Abstract
BACKGROUND AND OBJECTIVES:
One out of five Saudi diabetics develops end-stage renal disease (ESRD). Factors associated with progressive loss of renal function have not been extensively studied and reported in our community. We sought to evaluate the pattern and progression in glomerular filtration rate (GFR) and investigate the potential risk factors associated with progression to diabetic nephropathy (DN) among Saudi patients.
DESIGN AND SETTING:
Hospital-based retrospective analysis of type 2 diabetic patients seen between January 1989 and January 2004 at Security Forces Hospital and King Saud University in Riyadh, Saudi Arabia.
PATIENTS AND METHODS:
DN was defined as persistent proteinuria assessed by urine dipstick [at least twice for at least two consecutive years and/or serum creatinine >130 μmol/L; and/or GFR <60 mL/min/1.73m2].
RESULTS:
Of 1952 files reviewed, 621 (31.8%) met the criteria for DN, and 294 (47%) were males. The mean (SD) age of the patients at baseline was 66.9 (11.4) years, and mean duration of diabetes was 15.4 (7.5) years. GFR deteriorated from a baseline value of 78.3 (30.3) mL/min/1.73m2 to 45.1 (24.1) mL/min/1.73m2 at the last visit, with a mean rate of decline in GFR of 3.3 mL/min/year. Progression of nephropathy was observed in 455 (73.3%) patients, with 250 (40.3%) patients doubling their first–hospital-visit serum creatinine level in a mean of 10.0 (6.0) years. At the end of the study, 16.5% of the cohort developed ESRD and were dialyzed. GFR >90 mL/min/1.73m2 at the first hospital visit; duration of diabetes >10 years; persistent proteinuria; systolic blood pressure >130 mm Hg; and presence of retinopathy were significant markers associated with progression of nephropathy.
CONCLUSION:
Diabetic nephropathy tends to be progressive among Saudis, with GFR deteriorating at a rate of 3.3 mL/year and with a doubling of serum creatinine level in 40.3% of patients in 9.9 years.
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease (ESRD).1,2 There are tremendously increasing trends in the incidence of diabetes and DN, signaling a medical catastrophe in dialysis units which results in a greater consumption of economic resources.3,4 In Saudi Arabia alone, approximately 11 000 patients have ESRD, and 20% is due to diabetes.4,5 The cost of care with dialysis in Saudi Arabia amounts to US$14 000 per patient per year and the total economic burden exceeds US$540 million.6 Unfortunately, all DN patients hardly achieve the international guideline-recommended target levels.7 Recently we reported that the complication rate associated with type 2 diabetes was 32% for renal and 23% for cardiovascular complications.8,9
Socioeconomic factors, age, gender, diet, obesity and the high incidence of hypertension play an important role in the progression of diabetic nephropathy. Uncontrolled blood pressure is known to be deleterious, but other factors may become more important once BP is treated.10–15 Several studies have identified age,16,17 male gender,17 lower initial glomerular filtration rate (GFR),14–18 first–hospital-visit fasting plasma glucose18 and concomitant presence of retinopathy19,20 as important risk factors for GFR decline. The proteinuria itself is a major risk factor for progression of DN.12–15,19,20 Above all, these risk factors for progression of chronic kidney disease (CKD) in type 2 diabetes mellitus have not been fully elucidated.
The decline in GFR is highly variable, ranging from 2 to 20 mL/min/year.8,9,13–21 Factors associated with progressive loss of renal function have not been studied extensively, though hypertension and proteinuria have been identified as important promoters; whereas glycemic control, the renoprotective effect of angiotensin receptor blockers and angiotensin converting enzyme inhibitors have been found to delay progression of nephropathy. The aim of our study was to evaluate the pattern and changes in GFR over time; and investigate the potential risk factors associated with enhanced loss of renal function and all-cause mortality among Saudis with type 2 diabetes and nephropathy.
PATIENTS AND METHODS
The study included all Saudi patients diagnosed with type 2 diabetes in the period from January 1989 to January 2004. The medical records were obtained from a database scan at the Medical Records Division of the Security Forces Hospital in Riyadh, Saudi Arabia. The inclusion criteria were that the patient should have had at least 1 year of active follow-up with at least three measurements of laboratory parameters, and a persistent urine-dipstick macroproteinuria (+1 or greater on urine dipstick test) for 2 consecutive years; and/or serum creatinine (SCr) of >130 μmol/L at the last visit; and/ or GFR of <60 mL/min/1.73m2 at the last visit. This was based on the WHO criteria for diabetic nephropathy.22 Patients who presented to the hospital for dialysis or reached end-stage renal disease (ESRD) (GFR less than 15 mL/min) and those patients with less than 1 year of follow-up were excluded. The patients were followed until the last visit to the hospital or death, and determinants of renal function loss and mortality were evaluated yearly.
Data on demographic and anthropometric characteristics of patients, the duration of diabetes, family history of diabetes, the duration of follow-up, and medications were collected and recorded. Data on the first hospital visit, follow-up and last hospital visit; serum creatinine levels; fasting blood sugar (FBS); results of urinary dipstick for microproteinuria; and serum cholesterol were extracted from the main database of the hospital computer and matched to the corresponding dates of hospital visits. First hospital visit and follow-up readings for blood pressure were also noted. GFR was estimated using the Cockcroft-Gault equation.23 Mean values of blood pressure, blood sugar, serum creatinine, GFR and serum cholesterol at follow-up were calculated as the average of at least three readings within a year of follow-up and the time course was recorded.
Diabetic nephropathy, defined as the presence of positive persistent macroproteinuria as assessed by urine dipstickat least twice for at least two consecutive years from the first hospital visit to the end of follow-up; and/or serum creatinine >130 μmol/L; and/or GFR <60 mL/min/1.73m2. Proteinuria, assessed by urine dipstick test measures; macroalbuminuria, defined as +1, +2 or greater, and representing a protein concentration greater than 0.30 g/L. Persistent proteinuria was defined as macroproteinuria for more than twice from first hospital visit to the end of at least 2 consecutive years of follow-up as assessed by urine dipstick. Based on the guidelines of European Society of Hypertension–European Society of Cardiology24 and the of the American Diabetic Association,25 the following definitions were used: hypertension as systolic blood pressure >130 mm Hg and/or diastolic blood pressure >80 mm Hg; dyslipidemia as serum cholesterol of >5.2 mmol/L and uncontrolled diabetes with FBS >7.0 mmol/L.
After estimating GFR by Cockcroft-Gault method, patients were grouped according to CKD stages based on the first–hospital-visit GFR levels. Progression of nephropathy was defined using the changes in GFR levels from the first hospital visit to the last hospital visit, as follows: non-progressors when GFR levels over follow-up visits remained stable or showed improvement in CKD stage compared to the first-hospital-visit GFR; and progressors when a patient develops low GFR and moves to the next stage of chronic kidney disease, i.e., from stage 1 to stage 2 CKD.
Data entry was done using Microsoft Excel for Windows XP Professional edition. Statistical analysis was done using SPSS version 16.0 (SPSS Inc., Chicago, USA). The data were tested for normality of distribution and were presented as mean and standard deviation. Demographic data was analyzed descriptively. Significance of measured changes was assessed using the Mann-Whitney U test for continuous variables and chi-square and cross-tabulations for categorical variables. Pearson correlation was done for bivariate analysis. Linear regression analysis was used to determine the rate of decline in GFR for each patient, using all GFR values measured during follow-up. Multiple linear regression models using backward selection were used to evaluate the impact of variables at first hospital visit and at follow-up visits on the progression of nephropathy. Differences were assumed to be statistically significant when the P value was <.05.
RESULTS
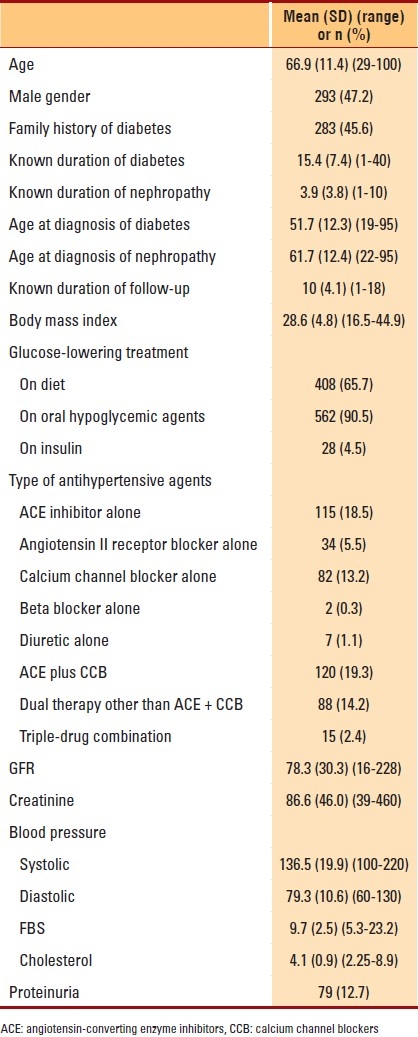
First–hospital-visit characteristics of the 621 diabetic nephropathy patients that met inclusion criteria are shown in Table 1. Renal function was evaluated over a mean period of 10 (4.1) years with 3.3 (3-33) determinations of GFR per patient. At the first hospital visit, concomitant hypertension was seen in 528 (84.4%) patients, of whom 465 (88.1%) were on medication; of these 465 patients, BP was controlled in 189 (35.8%) patients. Also, there were 92 (14.7%) patients who were dyslipidemic; of them, 51 (55.4%) were on statins. Forty-three (46.7%) had controlled serum cholesterol. At the last visit, 577 (92.2%) patients had hypertension; 263 (42.0%) with systolic hypertension only, 134 (21.4%) with diastolic hypertension only and 180 (28.7%) with both systolic and diastolic hypertension. Five hundred forty-three (94.1%) patients were taking medications, and BP was controlled in 289 (50.1%) of these patients. At the end of follow-up, systemic blood pressure was 134.5 (9.5) mm Hg/ 77.9 (5.4) mm Hg (110-160/60-100), mean serum creatinine was 197.3 (173.4) μmol/L (52-1, 698), cholesterol was 1.2 (0.4) mmol/L (1-2) and GFR was 45.1 (24.1) mL/min/1.73 m2 (5-163.5).
Table 1.
First–hospital-visit (baseline) characteristics of 621 type 2 diabetic nephropathy patients followed for a mean of 9.9 years at the security forces hospital, Riyadh, Saudi Arabia
Progression of nephropathy was observed in 455 (73.3%) patients. Among the progressors, 227 (49.9%) were females. Also, in this group of patients, obesity was seen in 364 (80.0%) patients; and hypertension in 421 (92.5%). Seventy-five (16.5%) of the progressors developed with ESRD and were eventually dialyzed. Among the 166 non-progressors, 100 (60.2%) patients were females, 115 (69.3%) were obese and 153 (92.2%) had hypertension. During follow-up, 250 (40.3%) patients doubled their first–hospital-visit serum creatinine in a mean duration of 9.98 (6.04 years) (range, 1-39 years). The mean rate of decline in GFR was 3.3 mL/min/year (Table 2).
Table 2.
Change from first hospital visit (baseline) to follow-up examinations 9.9 years later in progressors and non-progressors.
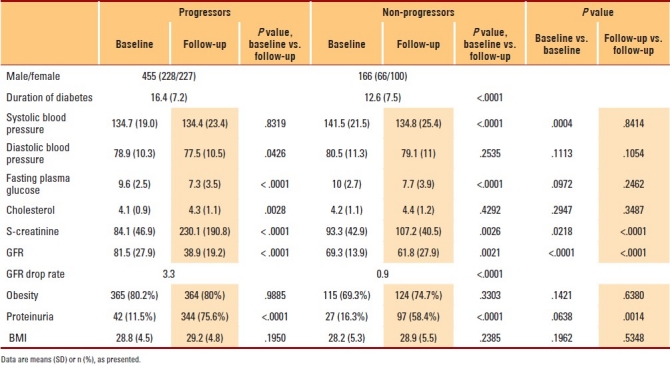
The mean duration of diabetes was significantly longer among the progressors compared to non-progressors. Duration of follow-up was significantly longer among the progressors compared to non-progressors (10.4 [3.9] years vs. 9.0 [4.4] years, P=.001). Diabetes was diagnosed significantly earlier among the progressors compared to non-progressors (50.9 [11.6] years vs. 53.7 [13.8] years, P=.023). Nephropathy was diagnosed at a significantly lower age among non-progressors compared to progressors (57.9 [15.0] years vs. 62.7 [11.3] years, P=.003).
GFR at first hospital visit was significantly lower among non-progressors compared to progressors. Mean SCr at first hospital visit was significantly higher among non-progressors compared to progressors. FBS level at first hospital visit was also significantly higher among the non-progressors compared to progressors. Last-visit SCr was significantly higher among progressors compared to non-progressors. Last-visit mean GFR was significantly higher among non-progressors compared to progressors. Drop in GFR/year was significantly higher among progressors compared to non-progressors (Table 2).
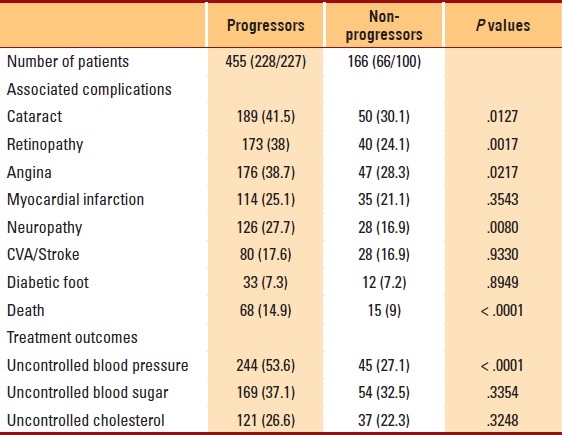
The prevalence of concomitant complications was significantly higher among progressors. Progressors had a significantly higher prevalence of cataract, retinopathy, angina and neuropathy compared to non-progressors (Table 3). Mortality from all causes was significantly greater among progressors compared to non-progressors (14.9% vs. 9.0%, P<.0001). The occurrences of myocardial infarction, stroke, diabetic foot infections and limb amputations were not significantly different between progressors and non-progressors (P>.05) (Table 3).
Table 3.
Frequency of associated complications and treatment outcomes among progressors and non-progressors in 621 diabetic nephropathy patients
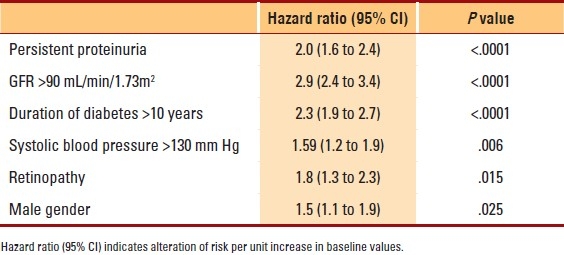
All variables thought to affect progression of diabetic nephropathy, such as age, gender, age of onset of diabetes, age of onset of nephropathy, age at diagnosis of diabetes and nephropathy, duration of diabetes, duration of nephropathy, initial serum creatinine level, initial GFR level, serum cholesterol, blood pressure and blood sugar level, were subjected to univariate and multivariate analyses to assess the determinants of progression of nephropathy. Body mass index (BMI) (t=2.895, P=.004), known age at diagnosis of diabetes (t=2.533, P=.012), duration of diabetes (t=5.650, P≤.0001), first-hospital-visit serum creatinine (t=2.212, P= .027), first-hospital-visit FBS level (t=2.049, P=.041), systolic blood pressure (t=3.850, P≤.0001) and duration of nephropathy (t=-6.387, P<.0001) together with male gender (t=5.009, P=.025), presence of cataract (t=6.698, P=.010), presence of retinopathy (t=6.427, P=.011), presence of neuropathy (t=7.642, P=.006) and persistent proteinuria (t=13.049, P<.0001) were significantly associated with an increased rate of decline in GFR and hastened progression of diabetic nephropathy. Figure 1 shows the variables that were independently analyzed and were found to be associated with progression of GFR such that, the presence of persistent proteinuria alone would cause a yearly drop in GFR of 3.9 mL/year; presence of retinopathy, of 3.8 mL/year; high systolic blood pressure (SBP), of 3.5 mL/year; and duration of diabetes beyond 10 years was found to decrease GFR by at least 3.3 mL/year. Diastolic blood pressure and first–hospital-visit cholesterol level were not associated with the increased rate of decline of GFR and progression of nephropathy and were excluded from the final model. Multivariate logistic regression analysis showed that first–hospital-visit GFR >90 mL/min/1.73 m2, duration of diabetes >10 years, persistent proteinuria, systolic blood pressure >130 mm Hg, presence of retinopathy and male gender are variables significantly associated with progression of nephropathy (Table 4).
Figure 1.
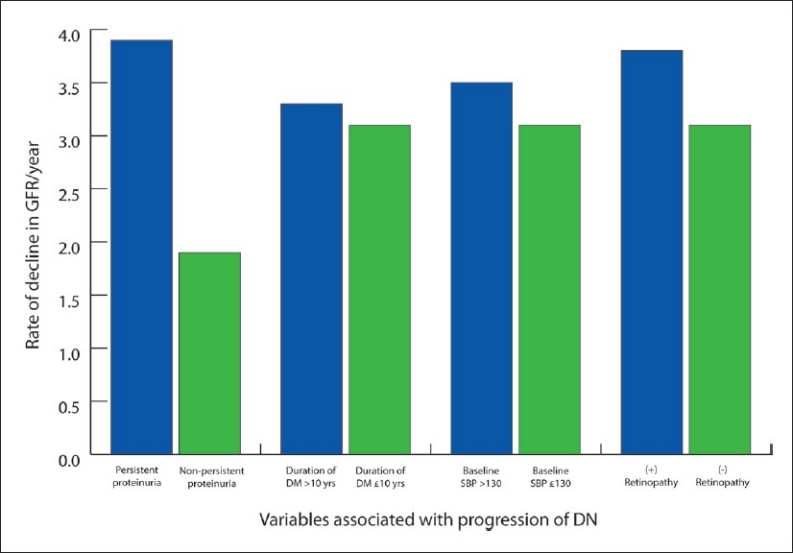
Rate of decline in GFR/year in relation to variables associated with progression of GFR and nephropathy in 621 diabetic nephropathy patients.
Table 4.
First–hospital-visit (baseline) and follow-up variables associated with progression of nephropathy in 621 type 2 diabetic patients with nephropathy followed for 9.9 years
DISCUSSION
Diabetic kidney disease is considered to be an irreversible and progressive disease. In previous studies on diabetic Pima Indians, up to 50% were found to develop nephropathy at 20 years of age.26 However, the incidence of diabetic ESRD was noted to have declined significantly to 15 cases per 1000 patients in 2002.27 In this study, only 12.7% of the patients showed proteinuria, but 31.8% developed nephropathy. Though this rate of incidence appears to be of lesser magnitude as compared to that among Pima Indians, the role and impact of genetic susceptibility and race have not been fully elucidated. This observation in such genetically disparate populations suggests a primary role for socioeconomic factors, such as diet and poor control of hyperglycemia, hypertension and obesity. Obesity is seen in more than 70% of our studied population, contributing to higher risk of nephropathy.
In diabetic nephropathy, hyperfiltration occurs for a variable period, followed by a progressive decline of GFR once overt proteinuria appears. Furthermore, the rate of deterioration was found to be higher among patients with high initial GFR. In these patients, the glomerular hyperfiltration that occurs initially increased the risk for diabetic renal disease; in comparison to those patients with lesser degree of hyperfiltration, who may have had a slower course, with lesser risk for ESRD. Variations in the rate of GFR decline in patients with type 2 diabetes with nephropathy have been described before in previous studies, ranging from 0.36 mL/min/1.73m2/year among the Japanese population11 to 4.7 mL/min/1.73m2/year among Brazilian subjects.19 Several other studies have also suggested an annual decline in GFR within this range.12,13,18,28 In our study, we showed an average of 3.3 mL/year drop in GFR and even greater among patients who reached ESRD (5.9 mL/year). Again, genetics, race, obesity, glycemic control and use of angiotensin-converting enzyme (ACE) inhibitors in the control of hypertension play an important role in the disparity of GFR drop rates. Furthermore, we have shown in our recently published studies among Saudi type 2 diabetics that renal complications go as high as 32%, although mortality is even higher with associated cardiovascular complications.8,9
The presence of persistent proteinuria was a strong risk factor for subsequent loss of GFR, as shown by our findings that 75.6% of our patients had proteinuria and 16.5% reached ESRD, re-emphasizing earlier reports that established the importance of sustained increases in urine albumin excretion in the pathogenesis and diagnosis of diabetic kidney disease.13–16,20 This is considerably higher than the reported rate in diabetic Pima Indians, with 15% progressing to ESRD at approximately 20 years of diabetes.26 This implies the pathophysiological effect of albuminuria on the future decline in kidney function, much more a risk for loss of kidney function; and eventually ESRD or proteinuria per se may contribute to glomerular and tubulointerstitial lesions, resulting in progressive renal disease.14,15,20
In this study, we identified several modifiable variables, including proteinuria, systolic blood pressure and obesity, to be associated with faster progression of renal disease. ACE inhibitors, controlling blood pressure and hypertension, controlling obesity, consumption of low-protein diet and control of blood sugar, can modify these identified factors. Furthermore, our study confirmed and extended the findings dealing with the impact of diabetic retinopathy on the progression of kidney disease in type 2 diabetics.19 When risk factors for progression in renal disease were evaluated, first–hospital-visit proteinuria was the most consistent independent risk factor for low time period to reach the composite end point of doubling of serum creatinine or the stage of developing ESRD.28 In our study, first–hospital-visit proteinuria was also associated with increased all-cause mortality. Surprisingly, though, we found no significant differences in the prevalence of cardiovascular diseases and stroke between progressors and non-progressors, we believe that those patients who did not progress had been in longstanding advanced CKD stage (Table 2). Furthermore, in our study, systolic but not diastolic blood pressure was associated with increased progression of diabetic nephropathy, which is in accordance with previously published data.11,19 This is likely due to the fact that type 2 diabetic patients primarily suffer from isolated systolic hypertension. However, our study was not designed to evaluate treatment effects. The renoprotective effects of angiotensin II receptor blockers have been demonstrated in several clinical trials.7,11
In conclusion, diabetic nephropathy among Saudis tends to be progressive with GFR decline at a rate of 3.3 mL/year with doubling of serum creatinine. Persistent proteinuria, duration of diabetes >10 years, uncontrolled systolic blood pressure (>130 mm Hg) and presence of retinopathy predict progression of nephropathy.
REFERENCES
- 1.Ritz E, Rychlík I, Locatelli F, Halimi S. End stage renal failure in type 2 diabetes: A Medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2003. US Renal Data System: USRDS 2003 Annual Data Report: Atlas of End Stage Renal Disease in the United States. [Google Scholar]
- 3.Al Zaid A, Sobki S, DeSalva V. Prevalence of micro albuminuria in Saudi Arabians with non insulin dependent diabetes mellitus: A clinic based study. Diabet Res Clin Practice. 1994;26:115–20. doi: 10.1016/0168-8227(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 4.Huraib S, Abu-Aisha H, Sulimani RA, Famuyiwa FO, Al-Wakeel J, Askar A, et al. The pattern of diabetic nephropathy among Saudi patients with non-insulin dependent diabetes mellitus. Ann Saudi Med. 1995;2:120–4. doi: 10.5144/0256-4947.1995.120. [DOI] [PubMed] [Google Scholar]
- 5.Alwakeel JS, Mitwalli AH, Abu-Aisha H, Tarif N, Memon N, Sulimani F, et al. Single center experience with pre-dialysis patients. Saudi J Kidney Dis Transplant. 2002;13:363–70. [PubMed] [Google Scholar]
- 6.Saudi Center for Organ Transplantation (SCOT) data. Annual Report of 2008. [Google Scholar]
- 7.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic Nephropathy: Diagnosis, prevention and treatment. Diabetes Care. 2005;28:164–76. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 8.Alwakeel JS, Sulimani R, Al-Asaad H, Al-Harbi A, Tarif N, Al-Suwaida A, et al. Diabetes complications in 1952 type 2 diabetes mellitus patients managed in a single institution in Saudi Arabia. Ann Saudi Med. 2008;28:260–6. doi: 10.5144/0256-4947.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alwakeel JS, Al-Suwaida A, Isnani AC, Al-Harbi A, Alam A. Concomitant macro and microvascular complications in diabetic nephropathy. Saudi J Kidney Dis Transpl. 2009;20:402–9. [PubMed] [Google Scholar]
- 10.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31:433–41. doi: 10.1291/hypres.31.433. [DOI] [PubMed] [Google Scholar]
- 11.Leehey DJ, Kramer HJ, Daoud TM, Chatha MP, Isreb MA. Progression of kidney disease in type 2 diabetes – beyond blood pressure control: An observational study. BMC Nephrol. 2005;6:8. doi: 10.1186/1471-2369-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–9. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- 13.Rossing P. Risk factors in the progression of diabetic nephropathies. Ugeskr Laeger. 2000;162:5057–61. [PubMed] [Google Scholar]
- 14.Christensen PK, Rossing P, Nielsen FS, Parving HH. Natural course of kidney function in type 2 diabetic patients with diabetic nephropathy. Diabet Med. 1999;16:388–94. doi: 10.1046/j.1464-5491.1999.00063.x. [DOI] [PubMed] [Google Scholar]
- 15.Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Premaratne E, Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith T, Jerums G. Renal hyperfiltration in type 2 diabetes: Effect of age-related decline in glomerular filtration rate. Diabetologia. 2005;48:2486–93. doi: 10.1007/s00125-005-0002-9. [DOI] [PubMed] [Google Scholar]
- 17.Eriksen BO, Ingebretsen OB. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–82. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 18.Murussi M, Gross JL, Silveiro SP. Glomerular filtration rate changes in normoalbuminuric and microalbuminuric type 2 diabetic patients and normal individuals.A 10-year follow-up. J Diabetes Complications. 2006;20:210–5. doi: 10.1016/j.jdiacomp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Trevisan R, Vedovato M, Mazzon C, Coracina A, Iori E, Tiengo A, et al. Concomitance of diabetic retinopathy and proteinuria accelerates the rate of decline of kidney function in type 2 diabetic patients. Diabetes Care. 2002;25:2026–31. doi: 10.2337/diacare.25.11.2026. [DOI] [PubMed] [Google Scholar]
- 20.Jerums G, Panagiotopoulos S, Tsalamandris C, Allen TJ, Gilbert RE, Comper WD. Why is proteinuria such an important factor for progression in clinical trials? Kidney Int Suppl. 1997;63:S87–92. [PubMed] [Google Scholar]
- 21.Coyne DW. Evaluation of the patient with renal disease. In: Carey CF, Lee HH, Woeltje KF, editors. 29th ed. Vol. 12. Washington: Manual of medical therapeutics; p. 227. [Google Scholar]
- 22. [Last accessed on 2010 Feb 18]. Available from: http://www.who.int/healthinfo/statistics/gbdestimatescasedefinitions.pdf .
- 23. [Last accessed on 2010 Feb 18]. Available from: http://www.eshonline.org/pdf/2003_guidelines.pdf .
- 24.Nelson RG, Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetic kidney disease in Pima Indians. Diabetes Care. 1993;16:335. doi: 10.2337/diacare.16.1.335. [DOI] [PubMed] [Google Scholar]
- 25.Pavkov ME, Knowler WC, Bennett PH, Looker HC, Krakoff J, Nelson RG. Increasing incidence of proteinuria and declining incidence of end stage renal disease in diabetic Pima Indians. Kidney Int. 2006;70:1840. doi: 10.1038/sj.ki.5001882. [DOI] [PubMed] [Google Scholar]
- 26.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes. 2006;55:1832–9. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 27.Poggio ED, Nef PC, Wang X, Greene T, Van Lente F, Dennis VW, et al. Performance of the Cockcroft-Gault and modification of diet and renal disease equation in estimating GFR in ill hospitalized patients. Am J Kidney Dis. 2005;46:242–52. doi: 10.1053/j.ajkd.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int. 2003;63:1499–507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]


