et al. 1996).
PS1 and PS2 each have nine transmembrane domains (TMD) and occur in ER, Golgi, and other vesicles in the secretory pathway, plasma membrane, and endosomes. Presenilin exists largely as a mature heterodimer of endoproteolytically generated fragments (NTF plus CTF) (Thinakaran et al. 1996). Studies in Caenorhabditis elegans led to the recognition that its presenilin (called SEL-12) is a facilitator of the Notch intercellular signaling pathway (Levitan et al. 1996). Accordingly, deleting the presenilin 1 gene in mouse causes profound Notch hypofunction (i.e., an embryonic lethal phenotype that includes severely disordered somitogenesis and axial skeletal development as well as abnormal neuronal differentiation in the forebrain [Shen et al. 1997; Wong et al. 1997]). In addition, the production of all Aβ peptides is markedly reduced in PS1 knockout embryos because of a loss of γ-secretase processing (De Strooper et al. 1998). APP was found by some investigators to interact with PS1 (Weidemann et al. 1997; Xia et al. 1997), suggesting that presenilin might either be a necessary cofactor of the γ-secretase reaction or might actually be γ-secretase itself. The latter possibility was furthered by the finding that inhibiting γ-secretase activity in cells with APP peptidomimetic transition-state analogs suggested that γ-secretase was an aspartyl protease (Wolfe et al. 1999a). Close inspection of the presenilin sequence revealed two aspartates in adjacent TMDs of all presenilins down to C. elegans, and these flanked the cytoplasmic loop in which presenilin is normally cleaved into its heterodimer (Fig. 3). Mutation of either of the aspartates completely prevented both this endoproteolysis of presenilin and the γ-secretase processing of C99 to Aβ and C83 to p3 (Wolfe et al. 1999b). These results suggested that presenilin was a first-in-class intramembrane aspartyl protease that cleaves itself (an autoactivation step) and can then cleave intramembrane substrates such as C83 and C99 of APP. Separate work showed that presenilin is also required for the normal intramembrane cleavage of Notch following binding of its extracellular ligand (De Strooper et al. 1999). Consequently, mutating either one of the PS aspartates is lethal to C. elegans (Brockhaus et al. 1998) and all other animals. It was soon shown that γ-secretase inhibitors mimicking the transition state of a substrate with an aspartyl protease bound directly to presenilin (Esler et al. 2000; Li et al. 2000). These and many subsequent studies firmly establish presenilin as the catalytic site of γ-secretase. Thus, missense mutations in either the protease (presenilin) or the substrate (APP) of the γ-secretase reaction elevate the relative levels of Aβ42 and result in an aggressive AD phenotype. These findings are assumed to be relevant to conventional (“sporadic”) forms of AD, which are usually indistinguishable neuropathologically from cases caused by PS mutations.
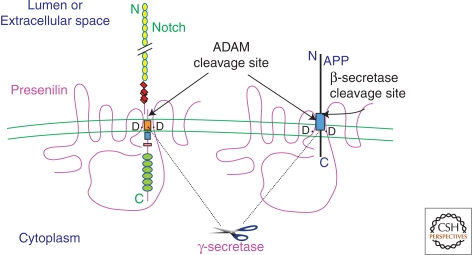
Figure 3.
Model of the role of presenilin (PS) in Notch and APP processing based on current information. Polytopic PS protein, which occurs principally as a cleaved heterodimer. Some Notch and APP molecules form complexes with PS. Two aspartates (D) in TM6 and TM7 of PS are required for the cleavages of Notch and APP within their TM domains, and these align with the respective sites of cleavage in the two substrates. PS-mediated proteolysis of both Notch and APP is preceded by ectodomain shedding by an ADAM family protease (“α-secretase”). Alternatively, APP can undergo ectodomain shedding by β-secretase. Several motifs are depicted in Notch: EGF-like repeats (yellow circles), LNG repeats (red diamonds), a single TM (orange box), the RAM23 domain (blue square), a nuclear localization sequence (pink rectangle), and six cdc10/ankyrin repeats (green ovals). Following the putative intramembranous cleavage mediated by PS, the Notch intracellular domain is released to the nucleus to activate transcription of target genes. APP contains the Aβ region (light blue box), which is released into the lumen after sequential cleavages of APP by β-secretase and then γ-secretase/PS. The APP intracellular domain is released into the cytoplasm.
GENETICALLY ENGINEERED MOUSE MODELS OF AD
Given the many obstacles to studying disease progression dynamically in the human brain, significant effort has been expended to create mouse models that replicate aspects of AD pathogenesis. A sizable number of mouse lines transgenic for human APP (either without or with human PS1 coexpression) have been generated (Games et al. 1995; Hsiao et al. 1996; Holcomb et al. 1998; Mucke et al. 2000; Oddo et al. 2003). Most of these models recapitulate some but not all neuropathological features of the human disease. Typically, Aβ42/40 peptide ratios are elevated and both diffuse and fibrillar (neuritic) plaques appear in an age-dependent fashion. No PHF in neurites or tangles are observed unless mutant human tau is also overexpressed. “Bigenic” mice expressing mutant human APP plus human tau bearing the P301L mutation that causes a form of frontotemporal dementia (Lewis et al. 2001) or “triple transgenic” mice expressing mutations in human APP, PS1, and tau (Oddo et al. 2003) develop NFTs reminiscent of those seen in AD and at a faster rate than mice expressing P301L tau alone.
In most APP transgenic models, significant neuronal loss is not observed (Irizarry et al. 1997). However, at least one mouse line (called APP23) that expresses mutant human APP shows quantifiable neuronal loss in the CA1 region of hippocampus at age 14–18 mo (Calhoun et al. 1998). In the latter mice, Aβ is deposited almost exclusively in the form of Thioflavin-positive (amyloid fibril–rich) plaques (Sturchler-Pierrat et al. 1997), and the cell loss is observed primarily in the vicinity of such hippocampal deposits. In accord, another study reported a reduction in neuronal density around Thioflavin S–positive Aβ deposits in both AD brain and the brains of 12-mo-old mice coexpressing mutant human PS1 plus APP (Urbanc et al. 2002).
Although fibrillar Aβ in mature AD plaques may contribute to neurotoxicity, such plaques are dynamic structures and likely act as local reservoirs of small, diffusible oligomers (Koffie et al. 2009). Furthermore, a mouse line transgenic for mutant APP took ∼12 mo to develop plaques, and yet the animals showed cognitive impairment and decreased hippocampal long-term potentiation (LTP) from 3 mo onward (Moechars et al. 1999). Another APP mouse was found to have enhanced paired pulse facilitation, distorted responses to high frequency stimulation, and impaired LTP at just age 4–5 mo (Larson et al. 1999; Shinsky et al. 2002). Analyses of other mice have confirmed morphological, biochemical, and electrophysiological changes well in advance of amyloid plaque deposition (Mucke et al. 2000; Wu et al. 2004). In addition, studies in a C. elegans model also suggest that small oligomers of Aβ can confer cytotoxicity (Cohen et al. 2006).
PREFIBRILLAR FORMS OF Aβ PERTURB SYNAPTIC FORM AND FUNCTION
Early evidence from human studies that soluble, nonfibrillar assemblies of Aβ might play a principal role in cognitive impairment came from analyses that showed statistical correlations between cortical levels of soluble Aβ and the extent of synaptic loss and severity of cognitive symptoms (Lue et al. 1999; McLean et al. 1999; Wang et al. 1999). In such studies, the term “soluble Aβ” is an operational definition, embracing all forms of Aβ that remain in aqueous solution following high-speed centrifugation of brain extracts (Kuo et al. 1996; Lue et al. 1999; McLean et al. 1999; Wang et al. 1999).
Experimental approaches to this concept in the investigator’s laboratory have confirmed that abundant SDS-stable Aβ dimers (∼8 kDa) and some SDS-stable trimers (∼12 kDa) are detectable in buffer-soluble extracts of postmortem AD cortex (Shankar et al. 2008). We subjected such extracts to size-exclusion chromatography, allowing the separation of monomers (∼4 kDa) from dimers. Application of the respective SEC fractions to normal mouse hippocampal slices showed that the dimers potently inhibited LTP, an electrophysiological correlate of aspects of synaptic plasticity during learning and memory in rodents (Shankar et al. 2008). The dimers could also facilitate long-term synaptic depression (LTD) in the hippocampus (Li et al. 2009). Monomers isolated simultaneously from the same AD brains were without effect. The dimers also decreased dendritic spine density; this is relevant to the strong correlations between AD neuropathology and synaptic loss in human brains. Monoclonal antibodies to Aβ (especially, to its free amino terminus) prevented these changes. Insoluble amyloid plaque cores isolated form the same AD brains did not impair LTP in hippocampal slices unless the cores were first solubilized in formic acid to release their constituent dimers (Shankar et al. 2008). Importantly, microinjection of the dimer-rich soluble AD extracts into the cerebral ventricles of healthy adult rats transiently impaired the memory of a learned behavior (Shankar et al. 2008). Further studies have recently shown that the isolated AD brain dimers potently induce hyperphosphorylation of endogenous tau in rat cortical neurons, followed by a progressive collapse of the neuritic cytoskeleton (M Jin and DJ Selkoe, unpublished data). Knocking down rat tau prevented the neuritic dystrophy, whereas expressing human tau accelerated it. Taken together, these findings support the hypothesis that small, soluble oligomers of human Aβ are sufficient to induce several features of the AD phenotype, including synaptic loss, tau hyperphosphorylation, neurofibrillary degeneration, and memory impairment, in the absence of amyloid plaques. Moreover, earlier in vivo studies that genetically deleted tau in APP transgenic mice strikingly protected these animals from Aβ-mediated behavioral deficits (Roberson et al. 2007).
Many studies employing supraphysiological concentrations of synthetic Aβ peptides in oligomeric form (generally larger than dimers/trimers) have also provided evidence of tau alteration (Zempel et al. 2010) and various neurotoxic effects. Because such synthetic intermediates can readily associate into higher order aggregates and fibrils can in turn dissociate, it is difficult to unambiguously ascribe cytopathological activity to a discrete species. Nonetheless, several groups have generated prefibrillar synthetic Aβ assemblies and probed their synaptotoxic activity. In 1998, Lambert and colleagues presented the first experimental evidence that certain soluble, nonfibril forms of synthetic Aβ (which they called Aβ-derived diffusible ligands, or ADDLs) could be neurotoxic (Lambert et al. 1998). ADDLs have been shown to cause neuronal death in culture, to block LTP (Lambert et al. 1998; Wang et al. 2004) and to inhibit reduction of MTT by neural cell lines (Lambert et al. 1998; Stine et al. 2003). Apparently distinct assembly intermediates of synthetic Aβ termed protofibrils (PFs) have been shown by other workers to alter neuronal function. Synthetic PFs range from spherical assemblies of ∼5 nm diameter to short, flexible rods of up to 200 nm in length (Harper et al. 1997; Walsh et al. 1997). Synthetic PFs appear to behave as fibril intermediates in vitro in that they can both form fibrils and dissociate to lower molecular weight species (Harper et al. 1999; Walsh et al. 1999). Among other neurotoxic effects, PFs can induce an increase in excitatory postsynaptic currents (EPSCs) in rat cortical neurons (Hartley et al. 1999). An alternative to the use of synthetic peptides is to analyze the conditioned media of certain cultured cell lines that express mutant human APP and secrete low-nanomolar amounts of small, soluble oligomers. When such cell-derived oligomers were injected intraventricularly into healthy rats, they inhibited hippocampal LTP (Walsh et al. 2002) and also impaired the memory of a learned behavior (Cleary et al. 2005). Finally, yet another approach has been to identify—and then isolate—Aβ assembly intermediates (e.g., a metastable docamer designated Aβ*56) from the brains of certain APP transgenic mice (Lesne et al. 2006). Aβ*56 has been shown to be associated with—and actually induce—transient learning deficits in water maze and other mouse behavioral assays (Lesne et al. 2006).
SEVERAL THERAPEUTIC OPPORTUNITIES ARE EMERGING FROM THE MECHANISTIC STUDY OF Aβ
Progress in deciphering the role of the presenilins in the proteolytic processing of APP, Notch and many other single-transmembrane proteins has provided a way of thinking about the question of how Alzheimer’s disease arose in the human population. The presenilins, and specifically their two intramembrane aspartates, were conserved during evolution because they confer a strong developmental advantage by mediating the Notch nuclear signaling pathway that is vital for cell fate decisions in all multicellular animals. But the similar processing of another, biologically less important substrate, APP, allows the lifelong production of Aβ, and this can lead in long-lived hosts to the accumulation of its most hydrophobic and oligomer-prone form (Aβ42). Because large numbers of humans now survive long past reproductive maturity, one sees a very substantial and rising prevalence of AD. A corollary of the latter concept is provided by the inheritance of missense mutations in either the substrate (APP) or the protease (presenilin) that biochemically accentuate—at least in relative terms—the cleavage of APP at the Aβ42-43 peptide bond, producing an early-onset form of AD.
Although many questions remain unanswered, sufficient progress in delineating the pathogenic cascade has been achieved to envision several discrete targets for treatment. Inhibitors of Aβ production—that is, small compounds that cross the blood–brain barrier and decrease but do not eliminate either β- or γ-secretase activity—could be therapeutic in the early clinical phases of the disease, namely in patients with minimal cognitive impairment or mild AD, and ultimately also in presymptomatic subjects with genetic predispositions. In the case of γ-secretase inhibitors, these could be designed to decrease APP cleavage by some 20% to 40%, but they must do so selectively, that is, without interfering in a quantitatively important way with Notch processing.
An alternate approach would be to use small molecules to bind Aβ monomers and prevent their assembly into potentially cytotoxic oligomers or else coat the small oligomers to mask their toxicity. However, if an antiaggregating compound solely blocked amyloid fibril formation, this could actually allow increased accumulation of metastable intermediates such as oligomers and might theoretically aggravate the disease. One advantage of a properly designed antioligomer agent is that one would be targeting a purely pathological event in the disease rather than interfering with normal metabolic reactions such as those of β- and γ-secretase.
A third general approach would be to administer anti-inflammatory drugs that could interfere with certain aspects of the responses by microglia, astrocytes, or bone marrow–derived macrophages that occur in the AD brain. It would be necessary to design novel compounds that interfere with one or more specific steps in the Aβ-induced inflammatory cascade of AD, rather than relying on conventional anti-inflammatory drugs that can have considerable toxicity when administered chronically to elderly humans.
One could also use a variety of antioxidants, free radical scavengers, calcium channel blockers, and modulators of certain signal transduction pathways that might protect neurons from the downstream effects of Aβ accumulation. The problem with this general approach may turn out to be that there are multiple ways in which neurons respond to Aβ and the associated inflammatory process, and blocking one or two of these might not significantly decrease overall neuronal dysfunction and loss. An increasingly interesting possibility is to lower or neutralize the effects of tau, given the striking protective effects against the neurotoxicity of Aβ that tau knockdown has shown in cell culture and mouse models (above). One can also consider the administration of neurorestorative factors, for example, neurotrophins and small compounds mimicking their actions that might rescue synapses and cell bodies undergoing active injury. However, this approach would need to operate successfully in the presence of ongoing new injury from the cytotoxic effects of Aβ.
Perhaps the clinically most advanced approach to lowering the levels of Aβ monomers, oligomers, and amyloid deposits is active and passive immunotherapy. This idea arose from studies in APP transgenic mice immunized with synthetic human Aβ peptide, leading to a humoral response and the movement of some of the antibodies across the blood brain barrier into the brain parenchyma, where they reduced both plaque burden and soluble Aβ levels (Schenk et al. 1999). A subsequent study infusing monoclonal antibodies to Aβ into transgenic mice also led to Aβ clearing (Bard et al. 2000). Many follow-up preclinical studies have robustly confirmed these effects and shown that abnormal behavior in APP mice can also be ameliorated. In one study, acute administration of a single injection of an antibody appeared to return certain cognitive deficits to baseline in older mice, obviously without reducing plaque burden (Dodart et al. 2002). The precise mechanisms of immunotherapy remain unclear but likely involve both the stimulation of plaque clearance by local microglia and/or exogenous macrophages (Bard et al. 2000) and the binding and neutralization of soluble forms of Aβ in the brain (Shankar et al Nat Med 2008) and perhaps in the systemic circulation (DeMattos et al. 2002). Success in APP transgenic mouse models led to initial human trials of active vaccination with a full-length Aβ42 peptide (plus adjuvant), but in a phase 2 trial, 6% of the 300 immunized subjects developed a self-limited sterile meningo-encephalitis, apparently because of the entry of T-cells auto-reactive to Aβ through the blood–brain barrier. Although this trial had to be halted, some subjects were followed and showed limited evidence of a biological effect, namely fewer declines than placebo-treated subjects on certain memory tests that correlated with their residual Aβ antibody titers, plus a lowering of CSF tau levels in a small subset of recipients (Gilman et al. 2005). Given this mixed outcome, the next clinical approach was passive infusion of antibodies directed to the free Aβ amino terminus, thus avoiding side effects of active vaccination. An 18-mo phase 2 trial again showed mixed results: (1) less declines in some cognitive measures in those who received all six antibody doses, again with lowering of mean CSF tau levels in the few subjects who had two lumbar punctures; and (2) the development in a small but significant percentage of a transient neuroradiological change called vasogenic edema, associated with transient worsening of the degree of dementia in a minority of these radiologically affected patients (Salloway et al. 2009). This amino-terminal antibody is now being tested in four large Phase 3 trials, and other passive and active immunotherapy trials are underway.
In the future, it is probable that individuals reaching their 50s or beyond will be offered a specific risk-assessment profile to determine their likelihood of developing AD. Such an assessment, modeled on that now widely used to judge the risk of serious atherosclerotic disease, would include inquiry about a positive family history of AD, identification of specific predisposing genetic factors, structural and functional brain imaging (including amyloid PET scans) to detect evidence of presymptomatic lesions, and measurement of Aβ42, tau, and other markers of the neuropathology in CSF and perhaps (in the case of Aβ) even in blood. Based on further epidemiologic experience with such assessment measures in large populations of healthy elderly, MCI and AD subjects, it should be possible to estimate—first crudely and later more accurately—the likelihood that an individual will develop AD. If this can be accomplished, then those at appreciable risk could be offered preventative treatments, assuming one or more of the agents contemplated in the previous paragraphs prove efficacious and safe. Although the achievement of an integrated diagnostic and therapeutic approach to this complex and devastating disorder may seem remote, the current rate of scientific progress and the likelihood of many additional clinical trials suggest that some level of practical success may come sooner than one might think.
ACKNOWLEDGMENTS
The author thanks the members of his laboratory and his collaborators for many helpful discussions that underlie the concepts in this paper. Supported by grants from the NIH (NIA) and the Foundation for Neurologic Diseases.
Footnotes
Editors: Richard Morimoto, Jeffrey Kelly, and Dennis Selkoe
Additional Perspectives on Protein Homeostasis available at www.cshperspectives.org
REFERENCES
- Anliker B, Muller U 2006. The functions of mammalian amyloid precursor protein and related amyloid precursor-like proteins. Neurodegener Dis 3: 239–246 [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. 1997. Lack of apolipprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet 17: 263–264 [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. 2000. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916–919 [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada C-M, Kim G, Seekins S, Yager D, et al. 1996. Familial Alzheimer’s disease–linked presenilin 1 variants elevate Aβ1-42/1-40 ratio in vitro and in vivo. Neuron 17: 1005–1013 [DOI] [PubMed] [Google Scholar]
- Brockhaus M, Grunberg J, Rohrig S, Loetscher H, Wittenburg N, Baumeister R, Jacobsen H, Haass C 1998. Caspasse-mediated cleavage is not required for the activity of presenilins in amyloidogenesis and NOTCH signaling. NeuroReport 9: 1481–1486 [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA 1998. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem 273: 27765–27767 [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchlerpierrat C, Staufenbiel M, Sommer B, Jucker M 1998. Neuron loss in APP transgenic mice. Nature 395: 755–756 [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, et al. 1997. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Med 3: 67–72 [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH 2005. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci 8: 79–84 [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A 2006. Opposing activities protect against age-onset proteotoxicity. Science 313: 1604–1610 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Gundula G, Annaert W, Von Figura K, Van Leuven F 1998. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391: 387–390 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. 1999. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522 [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM 2002. Brain to plasma amyloid-β efflux: A measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 295: 2264–2267 [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, et al. 2002. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci 5: 452–457 [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai J-Y, Rahmati T, Xia W, Selkoe DJ, Wolfe MS 2000. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat Cell Biol 2: 428–434 [DOI] [PubMed] [Google Scholar]
- Evans KC, Berger EP, Cho C-G, Weisgraber KH, Lansbury PT Jr 1995. Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatement of Alzheimer disease. Proc Natl Acad Sci 92: 763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. 1995. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373: 523–527 [DOI] [PubMed] [Google Scholar]
- Geser F, Lee VM-Y, Trojanowski JQ 2008. Frontotemporal Dementias. In The molecular and genetic basis of neurologic and psychiatric disease (ed. Rosenberg R, et al. ), pp. 330–338 Wolters Kluwer/Lippincott Williams & Wilkins, New York [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, et al. 2005. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64: 1553–1562 [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW 1984a. Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122: 1131–1135 [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW 1984b. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890 [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Rebeck GW, Vonsattel JPG, Gomez-Isla T, Hyman BT 1995. Apolipoprotein E e4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 38: 254–259 [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder LI 1986. Abnormal phosphorylation of the microtubule-associated protein τ (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci 83: 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Schlossmacher M, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski B, Lieberburg I, Koo EH, Schenk D, Teplow D, et al. 1992. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322–325 [DOI] [PubMed] [Google Scholar]
- Hansen LA, Masliah E, Galasko D, Terry RD 1993. Plaque-only Alzheimer disease is usually the Lewy body variant, and vice versa. J Neuropathol Exp Neurol 52: 648–654 [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT Jr 1997. Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem & Biol 4: 119–125 [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT 1999. Assembly of Aβ amyloid protofibrils: An in vitro model for a possible early event in Alzheimer’s disease. Biochemistry 38: 8972–8980 [DOI] [PubMed] [Google Scholar]
- Hartley D, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ 1999. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19: 8876–8884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Serneels L, Tolia A, Craessaerts K, Derks C, Filippov MA, Muller U, De Strooper B 2006. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep 7: 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks L, van Duijn CM, Cras P, Cruts M, Van Hul W, van Harskamp F, Warren A, McInnis MG, Antonarakis SE, Martin J-J, et al. 1992. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the β-amyloid precursor protein gene. Nature Genet 1: 218–221 [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, et al. 1998. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4: 97–100 [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. 2000. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci 97: 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, et al. 1998. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282: 1914–1917 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Ekman C, Harigaya Y, Younkin S, Yang F, Cole G 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274: 99–102 [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon C, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. 1998. Association of missense and 5′-splice-site mutations in tau with the inherited FTDP-17. Nature 393: 702–705 [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT 1997. Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci 17: 7053–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina H, Ihara Y 1994. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Aβ42(43). Neuron 13: 45–53 [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, Multhaup G, Beyreuther K, Muller-Hill B 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736 [DOI] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, et al. 2009. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci 106: 4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ 1986. Microtubule-associated protein, tau, is a major antigenic component of paired helical filaments in Alzheimer’s disease. Proc Natl Acad Sci 83: 4044–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF 2010. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. Embo J 29: 3020–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-M, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE 1996. Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem 271: 4077–4081 [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Iosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. 1998. Diffusible, nonfribrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci 95: 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Lynch G, Games D, Seubert P 1999. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res 840: 23–35 [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ 2001. Neurodegenerative tauopathies. Annu Rev Neurosci 24: 1121–1159 [DOI] [PubMed] [Google Scholar]
- Lemere CA, Blustzjan JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ 1996a. Sequence of deposition of heterogeneous amyloid β-peptides and Apo E in Down syndrome: Implications for initial events in amyloid plaque formation. Neurobiol Dis 3: 16–32 [DOI] [PubMed] [Google Scholar]
- Lemere CA, Lopera F, Kosik KS, Lendon CL, Ossa J, Saido TC, Yamaguchi H, Ruiz A, Martinez A, Madrigal L, et al. 1996b. The E280A presenilin 1 Alzheimer mutation produces increased Aβ42 deposition and severe cerebellar pathology. Nature Med 2: 1146–1150 [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH 2006. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440: 352–357 [DOI] [PubMed] [Google Scholar]
- Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I 1996. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci 93: 14940–14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GTAM, Luyendijk W, Frangione B 1990. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch-type. Science 248: 1124–1126 [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell H, Yu C, Jondro PD, Schmidt SD, Wang K, et al. 1995. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269: 973–977 [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, et al. 2001. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293: 1487–1491 [DOI] [PubMed] [Google Scholar]
- Li Y-M, Xu M, Lai M-T, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelli JG, et al. 2000. Photoactivated g-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405: 689–694 [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D 2009. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62: 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J 1999. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155: 853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Yee A, Brewer HB Jr, Das S, Potter H 1994. The amyloid-associated proteins a1-antichymotrypsin and apolipoprotein E promote the assembly of the Alzheimer β-protein into filaments. Nature 372: 92–94 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y 2009. Alzheimer disease: multiple causes, multiple effects of apolipoprotein E4, and multiple therapeutic approaches. Ann Neurol 65: 623–625 [DOI] [PubMed] [Google Scholar]
- Mann DM, Iwatsubo T, Fukumoto H, Ihara Y, Odaka A, Suzuki N 1995. Microglial cells and amyloid β protein (Aβ) deposition; association with Aβ40-containing plaques. Acta Neuropathol 90: 472–477 [DOI] [PubMed] [Google Scholar]
- Mann DMA, Iwatsubo T, Cairns NJ, Lantos PL, Nochlin D, Sumi SM, Bird TD, Poorkaj P, Hardy J, Hutton M, et al. 1996. Amyloid β protein (Aβ) deposition in chromosome 14–linked Alzheimer’s disease: Predominance of Aβ42(43). Ann Neurol 40: 149–156 [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K 1985. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci 82: 4245–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL 1999. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46: 860–866 [DOI] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, et al. 1999. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274: 6483–6492 [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L 2000. High-level neuronal expression of αβ1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci 20: 4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina N, Ihara Y 1986. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem 99: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM 2003. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 39: 409–421 [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT 1993. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 11: 575–580 [DOI] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, et al. 2007. The secreted β-amyloid precursor protein ectodomain APPs α is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci 27: 7817–7826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L 2007. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316: 750–754 [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holamn K, Tsuda T, et al. 1995. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376: 775–778 [DOI] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL 1989. Amyloid A4 protein and its precursor in Down’s syndrome and Alzheimer’s disease. N Engl J Med 320: 1446–1452 [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, et al. 2009. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73: 2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MachLachlan DR, Alberts MJ, et al. 1993. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43: 1467–1472 [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. 1999. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173–177 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. 1996. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nature Med 2: 864–870 [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD 1993. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci 90: 9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher MG, Whaley J, Swindlehurst C, et al. 1992. Isolation and quantitation of soluble Alzheimer’s β-peptide from biological fluids. Nature 359: 325–327 [DOI] [PubMed] [Google Scholar]
- Seubert P, Oltersdorf T, Lee MG, Barbour R, Blomqist C, Davis DL, Bryant K, Fritz LC, Galasko D, Thal LJ, et al. 1993. Secretion of β-amyloid precursor protein cleaved at the amino-terminus of the β-amyloid peptide. Nature 361: 260–263 [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. 2008. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14: 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S 1997. Skeletal and CNS defects in presnilin-1 deficient mice. Cell 89: 629–639 [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. 1995. Cloning of a novel gene bearing missense mutations in early onset familial Alzheimer disease. Nature 375: 754–760 [DOI] [PubMed] [Google Scholar]
- Shinsky N, Chen KS, Soriano F, Seubert P, Games D, Miljanich G, Freedman S, Schenk D 2002. Changes in LTP and other electrophysiological parameters found in hippocampal slices from PDAPP transgenic mice model of Alzheimer’s disease using A60-channel multi-electrode system. Soc Neurosci Abstr 28: 295–293 [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai X, McKay DM, Tintner R, Frangione B, et al. 1992. Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258: 126–129 [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, et al. 1999. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 402: 537–540 [DOI] [PubMed] [Google Scholar]
- Sisodia SS 1992. β-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci 89: 6075–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slunt HH, Thinakaran G, Von Koch C, Lo ACY, Tanzi RE, Sisodia SS 1994. Expression of a ubiquitous, cross-reactive homologue of the mouse β-amyloid precursor protein (APP). J Biol Chem 269: 2637–2644 [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B 1998. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci 95: 7737–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine WB Jr, Dahlgren KN, Krafft GA, LaDu MJ 2003. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Biol Chem 278: 11612–11622 [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD 1993. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci 90: 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abrahamowski D, Duke M, Wiederhold K-H, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, et al. 1997. Two amyloid precursor protein transgenic mouse models with Alzheimer disease–like pathology. Proc Natl Acad Sci 94: 13287–13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliavini F, Giaccone G, Frangione B, Bugiani O 1988. Preamyloid deposits in the cerebral cortex of patients with Alzheimer’s disease and nondemented individuals. Neurosci Lett 93: 191–196 [DOI] [PubMed] [Google Scholar]
- Terry RD, Hansen LA, DeTeresa R, Davies P, Tobias H, Katzman R 1987. Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropath Exp Neurol 46: 262–268 [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. 1996. Endoprotreolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17: 181–190 [DOI] [PubMed] [Google Scholar]
- Tokuda T, Fukushima T, Ikeda S, Sekijima Y, Shoji S, Yanagisawa N, Tamaoka A 1997. Plasma levels of amyloid β proteins Aβ1-40 and Aβ1-42(43) are elevated in Down’s syndrome. Ann Neurol 41: 271–273 [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT 2002. Neurotoxic effects of thioflavin S–positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci 99: 13990–13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. 1999. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286: 735–741 [DOI] [PubMed] [Google Scholar]
- Verbeek MM, de Wall RMW, Vinters HV, ed. 2000. Cerebral amyloid angiopathy in Alzheimer’s disease and related disorders. Kluwer Academic, Dordrecht [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Maggio JE, Condron MM, Teplow DB 1997. Amyloid β-protein fibrillogenesis: Detection of a protofibrillar intermediate. J Biol Chem 272: 22364–22374 [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB 1999. Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274: 25945–25952 [DOI] [PubMed] [Google Scholar]
- Walsh D, Klyubin I, Fadeeva J, William K, Cullen W, Anwyl R, Wolfe M, Rowan M, Selkoe D 2002. Naturally secreted oligomers of the Alzheimer amyloid β-protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539 [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM 1999. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol 158: 328–337 [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R 2004. Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24: 3370–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasco W, Bupp K, Magendantz M, Gusella J, Tanzi RE, Solomon F 1992. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease–associated amyloid β-protein precursor. Proc Natl Acad Sci 89: 10758–10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K 1989. Identification, biogenesis and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell 57: 115–126 [DOI] [PubMed] [Google Scholar]
- Weidemann A, Paliga K, Durrwang U, Czech C, Evin G, Masters CL, Beyreuther K 1997. Formation of stable complexes between two Alzheimer’s disease gene products: Presenilin-2 and β-amyloid precursor protein. Nat Med 3: 328–332 [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski BL, Rahmati T, Donkor IO, Selkoe DJ 1999a. Peptidomimetic probes and molecular modeling suggest Alzheimer’s γ-secretase is an intramembrane-cleaving aspartyl protease. Biochem 38: 4720–4727 [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ 1999b. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398: 513–517 [DOI] [PubMed] [Google Scholar]
- Wong P, Zhen H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Proce DL, Van der Ploeg LHT, Sisodia SS 1997. Presenilin 1 is required for Notch 1 and D111 expression in the paraxial mesoderm. Nature 387: 288–292 [DOI] [PubMed] [Google Scholar]
- Wu CC, Chawla F, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE 2004. Selective vulnerability of dentate granule cells prior to amyloid deposition in PDAPP mice: Digital morphometric analyses. Proc Natl Acad Sci 101: 7141–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Zhang J, Perez R, Koo EH, Selkoe DJ 1997. Interaction between amyloid precursor protein and presenilins in mammalian cells: Implications for the pathogenesis of Alzheimer’s disease. Proc Natl Acad Sci 94: 8208–8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Harigaya Y 1988. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol 77: 113–119 [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, et al. 1999. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 402: 533–537 [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ 2007. A critical function for β-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 27: 14459–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM 2010. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 30: 11938–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Koo EH 2006. The amyloid precursor protein: Beyond amyloid. Mol Neurodegener 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJS, Hopkins R, Smith DW, Heavesn RP, Dawson GR, Boyce S, Conner MW, et al. 1995. β-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81: 525–531 [DOI] [PubMed] [Google Scholar]