Abstract
The mechanisms underlying violence and aggression and its control remain poorly understood. Using the Resident-Intruder paradigm, we have discovered that resident mice with combined deletion of TNF receptor type 1 (TNF-R1) and type 2 (TNF-R2) genes show a striking absence of aggressive behavior, which includes fighting, sideways postures, and tail rattling. In parallel, resident TNF-R1 and TNF-R2 knockout mice show an increase in non-aggressive exploration of the intruder mice. Given the relationship between aggression and anxiety, we also measured anxiety-related behavior, as reflected by performance in the Open Field and the Light-Dark Choice Test. Compared with wild type mice, TNF-R1 and TNF-R2 deficient mice spent significantly more time and showed increased movement in the center of the Open Field and in the illuminated compartment of the light-dark box, suggesting an anxiolytic-like profile. Together, these data show that combined deletion of TNF-R1 and TNF-R2 results in a striking absence of aggressive behavior, an increase in non-aggressive exploration, and anxiolytic-like effects. These findings identify potent roles for TNF in regulating aggression and anxiety-related behavior, and suggest that TNF receptor signaling tonically modulates activity in brain regions underlying these behaviors.
Keywords: TNF, TNF receptors, TNF-R1, TNF-R2, aggression, anxiety, proinflammatory cytokines
1. Introduction
In recent years, several groups have reported that a positive relationship exists between pro-inflammatory cytokines and aggression/hostility (see Zalcman and Siegel, 2006). For example, Suarez and colleagues showed in healthy male subjects that scores on the Buss-Perry Aggression Questionnaire, which measures hostility, anger, and aggression, were associated with increased production of tumor necrosis factor (TNF)α (Suarez et al., 2002). A positive relationship between hostility and pro-inflammatory cytokine production was likewise found in a study of male Dutch military personnel before deployment (Mommersteeg et al., 2008) and in a study of healthy middle aged adults (Marsland et al., 2008). Studies using patients’ population, receiving cytokine immunotherapy, provide further support for the link between cytokines and anger/hostility (Kraus et al., 2003; McHutchison et al., 1998). A possible genetic basis for this relationship is suggested by a study by Petitto and colleagues who showed that cytokine production was higher in mice bred for high aggression than in mice bred for low aggression (Petitto et al., 1994). Together, these studies show that a positive association exists between pro-inflammatory cytokines and measures of aggressive behavior in patient and non-patient populations. It is also noteworthy that exposure to conflict in humans, and aggressive encounters or social disruption in rodents also modulate immune function (Glaser et al., 2005; Avitsur et al., 2005; Stefanski, 2001).
Studies conducted in our laboratory have focused on the role of pro-inflammatory cytokines and their receptors in the hypothalamus and midbrain periaqueductal gray (PAG) in modulating feline aggression (Zalcman and Siegel, 2006). We recently extended these studies to identify cytokines that mediate the effects of LPS on aggression in cats. We found that LPS-induced modulation of defensive rage is mediated, in part, through peripheral TNF-α (Bhatt et al., 2009). Immunocytochemical studies further revealed that TNF receptors were present in abundant quantities throughout defensive rage sites in the medial hypothalamus (unpublished finding). These studies suggest a role for TNF signaling in modulating aggressive behavior. Over the past decade, a number of studies have utilized TNF receptor deficient mice to study such processes as fear conditioning (Simen et al., 2006) and sickness behavior (Palin et al, 2009) which establishes the use of this model for the study of other related behavioral processes. Thus, to better understand the role played by TNF signaling in aggression, we sought to evaluate the effects of TNF receptor deletion on the varied components of aggressive behavior and related processes.
2. Material and Methods
2.1 Subjects
Male wild type (WT) (B6129SF2/J) (n=6) and combined TNF receptor type 1 and type 2 knockout (KO) mice [B6129S-Tnfrsf1atm1ImxTnfrsf1btm1Imx/J] (n=6) from different litters, were purchased from Jackson Laboratories, Bar Harbor, Maine at 5-wk of age. The mice were housed in standard polypropylene ‘shoebox’ cages in groups of four until one week prior to testing whereupon they were individually housed. The animals were maintained on a 12 hour light/12 hour dark light cycle (7 am – 7 pm), and permitted free access to standard laboratory chow and water. Changes in body weight occurring during the period beginning 1-week after arrival of the mice in our facility and ending upon termination of testing were as follows: WT mice gained 4.0 ± 0.25 g while TNFR-KO mice gained 4.0 ± 0.20 g. At the end of testing, the mice were euthanized by CO2.
2.2 Procedure
To study the role played by TNF receptors in aggression, we evaluated the varied components of aggressive behavior in wild type (WT) mice and combined TNFR KO mice in a resident-intruder paradigm. We conducted additional tests to determine the effects of TNFR gene deletion on related processes that may have contributed to or confounded effects on aggression, including anxiety-like behavior (open field center:margin time ratios, performance in the light-dark choice test) and social behavior (non-aggressive investigation of a sex matched conspecific). Testing began on PD60. The mice were exposed to tests with a 1-week inter-test interval. The mice were exposed to the resident-intruder test followed by the open-field test and the light-dark choice test. Studies in our laboratory have consistently found that the order of testing does not affect responses in these paradigms.
Aggressive Behavior
We used the resident-intruder paradigm to study intermale aggression. WT and combined TNF-R1 and TNF-R2 KO mice served as residents and non-aggressive Balb/c mice (Charles River Labs, MA) served as intruders. During the latter phase of the light cycle (i.e., approximately 4PM) an intruder mouse was introduced into the home cage of a resident mouse for 15-min. The session was videotaped and scored at a later date by an experienced rater. The following resident behaviors were measured: fighting (the incidence and duration of bouts), aggressive postures (sideways postures and tail rattling), and pursuit episodes. We also measured the time spent engaged in non-aggressive contact (exploratory sniffing) of the intruder.
Anxiety-related behavior
To determine whether TNF receptors affect anxiety related behavior, which is positively correlated with aggression, we determined whether combined TNF-R1 and TNF-R2 deletion affects responding in an open field and the Light-Dark Choice Test.
Open Field
The mice were individually placed into an open field (automated TruScan test arena, Coulbourn Inc., PA) for 2-hr in normal illumination. The test arenas are equipped with photobeam sensor rings that sense in the X-Y dimension and in the Z plane vertical dimension (for rearing). Precision is twice the arena’s beam resolution (.76 cm for beam spacing). Each arena is linked via a station interface box to a computer, which runs on Windows 98. The ratio of the time spent in the center and margins of the arena, which reflects, at least in part, anxiety-related responding, was subsequently determined. Mice typically spend more time along the margins of the open field. Thus, a decrease in the time spent in the center of the arena relative to the margins suggests anxiogenic-like behavior while an increase suggests anxiolytic-like behavior.
Light-Dark Choice Test
To better characterize anxiety-related behavior, we also exposed the mice to the light-dark choice test. When given a choice between a well illuminated area and a darkened area, mice typically spend more time in the darkened area. An increase in the time spent in the illuminated area is thought to reflect anxiolytic like responding. A black opaque box was placed into each of the automated TruScan test arenas thereby dividing the arena into ‘light’ and ‘dark’ compartments. The ‘light’ compartment was illuminated with a 40W bulb. The mice were individually placed into the dark compartment and behavior was recorded for 10-min. The following behaviors were measured: the number of moves, and movement time in each compartment.
3. Results
Aggressive Behavior
To evaluate the effects of TNF receptor deletion on the varied components of aggressive behavior, we tested mice in a Resident-Intruder paradigm, in which wild type and TNF receptor deficient mice served as residents, and a non-aggressive Balb/c mouse served as intruders. The results showed a significant effect of genotype on fighting (number of bouts) F (1, 10) = 16.33, p < .005 (Figure 1A).
Figure 1.
Measures of aggressive behavior (mean + s.e.m.) in Wild Type and TNF-R1 and TNF-R2 deficient mice (TNF-R1/TNF-R2 −/−) in the Resident-Intruder paradigm. A striking absence in the number of Bouts (A), Sideways Postures (B), and Tail Rattling (C), and a marked decrease in Pursuit Episodes (D) was evident in TNFR deficient mice. Non-aggressive exploratory sniffing was significantly increased (p <.05) in TNFR deficient mice.
Indeed, there was a striking absence of fighting in the TNFR deficient mice. Whereas WT mice had 7 + 1.7 bouts per 15-min test session, KO mice had 0 bouts. In WT mice, the mean duration of bouts was 41.6 + 6.8 s. Of further importance, there were significant between-group differences in aggressive postures, including sideway postures (F (1, 10) = 12.79, p = .005; Figure 1B), tail rattling (F (1, 10) = 12.62, p = .006; Figure 1C), and pursuit episodes (F (1, 10) = 5.69, p < .05), Figure 1D). It should be underscored that as was the case for fighting, a striking absence of sideways postures, and tail rattling was evident in TNFR deficient mice. Regarding pursuit, two-thirds of the TNFR deficient mice tested showed an absence of pursuit episodes. Parenthetically, data for pursuit are presented as percent control given the inter squad variability in responses (but not in direction of effect). It is also important to note that TNFR deficient mice spent significantly more time than WT mice in non-aggressive exploration of the intruder (Figure 1E). Thus, when confronted with an intruder mouse, resident TNF-R1 and TNFR2 deficient mice showed a lack of aggressive behavior and an increase in nonaggressive exploration.
Anxiety-Related Behavior
To study anxiety-related behavior, we evaluated performance in the Open Field and in the Light-Dark Choice Test. In the Open field, a decrease in the time spent in the center of the arena suggests anxiogenic-like responding while an increase in center time reflects anxiolytic-like responding. In the Light-Dark Choice Test, an increase in activity in the dark compartment is thought to reflect anxiogenic-like responding while increases in the illuminated compartment is thought to reflect anxiolytic-like effects.
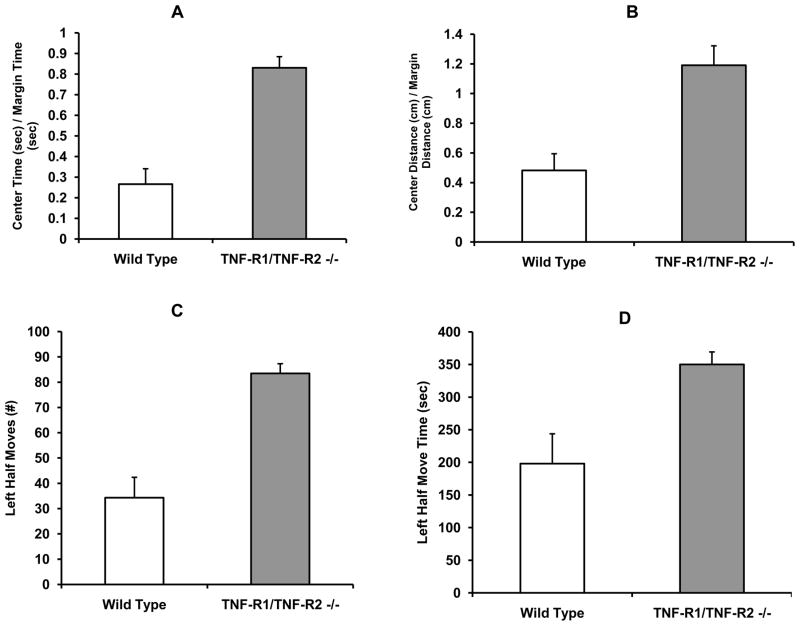
The ratios of the time spent and the distance traveled in the center of the open field relative to the margins were markedly higher in TNFR deficient mice than in WT mice, F (1,10) = 37.56, p = .0001 (Figure 2A), and F (1,10) = 16.93 (Figure 2B), respectively. In the Light-Dark Box, the number of moves (F (1, 10 = 30.46, p = .0002; Figure 2C) and move time (F (1, 10 = 9.39, p =. 01; Figure 2D) in the illuminated compartment were also significantly increased in TNFR deficient mice compared to WT mice. Thus, TNFR deficient mice displayed anxiolytic-like responding in both the open field and in the Light-Dark Box.
Figure 2.
Effects of TNF receptor deficiency on anxiety-related behavior (mean + s.e.m.) in the Open-Field and Light-Dark Choice Test. Compared with Wild Type mice, TNF-R1 and TNF-R2 deficient mice (TNF-R1/TNF-R2 −/−) exhibited an significant increase in anxiolytic-like behavior as reflected by an increase in the amount of time spent (A) and distance traveled (B) in the center relative to the margins of the open-field. Of further importance, the number moves (C) and amount of time (D) in the illuminated (Left Half) of the Light-Dark Box was significantly increased (p <.05) in TNFR deficient mice.
Collectively, these data show that deletion of TNF-R1 and TNF-R2 results in a striking lack of aggressive behavior, an increase in non-aggressive behavior, and anxiolytic-like effects. These findings support the view that activation of TNF receptors and thus TNF receptor signaling is associated with heightened aggression and related processes.
4. Discussion
We found that combined deletion of TNF-R1 and TNF-R2 results in a striking absence of aggressive behavior, which includes fighting and various aggressive postures. In parallel, increases in non-aggressive exploration and anxiolytic-like responding were evident in these mice. These findings provide support for the view that activation of TNF receptors potently regulates aggression and anxiety-related behavior in rodents.
This study did not permit any conclusions to be drawn regarding possible differential effects of TNF-R1 and TNF-R2 in their modulation of either aggression or anxiety-like behavior. Nonetheless, it is of interest to note that Simen et al. (2006) showed that deletion of TNF-R1 results in decreased fear conditioning. It is well established that fear and fear conditioning are clearly linked to aggressive behavior and, in fact, it is recognized and constitutes one of the early and fundamental models of aggression (Moyer, 1968; Siegel, 2005). Thus, in light of the fact that rodents showing increased fear conditioning likewise show increased aggression, it is tempting to speculate that TNF modulation of aggressive behavior is mediated through TNF-R1. Moreover, Simen and colleagues (2006) further showed that deletion of TNF-R1 or TNF-R2 does not appreciably affect anxiolytic-like responding. In view of the linkage between fear (anxiety) and aggression, and since TNF-R1 and TNF-R2 deficient mice presently showed anxiolytic-like behavior, we suggest that combined deletion of TNF-R1 and TNF-R2 is required to affect anxiety-related behavior. Of further importance, in light of the discovery made by Palin and colleagues that TNF-R1 mediates TNF-α-induced sickness behavior (Palin et al., 2009), we tentatively conclude that the present effects of TNFR deletion on non-aggressive exploration are likewise mediated by TNF-R1.
A separate issue that may be raised is whether the inhibitory effects of TNF deletion upon aggressive and anxiety related behaviors are due to non-specific factors. For example, it could be argued that TNF deletion causes a non-selective depression of motor responses. Such a possibility may be ruled out since such responses were clearly demonstrated in tests of anxiety and exploratory conditions (i.e., as shown in Fig. 2, TNFR-KO mice show increased motor activity in the Open Field (center:margin ratios), and an increase in the number of moves in the illuminated portion of the Light-Dark Box.). Consistent with this finding, TNFR-KO mice did not exhibit freezing behavior. Parenthetically, it has been shown that, anti-anxiety drugs (e.g., diazepam) may affect anxiety-related behavior in the open field without affecting total locomotion, suggesting that motor activity may be dissociated from anxiety-related behavior (Choleris et al., 2001). Anxiolytic-like responses are similarly not necessarily related to changes in motor activity. Moreover, deletion of TNF receptors has been shown to result in other abnormal behaviors or responses that may influence aggression, notably sleep disturbances (Deboer et al., 2002; Fang et al., 1997) and enhanced stress responses (Gimsa et al., 2009). However, such effects would be expected to potentiate aggressive behavior. Moreover, it is possible that TNFR deletion results in a release of other compensatory cytokines. It remains to be determined whether relevant site-specific compensatory increases in inflammatory cytokines occur in the CNS of unchallenged TNF receptor deficient mice, and whether possible changes modulate aggressive behavior. A possible role for IL-1 may be tentatively ruled out, however; at least in the cat, IL-1 in the medial hypothalamus and PAG facilitates aggressive behavior (see Zalcman and Siegel, 2006), although species differences with regards to cytokine effects on aggression need to be considered.
The neural substrates underlying the lack of aggressive behavior in TNF knockout receptor mice remain to be identified. Nonetheless, evidence from studies conducted in the cat suggest that pro-inflammatory cytokines and their receptors potently modulate aggression and rage in the hypothalamus and midbrain periaqueductal gray (PAG), which comprise basic components of the limbic-midbrain axis (Siegel et al, 1999). Specifically, it has been shown that cytokine receptor activation in the hypothalamus and PAG markedly alters response latencies for defensive rage behavior in cats (e.g., Bhatt and Siegel, 2006; Hassanain et al., 2003). Of particular importance with respect to these studies, the modulating effects of these cytokines upon the rage response were mediated through known neurotransmitter systems (see Zalcman and Siegel, 2006). The implication of these studies with respect to the present observations is that TNF receptor activation in the medial hypothalamus and PAG likely play principal roles in mediating the potentiating effects upon aggressive behavior seen in TNFR knockout mice.
Inasmuch as TNF receptors are found in the limbic-midbrain axis (Bette et al., 2003; Simen et al., 2006), future studies should aim to identify specific sites of action. It is reasonable to suggest that, based upon studies conducted in cats, affective aggression (expressed in the present study as fighting behavior) is generated through a mechanism situated in the medial hypothalamus and ultimately upon neurons in the midbrain PAG (Siegel et al, 1999; Siegel et al., 2007). It is of interest to note that a somewhat similar projection pattern was described in the rat concerning descending projections from the hypothalamus to the midbrain (Roeling, et al, 1994). Accordingly, we further suggest that such a mechanism involves the activation of TNF receptors present in the medial hypothalamus whose excitatory functions are mediated through known neurotransmitter systems (see Anisman et al., 2003; Dunn, 2006) in these regions of the brain such as 5-HT2, catecholamine receptors, or NK1 receptors, all of which are known to potentiate rage behavior (Siegel et al, 1999). Moreover, since it has been established that the output functions of the medial hypothalamus are mediated through excitatory NMDA receptors in the dorsal PAG in cats (Lu et al., 1992; Schubert et al, 1996), we speculate that a similar mechanism may likewise be present in the rodent, in which the midbrain PAG is then viewed as the most caudal region of the CNS linked to the integration of fighting and rage behavior.
In view of the similar effects of TNF upon both aggression and anxiety seen in the present study, it is possible that overlapping neural structures underlie TNF modulation of these processes. It has recently been discovered that anxiety and related behaviors are associated with the PAG (Cunha et al., 2009) and the amygdala (LeDoux, 1998; Roozendaal et al., 2009). Therefore, it is possible that TNF modulation of anxiety and aggression are mediated through actions upon receptors in these regions. Other components of the limbic-midbrain axis known to modulate aggression remain possibilities as well, including the hypothalamus, prefrontal cortex, hippocampal formation and septal area (Siegel et al., 1999).
In conclusion, the present data identifies for the first time a previously unknown potent role that TNF receptors play in the expression of aggressive behavior in rodents. That aggressive behavior was absent in TNFR deficient mice suggests that TNF normally exerts a tonic potentiating effect on aggression. Presumably, these effects are manifest through interactions with neurotransmitters in the medial hypothalamus and PAG. We suggest that a greater understanding of this phenomenon may be gained by identification of molecular mechanisms governing TNF potentiation of aggressive behavior.
Acknowledgments
Supported by NIH grant R01 MH74689 (SSZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Merali Z, Hayley S. Sensitization associated with stressors and cytokine treatments. Brain Behav Immun. 2003;17(2):86–93. doi: 10.1016/s0889-1591(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19(4):311–7. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Bette M, Kaut O, Schäfer MK, Weihe E. Constitutive expression of p55TNFR mRNA and mitogen-specific up-regulation of TNF alpha and p75TNFR mRNA in mouse brain. J Comp Neurol. 2003;465(3):417–30. doi: 10.1002/cne.10877. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Bhatt RS, Zalcman SS, Siegel A. Peripheral and central mediators of lipopolysaccharide induced suppression of defensive rage behavior in the cat. Neuroscience. 2009;10;163(4):1002–11. doi: 10.1016/j.neuroscience.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Siegel A. Potentiating role of interleukin 2 (IL-2) receptors in the midbrain periaqueductal gray (PAG) upon defensive rage behavior in the cat: role of neurokinin NK(1) receptors. Behav Brain Res. 2006;167(2):251–60. doi: 10.1016/j.bbr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001 May;25(3):235–60. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985 Spring;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Zanoveli JM, Ledvinka-Filho E, Brandão ML. l-Allylglycine dissociates the neural substrates of fear in the periaqueductal gray of rats. Brain Res Bull. 2009;16;81(4–5):416–23. doi: 10.1016/j.brainresbull.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Deboer T, Fontana F, Tobler I. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol. 2006;88:839–846. doi: 10.1152/jn.2002.88.2.839. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDA receptor fail to sleep more after TNFa treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa U, Kanitz E, Otten W, Tuchscherer M, Ibrahim S. Increased response to psychological stress in TNFG-recdeptor deficient mice. Brain Behav Immun. 2009;23(Suppl 1):S12–S13. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Zalcman S, Bhatt S, Siegel A. Interleukin-1β in the hypothalamus potentiates feline defensive rage:Role of serotonin-2 receptors. Neuroscience. 2003;120:227–33. doi: 10.1016/s0306-4522(03)00264-1. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schäfer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64(6):708–14. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The Mysterious Underpinnings of Emotional Life. New York: Simon and Shuster; 1998. The Emotional Brain. [Google Scholar]
- Lu CL, Shaikh MB, Siegel A. Role of NMDA receptors in hypothalamic facilitation of feline defensive rage elicited from the midbrain periaqueductal gray. Brain Res. 1992 May 22;581(1):123–32. doi: 10.1016/0006-8993(92)90351-9. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers of trait negative emotionality. Brain Behav Immun. 2008;22(5):753–61. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. New Eng J Med. 1998;339(21):1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PM, Vermetten E, Kavelaars A, Geuze E, Heijnen CJ. Hostility is related to clusters of T-cell cytokines and chemokines in healthy men. Psychoneuroendocrinology. 2008;33(8):1041–50. doi: 10.1016/j.psyneuen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Moyer KE. Kinds of aggression and their physiological basis. Communications in Behavioral Biology. 1968;2:65–87. [Google Scholar]
- Palin K, Bluthé RM, McCusker RH, Levade T, Moos F, Dantzer R, Kelley KW. The type 1 TNF receptor and its associated adapter protein, FAN, are required for TNFα-induced sickness behavior. Psychopharmacology (Berl) 2009;201(4):549–56. doi: 10.1007/s00213-008-1331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto JM, Lysle DT, Gariepy JL, Lewis MH. Association of genetic differences in social behavior and cellular immune responsiveness: effects of social experience. Brain Behav Immun. 1994;8(2):111–22. doi: 10.1006/brbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Schubert K, Shaikh MB, Siegel A. NMDA receptors in the midbrain periaqueductal gray mediate hypothalamically evoked hissing behavior in the cat. Brain Res. 1996 Jul 8;726(1–2):80–90. [PubMed] [Google Scholar]
- Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharmacol. 2007;5(2):135–47. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A. The neurobiology of aggression and rage. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brainstimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23(3):359–89. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiat. 2006;59(9):775–85. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress in laboratory rats: behavior, immune function, and tumor metastasis. Physiol Behav. 2001;73(3):385–91. doi: 10.1016/s0031-9384(01)00495-4. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun. 2002;16(6):675–84. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Zalcman SS, Siegel A. Neurobiology of aggression and rage: Role of cytokines. Brain, Behavior and Immunity. 2006;20:507–514. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]