Abstract
Chronic pruritus, one of the main symptoms in dermatology, is often intractable and has a high impact on patient's quality of life. Beyond dermatologic disorders, chronic pruritus is associated with systemic, neurologic as well as psychologic diseases. The pathogenesis of acute and chronic (>6 weeks duration) pruritus is complex and involves in the skin a network of resident (e.g., sensory neurons) and transient inflammatory cells (e.g., lymphocytes). In the skin, several classes of histamine-sensitive or histamine-insensitve C-fibers are involved in itch transmission. Specific receptors have been discovered on cutaneous and spinal neurons to be exclusively involved in the processing of pruritic signals. Chronic pruritus is notoriously difficult to treat. Newer insights into the underlying pathogenesis of pruritus have enabled novel treatment approaches that target the pruritus-specific pathophysiological mechanism. For example, neurokinin-1 antagonists have been found to relieve chronic pruritus.
Keywords: Atopic dermatitis, Itch, Pathophysiology, Pruritus, Therapy
INTRODUCTION
Pruritus (syn.: itch) is an unpleasant sensory perception which causes an intense desire to scratch and which has a high impact on the quality of life. Pruritus is the most frequent symptom in dermatology and can occur in acute or chronic forms (over 6 weeks in duration)1,2. Further, the acute induction of pruritus, e.g. in an experimental model through a histamine stimulus, must be differentiated from the mechanisms that play a role in clinical forms of chronic pruritus2. Pruritus can have its origin directly in the skin or can develop in the central nervous system via haematogenic or neurogenic mediators. With this background the pathogenesis of pruritus is quite complex. In recent years fundamental new insights have been gained into pruritus induction and transmission as well as involved nerves, receptors and mediators. The understanding of the pathogenesis of pruritus has increased; the clinical assignment of the individual mechanism to the different pruritic diseases remains a task for future research.
FREQUENCY OF CHRONIC PRURITUS
According to a cross-sectional study in Oslo, acute pruritus affects 8.4% of the general population, while in a large French study 42% of patients with skin diseases stated that they experienced pruritus3,4. In a German pilot study a life-time prevalence of 22.6% was surveyed5. A recent cross-sectional observational study in >11,000 employees of 144 German companies demonstrated a point prevalence of 17% among German employees6. A quarter of the affected persons had suffered from pruritus for more than 5 years. 47% had never sought medical advice; 94% had undergone no treatment. Although gender differences have been little explored, the higher level of pruritus among females has been reported5,7. Focused on elderly people, only a small number of difficult-to-match studies have investigated prevalence and incidence of chronic pruritus. The German study showed that prevalence increased with age from 12.3% (16~30 years) to 20.3% (61~70 years)6. According to a Turkish study with 4,099 elderly dermatological patients, 11.5% reported pruritus, whereas the highest prevalence was in patients older than 85 years8. In a Thai-study with 149 elderly patients pruritic diseases were the most common ones (41%)9.
PRURITUS CLASSIFICATION
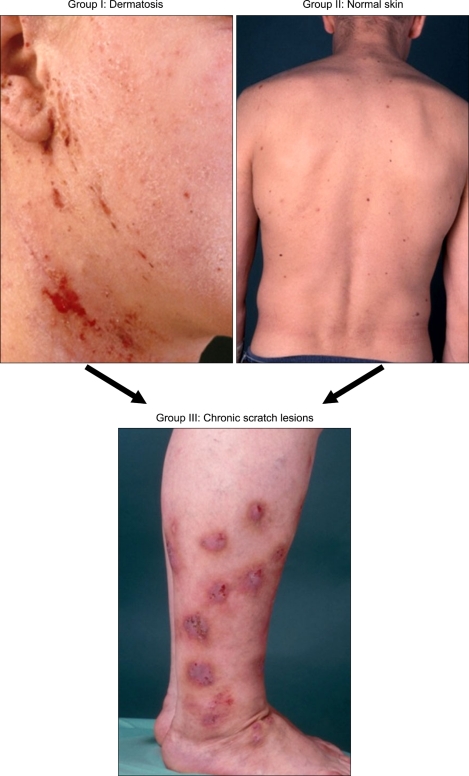
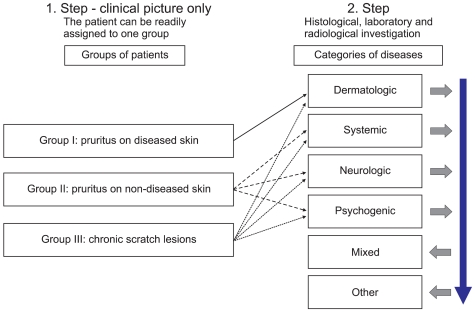
Several classifications have been published, considering these different aetiologies and clinical morphologies of chronic pruritus1,10,11. The International Forum for the Study of Itch (IFSI; http://www.itchforum.net) has proposed a classification, which considers clinical as well as differential diagnostic reasons. One part of the classification encounters the presence of skin changes: (I) "pruritus on primary diseased, inflamed skin" like in inflammatory, infectious, autoimmune disorders, lymphomas or drug reactions or (II) "pruritus on primary non-diseased, non-inflamed skin" like in neurologic or psychiatric origin. Given that scratching damages the skin, consequently maintaining and reinforcing the inflammatory processes which induce further pruritus and predominating the clinical picture a third group was defined: (III) "secondary scratch lesions", which includes patient's with excoriations, crusts, papules, nodules and chronic secondary scratch lesions like prurigo nodularis (Table 1, 2, Fig. 1). A vicious circle may result between pruritus and mechanical stimulus responses. However, the presence or absence of secondary scratch lesions does not allow the determination of origin. Thus, for differential diagnostic purposes, a categorization of underlying pruritogenic diseases has been proposed including dermatological, systemic, neurological and psychiatric/psychosomatic diseases. Patients, with more than one underlying disease should be categorized as "mixed". If no underlying disease can be identified, pruritus should be classified as "pruritus of undetermined origin" (PUO) (Fig. 2)1.
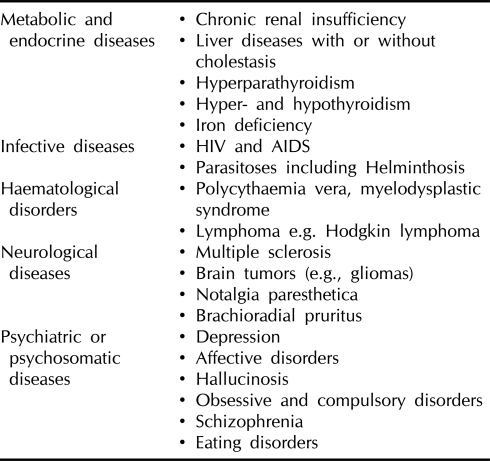
Table 1.
Systemic diseases that can induce pruritus (examples)
Table 2.
Generalised pruritus on primarily inflammatory skin
Fig. 1.
Clinical groups of patients with chronic pruritus. Chronic pruritus occurs on primarily diseased skin (i.e., dermatosis, group 1) or on normal skin (group 2). Chronic scratch lesions may be predominant and alter the clinical picture (group 3).
Fig. 2.
Clinical classification in the management of chronic pruritus patients (1). As a first step, patients were grouped according to the clinical picture. Subsequent clinical, laboratory and radiological investigation enables categorization of patient concerning the underlying origin. If several diseases were found (e.g., dry skin and chronic renal insufficiency) the patient has a mixed (multifactorial) origin. If no causal disease is identified, the patient has a pruritus of unknown/undetermined origin (PUO).
PRURITUS PATHOPHYSIOLOGY
Although pruritus is a frequent symptom of dermatological, systemical, psychogenic and neurogenic diseases, its pathophysiology is still not fully understood. As a cutaneous sensory perception, itch is excited on neuropeptide-containing unmyelinated nerve fibers in the papillary dermis and epidermis. In 1997 Schmelz et al.12 demonstrated that a subgroup of unmyelinized, slowly transmitting, sensory C-fibers are mechano-insensitive and were therefore termed CMi-fibers (C-fibers, mechano-insensitive). Some of these fibers were also stimulated by heat. In subsequent experiments on animal models it was shown that the expression of the heat receptor TRPV1 on the mechano-insensitive nerve fibers is needed for pruritus induction by histamine13. A further subgroup of C-fibers, the polymodal nociceptors (CMH), i. e. C-fibers that are mechano- and heat-sensitive, demonstrated no reaction to histamine and were considered pain fibers. This also was revised in recent studies. It has been known since the 1950s that the enzyme mucunain contained in the hairs of cowhage (Mucuna pruriens) can induce pruritus on contact with the skin. Interestingly, it was shown that cowhage activates the CMH-fibers causing pruritus with burning without simultaneous erythema or edema14. Just the different involved sensory C-nerve fiber classes and receptors illustrate how complex pruritus induction on the cutaneous sensory nerve endings is. An assignment of involved nerve fibers to the clinical diseases is still lacking at present just as is a clinical assignment of involved sensory receptors. The receptors and their ligands, however, are better characterized and are already useful in the treatment of pruritus. Several mediators such as neuropeptides and interleukins are known to provoke itch by direct binding to itch receptors or indirectly via release of histamine. Some recent examples are presented.
HISTAMINE 1 AND 4 RECEPTOR
Many mediators triggering itch have been investigated. Among them, histamine has been a persistent candidate and is the most thoroughly studied pruritogen for decades. Histamine binds to H1 receptor expressed on sensory nerve fibers and endothelial vessel walls. Intradermal injections of histamine provoke vasodilation with redness, wheal and flare (the so-called triple response of neurogenic inflammation) accompanied with pruritus. This model is still in use in pruritus research to induce acute itch. Furthermore, intradermally injected substance P (SP) releases histamine and provokes itch. However, antihistamines in normal dosages are of weak efficacy in several forms of chronic pruritus, as demonstrated in experimental studies as well as double-blind, cross-over trials15.
Recently, histamine 4 receptors were found on inflammatory cells mainly mast cells, eosinophilic granulocytes and T-lymphocytes16. Gutzmer et al.17 showed that the Th2 lymphocytes of atopic dermatitis (AD) patients express a functionally active H4 receptor. Stimulation of H4 receptor leads to up-regulation of the pruritogenic interleukin IL-31 (see below). This newly found mechanism may explain the quick increase of pruritus intensity during flaring up of AD patients. Interestingly, a mouse model suggests that a combination of H4 and H1 receptor antagonism might be a new strategy to treat pruritus related to allergic diseases. In their experiments, the authors showed that H4 receptor antagonism fails to reduce the allergic inflammatory response but strongly inhibits allergen-induced itch18. In sum, these results support the idea that histamine and the histamine 4 receptor but to a lesser degree also histamine 1 receptor may play a role in chronic pruritus pathophysiology.
INTERLEUKIN 31 (IL-31)
The newly discovered interleukin, IL-31 might have a major role in pathophysiology of pruritus, especially in atopic dermatitis19. For example, IL-31 mRNA in the skin of NC/Nga mice (atopic dermatitis mouse model) with scratching behavior was found to be significantly higher than that in NC/Nga mice without scratching behaviour19. IL-31 binds to a heterodimeric receptor consisting of the IL-31 receptor A and the oncostatin-M receptor. In the skin it is found on sensory C-fibers and keratinocytes and in the dorsal spinal ganglia, where it probably contributes to the transmission of the signal pruritus. IL-31 was shown to be significantly overexpressed in human AD skin compared with nonpruritic psoriatic skin inflammation20. The concentration of IL-31 in the peripheral blood is also elevated in patients with AD and correlates with disease severity of AD21. A link between bacterial colonization and induction of pruritus was also demonstated. Staphylococcal superantigen rapidly induced IL-31 expression in atopic individuals20. In vitro, staphylococcal enterotoxin B but not viruses or T(H)1 and T(H)2 cytokines induced IL-31 in leukocytes. In patients with AD, activated leukocytes expressed significantly higher IL-31 levels compared with control subjects. IL-31 receptor A showed most abundant expression in dorsal root ganglia representing the site where the cell bodies of cutaneous sensory neurons reside. These results suggest a direct link among staphylococcal colonization, subsequent T-cell recruitment/activation, and pruritus induction in patients with AD20. In sum, IL-31 represents a new and promising target in pruritus therapy especially in AD.
NEUROPEPTIDES
Several observations support the idea of an important role of neuropeptides for the pathophysiology of pruritus in various skin diseases. Neuropeptides such as SP, vasoactive intestinal peptide (VIP), somatostatin, and neurotensin provoke itch along with the characteristics of neurogenic inflammation such as erythema, wheal and flare. SP induces itch responses in human and mice which are mediated via activation of the neurokinin 1 receptor (NK1R) on mast cells and keratinocytes resulting in enhancement of inflammatory responses22 supporting a indirect effect of SP in mediating pruritus. Targeting the neuropeptides is a new concept in the treatment of itch. For example, a case series applying the NRK1 antagonstis aprepitant showed significant antipruritic effects in patients with prurigo nodularis and atopic predisposition23. Controlled trials are pending.
PATIENT'S HISTORY, EXAMINATION AND CLINICAL CHARACTERISTICS OF PRURITUS
Patients with chronic pruritus may present with a broad variety of symptoms and diagnostic management is challenging24. It is also important to ask about preexisting diseases, allergies, atopic diathesis and drug intake (Table 3). A great deal of helpful information can be obtained using questionnaires. There are no definite clinical findings related to specific pruritic diseases, but awareness of the history and clinical findings as follows may help with the diagnosis of the cause of pruritus: When several family members are affected, scabies or other parasites should be considered. Pruritus provoked by contact with water should prompt consideration of aquagenic pruritus. It may be associated or precede polycythemia vera, and screening for these diseases should be performed intermittently. Generalized pruritus associated with chills, fatigue, tiredness, weight loss, fever and nocturnal sweating raises the possibility of Hodgkin's disease. Somatoform pruritus rarely disturbs sleep; most other pruritic diseases cause nocturnal wakening.
Table 3.
Diagnostic assessment of patients with chronic pruritus
There is no standardized method of documenting pruritus. The sensation of pruritus is subject to much inter- and intra-individual variation due to tiredness, anxiety, stress24. To assess itch intensity, a visual analogue scale (VAS) is the most often used method. The patient indicates the intensity of pruritus on a line, with extremes from "0: no pruritus at all" to "10: the worst pruritus you can imagine"25. This method can easily be automated, allowing the analysis of a day- profile26. However, this method often fails to consider the frequency of itch attacks and qualities. For patients with severe pruritus of unknown origin, it can be helpful to keep a diary in order to allow for clearer attribution of the symptoms. A detailed history of the itch episodes should begin with the inquiries into onset, location, diurnal rhythm and alleviating or aggravating factors surrounding an excoriation period.
A full physical and dermatologic examination should be conducted to survey for excoriations, ulcerations, erythema, scars, and any evidence of infections or parasitosis to discover any underlying disease possibly causing chronic pruritus. It is also important to consider the methods used to relieve pruritus, e.g. brushes. This helps with the interpretation of clinical findings such as the absence of secondary skin lesions in the mid-back known as the "butterfly sign" which indicates that the patient cannot reach this area by hand and is thus unable to scratch it24.
Before starting any therapy a substantial history and thorough physical examination followed by laboratory investigation with a complete blood count, erythrocyte sedimentation rate, renal, liver and thyroid function should be performed (Table 3).
Severe pruritus may lead to considerable psychological distress. This should not be underestimated by the physician and should be addressed directly. Chronic pruritus can be accompanied by behavioural/adjustment disorder and a withdrawal from social and work life27. In these cases, psychosomatic counseling is required. Pruritus with excoriations sometimes progressing to self-mutilation can be caused by psychiatric disease such as delusional parasitosis. Such patients need psychiatric examination and if necessary treatment. A solely psychological cause of pruritus should not be diagnosed without psychiatric examination.
THERAPY: PRINCIPLES AND GUIDELINE
Pruritus management is challenging, as many therapies fail. Because chronic pruritus is a complex, multifactorial phenomenon, no generally accepted concept exists, and many circumstances prevent optimal treatment24,28. The skin and skin conditions (dermatosis and/or scratch induced lesions) need to be closely monitored. Elderly patients often complain of numerous co-morbidities that complicate therapeutic attempts. Due to the huge variety of systemic origins of pruritus, a successful therapeutic regimen implies a thorough screening for any underlying disease. An individualized approach, which combines systemical and topical therapy, is necessary and described in the German Guideline for the diagnostics and therapy of chronic pruritus24. Depending on the underlying cause, causal therapies range from the specific treatment of a primary dermatological disorders, avoidance of a contact allergen, discontinuation of a medication, specific internal, neurological and psychiatric therapies up to the surgical therapy of neoplasms. A targeted therapy of the underlying disease often results in a relevant relief of pruritus e.g. during/after chemotherapy in Hodgkin's disease, though many patients have a need for additive antipruritic therapies. Beyond, superinfected dermatoses or secondary scratch lesions may require anti-infectants and antimicrobiotic therapy. However, immediate relief of pruritus has to be an initial treatment goal.
TOPICAL THERAPY
First of all, triggers and harmful regimens like alcoholic compresses or irritating tinctures have to be avoided. An appropriate short-time acting approach to relief acute pruritus attacks includes moisturizing with lotions and other lubricants (occlusive oily emollients, humectants like urea), which can help with xerosis which often aggravates the pruritic condition24. Additionally, cooling with menthol- or camphor29 creams or local anaesthetics containing creams like polidocanol can temporarily reduce pruritus. Patients can apply these therapies as necessary particularly during pruritic exacerbation or in case of nocturnal pruritus. If pruritus still persists, a combined or consecutive, step-by-step, symptomatic treatment is necessary (Table 4). Therefore, symptom- and etiology-related therapies as well as additional topical and systemic therapies have to be applied24.
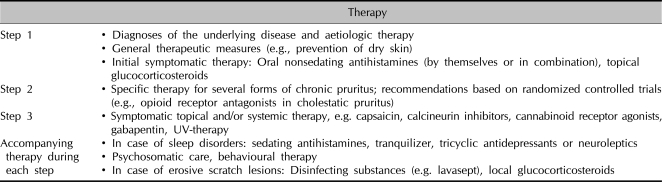
Table 4.
Therapeutic approach in chronic pruritus by step
Step 1 through 3 can be administered additively (e.g. antihistamines and ketotifen and calcineurin inhibitors in case of renal pruritus) or consecutively.
An appropriate topical therapy is essential for a successful treatment of different forms of pruritus. The patient's individual requirements, e.g. the body region to be treated, and the state of the patient's skin including underlying dermatosis have to be considered. Hence, a suitable vehicle (lotion, gel, cream, ointment) should be chosen. Though this topical therapy is essential in almost all geriatric patients complaining of chronic pruritus, there is a high rate of incompliance. Immobility and physical limitations in elderly complicate effective topical care of all affected skin areas, especially if the therapy has to be applied more than once a day.
Topical corticosteroids may obtain a fast relief even of severe pruritus in steroid responsive dermatosis. However, studies which give evidence of a significant antipruritic effect in the treatment of pruritus of different aetiology hardly exist30. If pruritus is one parameter among others suggesting an underlying inflammatory disorder, topical glucocorticosteroids with a favourable side effect profile (e.g. fluticasonpropionate, ethylprednisolonaceponate or mometasonfuroate) are helpful but not sufficient to completely abolish pruritus. Nevertheless, increased skin fragility and petechia can be induced by the prolonged use of topical steroids especially in aged skin. Therefore, single application of topical glucocorticosteroids for treating the symptom of pruritus is not advised, although they can be effectively applied in secondary pruritic scratch lesions.
The active ingredient of chilli peppers, the vanilloid-alkaloid capsaicin, owes its antipruritic properties to desensitization of the sensory nerve fibre and interrupts the conduction of cutaneous pruritus and burning pain. It directly affects polymodal nerve fibers (fully ligand at the TRPV1 receptor), and is also effective in non histamine-induced pruritus. Therefore, topical applied capsaicin has been reported to be effective in postherpetic neuralgia, notalgia paresthetica and brachioradial pruritus, PUVA itch-and pain, aquagenic pruritus and prurigo nodularis31,32. In cases of pruritus associated with dermatoses as well as on primarily non-inflammatory skin the effect of capsaicin generally starts within days. Capsaicin needs to be applied several times daily (3 to 6 times daily) which limits its use in generalised pruritus especially for physically or mentally limited geriatric patients. It is important to start this therapy gradually increasing the concentration (0.025~0.05% to 0.075~0.1%) to attenuate the initial neurogenic inflammation with burning or pruritus.
The topical calcineurin inhibitors, pimecrolimus and tacrolimus were initially developed for the treatment of atopic dermatitis. In addition to relief of skin lesions, a very good antipruritic effect of has been observed. Comparable with capsaicin, an initial burning and pruritus may occur, which may give clinical evidence for assumed TRPV1 binding. Nowadays, pimecrolimus and tacrolimus have been proven to be effective for other pruritic dermatoses such as prurigo nodularis, lichen sclerosus et atrophicus, and genital chronic pruritus33. Due to the more favourable side effect profile, compared with topical corticosteroids and capsaicin, calcineurin inhibitors can be recommended for the treatment of chronic pruirtus and pruritic inflammatory dermatosis; however, a long-term therapy is associated with high costs.
Endogenous as well as synthetic cannabinoids are known for their analgetic potency upon systemic administration. Both cannabinoid receptors CB1 and CB2 were found to be present on cutaneous sensory nerve fibers, which suggest a rational basis for the use of cannabinoid receptor agonists as topical therapy. Their activation by cutaneous application of a cannabinoid agonist could significantly suppress experimentally induced pain as well as itch sensation and erythema34. Up to now, several papers report on case series applying a cream containing low concentration of N-palmitoylethanolamine which relieved pruritus due to hemodialysis35, atopic dermatitis36, pruritus of unknown origin and pruritus in prurigo nodularis and lichen simplex37. It may be speculated that creams with another concentration or another type of cannabinoid will have analogic or even higher antipruritic properties.
SYSTEMICAL THERAPY
When choosing a therapy, a step-by-step approach depending on the severity of pruritus, expected side-effects and the general condition of the patient has to be considered (Table 4). Systemic therapies should be initiated, if topical therapy or compliance fails24,28.
ANTIHISTAMINES
Most of the known pruritus mediators are mast cell and histamine liberators. Antihistamines have been the only available antipruritic therapy in various types of pruritus. They have to be shown to be very effective when the itch sensation is mediated by histamine, like in urticaria. In chronic pruritus of any pruritus, antihistamines are of limited efficacy in low dosage. Single studies detected antipruritic effect, e.g. terfenadine or azelastine in uraemic pruritus while antihistamines as cetirizine showed no efficacy in uraemic pruritus38. Beyond its antihistaminic effects, antihistamines modulate immunological responses, such as mediator release, adhesion molecule expression, cytokines, chemokines and cell recruitment. Accordingly, it could be shown that azelastine had a clearly antipruritic effect by blocking of leukotriene B4 and substance P39. However, recent case series suggest that high dosage or combination of antihistamines have good antipruritic effects in patients with dermatosis and patients with unknown origin40.
CYCLOSPORIN A
Cyclosporin A is known for its potent immunosupressive effects. Apart from in transplant medicine or rheumatology, it is also used in severe dermatosis like psoriasis, atopic dermatitis, pyoderma gangrenosum41. It also has been reported to have significant antipruritic effect in prurigo nodularis42, whereas the antipruritic mechanism is assumed to be symptomatic due to anti-inflammatory effects of cyclosporine A and non causal. However, cyclosporin A has several side-effects like nephrotoxicity, drug interactions and increasing blood pressure limiting its use especially in elderly.
UV-PHOTOTHERAPY
Many clinical studies demonstrate the efficacy of different UV therapy regimes in dermatoses associated with pruritus. However, a study on chronic pruritus not relating to the underlying dermatosis is not known. The modulation of anti-inflammatory and immunosuppressive factors as well as antiproliferative effects and mast-cell apoptosis43 can be assumed as significant factors of UV-induced antipruritic effect. Several studies describe the effects of UV-irradiation in the treatment of polycythemia vera (narrow-band UVB), Hodgkin's disease, and generalised pruritus of unknown origin. PUVA therapy was reported successfully in prurigo nodularis and aquagenic pruritus for the duration of therapy, though controlled studies are lacking. Due to its positive antipruritic effects, UV-phototherapy could be a rational therapy for elderly patients.
Current concepts of pruritic pathways involve peripheral nerve fibres and central nervous structures, which play a central role in pruritic sensory perception. Pruritus-specific receptors were observed; however, pain afferences may communicate and interact with pruritus neurons28. Altogether, these results encouraged the introduction of centrally acting antipruritic substances.
ANTICONVULSANTS AND PAIN MODULATORS
Anticonvulsants and pain modulators such as gabapentin or pregabalin are known to modulate various receptor sides of dopamin, serotonin and noradrenaline metabolism. Gabapentin and pregabalin are used for neuropathic pain and also have antipruritic effects44. The exact antipruritic mechanism is unknown, but gabapentin has been demonstrated to be effective particularly in the treatment of neuropathic pruritus such as brachioradial pruritus. Furthermore, a significant efficacy has been reported in uremic pruritus45. Both gabapentin and pregabalin, which is structurally related to gabapentin, have to be titrated up to an effective dose starting with an low initial dose (Gabapentin initial 100 to 300 mg/d; maximum dosage up to 900 mg/d; pregabalin initial 25 to 75 mg; 300 mg maximum dose)28. In elderly, lower dosages are sufficient (up to 600 mg/d). Especially in elderly side effects may occur including dizzines, peripheral edema, worsening of diabetes mellitus which besides renal impairment may limit the use in elderly.
OPIOID RECEPTOR -ANTAGONISTS; -AGONISTS
Opiate use is associated with the appearance of pruritus, thus (mu)-opioid antagonists were tested for its antipruritic capacity. Several clinical trials have proven a positive effect of opioid receptor antagonists in different pruritic dermatoses as prurigo nodularis, atopic dermatitis or bullous pemphigoid. Double-blind controlled studies in cholestatic pruritus showed a significant decrease of pruritus after the application of naloxone, nalmefene as well as naltrexone [review in: 46]. Naltrexone has the advantage of oral application. Studies focussing on renal pruritus were leading to contradictory results46. Side-effects like nausea, vomiting, dizziness and tiredness are reported during the first days of therapy with opioid-antagonists, which may need inpatient care especially in elderly with impaired mobility. Furthermore therapeutic costs are high, so that opioid antagonists are mainly used as second line therapy.
Nalfurafine binds selectively to kappa-opioid-receptors and was synthesized as analgesic. It inhibits substance-P and histamine-induced scratching and has been demonstrated to significantly reduce ureamic pruritus in recent clinical trials46. Currently, nalfurafine is approved in Japan only what limits the use of the new and promising drug.
ANTIDEPRESSANTS
Antidepressants directly influence central pruritus perception. Tricyclic and tetracyclic antidepressants as well as selective serotonin re-uptake inhibitors were reported to have beneficial effects, whereas the exact antipruritic mechanism is unknown.
Amitryptylin was reported to be useful in some cases of neuropathic pruritus. The initial dose is 10 mg three times daily or 25 mg once a day at bedtime. Another tricyclic antidepressant, doxepin shares its antipruritic, antihistaminic and antidepressant, and sedating properties. Therefore, it has been used for particularly psychogenic and neuropathic types of pruritus47. Its doses range from 25 mg up to 150 mg at bedtime. Typically 6-8 weeks of therapy is needed before results are seen. Mirtazapine is a tetracyclic antidepressant with antihistaminic and serotonin-antagonistic effects48. Doses of 15 mg have been used successfully in cholestatic, uraemic, neuropathic and paraneoplastic pruritus, higher doses do not seem to be of any additional effect and cause more side effects.
Nowadays, modern drugs, like selective serotonin re-uptake inhibitors (SSRI), are available which has similar effects, but a better safety profile. Paroxetine (20 mg/d) was reported to have a very good antipruritic effect in patients with polycythemia vera, psychogenic and paraneoplastic pruritus as well as in pruritus of non-dermatologic origin49. A recent study confirms that paroxetine and fluvoxamine have beneficial effects in different types of pruritus50. As severe cardiac and central nervous side effects like drowsiness, vertigo and fatigue have been described especially in elderly patients this therapy should be used with caution until ongoing studies have been completed.
PSYCHOSOMATIC THERAPY
Beyond somatic therapy, psychosomatic therapies, e.g. behavioural therapy, have to be initiated in time to break the vicious circle of itching and scratching51. Especially patients with prurigo nodularis show an unconscious automatic scratching behaviour. Counselling with a psychotherapist may be important in disorders of mood and personality or in dealing with social tensions. In patients with a coexisting depression, psychotherapy in combination with a psychopharmacologic therapy can be helpful to treat pruritus.
CONCLUSIONS
In summary, the understanding of the pathogenesis of acute and chronic pruritus has improved significantly in recent years. Through this already new concepts in pruritus therapy have been developed. At present pruritus therapy is still based on conventional dermatologic therapies, some specific topical agents, which act directly on nerves in the pathogenesis of pruritus (e.g. capsaicin, cannabinoid agonists), and substances acting not primarily on neurons, that demonstrated efficacy in case series and individual studies. This underscores the need for the development of new substances that intervene specifically in the pathogenesis of pruritus.
References
- 1.Ständer S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291–294. doi: 10.2340/00015555-0305. [DOI] [PubMed] [Google Scholar]
- 2.Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 3.Dalgard F, Svensson A, Holm JØ, Sundby J. Self-reported skin morbidity in Oslo. Associations with sociodemographic factors among adults in a cross-sectional study. Br J Dermatol. 2004;151:452–457. doi: 10.1111/j.1365-2133.2004.06058.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolkenstein P, Grob JJ, Bastuji-Garin S, Ruszczynski S, Roujeau JC, Revuz J. French people and skin diseases: results of a survey using a representative sample. Arch Dermatol. 2003;139:1614–1619. doi: 10.1001/archderm.139.12.1614. discussion 1619. [DOI] [PubMed] [Google Scholar]
- 5.Matterne U, Strassner T, Apfelbacher CJ, Diepgen TL, Weisshaar E. Measuring the prevalence of chronic itch in the general population: development and validation of a questionnaire for use in large-scale studies. Acta Derm Venereol. 2009;89:250–256. doi: 10.2340/00015555-0641. [DOI] [PubMed] [Google Scholar]
- 6.Ständer S, Schäfer I, Phan NQ, Blome C, Herberger K, Heigel H, et al. Prevalence of chronic pruritus in Germany - results of a cross-sectional study in a sample working population of 11,730. Dermatology. 2010;221:229–235. doi: 10.1159/000319862. [DOI] [PubMed] [Google Scholar]
- 7.Meding B, Lidén C, Berglind N. Self-diagnosed dermatitis in adults. Results from a population survey in Stockholm. Contact Dermatitis. 2001;45:341–345. doi: 10.1034/j.1600-0536.2001.450604.x. [DOI] [PubMed] [Google Scholar]
- 8.Yalçin B, Tamer E, Toy GG, Oztaş P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45:672–676. doi: 10.1111/j.1365-4632.2005.02607.x. [DOI] [PubMed] [Google Scholar]
- 9.Thaipisuttikul Y. Pruritic skin diseases in the elderly. J Dermatol. 1998;25:153–157. doi: 10.1111/j.1346-8138.1998.tb02371.x. [DOI] [PubMed] [Google Scholar]
- 10.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, et al. Itch: scratching more than the surface. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 11.Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288–291. doi: 10.1111/j.1529-8019.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522–1525. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 16.Dijkstra D, Stark H, Chazot PL, Shenton FC, Leurs R, Werfel T, et al. Human inflammatory dendritic epidermal cells express a functional histamine H4 receptor. J Invest Dermatol. 2008;128:1696–1703. doi: 10.1038/sj.jid.5701250. [DOI] [PubMed] [Google Scholar]
- 17.Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on T(H)2 cells. J Allergy Clin Immunol. 2009;123:619–625. doi: 10.1016/j.jaci.2008.12.1110. [DOI] [PubMed] [Google Scholar]
- 18.Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, et al. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 19.Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516:180–181. doi: 10.1016/j.ejphar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Raap U, Wichmann K, Bruder M, Ständer S, Wedi B, Kapp A, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122:421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–1145. [PubMed] [Google Scholar]
- 23.Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ständer S, Streit M, Darsow U, Niemeier V, Vogelgsang M, Ständer H, et al. Diagnostic and therapeutic procedures in chronic pruritus. J Dtsch Dermatol Ges. 2006;4:350–370. doi: 10.1111/j.1610-0387.2006.05887.x. [DOI] [PubMed] [Google Scholar]
- 25.Wahlgren CF. Itch and atopic dermatitis: clinical and experimental studies. Acta Derm Venereol Suppl (Stockh) 1991;165:1–53. [PubMed] [Google Scholar]
- 26.Hägermark O, Wahlgren CF. Some methods for evaluating clinical itch and their application for studying pathophysiological mechanisms. J Dermatol Sci. 1992;4:55–62. doi: 10.1016/0923-1811(92)90059-k. [DOI] [PubMed] [Google Scholar]
- 27.Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Ständer S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol. 2006;31:762–767. doi: 10.1111/j.1365-2230.2006.02211.x. [DOI] [PubMed] [Google Scholar]
- 28.Ständer S, Weisshaar E, Luger TA. Neurophysiological and neurochemical basis of modern pruritus treatment. Exp Dermatol. 2008;17:161–169. doi: 10.1111/j.1600-0625.2007.00664.x. [DOI] [PubMed] [Google Scholar]
- 29.Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187:157–160. doi: 10.1016/0304-3940(95)11362-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhai H, Frisch S, Pelosi A, Neibart S, Maibach HI. Antipruritic and thermal sensation effects of hydrocortisone creams in human skin. Skin Pharmacol Appl Skin Physiol. 2000;13:352–357. doi: 10.1159/000029943. [DOI] [PubMed] [Google Scholar]
- 31.Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471–478. doi: 10.1067/mjd.2001.110059. [DOI] [PubMed] [Google Scholar]
- 32.Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol. 1995;32(2 Pt 1):287–289. doi: 10.1016/0190-9622(95)90152-3. [DOI] [PubMed] [Google Scholar]
- 33.Ständer S, Luger TA. Antipruritic effects of pimecrolimus and tacrolimus. Hautarzt. 2003;54:413–417. doi: 10.1007/s00105-003-0521-6. [DOI] [PubMed] [Google Scholar]
- 34.Dvorak M, Watkinson A, McGlone F, Rukwied R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm Res. 2003;52:238–245. doi: 10.1007/s00011-003-1162-z. [DOI] [PubMed] [Google Scholar]
- 35.Szepietowski JC, Reich A, Szepietowski T. Emollients with endocannabinoids in the treatment of uremic pruritus: discussion of the therapeutic options. Ther Apher Dial. 2005;9:277–279. doi: 10.1111/j.1774-9987.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 36.Eberlein B, Eicke C, Reinhardt HW, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study) J Eur Acad Dermatol Venereol. 2008;22:73–82. doi: 10.1111/j.1468-3083.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 37.Ständer S, Reinhardt HW, Luger TA. Topical cannabinoid agonists. An effective new possibility for treating chronic pruritus. Hautarzt. 2006;57:801–807. doi: 10.1007/s00105-006-1180-1. [DOI] [PubMed] [Google Scholar]
- 38.Weisshaar E, Dunker N, Rohl FW, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol. 2004;13:298–304. doi: 10.1111/j.0906-6705.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 39.Andoh T, Kuraishi Y. Inhibitory effects of azelastine on substance P-induced itch-associated response in mice. Eur J Pharmacol. 2002;436:235–239. doi: 10.1016/s0014-2999(01)01617-x. [DOI] [PubMed] [Google Scholar]
- 40.Schulz S, Metz M, Siepmann D, Luger TA, Maurer M, Ständer S. Antipruritic efficacy of a high-dosage antihistamine therapy. Results of a retrospectively analysed case series. Hautarzt. 2009;60:564–568. doi: 10.1007/s00105-009-1730-4. [DOI] [PubMed] [Google Scholar]
- 41.Wahlgren CF, Scheynius A, Hägermark O. Antipruritic effect of oral cyclosporin A in atopic dermatitis. Acta Derm Venereol. 1990;70:323–329. [PubMed] [Google Scholar]
- 42.Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6:941–946. doi: 10.1111/j.1610-0387.2008.06745.x. [DOI] [PubMed] [Google Scholar]
- 43.Szepietowski JC, Morita A, Tsuji T. Ultraviolet B induces mast cell apoptosis: a hypothetical mechanism of ultraviolet B treatment for uraemic pruritus. Med Hypotheses. 2002;58:167–170. doi: 10.1054/mehy.2001.1505. [DOI] [PubMed] [Google Scholar]
- 44.Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491–495. doi: 10.1046/j.1365-4362.2003.01831.x. [DOI] [PubMed] [Google Scholar]
- 45.Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19:3137–3139. doi: 10.1093/ndt/gfh496. [DOI] [PubMed] [Google Scholar]
- 46.Phan NQ, Bernhard JD, Luger TA, Ständer S. Antipruritic treatment with systemic µ-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63:680–688. doi: 10.1016/j.jaad.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 47.Gupta MA, Guptat AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol. 2001;15:512–518. doi: 10.1046/j.1468-3083.2001.00278.x. [DOI] [PubMed] [Google Scholar]
- 48.de Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacol. 1995;10(Suppl. 4):19–23. doi: 10.1097/00004850-199512004-00004. [DOI] [PubMed] [Google Scholar]
- 49.Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26:1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Ständer S, Böckenholt B, Schürmeyer-Horst F, Weishaupt C, Heuft G, Luger TA, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45–51. doi: 10.2340/00015555-0553. [DOI] [PubMed] [Google Scholar]
- 51.Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther. 1981;19:313–318. doi: 10.1016/0005-7967(81)90052-8. [DOI] [PubMed] [Google Scholar]