Abstract
Deoxycytidine kinase (dCK) is a rate-limiting enzyme in the deoxyribonucleoside salvage pathway and a critical determinant of therapeutic activity for several nucleoside analog pro-drugs. We have previously reported the development of 18F-FAC, (1-(2′-deoxy-2′-18F-fluoro-β-D-arabinofuranosyl) cytosine), a new probe for PET imaging of dCK activity in immune disorders and certain cancers. The objective of the current study was to develop PET probes with improved metabolic stability and specificity for dCK. Towards this goal, several candidate PET probes were synthesized and evaluated in vitro and in vivo.
Methods
High pressure liquid chromatography was used to analyze the metabolic stability of 18F-FAC and of several newly-synthesized analogs with the natural D-enantiomeric sugar configuration or the corresponding unnatural L-configuration. In vitro kinase and uptake assays were used to determine the affinity of the 18F-FAC L-nucleoside analogs for dCK. The biodistribution of selected L- analogs in mice was determined by microPET/CT imaging.
Results
Candidate PET probes were selected using the following criteria: low susceptibility to deamination, high affinity for purified recombinant dCK, high uptake in dCK expressing cell lines and biodistribution in mice reflective of the tissue expression pattern of dCK. Amongst the ten newly-developed candidate probes, 1-(2′-deoxy-2′-18F-fluoro-β-L-arabinofuranosyl) cytosine (L-18F-FAC) and 1-(2′-deoxy-2′-18F-fluoro-β-L-arabinofuranosyl)-5-methylcytosine (L-18F-FMAC) most closely matched the selection criteria. The selection of L-18F-FAC and L-18F-FMAC was validated by showing that these two PET probes can be used to image animal models of leukemia and autoimmunity.
Conclusion
Promising in vitro and in vivo data warrant biodistribution and dosimetry studies of L-18F-FAC and L-18F-FMAC in humans.
Keywords: Deoxycytidine kinase (dCK), cytidine deaminase (CDA), PET probes, deoxyribonucleoside salvage, autoimmune and malignant lymphoproliferation
Introduction
Deoxycytidine kinase (dCK) catalyzes a rate-limiting phosphorylation step in the deoxyribonucleoside salvage pathway (1). dCK is unique among salvage enzymes due to its ability to provide cells with all four deoxyribonucleoside triphosphates (dNTPs) via direct (dCTP, dATP and dGTP) and indirect (dTTP) mechanisms (Supplemental Figure 1, reviewed in (1)). Highest levels of dCK are found in lymphocytes (2, 3), particularly during lymphopoiesis (reviewed in (1)). dCK expression is upregulated in activated T cells (4). dCK is also expressed in most lymphoid and myeloid malignancies and in some solid tumors (reviewed in (1)).
We have recently demonstrated a critical requirement for dCK in normal lymphocyte development in vivo (5). This requirement suggests that the rate-limiting function of dCK in deoxyribonucleoside phosphorylation is biologically significant. Moreover, dCK is therapeutically important. dCK phosphorylates and activates cytarabine, fludarabine, gemcitabine, cladribine, decitabine and clofarabine (reviewed in (6)). These nucleoside analogs are mainstay cytotoxic pro-drugs in the treatment of hematologic and solid tumors. Cladribine has completed phase III clinical trials in multiple sclerosis (7). Lamivudine and Emtricitabine, two other dCK-dependent nucleoside analogs, are approved by the FDA for therapy of HIV infections (8).
A common thread amongst dCK-dependent pro-drugs is the high degree of variability in their therapeutic responses. This heterogeneity may reflect genetic variations in deoxyribonucleoside metabolism (9) which confer resistance to therapy. Since decreased dCK activity is a common drug-resistance mechanism (reviewed in (10)), dCK-specific PET probes may enable patient stratification into likely responders and non-responders to dCK-activated pro-drugs.
We have recently described the development of 18F-FAC (Figure 1A) (4, 11), a new probe for PET imaging of the deoxyribonucleoside salvage pathway. In mice, 18F-FAC microPET enables imaging of adaptive immunity and of several types of cancer (4). 18F-FAC accumulation in tumor tissues is predictive of responses to gemcitabine (12). Here we analyzed the metabolic stability of 18F-FAC. In mice, 18F-FAC was found to rapidly undergo deamination to 1-(2′-deoxy-2′-18F-fluoro-β-D-arabinofuranosyl) uracil (18F-FAU). Deamination confounds the specificity of 18F-FAC for dCK and it may decrease its sensitivity in species with high deaminase activity such as humans (13). To bypass tracer deamination, a series of 18 F-FAC analogs were synthesized and evaluated. These studies indicated that 18F-FAC analogs with the unnatural L-enantiomeric sugar configuration resist deamination and allow PET imaging of dCK activity in vivo.
FIGURE 1. In vivo metabolite analysis of 18F-FAC.
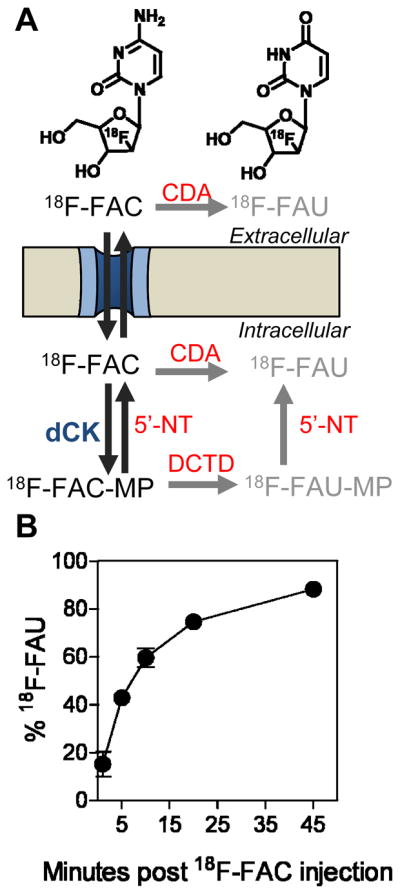
(A) Chemical structures of 18F-FAC and 18F-FAU and schematic showing the extracellular and intracellular routes by which 18F-FAC can be deaminated to 18F-FAU. CDA, cytidine deaminase; 5′-NT, 5′nucleotidase; DCTD, cytidylate deaminase (catabolic enzymes are shown in red fonts); (B) Percentage of total detected radioactivity in plasma that is attributable to 18F-FAC over time as determined by HLPC analysis. Analysis is carried out following intravenous injection of 18F-FAC in mice.
Materials and Methods
Radiochemical synthesis of 18F-labeled PET probes
18F-FAC was synthesized as previously described (4, 11). The radiochemical syntheses of the new probes are described in the Supplementary Materials and Methods section.
PET probe metabolite analyses
Mice were injected intravenously with 18F-labeled probes and allowed up to 45 min uptake. Tissues were removed and homogenized. Whole blood sampling was collected via retro-orbital eye bleeding, followed by centrifugation to obtain plasma. Tissues and plasma were treated with a mixture of methanol:acetonitrile (1:9) to extract nucleosides. Following centrifugation, supernatants were evaporated at 50°C under nitrogen. The residue is dissolved in 100 mL of mobile phase (5 mM Pentane-1-sulfonic acid, pH 3.1 and methanol (96:4)), filtered and injected into a Waters microBondapak C18 column with a flow rate of 1.5 ml/min. Peak-radioactivity is measured using a Bioscan coincidence detector.
In vitro enzymatic assays
Production of recombinant human cytidine deaminase (CDA) and dCK is described in the Supplementary Materials and Methods section. The deamination assay solution contained purified CDA (0.05 μg/μL) and 140 μM nucleoside analog in 0.125 M phosphate buffer, pH 7.6. The assay was carried out at 37ºC for 1 hour; products were resolved via HPLC using a Waters C18 μBondapak column (3.9 × 300 mm), as previously reported (14). Elution was isocratic using 3% methanol in 0.l M phosphate buffer (pH 5.5) at a flow rate of 1 mL/min. The rate of dCK-catalyzed phosphorylation was measured continuously at 340 nm using an iEMS Reader MF (LabSystems) system and a modification of a previously described spectrophotometric assay consisting of a coupled lactate dehydrogenase (LDH)/pyruvate kinase (PK) reaction (15). Reactions were carried out in triplicates at 37°C with 50 mM Tris-HCl pH 7.6, 50 mM KCl, 10 mM MgCl2, 5 mM ATP, 0.2 mM NADH, 1 mM phosphoenolpyruvate, 1 mM DTT, 1.4 μM dCK and PK 10 U/mL (Sigma) and LDH 15 U/mL (Sigma). Kinetic constants were calculated using the GraphPad Prism 5 software.
Cell-based phosphorylation and uptake competition assays using 3H-labeled deoxycytidine (3H-dC)
L1210 cells (12) were lysed by 3 cycles of freeze-thawing in 50 mM Tris-HCl, pH 7.6, 2 mM DTT, 20% glycerol, 0.5% Nonident P40. The lysate was clarified by centrifugation (15,000 g for 10 min) and the protein concentration in the supernatants was determined using the Bradford assay. The kinase reaction mixture contained 4.2 μg of L1210 lysate, 3 μM 3H-dC (Moravek Biochemicals, specific activity 35 Ci/mmol), 50 mM Tris-HCl, pH 7.6, 5 mM MgCl2, 1 mM UTP (Sigma), 2 mM DTT, 10 mM NaF, 1 mM thymidine (to inhibit thymidine kinase 2) and 30 μM nucleoside analog. The reaction was incubated at 37ºC for 20 min and was stopped by the addition of 30 μL of ice cold water followed by heating at 90ºC for 3 min. The reaction was then spotted on Whatmann DE81 filter disks; these were washed 3 times with 4 mM ammonium formate and twice with ethanol. Disks were placed in scintillation vials and counted using a Beckman Liquid Scintillation counter. For uptake assays, 1.05 of μCi of 3H-dC were added to L1210 cells (105 cells/well in 96-well plates) followed by 3 μL of 100 μM stocks of each nucleoside analog (at a final volume of 100 μL/well). After 1 hour at 37ºC, wells were washed 3 times with ice-cold PBS using a vacuum filtration system (Millipore). Plates were oven-dried at 45–50ºC for 10 minutes, and 200 μL of scintillation fluid were added/well. Radioactivity was measured using a Trilux® MicroBeta scintillation counter (Perkin Elmer).
MicroPET/CT imaging studies in mice
In vivo studies followed the guidelines of the Department of Laboratory Animal Medicine at UCLA and were performed as previously described (4, 12) (see the Supplementary Materials and Methods section). Autoimmune B6.MRL-Faslpr/J mice were obtained from The Jackson Laboratory (stock number: 000482).
Statistical Analysis
Data are presented as means ± SD (standard deviation). All P values are two-tailed and P values of <0.05 are considered to be statistically significant. Graphs were generated using the Prism 5 software.
Results and Discussion
18F-FAC is rapidly deaminated in vivo
With the exception of the fluorine substitution of the ara-2′ hydrogen, FAC is identical to deoxycytidine (dC). It is therefore likely that 18F-FAC and dC are competitive substrates and share the same metabolic pathway. According to the model shown in Figure 1A, 18F-FAC is either phosphorylated by cytosolic dCK to 18F-FAC-monophophate (18F-FAC-MP) or is converted to 18F-FAU by extracellular or cytosolic cytidine deaminase (CDA). 18F-FAU, a weak substrate for thymidine kinase 1 (TK1) (16), cannot be phosphorylated by dCK. 18F-FAU can also be produced from 18F-FAC by a CDA-independent mechanism involving cytidylate deaminase (which converts 18F-FAC-MP to 18F-FAU-MP) and 5′nucleotidase (which dephosphorylates 18F-FAU-MP producing 18F-FAU, Figure 1A). To validate this model, mice were intravenously injected with 18F-FAC and plasma samples were analyzed by HPLC. 18F-FAU was the only 18F-FAC metabolite detected in plasma by this HPLC method (Supplemental Figure 2A). As early as 1 minute post injection, 18F-FAU contributed to ~14% of the total radioactivity in the plasma (Figure 1B). Ten minutes later, the contribution of 18F-FAU increased to ~60% and reached ~90% at 45 minutes. The plasma clearance rate of 18F-FAU was significantly slower than that of 18F-FAC (Supplemental Figure 2B). Similar to plasma, thymus and liver samples from 18F-FAC-injected mice also contained high concentrations of 18F-FAU (Supplemental Figure 2C) at 45 minutes after injection.
18F-Clofarabine (2-chloro-2′-deoxy-2′-18F-fluoro-9-β-D-arabinofuranosyl-adenine, 18F-CA), a potential dCK-specific PET probe that resists deamination
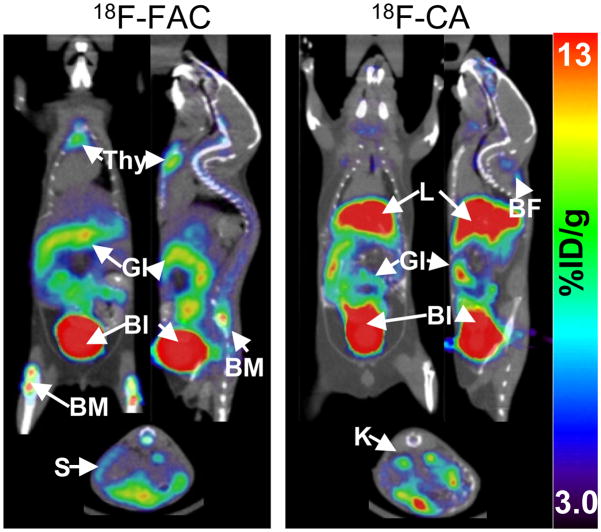
The fast exponential decline of 18F-FAC in plasma and the accumulation of the 18F-FAU metabolite in plasma and tissues reduce the specificity of 18F-FAC. They may also affect the sensitivity of 18F-FAC PET in humans who express twice as much deaminase activity than mice in most tissues (13). This problem can be solved by identifying dCK substrates that are amenable to 18F labeling and have low affinity for deaminase enzymes. Clofarabine (Supplemental Figure 3), is a dCK-dependent pro-drug with excellent metabolic stability (reviewed in (17)). The 2-halogenated aglycone of clofarabine confers resistance to deamination by adenosine deaminase. The 2′ fluorine atom blocks cleavage of the glycosidic bond by bacterial purine nucleoside phosphorylase (17). Clofarabine is efficiently phosphorylated by dCK (18) and is amenable to 18F labeling (Supplemental Figure 3). Surprisingly, 18F-FAC and 18F-labeled clofarabine (18F-CA) showed distinct biodistribution patterns in immunocompetent (C57/BL6) mice (Figure 2). In contrast to 18F-FAC (4), 18F-CA accumulation in expected target organs such as thymus, bone marrow and spleen was undetectable. Whether the differences in the biodistribution of 18F-FAC and 18F-CA are unique to mice or can also be found in other species, including humans, will be addressed in future studies.
FIGURE 2.
18F-FAC and 18F-CA microPET/CT scans of C57/BL6 mice (images are representative of the pattern observed in 3 mice scanned with each probe). Bl, bladder; BM, bone marrow; GI, gastrointestinal tract; L, liver; S, spleen; SG, salivary gland; Thy, thymus; K, kidney, BF, brown fat.
FAC analogs as potential dCK-specific probes
The disappointing performance of 18F-CA in mice indicated that a different approach was required to solve the tracer deamination problem. Towards this end, the FAC pharmacophore was explored to seek pyrimidine analogs matching three criteria: (i) amenability to routine 18F-radiolabeling with high specific activity; (ii) resistance to deamination; (iii), affinity for dCK comparable to that of 18F-FAC. Nine potential dCK-specific probes (Figure 3) were designed and synthesized. The rationale for selecting these compounds was twofold. First, modifications of deoxycytidine at position 5 of the nucleobase have been shown to reduce susceptibility to deamination (19). Second, dCK has the unusual property of phosphorylating both D-enantiomers (the natural configuration of nucleosides), and unnatural L-enantiomers (20, 21). In contrast, CDA has a strong preference for D- over L- deoxycytidine analogs (20). The susceptibility of FAC analogs to deamination was determined by incubating them with purified recombinant human CDA followed by HPLC analysis to determine the formation of deaminated products. Figure 3 shows that the nucleosides with the D-chirality were completely deaminated while the unnatural L-nucleosides were resistant to deamination. While these modifications solve the deamination problem it is possible that they also reduce the affinity of the FAC analogs for dCK. This possibility was addressed using several assays.
FIGURE 3. Candidate dCK-specific PET probes that resist deamination.
(A) Chemical structures of deoxycytidine analogs amenable to 18F-labeling. (B) In vitro deamination assay. Following incubation at 37°C for 1 hour in the presence (red solid traces) or absence (blue dashed traces) of recombinant purified CDA, candidate probes were analyzed on HPLC. D-analogs are shown in the top row and the L-analogs are shown in the bottom row.
First, a lactate dehydrogenase (LDH)/pyruvate kinase (PK)-coupled kinase reaction (15) was used to determine the kinetic parameters of human recombinant dCK for L-analog candidate probes. Kinetic parameters for three of the five L-analogs were obtained (Table 1). L-FMAC and L-FCAC had similar kinetics with Km values of 1.0 μM and 0.6 μM, respectively. These Km values were similar to that of FAC (0.8 μM). L-FMAC and L-FCAC also had specificity constants (kcat/Km) for dCK that were similar to FAC (0.96–1.2 times that of FAC). However, this was not the case for L-FBAC which had a higher Km and a lower specificity constant than FAC.
TABLE 1.
Kinetic parameters for FAC and its unnatural analogs with L-chirality. The kcat and specificity constant (kcat/Km) were determined using purified recombinant human dCK, and the values are given relative to FAC.
| Probe | Km (μM) | Relative kcat | Relative kcat/Km |
|---|---|---|---|
| FAC | 0.76 ± 0.15 | 1.0 | 1.0 |
| L-FAC | ND* | ND | ND |
| L-FMAC | 1.02 ± 0.15 | 1.0 | 0.96 |
| L-FFAC | ND | ND | ND |
| L-FCAC | 0.61 ± 0.09 | 0.96 | 1.20 |
| L-FBAC | 6.54 ± 0.99 | 2.70 | 0.31 |
ND = Not Determined.
The LDH/PK assay only determines the kinetics for phosphorylation reactions that are faster than the UV-induced decomposition of NADH (22). The inability to obtain the kinetic parameters for L-FAC and L-FFAC using the LDH/PK assay indicated that these nucleoside analogs are either high affinity substrates for dCK with low enzymatic turnover, or that they lack affinity for dCK. To determine if L-FAC and L-FFAC were phosphorylated by dCK, kinase assays (23) were performed using 18F-labeled probes (synthesized according to the scheme shown in Supplemental Figure 4) and purified recombinant human dCK as well as lysates from L1210 dCK positive and negative cell lines (12). The kinase assays showed conclusively that L-FAC and L-FFAC are indeed dCK substrates (Supplemental Figure 5).
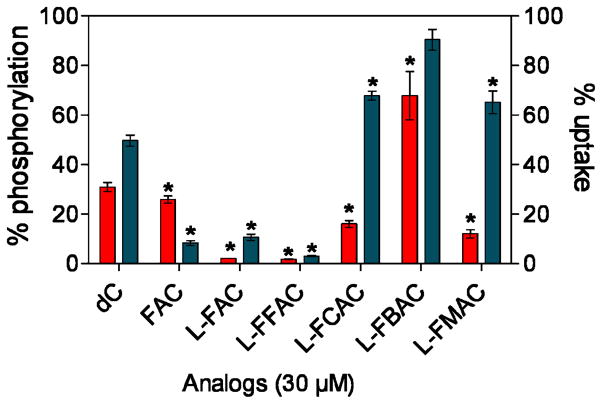
Two cell-based assays were then used to determine whether the L-analogs can compete with the endogenous dCK substrate deoxycytidine (dC). In a kinase assay using whole-cell lysates of the dCK positive murine leukemia cell line L1210 (12), L-FAC and L-FFAC outperformed the other nucleoside analogs by inhibiting 3H-dC phosphorylation by over 95% (Figure 4). The enhanced activity of dCK towards L-FAC and L-FFAC compared to FAC is not entirely surprising as the enzyme has been previously shown to favor the L-conformation of other pyrimidine analogs (21, 24). Confirming the results from the LDH/PK assay, L-FBAC inhibited dC phosphorylation by only 32%. The ability of L-nucleosides to competitively inhibit 3H-dC uptake by L1210 cells was also determined (Figure 4). In addition to dCK-mediated phosphorylation, the uptake assay takes into account the efficiency of transport across the cell membrane. L-FAC and L-FFAC reduced the uptake of 3H-dC by at least 90%. The inhibition by L-FCAC and L-FMAC was 32–35%.
FIGURE 4. Analyses of the affinity of the L-analogs for dCK.
These were tested for their ability to competitively inhibit the phosphorylation (left axis) and uptake (right axis) of tritium-labeled deoxycytidine (3H-dC) using the dCK-expressing L1210 cells. Results are representative of 2 independent experiments. (P values are calculated relative to water (n=3). * = P<0.05.
Biodistribution of the 18F-labeled L-nucleosides in immunocompetent C57/Bl6 mice
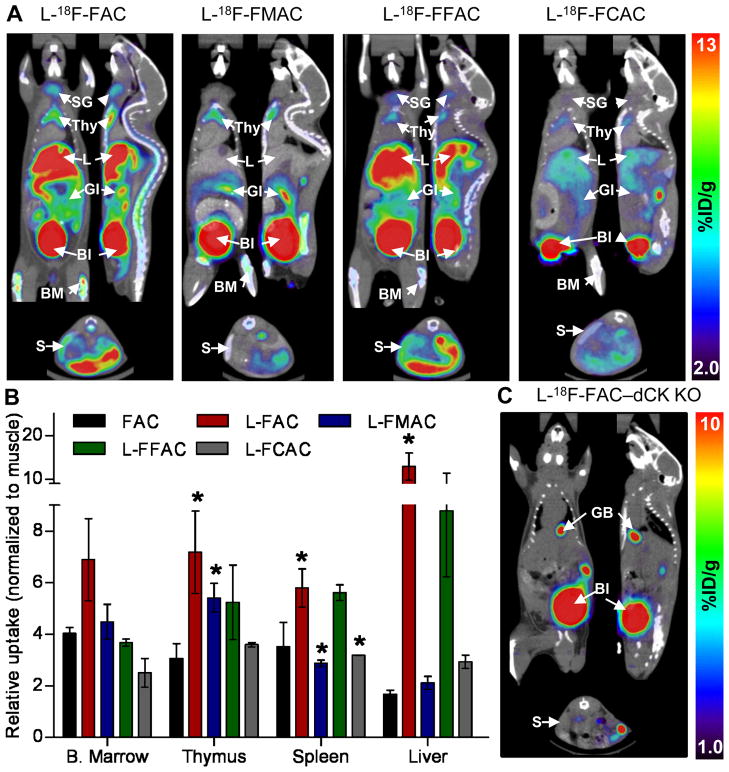
Results from the in vitro phosphorylation and uptake assays narrowed down the selection of candidate L-enantiomer probes to L-18F-FAC, L-18F-FMAC, L-18F-FFAC and L-18F-FCAC. Similar to 18F-FAC and unlike 18F-CA, all four candidate probes accumulated in the thymus, and to varying degrees in the bone marrow and spleen (Figure 5A). Figure 5B shows the relative probe uptake values in dCK positive tissues normalized to uptake in the muscle (a dCK negative tissue); absolute probe uptake values are shown in Supplemental Figure 6. Amongst the tested L-nucleosides, L-18F-FCAC had the lowest uptake in lymphoid tissues while L-18F-FAC had the highest uptake in thymus and spleen. An intermediate profile was observed for L-18F-FMAC, which had higher uptake than 18F-FAC in the thymus and lower uptake in the spleen.
FIGURE 5. Biodistribution of the 18F-labeled unnatural nucleosides in mice.
(A) MicroPET/CT scans of C57/BL6 mice; Bl, bladder; BM, bone marrow; GI, gastrointestinal tract; L, liver; S, spleen; SG, salivary gland; Thy, thymus. (B) Quantification of PET imaging data. Probe uptake was normalized to the muscle background (absolute uptake values are shown in Supplemental Figure 6). (P values are calculated relative to FAC for each specific tissue. * = P<0.05, n=5 (18F-FAC), n=3 (L-18F-FAC), n=3 (L-18F-FMAC), n=2 (L-18F-FFAC), n=2 (L-18F-FCAC)). (C) L-18F-FAC microPET/CT scan of a dCK knockout mouse.
In addition to bone marrow and thymus, L-18F-FAC and L-18F-FFAC are also retained in the liver. Similar to 18F-3′-deoxy-3′-fluorothymidine (18F-FLT) (25), these probes accumulate in the liver by a non-specific mechanism (26). Alternatively, the liver uptake may be dCK-specific. A novel dCK knockout (KO) mouse (5) can be used to evaluate these possibilities. L-18F-FAC microPET/CT scans (Figure 5C and Supplemental Figure 7) indicate that, with the exception of excretory organs such as gall bladder and bladder, the L-18F-FAC organ distribution typically observed in wild type mice (Figure 5A) was absent in the dCK KO mouse. While these data demonstrate that the liver trapping of L-18F-FAC requires dCK expression, other mechanisms such as uptake via liver-specific transporters for unnatural L-nucleosides may also play a role.
L-18F-FAC and L-18F-FMAC microPET/CT imaging of malignant and autoimmune lymphoproliferative disorders in mice
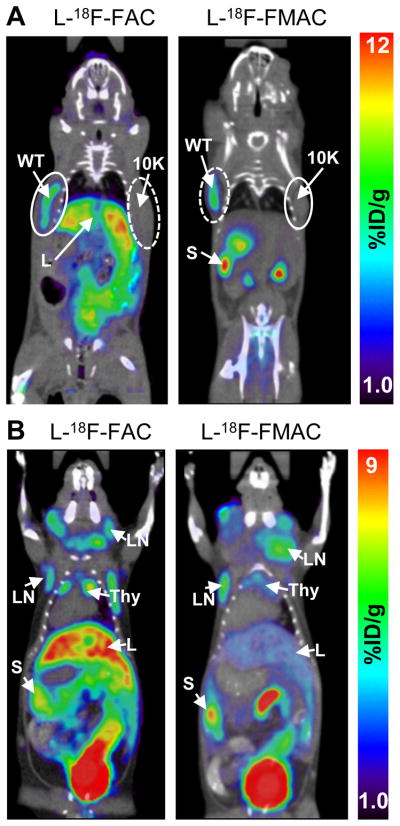
Biodistribution studies in healthy mice indicate that the deamination-resistant probes L-18F-FAC, L-18F-FFAC and L-18F-FMAC compare favorably with 18F-FAC. While L-18F-FMAC had a slightly lower sensitivity than L-18F-FAC and L-18F-FFAC, its low liver uptake may be advantageous in certain applications. Since the biodistribution of L-18F-FFAC was similar to that of L-18F-FAC, this probe was not selected for further evaluations. L-18F-FMAC and L-18F-FAC, the two remaining candidate probes, were compared in two mouse models of cancer and autoimmunity, previously used to evaluate 18F-FAC (4, 12). The first test was to determine whether L-18F-FAC and L-18F-FMAC can distinguish between isogenic murine L1210 leukemia cells that either express dCK (wild type cells) or lack this enzyme (L1210-10K cells) (12). As shown in Figure 6A, dCK positive tumors were detected with both L-18F-FAC and L-18F-FMAC. Neither of these probes accumulated in the dCK negative L1210-10K tumors. L-18F-FAC and L-18F-FMAC were then evaluated in the B6.MRL-Faslpr/J autoimmune mice (27),(28). Figure 6B shows that both probes detected the cervical, axillary and brachial lymphadenopathies characteristic of the Faslpr model.
FIGURE 6. L-18F-FAC and L-18F-FMAC microPET/CT scans of malignant and autoimmune lymphoproliferative disorders.
(A) L-18F-FAC and L-18F-FMAC microPET/CT imaging of L1210 lymphoma tumors. The L1210 parental cell line (WT, solid-lined circle) and the dCK-deficient variant L1210-10K (10K, dash-lined circle) were injected subcutaneously under the left and right shoulder of the mouse, respectively. Only the parental cell line accumulated both probes; (B) L-18F-FAC and L-18F-FMAC microPET/CT imaging of autoimmune B6.MRL-Faslpr/J mice. Both probes detected cervical, axillary and brachial lymphadenopathy in these mice. L, liver; LN, lymph nodes; S, spleen; Thy, thymus; number of mice per probe ≥ 3.
Factors that may affect the performance of 18F-FAC, L-18F-FAC and L-18F-FMAC in preclinical and clinical applications
Despite its rapid deamination in vivo (Figure 1B), 18F-FAC performs well in mice (4, 12). A potential limitation of 18F-FAC concerns its muscle background which is the highest amongst tested compounds (Supplementary Figure 6). In applications in which muscle background may interfere with specific signals, 18F-FAC could be replaced by L-18F-FMAC, a probe with a similar biodistribution pattern and sensitivity but with a lower non-specific uptake in the muscle. Regarding L-18F-FAC, its utility in mice may be limited by the high liver uptake. Furthermore, it is conceivable that, because of their unnatural L-chirality, L-18F-FAC and L-18F-FMAC may be transported less efficiently than 18F-FAC across the plasma membrane.
In the context of clinical applications, the deamination resistant probes L-18F-FAC and L-18F-FMAC may have an advantage over 18F-FAC given the high deamination activity in certain human tissues (13). Concerning the utility of the FAC probes for treatment stratification, 18F-FAC could be the best probe to use for predicting responses to deamination-susceptible pro-drugs such as cytarabine, gemcitabine and decitabine. In contrast, L-18F-FAC and L-18F-FMAC may be more suitable for predicting responses to deamination resistant drugs such as cladribine and clofarabine.
Conclusions
PET imaging of TK1 and dCK, the two rate-limiting enzymes in the deoxyribonucleoside salvage pathway, are now possible due to the development of 18F-FLT by Shields and colleagues in 1998 (26), followed by the identification of 18F-FAC in 2008 (4) and of optimized FAC analogs described herein. Since the high levels of endogenous thymidine in rodent serum compete with 18F-FLT and reduce its sensitivity in mice (29), direct comparisons between 18F-FLT and the FAC series of probes have to be conducted in other species. Nonetheless, it is likely that PET measurements of TK1 and dCK provide non-overlapping information. Thus, while 18F-FLT provides measurements of DNA synthesizing activity and cell proliferation (30), dCK-specific probes may enable PET-guided identification of cancer patients who are more likely to respond to cytotoxic chemotherapy nucleoside analog pro-drugs. Furthermore, a better understanding of the deoxyribonucleoside salvage pathway in terms of its physiological function and potential role in cancer development and progression may expand the utility of TK1 and dCK-specific PET probes beyond their current status of surrogate markers for cell proliferation, immune activation, treatment stratification and monitoring. In this context, recently described mouse genetic models of TK1 (31) and dCK deficiency (5) provide invaluable tools to enable the identification of biological processes that are critically dependent on the activity of these two cytosolic deoxyribonucleoside kinases.
Supplementary Material
Acknowledgments
We thank David Stout, Waldemar Ladno, and Judy Edwards for microPET/CT imaging, Rachel Laing for helping with experiments and the cyclotron group for the production of PET probes. This work was supported by the In Vivo Cellular and Molecular Imaging Centers Developmental Project Award NIH P50 CA86306 (to Caius Radu and Jason Lee), and R24CA92865, U.S. Department of Energy Contract DE-FG02-06ER64249 (to Michael Phelps), RT1-01126-1 California Institute for Regenerative Medicine (to Michael Phelps, Owen Witte and Caius Radu) and the Dana Foundation (to Caius Radu). Owen Witte is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: Caius Radu, Owen Witte and Johannes Czernin are among the inventors of the national and PCT patent applications for the FAC technology referred to in the article. That patent application was filed on September 19, 2008. A group of UCLA faculty members including Caius Radu, Johannes Czernin, Michael Phelps and Owen Witte are involved in Sofie Biosciences, a startup company that has licensed this intellectual property.
Contributor Information
Chengyi J. Shu, Email: jennyshu@gmail.com.
Dean O. Campbell, Email: DCampbell@mednet.ucla.edu.
References
- 1.Staub M, Eriksson S. The Role of Deoxycytidine Kinase in DNA synthesis and Nucleoside Analog Synthesis. In: Peters GJ, editor. Cancer Drug Discovery and Development: Deoxynucleoside Analogs in Cancer Therapy. Totowa, NJ: Humana Press Inc; 2006. pp. 29–52. [Google Scholar]
- 2.Eriksson S, Arner E, Spasokoukotskaja T, et al. Properties and levels of deoxynucleoside kinases in normal and tumor cells; implications for chemotherapy. Adv Enzyme Regul. 1994;34:13–25. doi: 10.1016/0065-2571(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 3.Durham JP, Ives DH. Deoxycytidine Kinase. I. Distribution in normal and neoplastic tissues and interrelationships of deoxycytidine and 1-beta-D-arabinofuranosylcytosine phosphorylation. Mol Pharmacol. 1969;5:358–375. [PubMed] [Google Scholar]
- 4.Radu CG, Shu CJ, Nair-Gill E, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat Med. 2008;14:783–788. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toy G, Austin WR, Liao H-I, et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0913900107. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewald B, Sampath D, Plunkett W. Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–6537. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 7.Giovannoni G, Comi G, Cook S, et al. A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple Sclerosis. N Engl J Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 8.Sabini E, Hazra S, Ort S, Konrad M, Lavie A. Structural basis for substrate promiscuity of dCK. J Mol Biol. 2008;378:607–621. doi: 10.1016/j.jmb.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamba JK, Crews K, Pounds S, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther. 2007;323:935–945. doi: 10.1124/jpet.107.128595. [DOI] [PubMed] [Google Scholar]
- 10.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues: mechanisms of drug resistance and reversal strategies. Leukemia. 2001;15:875–890. doi: 10.1038/sj.leu.2402114. [DOI] [PubMed] [Google Scholar]
- 11.Radu CG, Witte ON, Nair-Gill ED, Satyamurthy N, Shu CJ, Czernin J. The Regents of the University of California assignee. Positron Emission Tomography Probes for Imaging Immune Activation and Selected Cancers. 2009/0105184. US Patent Application Publication No.: US. 2009 April 23;
- 12.Laing RE, Walter MA, Campbell DO, et al. Noninvasive prediction of tumor responses to gemcitabine using positron emission tomography. Proc Natl Acad Sci U S A. 2009;106:2847–2852. doi: 10.1073/pnas.0812890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho DH. Distribution of kinase and deaminase of 1-beta-D-arabinofuranosylcytosine in tissues of man and mouse. Cancer Res. 1973;33:2816–2820. [PubMed] [Google Scholar]
- 14.Richards DA, Sherwood RA, Ndebele D, Rocks BF. Determination of plasma cytidine deaminase activity by HPLC. Biomed Chromatogr. 1987;2:148–151. doi: 10.1002/bmc.1130020404. [DOI] [PubMed] [Google Scholar]
- 15.Krenitsky TA, Tuttle JV, Koszalka GW, et al. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976;251:4055–4061. [PubMed] [Google Scholar]
- 16.Sun H, Collins JM, Mangner TJ, Muzik O, Shields AF. Imaging the pharmacokinetics of [F-18] FAU in patients with tumors: PET studies. Cancer Chemother Pharmacol. 2006;57:343–348. doi: 10.1007/s00280-005-0037-0. [DOI] [PubMed] [Google Scholar]
- 17.Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 18.Lotfi K, Mansson E, Spasokoukotskaja T, et al. Biochemical pharmacology and resistance to 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine, a novel analogue of cladribine in human leukemic cells. Clin Cancer Res. 1999;5:2438–2444. [PubMed] [Google Scholar]
- 19.Fanucchi MP, Watanabe KA, Fox JJ, Chou TC. Kinetics and substrate specificity of human and canine cytidine deaminase. Biochem Pharmacol. 1986;35:1199–1201. doi: 10.1016/0006-2952(86)90160-7. [DOI] [PubMed] [Google Scholar]
- 20.Maury G. The enantioselectivity of enzymes involved in current antiviral therapy using nucleoside analogues: a new strategy? Antivir Chem Chemother. 2000;11:165–189. doi: 10.1177/095632020001100301. [DOI] [PubMed] [Google Scholar]
- 21.Sabini E, Hazra S, Konrad M, Lavie A. Nonenantioselectivity property of human deoxycytidine kinase explained by structures of the enzyme in complex with L- and D-nucleosides. J Med Chem. 2007;50:3004–3014. doi: 10.1021/jm0700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiianitsa K, Solinger JA, Heyer WD. NADH-coupled microplate photometric assay for kinetic studies of ATP-hydrolyzing enzymes with low and high specific activities. Anal Biochem. 2003;321:266–271. doi: 10.1016/s0003-2697(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 23.Johansson M, van Rompay AR, Degreve B, Balzarini J, Karlsson A. Cloning and characterization of the multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster. J Biol Chem. 1999;274:23814–23819. doi: 10.1074/jbc.274.34.23814. [DOI] [PubMed] [Google Scholar]
- 24.Shewach DS, Liotta DC, Schinazi RF. Affinity of the antiviral enantiomers of oxathiolane cytosine nucleosides for human 2′-deoxycytidine kinase. Biochem Pharmacol. 1993;45:1540–1543. doi: 10.1016/0006-2952(93)90058-5. [DOI] [PubMed] [Google Scholar]
- 25.Shields AF, Grierson JR, Muzik O, et al. Kinetics of 3′-deoxy-3′-[F-18] fluorothymidine uptake and retention in dogs. Mol Imaging Biol. 2002;4:83–89. doi: 10.1016/s1095-0397(01)00070-x. [DOI] [PubMed] [Google Scholar]
- 26.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18] FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 27.Morse HC, 3rd, Davidson WF, Yetter RA, Murphy ED, Roths JB, Coffman RL. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982;129:2612–2615. [PubMed] [Google Scholar]
- 28.Kelley VE, Roths JB. Interaction of mutant lpr gene with background strain influences renal disease. Clin Immunol Immunopathol. 1985;37:220–229. doi: 10.1016/0090-1229(85)90153-9. [DOI] [PubMed] [Google Scholar]
- 29.van Waarde A, Cobben DC, Suurmeijer AJ, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45:695–700. [PubMed] [Google Scholar]
- 30.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49 (Suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 31.Dobrovolsky VN, Bucci T, Heflich RH, Desjardins J, Richardson FC. Mice deficient for cytosolic thymidine kinase gene develop fatal kidney disease. Mol Genet Metab. 2003;78:1–10. doi: 10.1016/s1096-7192(02)00224-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.