Summary
Cycling glial precursors - “NG2-glia” - are abundant in the developing and mature central nervous system (CNS). During development they generate oligodendrocytes. In culture, they can revert to a multipotent state, suggesting that they might have latent stem cell potential that could be harnessed to treat neurodegenerative disease. This hope has been subdued recently by a series of fate mapping studies that cast NG2-glia as dedicated oligodendrocyte precursors in the healthy adult CNS - though rare neuron production in the piriform cortex remains a possibility. Following CNS damage, the repertoire of NG2-glia expands to include Schwann cells and possibly astrocytes – but so far not neurons. This confirms the central role of NG2-glia in myelin repair. The realization that oligodendrocyte generation continues throughout normal adulthood has seeded the idea that myelin genesis might also be involved in neural plasticity. We review these developments, highlighting areas of current interest, contention and speculation.
Keywords: oligodendrocyte, precursor/stem cell, cell fate, transgenic mice, Cre-lox, motor neuron disease, amyotrophic lateral sclerosis, multiple sclerosis, experimental autoimmune encephalomyelitis, EAE, spinal injury, axotomy, demyelination, motor skills, behaviour
NG2-glia in perspective: the neuron-glial dichotomy
The story of NG2-glia begins nearly thirty years ago, with the discovery of a class of glial progenitors – “O-2A progenitors” - that could generate oligodendrocytes or type-2 astrocytes in cultures of perinatal rat optic nerve cells (Raff et al., 1983). [Two types of glial fibrillary acidic protein (GFAP)-expressing astrocytes, type-1 and type-2, were recognized in culture; only type-2 astrocytes arose from O-2A progenitors.] O-2A progenitors express a range of defining molecular markers including the nerve/glial antigen-2 (NG2, a proteoglycan core protein) and the platelet-derived growth factor receptor (alpha subunit, PDGFRa). Using these and other markers, the natural history of O-2A progenitors began to be revealed. It was shown that O-2A progenitors first develop in the ventricular germinal zones of the embryonic spinal cord and brain and disseminate through the developing CNS by proliferation and migration, becoming more-or-less uniformly distributed throughout the CNS soon after birth in rodents (reviewed by Miller, 1996; Richardson et al., 2006). After birth, O-2A progenitors associate with axons and generate myelinating oligodendrocytes, which are required for fast and efficient propagation of action potentials.
Cells with an antigenic phenotype that closely resembles perinatal O-2A progenitors were also identified in adult optic nerves (ffrench-Constant and Raff, 1986; Wolswijk and Noble, 1989). These behaved as bipotential oligodendrocyte-astrocyte precursors in culture, just like their perinatal counterparts, but were found to divide, migrate and differentiate more slowly (Wren et al., 1992). The existence of these “adult O-2A progenitors” was immediately recognized to have important implications for the repair of demyelinating damage such as occurs during multiple sclerosis. Cells that express Pdgfra mRNA, presumed to correspond to adult O-2A progenitors, were also visualized throughout the mature brain in situ (Pringle et al., 1992). These were surprisingly numerous – around 5% of all cells in the CNS (Pringle et al., 1992; Dawson et al., 2003). Using antibodies against NG2 (Stallcup and Beasley, 1987; Diers-Fenger et al., 2001), a continuous network of NG2 immuno-positive cells and cell processes was revealed, extending through all parts of the adult brain and spinal cord (Butt et al., 1999; Ong and Levine, 1999; Nishiyama et al., 1999; Chang et al., 2000; Horner et al., 2000; Diers-Fenger et al., 2001; Dawson et al., 2003). The abundance and ubiquitous distribution of these NG2+ cells was visually striking – shocking, even - and they came to be regarded as a novel “fifth neural cell type” after neurons, oligodendrocytes, astrocytes and microglia (Nishiyama et al., 1999; Chang et al., 2000; Butt et al., 2002; Dawson et al., 2003; Peters, 2004; Butt et al., 2005). NG2 and PDGFRa are also expressed by pericytes associated with the CNS vasculature (NG2+ and PDGFRa+ pericytes appear to be distinct). However, double immunolabelling has shown that PDGFRa+ and NG2+ non-vascular cells are essentially one and the same population (e.g. Nishiyama et al., 1996; Diers-Fenger et al., 2001; Dawson et al., 2003; Rivers et al., 2008). Therefore, in this review we refer to the latter as “NG2-glia” to distinguish them from pericytes.
In the meantime, attempts to identify type-2 astrocytes in the developing CNS in vivo had stalled, so a consensus arose that type-2 astrocytes were an artifact of culture. The term “O-2A progenitor” gradually passed out of general use and was replaced by “oligodendrocyte precursor” (OLP) or “oligodendrocyte precursor cell” (OPC) to reflect the then-prevailing view (in the 1990s) that these cells are dedicated mainly or exclusively to oligodendrocyte production during normal development and presumably also in the adult. The nature of type-2 astrocytes and their relationship to real cells in vivo was - and still is - an interesting conundrum. The relationship between OLPs in the perinatal CNS and NG2-glia in the adult was also not immediately obvious. Although it seemed likely that the adult cells were descended by lineage from their perinatal counterparts this was not formally demonstrated until later, with the advent of genetic fate-mapping approaches in transgenic mice (see below). In this article we refer to both the perinatal and adult cells as NG2-glia.
The sheer number of NG2-glia in the adult brain and their uniform distribution in both grey and white matter seemed counter-intuitive. Given their presumed role as oligodendrocyte precursors, should they not be concentrated in white matter where they would presumably be in most demand for myelinating axons? Why should so many precursor cells persist in the mature adult brain in any case? Moreover, the complex process-bearing morphology of NG2-glia in vivo seemed more in keeping with differentiated cells than immature precursors. Perhaps NG2-glia served a dual purpose - as a source of oligodendrocytes during development but fulfilling some more homeostatic or “functional” role in the adult (Nishiyama, 1999; Butt et al., 2002; Wigley et al., 2007; Nishiyama et al., 2009). Anatomical studies revealed that NG2-glia form close contacts with neurons - with axons at nodes of Ranvier and in close proximity to synapses at neuronal cell bodies (Butt et al., 1999; Butt et al., 2002; Wigley and Butt, 2009). The hypothesis was born that NG2-glia, or a subset of them, might be involved in some aspects of information processing, in partnership with neurons.
This idea took off - and NG2-glia became really “exciting” – when electrophysiologists weighed in. It was already known that NG2-glia express some ion channels and neurotransmitter receptors and that glutamate can influence their proliferation and differentiation in culture (Barres et al., 1990; Patneau et al., 1994; Gallo et al., 1996). However, the first demonstration that NG2-glia in the hippocampus receive long-range synaptic input from neurons in vivo sent waves through the research community (Bergles et al., 2000). Synaptic communication between neurons and NG2-glia, both glutamatergic and GABAergic, was subsequently demonstrated in the cerebellum and cerebral cortex, both in grey and white matter (Lin and Bergles, 2002; Chittajallu et al., 2004; Karadottir et al., 2005; Lin et al., 2005; Salter and Fern, 2005; Paukert and Bergles, 2006; Kukley et al., 2007; Ziskin et al., 2007; Hamilton et al., 2009). Physical synapses were identified between NG2 glia and unmyelinated axons in the corpus callosum (Kukley et al., 2007; Ziskin et al., 2007). Some NG2-glia were found to display spiking sodium currents in response to an initial depolarization (Chittajallu et al., 2004; Karadottir et al., 2008; Mangin et al., 2008; De Biase et al., 2010). Suddenly, NG2-glia appeared exotic, ambiguous – glial in form (since they do not possess axons) but with some electrical properties akin to neurons. Their chimeric nature also contributed to the idea that NG2-glia, in their “other” role as precursor cells, might be more malleable than previously imagined and perhaps capable of transforming into neurons as well as glia.
NG2-glia as pluripotent neural stem cells?
One study in particular launched the idea of NG2-glia as latent neural stem cells. This was the demonstration that NG2-glia purified from early postnatal (P6) rat optic nerves can apparently be re-programmed into pluripotent stem cells by first treating with fetal calf serum (FCS) or bone morphogenetic proteins (BMPs) to generate type-2 astrocytes, followed by growth in basic fibroblast growth factor (bFGF) to generate free-floating balls of cells (neurospheres) containing neural stem cells (Kondo and Raff, 2000). Individual cells from these neurospheres could give rise to colonies containing a mixture of neurons, type-1 astrocytes and oligodendrocytes, judged by immunolabeling with cell type-specific antibodies (Kondo and Raff, 2000). This study suggested that NG2-glia are more plastic than previously believed and suggested an explanation for previous reports that newborn rat optic nerve cells can generate neurons in culture, even though optic nerves do not normally contain neurons (Omlin and Waldmeyer, 1989).
These developments took place against a backdrop of exploding interest in stem cells of all sorts and neural stem cells in particular. A landmark series of papers had shown that subependymal astrocytes (“type-B cells”) in the subventricular zone (SVZ) of the adult rodent forebrain are in fact neural stem cells that generate migratory neuroblasts (“type-A cells”) destined for the olfactory bulb (Doetsch et al., 1999). These neuroblasts follow the “rostral migratory stream” (RMS) from the SVZ to the olfactory bulb, where they differentiate into new olfactory interneurons of various types throughout adult life. It seemed (and still seems) possible that the type-2 astrocytes formed by culturing optic nerve NG2-glia with FCS or BMPs (Kondo and Raff, 2000) might be functionally analogous to the subependymal astrocytes (stem cells) of the SVZ. SVZ stem cells, hippocampal stem cells and cultured type-2 astrocytes all express the glial fibrillary acidic protein (GFAP), for example, and all generate neurosphere-like bodies when cultured in the presence of bFGF. Subsequent reports that SVZ stem cells and their immediate progeny express NG2 and PDGFRa – as also do type-2 astrocytes - lent support to this interpretation (Belachew et al., 2003; Aguirre and Gallo, 2004; Jackson et al., 2006). Taken together, these observations implied that SVZ stem cells, type-2 astrocytes and parenchymal NG2-glia might all be close relatives.
The world of glia was up-ended. No longer were glial cells simply the “support cells of neurons” but rather the precursors of neurons, with neuron-like character of their own. Revolutionary ideas need firm foundations, so several labs geared up to test the differentiation potential of NG2-glia directly in vivo, using Cre-lox technology in transgenic mice. The first wave of such studies is now published and the conclusion is clear, if chastening; by far the most common differentiation products of parenchymal NG2-glia are oligodendrocytes, both in the normal and injured adult CNS. Some NG2-expressing cells produce astrocytes during embryonic development but not in the normal adult CNS. In the damaged CNS the situation is a little more encouraging; following focal demyelination, for example, NG2-glia can generate remyelinating Schwann cells and possibly some astrocytes in addition to oligodendrocytes. However, the notion of NG2-glia as neuronal precursors has taken a significant blow. Although NG2-glia have some limited lineage plasticity – a source of continuing optimism for therapeutic applications - they are, by and large, precursors of myelinating cells. This shifts attention back to the therapeutic potential of NG2-glia in demyelinating conditions such as multiple sclerosis and spinal cord injury. It also raises a raft of intriguing new questions concerning the role of myelination during normal adulthood.
Cre-lox fate mapping: potential pitfalls
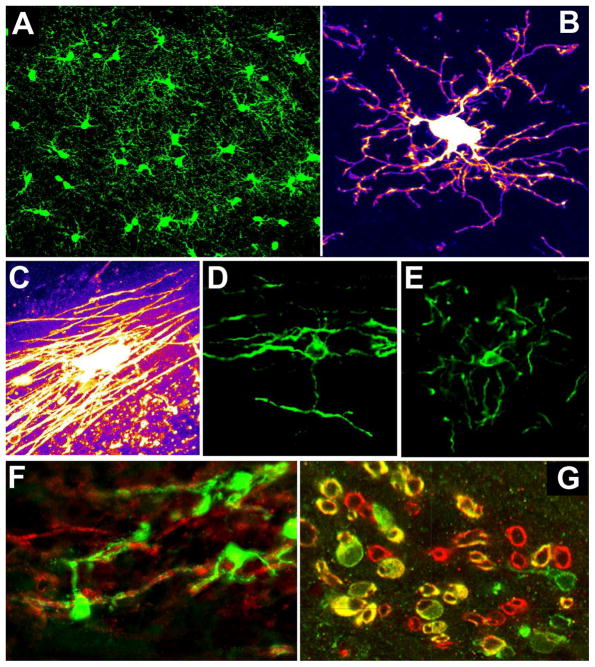
The general principles of Cre-lox fate mapping are as follows. Mice expressing Cre recombinase under transcriptional control of a gene that is active in NG2-glia (e.g. Pdgfra, NG2, Olig2, Plp1) are generated by conventional transgenesis using a plasmid or bacterial artificial chromosome (BAC), or else by homologous recombination in ES cells (knock-in). These are crossed with a Cre-conditional reporter line – e.g. Rosa26-lox-STOP-lox-GFP, where Rosa26 is a ubiquitously active promoter, lox the recognition site for Cre recombinase, STOP a series of four cleavage/polyadenylation sites (which effectively stop mRNA production) and GFP a cassette encoding green fluorescent protein. In double-transgenic offspring (e.g. NG2-Cre : Rosa26-GFP), Cre-driven recombination within the reporter transgene activates expression of GFP irreversibly in NG2-expressing cells and all of their descendants, which are identified retrospectively by immunolabeling for GFP together with cell type-specific markers. This version of the technique, using standard Cre, labels NG2-glia as they come into existence during early development and therefore labels all of the progeny of NG2-glia up to the time of analysis. An important modification is to use CreER*, a fusion between Cre and a mutated form of the estrogen receptor (ER*) that no longer binds estrogen at high affinity but can bind 4-hydroxy tamoxifen (4HT), a metabolite of the anti-cancer drug, tamoxifen. After binding 4HT, CreER* translocates from the cytoplasm (where unliganded ER is normally sequestered) to the nucleus, triggering recombination and reporter gene activation. This version of the technique allows NG2-glia to be labeled inducibly (by administering tamoxifen or 4HT to the mice) at a defined stage of development or adulthood, and the course of division and differentiation of the NG2-glia charted subsequently (Figure 1).
Figure 1.
Compendium of images of NG2-glia and their differentiated progeny in transgenic mice. (A) Low magnification epifluorescence image of the cerebral cortex of a P13 Sox10-GFP mouse showing the near-uniform distribution of oligodendrocyte lineage cells, mainly NG2-glia (R.B.T. unpublished). (B) A single NG2-glial cell in the cerebellum of a P45 Pdgfra-CreER* : Rosa26-YFP mouse, identified by intrinsic fluorescence in a vibratome section, dye-filled through a micropipet with Alexa Fluor 488 to reveal whole cell morphology and imaged by two-photon microscopy (Rivers et al., 2008; M.Rizzi unpublished). (C) A dye-filled myelinating oligodendrocyte in the corpus callosum of a Pdgfra-CreER* : Rosa26-YFP mouse, induced with tamoxifen at P45 and examined 28 days later (P45+28) in the two-photon microscope (Rivers et al., 2008). This adult-born oligodendrocyes has >50 internodes (D) A myelinating oligodendrocyte in the optic nerve of a Pdgfra-CreER* : Tau-mGFP mouse at P60+28. Membrane-tethered mGFP reveals full cell morphology including the myelin sheaths (K.M.Y. unpublished). (E) A myelinating oligodendrocyte in the cortical grey matter of a Pdgfra-CreER* : Tau-mGFP mouse, showing many short randomly-orientated myelin internodes that contrast with the multiple aligned internodes in white matter (K.M.Y. unpublished). (F) Remyelinating oligodendrocytes in a Pdgfra-CreER* : Rosa26-YFP focally-demyelinated spinal cord (Zawadzka et al., 2010). The mice were induced with tamoxifen on four consecutive days starting at ~P75, lysolecithin (1% v/v) was injected stereotaxically four days after the final dose and the animals were analyzed 3 weeks later by epifluorescence microscopy. Oligodendrocyte cell bodies are visualized with anti-GFP (green) and myelin sheaths with anti-PLP (red). Note that cytoplasmic YFP does not readily enter compact myelin, presumably for steric reasons. (G) Remyelinating Schwann cells within a focal ethidium bromide (0.1% w/v)-induced lesion in Pdgfra-CreER* : Rosa26-YFP spinal cord (Zawadzka et al., 2010). Schwann cells are identified by immunolabelling for the Schwann cell-specific protein Periaxin (red).
While this sounds straightforward, there are pitfalls. First among these is the transcriptional specificity of the Cre transgene, which rarely if ever targets exclusively the precursor cells of interest. Ideally, one should compare and integrate data from independent mouse lines that express Cre (or CreER*) under different transcriptional control – e.g. under either Pdgfra or NG2 control in the present context of NG2-glia. Another technical issue relates to the kinetics of Cre recombination – this depends on the structure of the reporter transgene (e.g. distance between lox sites) as well as the level and duration of Cre expression. Often, a larger proportion of the target cell population can be labeled using a “good recombiner” like Rosa26-YFP (Srinivas et al., 2001) compared to a “poor recombiner” like Rosa26-eGFP (Mao et al., 2001). The commonly used Z/EG (Novak et al., 2000) and Rosa26-LacZ (Soriano, 1999) somewhere between these. There seems to be a threshold of Cre expression, below which very little recombination of the reporter gene occurs; this threshold is lower for a good recombiner than a poor recombiner. This effect can introduce additional cell-type selectivity; for example, if a given mouse line expresses Cre in two types of cell, but more highly in one than the other, reporter gene activation can effectively be restricted to the more highly-expressing cells. This can be useful in some circumstances; for example, it is probably the reason that Olig2-CreER* drives recombination and reporter gene activation mainly in NG2-glia and not in differentiated oligodendrocytes (Dimou et al., 2008). However, in other situations it might introduce unwanted bias, e.g. by subdividing the NG2-glia population in some unpredictable way. In short, Cre-lox fate mapping studies need to be interpreted with care and an open mind, each study considered on its own merits.
It is worth noting here that differences in the tamoxifen induction protocol – whether tamoxifen or 4HT is used, whether it is administered by injection or gavage, or whether it is administered once or several times, for example - can also affect the efficiency of recombination independently of the reporter mouse line employed. While this will result in a greater or lesser fraction of NG2-glia becoming labeled, it will have no effect on the level of expression of the reporter in individual cells because that is determined purely by regulatory elements in the reporter transgene itself. The flip side of this is that the reporter transgene (e.g. Rosa26-based) is not necessarily expressed to the same high level in all cell types, which in principle could lead to under-estimation of certain cell types among the labeled progeny of NG2-glia, although there is no evidence that this has been a problem in the recent studies reviewed below.
NG2-glia generate oligodendrocytes but not astrocytes during normal adulthood
Initially, constitutively active Cre was used in NG2-Cre BAC transgenic mice to label the progeny of NG2 glia during brain and spinal cord development (Zhu et al., 2008a; 2008b). As expected, a large proportion of myelinating oligodendrocytes was found among the GFP-labeled progeny of perinatal NG2-glia. In addition, significant populations of GFP-labeled protoplasmic astrocytes were found in the grey matter of the ventral forebrain and spinal cord, though not in white matter. No other labeled cells were identified – in particular, no neurons. A follow-up study from the same group, using NG2-CreER* instead of NG2-Cre, allowed the progeny of NG2-glia to be traced in the postnatal as well as the embryonic brain (Zhu et al., 2011). When tamoxifen was administered at embryonic ages (E16.5), a similar result was obtained as before – NG2-glia generated mainly oligodendrocytes but also some protoplasmic astrocytes in ventral brain territories. Using a reporter line (Z/EG) that recombines inefficiently, Zhu et al. (2011) labeled a sparse subset of embryonic NG2-glia that over time generated discrete clusters (presumed clones) of sibling cells. They found that labeled cell clusters contained either astrocytes or oligodendrocyte lineage cells but not both, suggesting that different subsets of NG2-glia in the embryonic CNS are specialized for production of only ventral astrocytes or only oligodendrocytes. When tamoxifen was administered to postnatal mice (P2, P30 or P60) a different result was obtained - this time no astrocytes were found among the progeny of NG2-glia – concurring with previous experience from other labs that had used different CreER* lines (see below). These data imply that there are two distinct subtypes of NG2-glia – “astrogenic” and “oligogenic” – in the early developing CNS, the astrogenic population being depleted during late embryonic development. A feasible explanation might go as follows: neuroepithelial precursors (radial glia) in the ventral ventricular zone first divide asymmetrically to maintain their own numbers while giving rise to proliferative NG2-glia, which migrate away from the ventricular surface, generating oligodendrocytes during early postnatal development and persisting as oligogenic NG2-glia into adulthood. Then, just prior to birth, the remaining radial glia transform directly into astrocytes, expressing NG2 transiently as they do so; these astrocytes undergo limited cell division and settle in ventral territories close to their region of origin. Direct trans-differentiation of radial glia is a normal mode of astrocyte generation in the developing cortex, for example (Mission et al., 1991). The given scenario is consistent with a study using Olig2-CreER*, in which some astrocytes as well as oligodendrocytes (and motor neurons) were found among the progeny of Olig2-expressing neuropithelial precursors in the embryonic ventral spinal cord (Masahira et al., 2006).
Whatever the precise sequence of events during prenatal gliogenesis, it now seems likely that NG2-glia do not generate astrocytes during normal healthy adulthood. Several Cre-lox studies – using Pdgfra-CreER* (two independent lines: Rivers et al., 2008; Kang et al., 2010), NG2-CreER* (Zhu et al., 2011; see above) and Olig2-CreER* (Dimou et al., 2008) converge on that conclusion. While some reporter-positive astrocytes were observed in the cortical grey matter in the Olig2-CreER* line, their number did not increase significantly between 8 and 65 days post-tamoxifen administration, indicating that they were not generated continuously from dividing NG2-glia (Dimou et al., 2008). It appears that the Olig2-CreER* transgene is expressed in some protoplasmic astrocytes in the normal grey matter, resulting in labeling of some of these in addition to NG2-glia. A subsequent study from the same lab (Simon et al., 2011) marked NG2-glia in a different way, by long-term BrdU labeling of 2–3 month old mice, and confirmed that no astrocytes were found among their differentiated progeny.
Neuron genesis from NG2-glia – fact or fantasy?
NG2-glia exposed to appropriate environmental signals in a culture dish appear to revert to a multipotent state, from which they can generate neurons as well as oligodendrocytes and astrocytes (Kondo and Raff, 2000). This sparked the widespread hope that NG2-glia can be a regenerative resource for neurodegenerative diseases that involve neuronal as well as glial loss. A number of studies have encouraged this hope by describing neuronogenic properties of NG2-glia in the normal rodent CNS. For example, NG2-glia in the neocortex and piriform cortex have been reported to express Doublecortin (Dcx), an established marker of migratory neuronal progenitors in the forebrain SVZ/RMS and hippocampus (Tamura et al., 2007; Guo et al., 2010). Some NG2+ cells in the piriform cortex have been found to express Sox2 and Pax6 (Guo et al., 2010), two more neural stem cell markers. Conversely, SVZ and hippocampal stem cells have been reported to express NG2 (Belachew et al., 2003; Aguirre and Gallo, 2004) and PDGFRa (Jackson et al., 2006) and to actively transcribe a CNP-GFP transgene (Belachew et al., 2003; Aguirre and Gallo, 2004; Aguirre et al., 2004). However, not all of these observations have survived scrutiny. For example, other labs have failed to confirm NG2 or PDGFRa antibody labeling of SVZ or hippocampal stem cells (Komitova et al., 2009), or to detect NG2 or PDGFRa promoter activity in these stem cell populations in BAC transgenic mice (Rivers et al., 2008; Zhu et al., 2008a; Kang et al., 2011). While antibody labeling experiments are notoriously difficult and artifact-prone, genetic labeling should be more predictable – so one might imagine - and therefore capable of providing an unequivocal answer to the question “do NG2-glia generate neurons?” However, Cre-lox fate mapping studies have still not completely eliminated the controversy around this question.
Using Pdgfra-CreER* : Rosa26-YFP mice, our lab found that although NG2-glia generate predominantly Sox10-positive oligodendrocyte lineage cells during normal adulthood, some Sox10-negative, YFP+ cells appeared and accumulated in layers 2 and 3 of the anterior piriform cortex (aPC) (Rivers et al., 2008). The cells acquired NeuN reactivity and morphologically resembled piriform projection neurons. The scale of neuron genesis was small; we estimated that only ~1.4% of the neurons present in layers 2/3 of the aPC were generated in the ~200 days after P45 (Rivers et al., 2008). Two things pointed to their being generated continually from precursor cells: 1) they were not observed until around one month after tamoxifen administration, suggesting that they differentiated slowly from NeuN-negative precursors and 2) they steadily increased in number between 28 and 210 days post-tamoxifen. It is difficult to imagine how YFP-labeled PC neurons could continue to accumulate months after tamoxifen administration had ceased, unless they were generated from a population of precursor cells that had recombined the YFP reporter gene at the time of tamoxifen addition. They could not have been generated from SVZ stem cells because no YFP+ PC neurons were found in Fgfr3-CreER* :Rosa26-YFP mice, which marks all GFAP+ SVZ stem cells and their neuronal progeny in the olfactory bulb, for example (Rivers et al., 2008; Young et al., 2010). We were unable to co-label the YFP+ neurons with BrdU, even after months (100 days) of BrdU exposure via the drinking water, indicating that they might have formed by direct transformation of long-term-quiescent precursors. Since we found that ~50% of NG2-glia did not incorporate BrdU over the same time scale, we suggested that the new PC neurons were formed by trans-differentiation of post-mitotic NG2-glia (Rivers et al., 2008; Psachoulia et al., 2009). Another possibility is that the new aPC neurons were produced from some other pool of Pdgfra-expressing precursors, as yet unidentified. Pdgfra is expressed by large numbers of cells outside of the CNS so the new neurons could conceivably originate from precursors that enter the CNS via the circulatory system. Alternatively, they might be generated from Pdgfra+ perivascular cells within the CNS. (NG2+, PDGFRb+) perivascular pericytes have recently been reported to generate neurons and glia in culture in response to bFGF (Dore-Duffy et al., 2006), so it is conceivable that PDGFRa+ pericytes might have similar stem cell-like properties.
One other study has reported PC neurons from NG2-glia (Guo et al., 2010). This study used Plp1-CreER* : Rosa26-YFP mice to follow fates of NG2-glia in the healthy adult CNS. Plp1 is strongly expressed in differentiated oligodendrocytes and less strongly in NG2-glia, so Guo et al. (2010) could not address questions about new oligodendrocyte production; however, like Rivers et al. (2008), they did observe YFP-labeling of PC projection neurons. Their labeled neurons first became apparent 17 days post-tamoxifen and increased in number for at least 180 days. Control experiments using GFAP-CreER* : Rosa26-YFP mice to mark SVZ stem cells ruled out the possibility that the newly-labeled PC neurons were SVZ-derived. Thus, there are strong parallels between the experiments and data of Guo et al. (2010) and our own (Rivers et al. 2008), except that Guo et al. (2010) described their neurons in the posterior piriform cortex (pPC) (Bregma levels −2.3 mm to −1.1 mm), whereas ours were predominantly in the aPC.
Using their own independently-generated line of Pdgfra-CreER* mice, Kang et al. (2010) failed to detect production of long-term surviving GFP+ neurons in the PC or elsewhere in the forebrain. The reason for this difference between their study and ours (Rivers et al., 2008) is not clear. Since the Pdgfra BACs used for transgenesis were different (ours contained ~55 kb upstream and ~74 kb downstream of the Pdgfra gene, theirs ~70 kb upstream and ~40 kb downstream) it is conceivable that they might have inherently different transcriptional specificity – e.g. our BAC but not theirs might contain a regulatory element required for expression of the Pdgfra-CreER* transgene in a particular set of Pdgfra-expressing neuronal precursors. This presupposes that the putative Pdgfra+ precursors are something other than NG2-glia, since both Pdgfra-CreER* transgenes are demonstrably expressed in NG2-glia. Alternatively, it might depend on where one looks – we quantified piriform neuron production in the aPC (Bregma levels +0.22 mm to +1.2 mm) (Rivers et al. 2008 and unpublished) whereas Kang et al. (2010) examined pPC (Bregma −1.9 mm to +0.5 mm).
Other groups have reported small numbers of reporter-positive neurons (NeuN+) throughout the forebrain - particularly the ventral forebrain – at short times after CreER* induction, but discounted these as probably resulting from sporadic CreER* expression in the neurons themselves (Dimou et al., 2008; Kang et al., 2010; Guo et al., 2010; Zhu et al., 2011). Strangely, in one study YFP+, NeuN+ neurons were observed for only a few days following 4HT injection into NG2-CreER* : Rosa26-YFP mice (Zhu et al., 2011), suggesting that these neurons were eliminated from the CNS after a short time, or else became NeuN-negative (or YFP-negative). The aPC neurons that we observed (Rivers et al., 2008) are distinct from these and, whatever their origin, cannot easily be explained by sporadic activation of the CreER* transgene in neurons (Kang et al., 2010; Zhu et al., 2011), or by some aspect of the tamoxifen protocol (Simon et al., 2011).
Further experiments will be required to resolve the ambiguity around PC neuron genesis. For example, it will be useful to establish whether there is a real difference between the two Pdgfra-CreER* lines (Rivers et al., 2008; Kang et al., 2010). If there is, then differences in the transcriptional specificity of the two transgenes might give clues to the origin of the PC neurons observed by Rivers et al. (2008). The transcriptional specificity of the Plp1 promoter-proximal fragment used in Plp1-CreER* (Guo et al., 2010) also needs to be examined. This is a fragment of the Plp1 gene (2.4 kb of 5' sequence plus exon1 and intron1) (Doerflinger et al., 2003) and it cannot be assumed to exactly mimic endogenous Plp1 expression - which itself is not entirely oligodendrocyte lineage-specific, being expressed in a subset of multipotent precursors during development (Delaunay et al., 2009; Guo et al., 2009; Kang et al., 2010) as well as in some differentiated neurons (Nery et al., 2001; Miller et al., 2009). This potentially complicates interpretation of fate mapping studies (Guo et al., 2009; 2010). It is quite important to get to the bottom of these discrepancies because, at the very least, it will refine our understanding of the Cre-lox system and its potential shortcomings.
In any case there seems to be something interesting going on in the PC. There has been a steady trickle of evidence for neuronal progenitors/immature neurons residing there. For example, cells in the PC have been reported to express Doublecortin, polysialated NCAM, Sox2 and other markers of neural precursor cells (Seki and Arai, 1991; Hayashi et al., 2001; Nacher et al., 2001; Nacher et al., 2002; Pekcec et al., 2006; Shapiro et al., 2007; Bullmann et al., 2010; Guo et al., 2010). There have also been reports of continued neuron genesis in the adult rodent and primate PC and its modulation by olfactory stimulation (Bernier et al., 2002; Pekcec et al., 2006; Shapiro et al., 2007; Arisi et al., 2011). Another possibility is that the immature neuron markers might be indicative of neuronal de-differentiation and remodeling in response to changing inputs from the olfactory bulb (OB) (Seki and Arai, 1993; Nacher et al., 2001), since the aPC (otherwise known as the primary olfactory cortex) is the primary target for output neurons (mitral cells) of the OB. The internal OB circuitry is continually changing throughout life, due to the addition of new OB interneurons from the SVZ, so perhaps the whole olfactory system including the aPC is in a constant state of flux.
Do NG2-glia generate reactive astrocytes after injury?
NG2-glia are known to react to injury by proliferating, up-regulating NG2 expression and generating remyelinating oligodendrocytes when required (reviewed by Levine et al., 2001). Since their differentiation potential is known to be influenced by their environment in vitro (Kondo and Raff, 2000), it is possible that they might display a broader lineage potential following CNS injury or disease, when their microenvironment is likely to be altered by inflammatory cells and possibly through breach of the blood-brain barrier. Therefore, it is of great interest to discover the fates of NG2-glia in various experimental models of disease or traumatic injury.
There has now been a handful of genetic fate mapping studies of NG2-glia during various experimental pathologies in mice. These include experimental autoimmune encephalomyelitis (EAE) (Tripathi et al., 2010), acute gliotoxin-induced focal demyelination (Zawadzka et al., 2010), spinal cord section (Barnabe-Heider et al., 2010), cortical stab wound (Dimou et al., 2008; Komitova et al., 2011), and a mouse model of inherited amyotrophic lateral sclerosis (ALS; motor neuron disease) (Kang et al., 2010). All these studies found that NG2-glia respond to injury by proliferating and accumulating at the site(s) of damage, and that their major differentiation products are new oligodendrocytes. In addition to oligodendrocytes, modest astrocyte production was also reported in some but not all of these studies – the main source of reactive astrocytes being pre-existing astrocytes, not NG2-glia. One study does not conform to this pattern. This is a study of cell generation following a cold-induced injury to the cerebral cortex (Tatsumi et al., 2008), in which the major product of NG2-glia appeared to be protoplasmic “bushy” astrocytes, not oligodendrocytes (see below). NG2-glia derived neurons were not found in any of these studies, however. (The main features of all the fate mapping studies discussed in this review are summarized in Table 1.)
Table 1.
Summary of Cre-lox fate mapping studies discussed in this article. Abbreviations: R-YFP, Rosa26-YFP etc. (see text for references); SC, spinal cord; ALS amyotrophic lateral sclerosis, motor neuron disease; aPC, anterior piriform cortex; pPC posterior piriform cortex; nd, not done. (+/−) indicates that some astrocytes were found but that these were infrequent (<5% of reporter-positive cells) and/or could be explained by direct recombination in a subset of astrocytes after tamoxifen administration.
| CreER* | inducer | reporter | condition | % of NG2-glia labeled | cell types generated | |||
|---|---|---|---|---|---|---|---|---|
| OL | AS | N | other | |||||
| Pdgfra1 | tam | R-YFP | normal | 45–50 | +++ | − | + (aPC) | |
| Pdgfra2 | 4HT | Z/EG | normal | 40–45 | +++ | − | − | |
| R-YFP | normal | ~90 | nd | nd | − | |||
| Z/EG | ALS (SC) | ? | +++ | − | − | |||
| Pdgfra3 | tam | R-YFP | EAE (SC) | ~30 | +++ | +/− | − | unidentified |
| Pdgfra4 | tam | R-YFP | focal demyelin. | ~40 | +++ | +/− | − | Schwann cells |
| NG25 | 4HT | Z/EG | normal | <2 | +++ | − | − | |
| R-YFP | normal | ~45 | +++ | − | − | |||
| NG26 | 4HT | Z/EG | cortical stab | <2 | +++ | +/− | nd | |
| Olig27 | tam | Z/EG+R-GFP | normal | ? | +++ | +/− | − | |
| Z/EG+R-GFP | cortical stab | ? | +++ | +/− | − | |||
| Olig28 | 4HT | R-GAP43-GFP | cryoinjury | ? | − | + | − | |
| Olig29 | tam | R-LacZ, R-YFP | axotomy (SC) | ~40 | +++ | − | − | |
| Plp110 | tam | R-YFP | normal | 10 | nd | nd | + (pPC) | |
Following a cortical (grey matter) stab injury in adult Olig2-CreER* : Z/EG mice, Dimou et al. (2008) reported oligodendrocyte generation but little or no astrocyte production. An accumulation of GFAP+ BrdU+ reactive astrocytes was found in the vicinity of the lesion, as expected, but these were mostly reporter-negative (i.e. not NG2-glia derived). Very similar results to these were reported following cortical stab wounds in NG2-CreER* : Rosa26-YFP mice (Komitova et al., 2011). A subsequent BrdU fate mapping study (Simon et al., 2011) failed to find evidence for any astrocyte production from dividing NG2-glia after cortical stab injury. The emerging consensus from these studies is that the reactive (hypertrophic, strongly GFAP+) astrocytes that form the glial “scar” around sites of injury in the cortex are derived predominantly or exclusively from pre-existing astrocytes, not from NG2-glia. This conclusion has been supported by complementary experiments in which astrocytes were labeled specifically by injecting a GLAST-CreER* lentiviral vector into the cortex of reporter mice, and their fates followed before and after cortical stab injury (Buffo et al., 2008). Before injury, the labeled astrocytes were quiescent (did not incorporate BrdU after a long label) but, after injury, they started dividing and generated many new astrocytes, but not other cell types, at the site of the wound.
This also seems to be what happens after spinal cord injury. Barnabe-Heider et al. (2010) made a transverse cut through the dorsal funiculus of the spinal cord, severing the ascending and descending axon tracts. They observed new oligogenesis but insignificant astrocyte production from NG2-glia (marked using Olig2-CreER*), despite a robust astrocytic reaction/gliosis. Most interestingly, they identified two separate components of the astrocytic reaction – a localized accumulation of GFAP+ astrocytes at the core of the lesion site in the dorsal funiculus and a more diffuse accumulation/gliosis around the lesion site and throughout the spinal cord at the level of the injury. These two components had different cellular origins; the “core” astrocytes were derived from multipotent stem cells in the ependymal zone surrounding the central canal of the cord (labeled with FoxJ1-CreER*), whereas the “diffuse” astrocytes were derived from pre-existing astrocytes in the parenchyma (labeled with Connexin30-CreER*). It is curious that Barnabe-Heider et al (2010) found that all protoplasmic astrocytes in the spinal cord were Olig2-immunoreactive in their experiments. Despite this, the Olig2-CreER* transgene did not drive recombination in astrocytes, perhaps because the level of CreER* expression was below threshold (see above, under heading “Cre-lox fate mapping: potential pitfalls”). While this worked out well for Barnabe-Heider et al. (2010), it does raise the possibility that Olig2-CreER* might trigger recombination in astrocytes in addition to oligodendrocyte lineage cells in some circumstances.
In marked contrast to the above is a study by Tatsumi et al. (2008), who followed the fates of NG2-glia following freeze-thaw lesions in the cerebral cortex. They used Olig2-CreER* mice [the same line used by both Dimou et al. (2008) and Barnabe-Heider et al. (2010)] to mark presumptive NG2-glia prior to injury and reported a robust proliferative response followed by production of “bushy” protoplasmic astrocytes between one and two weeks post-injury. Astrocytes appeared to be the major differentiated product of NG2-glia in this injury model; oligodendrocytes were not observed. However, in this study, as in that of Dimou et al. (2008), Olig2-CreER* triggered recombination in some protoplasmic astrocytes in addition to NG2-glia in the uninjured cortex (~20% of reporter-positive cells were astrocytes at short times after 4HT administration; Tatsumi et al., 2008). This leaves open the possibility that the reactive astrocytes formed after injury were derived from division of pre-existing astrocytes. Tatsumi et al. (2008) discounted this idea because they failed to find BrdU-labeled GFAP+ astrocytes shortly after injury but it is possible that there could have been a delayed mitogenic response of astrocytes, or slow up-regulation of GFAP in previously GFAP-negative astrocytes, either of which might have obscured a transient population of BrdU+ astrocytes. Nevertheless, the apparent absence of oligodendrocyte production in the experiments of Tatsumi et al. (2008) marks their study out from the others; perhaps the particular environment of the freeze-thaw injury, as compared to stab injury for example, inhibits NG2-glia from differentiating into oligodendrocytes. It is important to confirm or refute this observation through cryo-lesioning experiments in different CreER* mouse lines, because it could perhaps provide a link to late-stage multiple sclerosis lesions, in which inhibition of oligodendrocyte differentiation is thought to contribute to remyelination failure .
Other researchers have examined the response of NG2-glia during experimentally-induced demyelination. In a gliotoxin-induced focal demyelination model, Zawadzka et al. (2010) found robust remyelination from NG2-glia (labeled using Pdgfra-CreER* : Rosa26-YFP; Figure 1F) - as expected from previous studies (Redwine and Armstrong, 1998; Watanabe et al., 2002; Dawson et al., 2003; reviewed by Franklin and ffrench-Constant, 2008). A small proportion of YFP+ cells were Aquaporin-4+ astrocytes (~3%), but the great majority of reactive astrocytes were derived from Fgfr3-expressing cells (ependymal cells and/or pre-existing astrocytes) (Young et al., 2010), because they were YFP-labeled in Fgfr3-CreER* : Rosa26-YFP mice (Zawadzka et al., 2010).
Schwann cells, the myelinating cells of the peripheral nervous system (PNS), are commonly found in remyelinating CNS lesions including some human multiple sclerosis lesions. Often these remyelinating Schwann cells surround blood vessels, which in the past has been taken to suggest that they enter the CNS from the PNS, using the vessels as a migration route. However Zawadzka et al. (2010) found that most remyelinating Schwann cells (Periaxin+) in their CNS lesions were YFP+ in Pdgfra-CreER* : Rosa26-YFP mice, suggesting that they were derived from NG2-glia (Figure 1G). In strong support of this, the CNS-resident Schwann cells were also labeled in Olig2-Cre : Rosa26-YFP animals – Olig2 is not thought to be expressed outside of the CNS. Moreover, almost no CNS Schwann cells, but many Schwann cells in peripheral nerves, were labeled in Pzero-CreER* : Rosa26-YFP mice. (Pzero is expressed in migrating neural crest and differentiated Schwann cells, but not in the oligodendrocyte lineage.)
Schwann cells were not a minor side-product of NG2-glia because 56% of all YFP+ cells in ethidium bromide-induced lesions were Periaxin+ Schwann cells. (Despite this, most new myelin is oligodendrocyte-derived, because Schwann cells each remyelinate only a single internode whereas oligodendrocytes remyelinate many.) To our knowledge, this is the clearest example to date of lineage plasticity among NG2-glia in vivo. Since both oligodendrocytes and Schwann cells are myelinating cells, relatively subtle re-programming might be required to cross between them.
In a different demyelinating model – experimental autoimmune encephalomyelitis (EAE), which causes more diffuse and widespread demyelination than gliotoxin injection - Tripathi et al. (2010) found robust production of NG2-glia derived oligodendrocytes but very few Schwann cells. In EAE there is a strong inflammatory component to the pathology that is not present in gliotoxin-induced demyelination, suggesting that the local microenvironment in demyelinated lesions exerts a strong influence on the direction of differentiation of NG2-glia. Only a small fraction of YFP+ cells (1–2%) were GFAP+ astrocytes in EAE, in keeping with the results from focal demyelination (Zawadzka et al., 2010). A relatively high proportion (~10%) of YFP+ cells in this EAE study could not be identified, despite much effort with antibodies against microglia, macrophages, B- or T-cells, neutrophils, vascular endothelial cells, pericytes, neurons, astrocytes, oligodendrocytes and Schwann cells.
No astrocyte production from NG2-glia was detected in a mouse model of hereditary ALS (Kang et al., 2010), despite extensive astrocytosis in the ventral horns of the spinal cord where motor neuron degeneration occurs. These authors did, however, observe proliferation and accumulation of NG2-glia and differentiated oligodendrocytes – an unexpected result, since oligodendrocyte involvement in ALS pathology was not previously suspected. Whether reactive NG2-glia are specifically involved in myelin repair in ALS, or an incidental by-product of tissue damage or inflammation, is not known.
The gathering consensus seems to be that NG2-glia remain largely committed to the oligodendrocyte lineage in the healthy CNS and in most pathological situations. Exceptions are 1) the still-unresolved question of low-level neuron genesis in the piriform cortex during normal adulthood, 2) robust Schwann cell generation following gliotoxin-induced demyelination and 3) production of a few GFAP+ astrocytes in some but not all injury studies. Overall, lineage flexibility seems to be strongly biased towards myelinating lineages. This injects a healthy dose of realism and tempers our hopes for NG2-glia as a panacea for neurodegenerative disease. It remains possible that NG2-glia might have the potential to generate neurons but do not readily reveal that potential in the environment of the damaged CNS – at least not those conditions that have been examined so far. Pharmacological interventions that can redirect differentiation towards neurons might be found in the future, but it will not be an easy fix. On the other hand, the data reaffirm the central role of NG2-glia in myelin repair, in demyelinating conditions such as multiple sclerosis or spinal cord injury. It has also been useful to learn that the great majority of reactive astrocytes are in most cases not descended from NG2-glia, but from parenchymal astrocytes that re-enter the cell cycle and, in the spinal cord, from ependymal cells around the central canal. The latter cells represent a relatively unexplored population that is a key target for future investigation. It will be important to discover whether these cells retain, or can be induced to recapitulate, some of the neuronogenic flavor of their forebears in the embryonic neuroepithelium.
Oligodendrocyte generation and myelin dynamics in the adult
Most newly-formed oligodendrocytes in the postnatal forebrain survive long-term and myelinate axons. Myelin formation has been demonstrated by microinjecting live YFP+ cells in tissue slices with a fluorescent dye that can spread throughout the cell and expose its full morphology. Like this, newly-formed mature oligodendrocytes that elaborate up to ~50 internodes have been visualized in the adult corpus callosum (Rivers et al., 2008) (Figure 1C). Newly-formed oligodendrocytes with myelinating morphology were also identified using reporter mice that express a membrane-tethered form of GFP (Kang et al., 2010; Zhu et al., 2011; Figure 1D). That most new oligodendrocytes survive long-term (at least in the corpus callosum) can be inferred by comparing the rate of new cell production (through division of NG2-glia) with the rate of accumulation of differentiated oligodendrocytes, given that the absolute number and density of NG2-glia does not change dramatically during the first year of life (Psachoulia et al., 2009). The cell cycle dynamics of NG2-glia is known from cumulative BrdU and EdU labeling experiments (Psachoulia et al., 2009; Simon et al., 2011; see below).
The scale of adult oligogenesis has been something of a surprise. Rivers et al. (2008) calculated that ~29% of all differentiated oligodendrocytes (identified by CC1 immunolabeling) that are present in the corpus callosum of ~8 month old mice are generated in the 210 days after P45 (Pdgfra-CreER*: Rosa26-YFP). Zhu et al. (2011) found that ~30% of CC1-positive oligodendrocytes in the corpus callosum of ~4 month old mice were formed in the 60 days after P60 (NG2-CreER*: Rosa26-YFP). Comparing these estimates, one might conclude that no more oligodendrocytes are formed after 4 months of age but Psachoulia et al. (2009) showed clearly that new myelinating oligodendrocytes are still being formed at a low rate even at 8 months. There are a lot of uncertainties in such calculations, (e.g. potential variation in recombination efficiencies from experiment to experiment and at different ages) but, nevertheless, it is clear that oligodendrocyte differentiation continues well into adulthood, though at a steadily decreasing rate (Rivers et al., 2008; Lasiene et al., 2009; Psachoulia et al., 2009; Kang et al., 2010; Simon et al., 2011; Zhu et al., 2011). NG2-glia in the cortical grey matter also continue to generate oligodendrocytes into adulthood (Figure 1E), although the overall rate of oligogenesis in the cortex is considerably less than in the corpus callosum at most ages (Rivers et al., 2008; Kang et al., 2010; Simon et al., 2011; Zhu et al., 2011).
It is not known yet whether adult myelin genesis is required to replace myelin that degenerates through normal “wear and tear”, or whether it adds to existing myelin. Only around 30% of axons in the corpus callosum of 8 month-old mice are fully myelinated (Sturrock, 1980), so there is plenty of scope there and in other major white matter tracts for de novo myelination of previously naked axons. There is evidence from cumulative [3H]-thymidine labeling that oligodendrocytes accumulate modestly in the mouse corpus callosum during the first year, without significant turnover, supporting the idea of de novo myelination (McCarthy and Leblond, 1988). Electron microscopy also shows that the number of myelinated axons in the rodent corpus callosum increases well into adulthood (Nunez et al., 2000; Yates and Juraska, 2007). If adult-born myelin is laid down exclusively on naked axons, we would expect not to find many new myelinating oligodendrocytes in fiber tracts that consist predominantly of myelinated axons, such as the optic nerve (Honjin et al., 1977); this remains to be tested. On the other hand, myelin turnover is suggested by the observation that average internode length decreases with age, shorter internodes being regarded as a hallmark of remyelination following myelin loss (Lasiene et al., 2009). Perhaps de novo myelination and myelin replacement go on concurrently in different parts of the CNS or within axon tracts, such as the corpus callosum, that contain a mixture of myelinated and unmyelinated axons. If myelin turnover turns out to be commonplace, how neural pathways can cope with continual loss and replacement of oligodendrocytes would need to be understood, because the loss of even one myelin internode has been predicted to cause conduction block (Koles and Raminsky, 1972; Waxman and Brill, 1978). Whether action potentials are blocked or delayed will depend on the geometry of the affected fibers, including internode length and axon diameter (e.g. Bostock and Sears, 1976; Waxman and Brill, 1978; Smith et al., 1982). Nevertheless, given that one oligodendrocyte usually myelinates many axons, significant problems might be anticipated from oligodendrocyte turnover. Perhaps new internodes can intercalate between existing internodes - i.e. remyelination might initiate at nodes of Ranvier and gradually expand length-wise, pushing aside the existing internodal sheath(s) while maintaining continuity of myelin. This brief discussion exposes gaps in our knowledge of basic myelin dynamics that need to be filled before we can hope to understand myelin maintenance and plasticity.
Speculations on the role of adult myelination in learning and memory
Personal experience tells us that learning a complex motor skill - riding a bicycle, playing a musical instrument, learning a dance step or a sporting activity – requires a great deal of time and practice. On the other hand a motor skill, once learned, is difficult to lose and stays with us throughout our active life. The extended learning experience and long decay time seem consistent with the production and long-term survival of new cells. Could new myelin formation during postnatal life play a part in motor learning? Motor learning is an example of unconscious or “non-declarative” learning, which includes habituation and classical conditioning (e.g. fear conditioning and Pavlovian conditioning). Non-declarative learning and memory is an ancient system that is well developed in invertebrate animals – for example, the gill retraction reflex that has been studied in Aplysia and other marine molluscs. Studies of this and related phenomena have established that even very small nervous systems have the capacity to learn and remember past experience and that such memories are an intrinsic part of the circuits involved in the behavioral response, not something that is generated or stored remotely (Carew and Sahley, 1986). The general idea is that, during repetitive use, circuit connectivity becomes strengthened so that the neurons comprising the circuit acquire a lower threshold for firing and become more likely to fire again in future – so-called “Hebbian learning”. How does this happen at a cellular and molecular level? It is well established that repetitive firing can induce changes to the molecular composition of the active synapses and that this can increase the strength of communication between pre-and post-synaptic cells (Milner et al., 1998). Synaptic strengthening is an established mechanism of long-term potentiation (LTP), a cellular correlate of learning and memory in both invertebrates and vertebrates (Bliss and Collingridge, 1993). With the evolution of myelin, vertebrates might have acquired an additional way of modulating circuit activity – by myelinating the interconnecting axons, if previously unmyelinated. New myelination would be expected to increase dramatically the speed of transmission of action potentials and alter the intrinsic circuit properties. Myelination would also provide neurotrophic and physical support to the circuit neurons and make for long-term survival.
There is some evidence that adult myelin genesis might contribute to motor learning in humans. For example, it has been reported that extensive piano practice (Bengtsson et al., 2005) or juggling (Scholz et al., 2009) can cause long-term changes to the structure of white matter tracts, including parts of the corpus callosum, as revealed by magnetic resonance imaging (MRI). It has also been reported that white matter structure is altered in children skilled in abacus use, which involves actual and imagined visuo-motor activity (Hu et al., 2011). There is also evidence that training in working memory tasks results in changes in the structure of frontoparietal white matter (Takeuchi et al., 2010) (for reviews see Fields, 2008; Ullen, 2009).
For new myelin to be involved in activity-dependent learning, there needs to be a mechanism for regulating oligodendrocyte generation and myelination according to circuit activity. Such a mechanism seems to exist. Recently, Li et al. (2010) showed that electrical stimulation of neurons in the motor cortex led to activity-dependent stimulation of proliferation of NG2-glia in the descending pyramidal (corticospinal) tract. Previously, Barres and Raff (1993) had shown that silencing retinal ganglion neurons, by injecting tetrodotoxin into the developing eye, inhibited proliferation of NG2-glia in the newborn rat optic nerve. Inhibition could be overcome by implanting PDGF-expressing cells next to the nerve, suggesting that electrical activity in retinal ganglion cell axons might normally regulate the supply of mitogens to NG2-glia - possibly by triggering its release from optic nerve astrocytes (Barres and Raff, 1993). This suggests one mechanism by which NG2-glia might sense electrical activity, which, at some threshold, might trigger them to divide and differentiate into myelinating oligodendrocytes. A more direct mechanism involving synaptic communication between axons and NG2-glia can also be envisaged - for example, synaptic signaling might regulate the intrinsic response of NG2-glia to PDGF or other mitogens. Testing these ideas will be an important task for the future.
Does the decreasing rate of new myelin genesis contribute to age-related cognitive decline?
There is an accumulating body of evidence that some measures of general cognitive ability correlate with white matter volume and integrity. For example, cognitive ability and white matter volume increase in parallel into the fourth decade of life and both decline thereafter (Bartzokis et al., 2001; Mabbott et al., 2006; Hasan et al., 2008; Ullen et al., 2008; Zahr et al., 2009; Bartzokis et al., 2010). The reasons behind these age-related changes are unknown but they could conceivably relate to changes in the ability of NG2-glia to proliferate and generate new oligodendrocytes as the brain matures and ages.
We recently measured the cell cycle time (Tc) of NG2-glia in the postnatal mouse brain by cumulative BrdU labeling (Psachoulia et al., 2009) and reported that Tc increases dramatically with age, from ~2 days on postnatal day 6 (P6) to >70 days at P240 (8 months of age) in the cerebral cortex. An age-related increase in the cell cycle time of NG2-glia in the mouse spinal cord has also been reported (Lasiene et al., 2009). The lengthening cell cycle results from the cells’ spending more and more time in the early G1 phase of the cycle (Geha et al., 2010; Simon et al., 2011). The decreasing rate of cell division correlates well with the decreasing rate of oligodendrocyte production with age (Psachoulia et al., 2009) – as expected, since new oligodendrocytes must ultimately come from precursor cell divisions. If we assume that oligodendrocytes have a long but finite lifetime in vivo, it could be that as the division rate of NG2-glia decelerates and, with it, the rate of oligodendrocyte production, a critical age is reached beyond which the rate of new myelin production does not keep pace with accelerating myelin loss. If so, finding a way to maintain the proliferative rate of NG2-glia in old age might help maintain white matter integrity and slow down age-related mental decline.
Conclusion
Recent experiments indicate that NG2-glia are, first and foremost, oligodendrocyte precursors in the healthy adult CNS. Thus, it is clear that NG2-glia are distinct from neural stem cells that generate hippocampal or olfactory neurons throughout life. Whether they can generate rare neurons in the piriform cortex, as reported by two labs recently, is still unresolved. Following CNS injury, NG2-glia undergo a burst of local proliferation before giving rise to oligodendrocytes and possibly some astrocytes. Following gliotoxin-induced focal demyelination in the spinal cord, they also generate significant numbers of remyelinating Schwann cells. The great majority of reactive astrocytes at sites of damage are not derived from NG2-glia, but from pre-existing astrocytes that re-enter the cell cycle and – in spinal cord – from stem-like cells in the ependymal zone around the central canal. The finding that NG2-glia are predominantly precursors of myelin-forming cells re-focuses our attention on their crucial role in remyelination, following demyelinating disease or injury. We are also drawn to the role of myelin genesis during normal healthy adulthood, which might play a role in some forms of neural plasticity – motor skills learning, for example. The fact that NG2-glia react rapidly to injury suggests that they might also respond to systemic modulation. Indeed, their cell cycle and/or differentiation rate can be influenced by prolactin levels during pregnancy (Gregg et al., 2007) or by physical exercise (Simon et al., 2011). Therefore, a key research focus for the future is the potential role of adult myelination in learning and memory and how that might be affected by the environment.
Acknowledgments
We thank David Attwell (UCL) and three anonymous reviewers for their constructive comments and suggestions for improvement. Work in the authors' laboratory was supported by the UK Medical Research Council (MRC), The Wellcome Trust and the National Institutes of Health, USA. I.McK. was the recipient of a Royal Society USA/Canada Exchange Fellowship. K.M.Y. is supported by the BUPA Foundation and the Alzheimer's Society, UK.
References
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisi GM, Foresti ML, Mukherjee S, Shapiro LA. The role of olfactory stimulus in adult mammalian neurogenesis. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.03.050. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bostock H, Sears TA. Continuous conduction in demyelinated mammalian nerve fibres. Nature. 1976;263:786–787. doi: 10.1038/263786a0. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmann T, Hartig W, Holzer M, Arendt T. Expression of the embryonal isoform (0N/3R) of the microtubule-associated protein tau in the adult rat central nervous system. J Comp Neurol. 2010;518:2538–2553. doi: 10.1002/cne.22351. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Sahley CL. Invertebrate learning and memory: from behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30:3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay D, Heydon K, Miguez A, Schwab M, Nave KA, Thomas JL, Spassky N, Martinez S, Zalc B. Genetic tracing of subpopulation neurons in the prethalamus of mice (Mus musculus) J Comp Neurol. 2009;512:74–83. doi: 10.1002/cne.21904. [DOI] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34:213–228. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van BE. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, Chneiweiss H, umas-Duport C, Varlet P. NG2+/Olig2+ Cells Are the Major Cycle-Related Cell Population of the Adult Human Normal Brain. Brain Pathol. 2010;20:399–411. doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, Weiss S. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27:1812–1823. doi: 10.1523/JNEUROSCI.4441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Hubbard PS, Butt AM. Effects of glutamate receptor activation on NG2-glia in the rat optic nerve. J Anat. 2009;214:208–218. doi: 10.1111/j.1469-7580.2008.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Kramer LA, Papnicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res. 2008;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Seki T, Sato K, Iwai M, Zhang WR, Manabe Y, Abe K. Expression of polysialylated neural cell adhesion molecule in rat brain after transient middle cerebral artery occlusion. Brain Res. 2001;907:130–133. doi: 10.1016/s0006-8993(01)02543-4. [DOI] [PubMed] [Google Scholar]
- Honjin R, Sakato S, Yamashita T. Electron microscopy of the mouse optic nerve: a quantitative study of the total optic nerve fibers. Arch Histol Jpn. 1977;40:321–332. doi: 10.1679/aohc1950.40.321. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Geng F, Tao L, Hu N, Du F, Fu K, Chen F. Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp. 2011;32:10–21. doi: 10.1002/hbm.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles ZJ, Raminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972;227:351–364. doi: 10.1113/jphysiol.1972.sp010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Serwanski DR, Richard LQ, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia. 2011;59:800–809. doi: 10.1002/glia.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Zhu X, Serwanski DR, Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol. 2009;512:702–716. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Lasiene J, Matsui A, Sawa Y, Wong F, Horner PJ. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell. 2009;8:201–213. doi: 10.1111/j.1474-9726.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett. 2010;479:128–133. doi: 10.1016/j.neulet.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol. 1988;271:589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. NeuroImage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008;28:7610–7623. doi: 10.1523/JNEUROSCI.1355-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Kangas CD, Macklin WB. Neuronal expression of the proteolipid protein gene in the medulla of the mouse. J Neurosci Res. 2009;87:2842–2853. doi: 10.1002/jnr.22121. [DOI] [PubMed] [Google Scholar]
- Miller RH. Oligodendrocyte origins. Trends Neurosci. 1996;19:92–96. doi: 10.1016/s0166-2236(96)80036-1. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Mission JP, Takahashi T, Caviness VS., Jr Ontogeny of radial and other astroglial cells in murine cerebral cortex. Glia. 1991;4:138–148. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell D, McEwen B. PSA-NCAM expression in the piriform cortex of the adult rat. Modulation by NMDA receptor antagonist administration. Brain Res. 2002;927:111–121. doi: 10.1016/s0006-8993(01)03241-3. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Nunez JL, Nelson J, Pych JC, Kim JH, Juraska JM. Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Res Dev Brain Res. 2000;120:87–90. doi: 10.1016/s0165-3806(99)00193-5. [DOI] [PubMed] [Google Scholar]
- Omlin FX, Waldmeyer J. Differentiation of neuron-like cells in cultured rat optic nerves: a neuron or common neuron-glia progenitor? Dev Biol. 1989;133:247–253. doi: 10.1016/0012-1606(89)90315-1. [DOI] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Pekcec A, Loscher W, Potschka H. Neurogenesis in the adult rat piriform cortex. Neuroreport. 2006;17:571–574. doi: 10.1097/00001756-200604240-00003. [DOI] [PubMed] [Google Scholar]
- Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle NP. Oligodendrocyte Wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers L, Young K, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2-positive glia generate myelinating oligodendrocytes and cortical projection neurons in the adult mouse CNS. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white matter architecture. Nat Neurosci. 2009;12:1367–1368. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat Embryol (Berl) 1991;184:395–401. doi: 10.1007/BF00957900. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res. 1993;17:265–290. doi: 10.1016/0168-0102(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ng KL, Zhou QY, Ribak CE. Olfactory enrichment enhances the survival of newly born cortical neurons in adult mice. Neuroreport. 2007;18:981–985. doi: 10.1097/WNR.0b013e3281532bc1. [DOI] [PubMed] [Google Scholar]
- Simon C, Götz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59:869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Bostock H, Hall SM. Saltatory conduction precedes remyelination in axons demyelinated with lysophosphatidyl choline. J Neurol Sci. 1982;54:13–31. doi: 10.1016/0022-510x(82)90215-5. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol Appl Neurobiol. 1980;6:415–420. doi: 10.1111/j.1365-2990.1980.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, Wanaka A. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res. 2008;86:3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullen F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biol. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- Ullen F, Forsman L, Blom O, Karabanov A, Madison G. Intelligence and variability in a simple timing task share neural substrates in the prefrontal white matter. J Neurosci. 2008;28:4238–4243. doi: 10.1523/JNEUROSCI.0825-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Brill MH. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J Neurol Neurosurg Psychiat. 1978;41:408–416. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210:661–670. doi: 10.1111/j.1469-7580.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley R, Butt AM. Integration of NG2 cells (synantocytes) into the neuroglial network. Neuron Glia Biol. 2009;5:21–28. doi: 10.1017/S1740925X09990329. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Wren D, Wolswijk G, Noble M. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol. 1992;116:167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Increases in size and myelination of the rat corpus callosum during adulthood are maintained into old age. Brain Res. 2007;1142:13–18. doi: 10.1016/j.brainres.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD. An Fgfr3-iCreER(T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia. 2010;58:943–953. doi: 10.1002/glia.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. NeuroImage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]