Abstract
There is evidence to suggest that alterations in neuropeptide Y (NPY) and corticotropin-releasing factor (CRF) contribute to the escalated voluntary ethanol intake seen following long term chronic ethanol exposure. The present study assessed whether the duration of chronic ethanol exposure and abstinence alters brain levels of NPY and CRF in adult Wistar rats. NPY-like immunoreactivity (NPY-LI) and CRF-LI were determined in the amygdala (AMYG), frontal cortex (FCTX), hippocampus (HPC) and parietal cortex (PCTX) of adult Wistar rats after chronic ethanol exposure, and 24-hr and 2-wk following withdrawal (WD). Chronic ethanol exposure consisted of either a 2-wk or an 8-wk ethanol vapor regimen. No change in brain levels of NPY-LI, CRF-LI and the NPY-LI/CRF-LI ratio were observed 2-wk following ethanol exposure, whereas, 8-wk of ethanol exposure produced a significant effect on NPY-LI expression in the AMYG and FCTX. Moreover, an 8-wk ethanol vapor regimen significantly increased CRF-LI levels in the HPC and PCTX. Findings from the present study suggest that a longer duration of ethanol vapor, similar to what is required to enhance voluntary drinking, is required to produce changes in NPY-LI and CRF-LI expression in the adult rat brain.
Keywords: Amygdala, CRF, Ethanol Withdrawal, Frontal Cortex, Hippocampus, NPY, Parietal Cortex
1. Introduction
Protracted withdrawal symptoms following chronic ethanol exposure are characterized by abnormalities in motor, sensory, endocrine, autonomic and central nervous system function (Heilig et al., 2010; Koob and Le Moal, 2008; Roberts et al., 2000). A model of protracted abstinence has been studied in rodents in order to characterize the neurobehavioral mechanisms associated with ethanol withdrawal following excessive ethanol drinking (Koob and Le Moal, 2008). This model has been also used to study the increase in voluntary consumption and self-administration of ethanol following periods of abstinence in animals with a history of prolonged vapor exposure (Rimondini et al., 2003; Thorsell et al., 2005a, 2005b). While findings from those studies have shown that chronic intermittent ethanol vapor exposure produces increases in voluntary ethanol consumption (Becker and Lopez, 2004; Dhaher et al., 2008; Finn et al., 2007; Lopez and Becker, 2005; Rimondini et al., 2002, 2003; Sommer et al., 2008; Thorsell et al., 2005a, 2005b) and ethanol self-administration (Chu et al., 2007; Lopez et al., 2008; O’Dell et al., 2004; Roberts et al., 1996, 2000) in rodents, the neural mechanisms underlying the short- and long-term consequences of this intermittent chronic ethanol vapor regimen and its relationship with protracted withdrawal symptoms are less well understood.
Studies characterizing neuroadaptive changes in rodent models with a history of ethanol exposure have provided insight into the neural mechanisms implicated in the protracted withdrawal syndrome (Heilig et al., 2010; Hoffman and Tabakoff, 1994; Koob, 2003; Koob and Le Moal, 2008; Kumar et al., 2009). There is evidence to suggest that alterations in neuropeptide Y (NPY) and corticotropin-releasing factor (CRF) systems following to chronic ethanol exposure could contribute to escalated ethanol intake (Funk et al., 2006, 2007; Gilpin et al., 2008; Thorsell et al., 2005a; Valdez et al., 2002). There is also evidence that both NPY and CRF are implicated in modulation of anxiety-related behaviors and stress responses (Dunn and File, 1987; Heilig et al., 1993, 1994; Koob et al., 1993; Sutton et al., 1982; Thatcher-Britton et al., 1986; Thorsell et al., 1998, 1999; Wahlestedt et al., 1993). While CRF has been shown to be a potent anxiogenic agent (Dunn and File, 1987; Sutton et al., 1982; Thatcher-Britton et al., 1986), NPY given intracerebrally has anxiolytic effects (Heilig et al., 1993; Wahlestedt et al., 1993). The interplay and/or balance between the opposing actions of these neuropeptides have been suggested to play an important role in the regulation of stress, anxiety, sleep and depression (Ehlers et al., 1997; Heilig et al., 1994; Yamada et al., 1996). These findings suggest a potential role for these neuropeptides on protracted symptoms associated with ethanol withdrawal.
Studies in rodent models have demonstrated that the duration of chronic ethanol exposure significantly impacts subsequent voluntary ethanol intake (Griffin III et al., 2009; Lopez and Becker, 2005). For instance, Lopez and Becker (2005) found that an increase in the number of cycles of repeated intermittent ethanol vapor exposure produced a significant increase in the duration and amount of voluntary ethanol drinking in mice. Recent studies in that rodent model found that while longer periods of ethanol vapor exposure increase voluntary ethanol drinking, longer periods of oral consumption of ethanol or gastric intubation of ethanol do not increase voluntary ethanol drinking (Griffin III et al., 2009). Taken together these studies suggest that the vapor inhalation procedure provides the required sustained blood ethanol concentrations (BEC) (> 175 mg/dl) and the duration of ethanol exposure to increase voluntary ethanol drinking in this rodent model (Griffin III et al., 2009).
We have previously shown that ethanol vapor treatment produces electrophysiological changes in several brain regions, including the amygdala (AMYG), frontal cortex (FCTX), hippocampus (HPC) and parietal cortex (PCTX) (Criado and Ehlers, 2010; Criado et al., 2008a, 2008b; Ehlers and Chaplin, 1991; Ehlers and Slawecki, 2000; Slawecki and Ehlers, 2005; Slawecki et al., 1999, 2000, 2001). Neurophysiological studies also demonstrated that intermittent exposure to ethanol vapor for 6-wk potentiated the effects of acute administration of NPY and CRF on FCTX, PCTX and AMYG in Wistar rats (Slawecki et al., 1999). There is evidence to suggest that the effects of chronic ethanol on brain levels of NPY and CRF may depend on the duration of ethanol exposure and the brain region affected (e.g., Ehlers et al., 1998; Slawecki et al., 2005; Walker et al., 2010; Zorrilla et al., 2001). However, it is not clear if the effects of ethanol on these peptides were triggered during chronic ethanol exposure or during the prolonged withdrawal period. It also remains unclear whether our previous studies showing ethanol-mediated alterations in neurophysiological function of cortical and limbic regions are mediated in part by the effects of chronic ethanol on NPY and CRF. Moreover, whether the duration of ethanol exposure and the period of abstinence from ethanol play a significant role on ethanol-mediated effects on the levels of NPY-LI and CRF-LI and on the NPY-LI/CRF-LI ratio in the brain have not been systematically assessed in the same subjects.
Determining whether the duration of both the chronic ethanol regimen and its withdrawal period differentially affect NPY and CRF levels in vivo could provide important information on the mechanism mediating symptoms associated with the effects of prolonged ethanol exposure. The objective of the present study was to determine whether the duration of intermittent ethanol vapor exposure differentially affect the brain levels of NPY-LI and CRF-LI in adult Wistar rats. The levels of NPY-LI and CRF-LI were determined in the AMYG, FCTX, HPC and PCTX at three different time points following 2-wk or 8-wk of chronic ethanol vapor or control treatments as follows: 8-wk exposure with no withdrawal (WD) period (chronic ethanol group), 24-hr withdrawal period (24-hr WD group) and 2-wk withdrawal period (2-wk WD group).
2. Materials and methods
2.1. Subjects
Male Wistar rats at postnatal day (PND) 90 (n = 124; Charles River, USA) were used in this study. Rats were housed two per cage in standard cages for the duration of the experiment. Rats were kept in a light/dark (12h light/12h dark, lights on at 06:00 a.m.) and temperature-controlled environment except for during ethanol vapor exposure (see below). Food and water were available ad libitum throughout the experiment, except where noted. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Scripps Research Institute and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
2.2 Ethanol Vapor Exposure
Adult rats (n = 125) were divided into two groups (2-wk chronic exposure, n = 62; 8-wk chronic exposure, n = 63). Adult rats (n = 62) in the 2-wk chronic ethanol treatment group were divided into two groups each (ethanol-exposed group; n = 32; control group, n = 30). At the start of the ethanol exposure, rats were 90 days old and exposure continued until they were 104 days old. Adult rats (n = 63) in the 8-wk chronic ethanol treatment group were divided into two groups each (ethanol-exposed group, n = 32; control group, n = 31). At the start of the ethanol exposure, rats were 91 days old and exposure continued until they were 147 days old.
Ethanol vapor exposure has been shown to reliably allow for the titration of blood alcohol levels (BALs) that are sufficient for inducing ethanol physical dependence as indicated by signs of withdrawal (O’Dell et al., 2004; Roberts et al., 1996, 2000). The ethanol vapor inhalation procedure and the chambers used in this study were previously described (Rogers et al., 1979, Slawecki, 2002, Slawecki et al., 2001). Ethanol-exposed rats were housed in sealed chambers, which were infused with vaporized 95% ethanol from 8 PM to 10 AM. For the remaining of the 10 hours of the day, ethanol vapor was not infused into the chamber. Ethanol vapor chambers (La Jolla Alcohol Research Inc, La Jolla, CA) were calibrated to produce high BALs between 175-225 mg/dL. Age-matched controls were handled identically to ethanol-exposed rats. Food and water were always available. Blood samples were collected from the tip of the tail three times per week to assess BALs (target: 150 to 200 mg/dl). BALs were determined using the Analox GM7 Micro-Stat (Analox Instr. Ltd., Lunenberg, MA). To control for the stress response during blood sampling in ethanol-exposed rats, the tip of the tail was also cut in control rats to collect tail blood. Control rats were handled identically to ethanol-treated rats.
Adult rats from each chronic ethanol treatment regimen (2-wk and 8-wk) were randomly subdivided into three groups each: the chronic ethanol treatment group (CET), the 24-hr ethanol withdrawal (WD) group, and the 2-wk ethanol WD group. In the CET group, rats were sacrificed and brains dissected immediately after chronic ethanol exposure. In the 24-hr WD group, brains were dissected 24 hours after ethanol exposure. In the 2-wk WD group, brains were dissected two weeks after ethanol exposure. Each ethanol group was compared to its respective control group. Age-matched control rats were sacrificed within two hours of their respective ethanol-treated groups. When ethanol exposure ended, rats from groups assigned to the withdrawal periods (24-hr WD and 2-wk WD) were maintained in the Scripps vivarium.
2.3. Tissue Dissection, Preparation and Analysis of NPY and CRF Levels
NPY and CRF levels were assessed in the AMYG, FCTX, HPC and PCTX. Rats were decapitated without anesthesia because the potential influence of anesthetics on brain systems regulating the stress response (Nellgard et al., 2000; Whitehouse et al., 1993). Brain regions of interest were microdissected over ice using a chilled metal rat brain matrix (Roboz Surgical Instruments Company, Inc., Gaithersburg, MD) as previously described (Ehlers et al., 1998). Tissue samples were weighed, frozen on dry ice and shipped to the Karolinska Institute for assessment of NPY- and CRF-like immunoreactivity (NPY-LI and CRF-LI, respectively), as previously described (Ehlers et al., 1998; Husum and Mathe, 2002; Mathe et al., 1997; Stenfors et al., 1989). In brief, samples were homogenized by ultrasonic dismembranation and extracted using 1 M acetic acid followed by neutral pH water extraction. Freeze-dried samples were reconstituted in a phosphate buffer. Immunoreactivity for each peptide was determined by competitive radioimmunoassay, as previously described (Mathe et al., 1998). NPY-LI was analyzed using antiserum provided by Dr. R. Ekman and Dr. M. Heilig. It cross-reacts 100% with NPY 2-36 and 5% with NPY 5-36 and >0.5% with shorter c-terminal NPY fragments. The lower level of sensitivity of this assay is 3.9 pmol/L. CRF-LI was analyzed using antiserum provided by Dr. P. Lowry. The lower level of sensitivity of this assay is 3.9 pmol/L. Intra-assay coefficients of variation typically ranged from 5% to 10%. All samples were assessed in duplicate.
2.4. Data and Statistical Analysis
Statistical analyses were performed by using SPSS (SPSS, Inc., Chicago, IL). Values are mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to determine the effects of chronic ethanol exposure on body weight and BALs. Ethanol and control groups were compared after termination of the chronic ethanol treatment and 2-wk after withdrawal from chronic ethanol (P < 0.05 for significance).
Brain regions (AMYG, FCTX, PCTX and HPC) and neuropeptides (NPY-LI and CRF-LI) were assessed independently. Data are expressed as mean pmol NPY-LI/g wet weight ± SEM and mean pmol CRF-LI/g wet weight ± SEM. Two-Way Between Subjects ANOVAs were used to assess the effects of 2-wk or 8-wk intermittent ethanol exposure on the levels of NPY-LI and CRF-LI at three different WD periods following chronic ethanol exposure: 0-hr (CET), 24-hr WD and 2-wk WD. Group (Ethanol vs. Control) and Time (CET, the 24-hr WD and the 2-wk WD) were assessed as between subject variables. Two-Way Between Subjects ANOVAs were also used to assess the effects of the intermittent chronic ethanol regimens on the NPY-LI/CRF-LI ratio. When appropriate, post hoc analysis of the ANOVA utilized independent one-way ANOVAs to assess group differences. P-values for all Two-Way ANOVAs and post hoc analyses were set at P < 0.05 to determine the levels of statistical significance. Independent One-Way ANOVA were used to assess differences in immunoreactivity in control groups across the three different time points. To correct for multiple comparisons, P value was set at P < 0.01 to determine the levels of statistical significance. Post hoc analysis of One-Way ANOVA utilized the Tukey’s HSD test. For these analyses, P-value was set at P < 0.05 to determine the levels of statistical significance. Nonparametric Spearman’s rho correlations were used to determine the relationship between increases in NPY-LI and CRF-LI among brain regions. These correlational analyses were restricted to changes in NPY-LI and CRF-LI levels found to be statistically significantly different from their respective control groups.
3. Results
3.1. Effects of 2-wk chronic ethanol exposure on NPY and CRF levels
3.1.1. Body weight and BALs
Results showed no differences in body weight between the ethanol (355 ± 4 g) and control (362 ± 3 g) groups prior to the initiation of the experiment. Blood alcohol levels (BALs) in adult rats were 197 ± 6 mg/dl. Rats showed significant differences in body weight during the ethanol exposure phase (Ethanol exposed: 387 ± 6 g; Control: 420 ± 4 g; F(1,41)=23.0, P < 0.01). Body weight in ethanol exposed and control adult rats after 2-wk of ethanol WD were not statistically different (Ethanol exposed: 477 ± 9 g; Control: 464 ± 8 g).
3.1.2. Effects of 2-wk Ethanol Exposure on NPY and CRF Expression
Two-way ANOVA revealed no significant main effect of Group (Ethanol vs. Control) in the levels of NPY-LI in the FCTX, PCTX and HPC (F’s(1,56) < 3.7; P’s > 0.05) and in the AMYG (F’s(1,55) < 0.8; P’s > 0.05). Two-way ANOVA showed no significant Group x Time interaction in the levels of NPY-LI in the FCTX, PCTX and HPC (F’s(2,56) < 2.0; P’s > 0.05) and in the AMYG (F(2,55) = 0.6; P > 0.05). One-way repeated measures ANOVA showed no significant differences in NPY-LI levels among control groups in the FCTX, PCTX, HPC and AMYG (F’s(2,27) < 4.0; P’s > 0.01).
Two-way ANOVA also showed no significant main effect of Group (Ethanol vs. Control) in the levels of CRF-LI in the FCTX, PCTX and HPC (F’s(1,56) < 3.3; P’s > 0.05) and in the AMYG (F’s(1,55) < 0.3; P’s > 0.05). No significant Group x Time interaction in the levels of CRF-LI was found in the FCTX, PCTX and HPC (F’s(2,56) < 0.7; P’s > 0.05) and in the AMYG (F(2,55) = 0.1; P > 0.05). Consistent with these results, the NPY-LI/CRF-LI ratio in the FCTX, PCTX, HPC and AMYG were not changed in adult rats exposed to 2-wk ethanol vapor (data not shown). One-way repeated measures ANOVA showed no significant differences in CRF-LI levels among control groups in the FCTX, PCTX, HPC and AMYG (F’s(2,27) < 4.6; P’s > 0.01).
3.2. Effects of 8-wk chronic ethanol exposure on NPY and CRF levels
3.2.1. Body Weight and BALs
No differences in body weight were found between the ethanol (383 ± 6 g) and control (388 ± 6 g) groups prior to the initiation of the 8-wk ethanol vapor regimen. Blood alcohol levels (BALs) in adult rats were 189 ± 13 mg/dl. No differences in body weight were observed between ethanol exposed and control rats following chronic ethanol exposure (Ethanol exposed: 521 ± 14 g; Control: 512 ± 10 g) and 2-wk after ethanol WD (Ethanol exposed: 559 ± 24 g; Control: 599 ± 22 g).
3.2.2. Effects of 8-wk Ethanol Exposure on NPY and CRF Expression
Two-way ANOVA revealed no significant main effect of Group (Ethanol vs. Control) in the levels of NPY-LI in the FCTX, PCTX and HPC (F’s(1,57) < 2.5; P’s > 0.05) and in the AMYG (F’s(1,56) < 2.8; P’s > 0.05). Two-way ANOVA showed significant Group x Time interaction in the levels of NPY-LI in the FCTX (Fig. 1A; F(2,57) = 4.8; P < 0.05). Post hoc assessment revealed that, compared to controls, NPY-LI levels in the FCTX decreased in the 24-hr WD group (F(1,20) = 4.3; P = 0.05), but increased in the 2-wk WD group (F(1, 19) = 5.1; P < 0.05). Significant Group x Time interaction was observed in the levels of NPY-LI in the AMYG (Fig. 1D; F(2,56) = 3.2; P < 0.05). Post hoc analysis showed that NPY-LI levels in the AMYG increased in the 2-wk WD group (F(1,19) = 6.3; P < 0.05). In contrast, Group x Time interaction in the levels of NPY-LI was not significant in the PCTX and HPC (Fig. 1B and 1C). One-way repeated measures ANOVA showed no significant differences in NPY-LI levels among control groups in the FCTX, PCTX and HPC (F’s(2,28) < 4.9; P’s > 0.01) and in the AMYG (F’s(2,27) < 1.2; P’s > 0.01).
Figure 1. Effects of 8 weeks of chronic ethanol treatment (CET) and ethanol withdrawal (WD) on NPY-LI levels in FCTX, PCTX, HPC and AMYG of adult rats.
(A) In FCTX, NPY-LI was significantly reduced at 24-hr WD, whereas it was increased at 2-wk WD, compared to control rats. CET, 24-hr and 2-wk WD had no effect on NPY-LI in the PCTX (B) and HPC (C) of adult rats. (D) In AMYG, NPY-LI was significantly increased at 2-wk WD, compared to control rats. CET and 24-hr WD had no effect on NPY-LI in AMYG. * indicates P < 0.05 for significant difference from control rats. NPY-LI levels are expressed as pmol/g. Error bars= S.E.M.
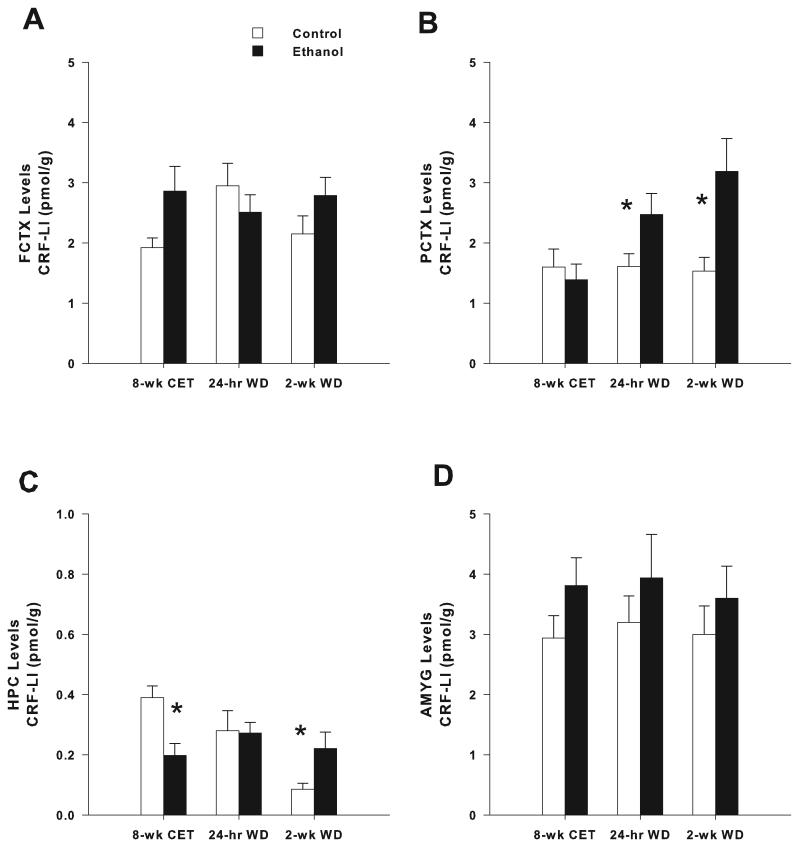
Two-way ANOVA also showed no significant main effect of Group (Ethanol vs. Control) in the levels of CRF-LI in the FCTX and HPC (F’s(1,57) < 2.2; P’s > 0.05) and in the AMYG (F’s(1,56) < 3.0; P’s > 0.05). In contrast, a significant main effect of Group was found in the PCTX (F(1,57) = 7.6; P < 0.01). CRF-LI levels in the PCTX were significantly higher in rats exposed to ethanol than in control rats (Ethanol exposed: 2.35 ± 0.2 pmol/g; Control: 1.58 ± 0.2 pmol/g). A significant Group x Time interaction in the levels of CRF-LI was found in the PCTX (Fig. 2B; F(2,57) = 3.6; P < 0.05). Post hoc assessment revealed that, compared to controls, CRF-LI levels in the PCTX increased in the 24-hr WD group (F(1,20) = 4.5; P < 0.05) and 2-wk WD groups (F(1, 19) = 7.3; P < 0.05). Significant Group x Time interaction was observed in the levels of CRF-LI in the HPC in adult rats (Fig. 2C; F(2,57) = 6.0; P < 0.005). Post hoc analysis showed that CRF-LI levels in the HPC decreased in the CET group (F(1,18) = 10.8; P < 0.005) and increased in the 2-wk WD group (F(1, 19) = 4.9; P < 0.05). In contrast, Group x Time interaction in the levels of CRF-LI was not significant in the FCTX and AMYG (Fig. 2A and 2D). One-way repeated measures ANOVA showed no significant differences in CRF-LI levels among control groups in the FCTX and PCTX (F’s(2,28) < 3.5; P’s > 0.01) and in the AMYG (F’s(2,27) < 0.2; P’s > 0.01). However, significant differences in CRF-LI levels were found among control groups in the HPC (F’s(2,28) = 10.0; P = 0.001). Post hoc analysis revealed that CRF-LI levels in the HPC in control rats were decreased in the 2-wk WD group (0.09 ± 0.02 pmol/g) compared to the CET group (0.39 ± 0.04 pmol/g) and 24-hr WD group (0.28 ± 0.07 pmol/g).
Figure 2. Effects of 8 weeks of chronic ethanol treatment (CET) and ethanol withdrawal (WD) on CRF-LI levels in FCTX, PCTX, HPC and AMYG of adult rats.
CET, 24-hr and 2-wk WD had no effect on CRF-LI in the FCTX (A) and AMYG (D) of adult rats. (B) In PCTX, CRF-LI was significantly increased at 24-hr and 2-wk WD, whereas it was not changed after CET, compared to control rats. (C) In HPC, CRF-LI was significantly reduced after CET, whereas it was increased at 2-wk WD, compared to control rats. 24-hr WD had no effect on CRF-LI in HPC. * indicates P < 0.05 for significant difference from control rats. CRF-LI levels are expressed as pmol/g. Error bars= S.E.M.
3.2.3. Association Between NPY and CRF After 8-wk Ethanol Exposure
The relationship between ethanol-induced changes in NPY-LI and CRF-LI was assessed among different brain regions. At 24-hr WD, the decrease in NPY-LI levels in the FCTX did not correlate with the increase in CRF-LI levels in the PCTX (Spearman’s rho = −0.345, P > 0.05). At 2-wk WD, the increase in NPY-LI in the FCTX did not correlate with the increase in CRF-LI in the HPC (Spearman’s rho = 0.436, P > 0.05) nor with the increase in CRF-LI in the PCTX (Spearman’s rho = −0.118, P > 0.05). The increase in NPY-LI levels in the AMYG at 2-wk WD did not correlate with the increase in CRF-LI in the HPC (Spearman’s rho = 0.527, P > 0.05) nor with the increase in CRF-LI in the PCTX (Spearman’s rho = −0.027, P > 0.05). Consistent with these findings, two-way ANOVA showed that the NPY-LI/CRF-LI ratio in the FCTX, PCTX, HPC and AMYG were not changed in adult rats exposed to 8-wk ethanol vapor (data not shown).
4. Discussion
In the present study, expression of NPY-LI and CRF-LI were determined in adult rats after 2-wk or 8-wk of ethanol vapor exposure, and 24 hr and 2 wk following withdrawal. Findings from this study revealed no change on brain levels of NPY-LI, CRF-LI and the NPY-LI/CRF-LI ratio after 2-wk ethanol exposure at any time point. In contrast, the 8-wk ethanol vapor regimen significantly reduced CRF-LI expression in the HPC, during ethanol exposure, but had no effects on NPY-LI. Eight weeks of ethanol exposure did have significant effects on NPY-LI expression in the FCTX and AMYG during the withdrawal period. NPY-LI levels in the FCTX were decreased after 24-hr WD, and increased after 2-wk WD. Consistent with these findings our data showed that 8-wk ethanol exposure also produced an increase in NPY-LI levels in the AMYG after 2-wk WD. Moreover, the 8-wk ethanol vapor regimen significantly increased CRF-LI levels in the PCTX following both WD periods and in the HPC following 2-wk WD. Findings from the present study suggest that a longer duration of ethanol vapor (8-wk vs. 2-wk) is necessary to produce changes in NPY-LI and CRF-LI in the adult rat brain. Additionally, the changes seen in peptide expression during withdrawal from 8-wk of vapor exposure are cyclical with decreases seen in NPY-LI at 24 hrs and increases in expression seen two weeks later.
Protracted withdrawal symptoms such as anxiety, depression and sleep disturbances have been hypothesized to play a role in the susceptibility to relapse in ethanol dependent individuals (De Soto et al., 1989; Miller and Harris, 2000; Mossberg et al., 1985). Previous studies in rodent models demonstrated that 4-wk of intermittent ethanol vapor produced an increase in anxiety-like behavior during the acute ethanol withdrawal phase (~ 8 hr) that returned to control levels during the early protracted withdrawal phase (2-wk WD) (Zhao et al., 2007). The authors proposed that these neuroadaptive changes may due in part to neurochemical changes in NPY and CRF systems (Zhao et al., 2007). Consistent with these results, findings from the present study demonstrated exposure to 8-wk ethanol vapor produced an increase in CRF levels in the PCTX 24 hr following withdrawal, whereas NPY levels in the FCTX were attenuated. These results are consistent with the anxiolytic and anxiogenic properties of NPY and CRF, respectively (Dunn and File, 1987; Heilig et al., 1993; Sutton et al., 1982; Thatcher-Britton et al., 1986; Wahlestedt et al., 1993). The evidence of increase anxiety-like behaviors during the acute ethanol withdrawal phase (Overstreet et al., 2004; Zhao et al., 2007) and findings from the present study showing a reduction of NPY levels in the FCTX also support the notion that reductions in NPY levels may play a role in the expression of spontaneous anxiety symptoms during ethanol withdrawal periods.
The present study found that while CRF levels in the HPC and PCTX were elevated 2-wk following withdrawal, NPY levels in the AMYG and FCTX also showed significant increase during this early protracted withdrawal phase. These findings are consistent with the remission of anxiety-like behavior 2-wk following withdrawal (Zhao et al., 2007) and supports the evidence that increase in NPY levels may play an anxiolytic role during ethanol withdrawal, and as a result, modulate ethanol-seeking behavior (Bison and Crews, 2003; Thorsell, 2007). Previous studies have shown that exposure to at least 2-wk of the intermittent vapor regimen used in the present study produce signs of physical dependence in rats (O’Dell et al., 2004; Roberts et al., 2000). We have previously shown that the 8-wk chronic ethanol vapor exposure regimen also increased ethanol intake in Wistar rats trained in a limited access paradigm (Thorsell et al., 2005a). We also found that intracerebroventricular (i.c.v.) administration of either NPY (10 μg) or CRF (1 μg) significantly reduced ethanol intake in Wistar rats exposed to an 8-wk ethanol exposure regimen (Thorsell et al., 2005a). Findings from the present study suggest neuroadaptations in the NPY and CRF systems in cortical and limbic regions during acute ethanol withdrawal and during the early protracted withdrawal phase (2-wk WD) that are consistent with biphasic changes in anxiety-like behavior following a similar ethanol exposure regimen in ethanol-dependent rats (Zhao et al., 2007). However, previous studies in rats have shown consistent effects of intermittent ethanol exposure increasing operant ethanol self-administration both during acute withdrawal (2-hr withdrawal) and after 2-wk of withdrawal (Roberts et al., 2000; Valdez et al., 2002). These findings suggest that not all the behavioral consequences of ethanol withdrawal following intermittent exposure exhibit a biphasic pattern. Further studies are needed to determine whether these neuroadaptations following ethanol vapor exposure may play an important role on the consequences of investigator-administered NPY and CRF on ethanol self-administration in dependent rats (Thorsell et al., 2005a).
We have previously shown a significant increase in NPY-LI in the hypothalamus (HYP) following one month of ethanol withdrawal in adult Wistar rats exposed to a 7-wk of intermittent ethanol exposure (Ehlers et al., 1998). In contrast, that ethanol vapor regimen had no effect on NPY-LI in the AMYG, FCTX and HPC of adult Wistar rats. While the present study did not test the effects of chronic ethanol vapor on the HYP of adult Wistar rats, results from the present study suggest that a similar ethanol vapor regimen (8-wk) increased NPY-LI in the FCTX and AMYG. These contrasting results could be explained by the shorter withdrawal times tested in the present study, in comparison to our previous study (24-hr and 2-wk WD vs. one month). These findings suggest that the increase in NPY-LI levels observed 2-wk following exposure to 8-wk ethanol vapor may return to baseline levels at one month after termination of chronic ethanol exposure. Further studies are needed to determine whether the effects of ethanol on FCTX and AMYG levels of NPY-LI found in the present study are resolved within one month after a prolonged ethanol exposure.
It was previously demonstrated that 10 days of ethanol vapor exposure, attaining significantly higher blood ethanol levels (250 mg%) than the present study, and 7-wk of withdrawal produces no changes in CRF-LI but produces a reduction in NPY-LI in the HPC in Sprague-Dawley rats (Slawecki et al., 2005). These findings suggest that higher levels of ethanol vapor are required to produce effects on NPY-LI during shorter duration periods, or that Sprague-Dawley rats are more sensitive to the effects of ethanol on NPY. These previous results together with our present findings suggest that the duration of ethanol vapor exposure and BAL play an important role modulating NPY-LI and CRF-LI in the rat brain. There is also evidence that the intensity and duration of chronic ethanol exposure plays an important role on subsequent voluntary ethanol drinking (Griffin III et al., 2009; Lopez and Becker, 2005). The present study did not determine whether the increase in voluntary ethanol drinking following chronic ethanol exposure is due to neuroadaptations in NPY and CRF systems during acute withdrawal or the early protracted withdrawal phase. However, our findings suggesting that a longer duration of ethanol vapor (8-wk vs. 2-wk) is necessary to produce changes in NPY-LI and CRF-LI in the adult rat brain are consistent with behavioral evidence that longer duration of ethanol exposure are required to increase ethanol intake after protracted abstinence. For instance, Rimondini and colleagues (2003) showed that 3-wk of abstinence following 7-wk of intermittent chronic ethanol exposure significantly increased voluntary ethanol consumption in a two-bottle free-choice paradigm in Wistar rats. In contrast, rats exposed to 2-wk of intermittent chronic ethanol exposure had no effect on ethanol self-administration nor ethanol preference (Rimondini et al., 2003).
The present study assessed whether the levels of NPY-LI and CRF-LI in control groups were similar across different WD time points (CET, 24-hr WD and 2-wk WD). Findings from the present study showed that NPY-LI and CRF-LI levels in control animals in the 2-wk chronic ethanol exposure group were not statistically significantly different across WD time points in all brain regions studied. These findings were also found for NPY-LI levels in control animals in the 8-wk chronic ethanol exposure group. In contrast, while CRF-LI levels in control animals were not statistically different across WD time points in the FCTX, PCTX and AMYG, significant differences in CRF-LI levels were found among control animals in the HPC in the 8-wk chronic ethanol group. Our results showed that CRF-LI levels in the HPC in control rats were decreased in the 2-wk WD group compared to the CET group and 24-hr WD group. These differences in CRF-LI levels in the HPC were not found among control animals in the 2-wk chronic ethanol group. Rats in the 2-wk chronic ethanol group were sacrificed at 104, 105 and 118 days old, whereas rats in the 8-wk chronic ethanol group were sacrificed at 147, 148 and 161 days old. It is unclear whether age-related factors are responsible for the the gradual decrease in control CRF-LI levels in the HPC in the present study. There is evidence to suggest age-related changes in the tissue levels of CRF in the HPC (Kowalski et al., 1992). This previous study found that CRF levels in the HPC increased in 18 month-old rats, compared to 4 month-old rats. However, it is not clear whether changes in CRF levels in the HPC take place between 4- to 18-month of age in Wistar rats. Findings from the present study showed that CRF-LI levels in the HPC decreased in 161 days old rats, compared to 147 and 148 days old rats. Discrepancies between studies may be due to the use of different tissue dissection techniques and immunoassays. Further research is necessary to determine whether differences in the control CRF-LI levels in the HPC of 161 days old rats are due to the consequences of normal aging.
Previous studies have suggested that the interplay and/or balance between the opposing actions of NPY and CRF may play an important role in the regulation of stress, anxiety, sleep and depression (Ehlers et al., 1997; Heilig et al., 1994; Yamada et al., 1996). To determine whether a shift in the NPY-LI to CRF-LI ratio is observed during ethanol withdrawal in this rodent model of chronic ethanol vapor exposure the NPY-LI/CRF-LI ratio was studied in rats exposed to ethanol for 2-wk and 8-wk and tested during different WD time points. Findings from the present study found that the NPY-LI/CRF-LI ratio in the FCTX, PCTX, HPC and AMYG was not changed in rats exposed to either 2-wk or 8-wk ethanol vapor. Consistent with these findings, we found that at 24-hr WD, the decrease in NPY-LI levels in the FCTX did not correlate with the increase in CRF-LI levels in the PCTX. No correlation between the effects of ethanol exposure on NPY-LI (FCTX and AMYG) and CRF-LI (PCTX and HPC) were also observed at 2-wk WD. These findings suggest that while 8-wk ethanol exposure produced changes in NPY-LI levels in the FCTX and AMYG and in CRF-LI levels in the HPC and PCTX, there is no evidence of an association between changes in NPY-LI and CRF-LI.
Other methods for ethanol administration and induction of ethanol dependence have been successfully used to study the consequences of chronic ethanol treatment (e.g., Becker, 2000; Griffin III et al., 2009). In addition to chronic ethanol vapor regimens, other ethanol treatments have also shown to produce changes in NPY-LI and CRF-LI levels in rats that are dependent on the time following ethanol withdrawal. For instance, Zorrilla and colleagues (2001) found that 3-4 wk exposure to a nutritionally balanced ethanol liquid diet decreased AMYG, HPC and FCTX CRF-LI 1-day after completion of the ethanol regimen in Wistar rats. Consistent with the findings in the present study Zorilla et al. (2001) found that CRF-LI levels increased 6-wk after ethanol exposure. Roy and Pandey (2002) showed that exposure to a 15-day Lieber-DeCarli ethanol diet had no effect on NPY protein expression in the FCTX, PCTX and central and medial nuclei of the AMYG of adult Sprague-Dawley rats. However, this study demonstrated that 24-hr withdrawal following chronic ethanol significantly reduced NPY protein levels in the FCTX, PCTX, pyriform cortex, AMYG and the paraventricular nucleus of the HYP (Roy and Pandey, 2002). Moreover, exposure to this chronic ethanol regimen and the 24-hr WD significantly reduced NPY protein levels in the arcuate nucleus of the HYP and in the cingulate gyrus (Roy and Pandey, 2002).
Using intragastric administration, Bison and Crews (Bison and Crews, 2003) showed that a 4-day, high dose, chronic ethanol treatment produced biphasic effects on NPY levels in the HPC. While chronic ethanol treatment significantly decreased the levels of NPY in the HPC and cortex, it increased it in the dentate, CA3 and CA2 of the hippocampus after 72 hr of ethanol withdrawal (Bison and Crews, 2003). The intermittent chronic ethanol vapor regimen used in the present study also showed increases in NPY in cortex during withdrawal although we did not look at the same time points. The ethanol vapor treatment used in the present study has been shown to produce behavioral, neurophysiological and neurochemical changes in Sprague-Dawley and Wistar rats (Criado and Ehlers, 2010; Criado et al., 2008a, 2008b; Pian et al., 2010; Slawecki et al., 2001; Slawecki and Ehlers, 2005). Findings from the present study may provide insight into the neural substrates mediating the short- and long-term consequences of this intermittent chronic ethanol vapor procedures.
It is important to consider that the observed changes in NPY-LI and CRF-LI levels found in the present study may have been associated with stress induced by the ethanol inhalation procedure and not by the effects of chronic ethanol exposure and withdrawal would. However, if the stress response produced by the alcohol vapor inhalation procedure was responsible for our present findings, then significant changes in NPY-LI and CRF-LI levels would have been expected in the CET group (no withdrawal). However, the effects of 8-wk ethanol vapor regimen on NPY-LI and CRF-LI levels were primarily found during the withdrawal periods in the AMYG (NPY-LI), FCTX (NPY-LI), HPC (CRF-LI) and PCTX (CRF-LI). Exposure to the 8-wk ethanol regimen without a withdrawal period reduced CRF-LI levels only in the HPC and had no effect on other brain regions. Our findings showed that rats exposed to this 8-wk chronic ethanol regimen without withdrawal had no changes in brain levels of NPY-LI. Finally, the present study found that a 2-wk ethanol vapor regimen had no effect on NPY-LI and CRF-LI levels in the brain. Since the effects of intermittent ethanol vapor were dependent on the duration of the ethanol vapor regimen, the brain region and the WD period, findings from this study were likely due to the pharmacological effects of ethanol and not mediated by the stress response associated with the ethanol inhalation procedure.
In summary, 8-wk intermittent ethanol vapor exposure, previously shown to increase ethanol intake in adult Wistar rats, significantly increased NPY-LI levels in the FCTX and AMYG after 2-wk WD of chronic ethanol exposure. This prolonged chronic ethanol regimen also significantly increased CRF-LI levels in the HPC and PCTX following 2-wk WD. In contrast, this study showed no change on adult brain levels of NPY-LI and CRF-LI after 2-wk intermittent ethanol exposure. Findings from the present study suggest that a longer duration of ethanol vapor (8-wk vs. 2-wk) is necessary to produce changes in NPY-LI and CRF-LI in the adult rat brain. However, whether the relationship between NPY-LI and CRF-LI expression in adult Wistar rats exposed to 8-wk ethanol vapor plays an important role increasing ethanol intake remains unclear. In the present study changes in NPY-LI and CRF-LI were interpreted as changes in the levels of these neuropeptides in brain tissue. However, determination of NPY-LI and CRF-LI expression in brain tissue does not provide accurate information to determine whether changes in the levels of these neuropeptides are due to altered release, synthesis, storage or metabolism. Therefore, the effects of chronic ethanol exposure on NPY-LI and CRF-LI levels may be mediated by several cellular mechanisms regulating NPY and CRF function. Further studies are needed to characterize further the neurochemical mechanisms mediating the findings from the present study and determine their role increasing ethanol intake and the expression of anxiety symptoms in rats exposed to chronic ethanol vapor.
The results of this study should be interpreted in light of several limitations. First, changes in the levels of both neuropeptides were measured at the regional level, thus limiting any interpretation on nucleus-specific changes in NPY-LI and CRF-LI levels. For instance, neuropeptide levels were measured from the whole amygdala, which include the central, medial, lateral and basolateral nuclei. Measurements were also obtained from the whole hippocampus, including both the dorsal and ventral hippocampi. Second, differences in the absolute levels of NPY-LI and CRF-LI in previous studies from our laboratory and other groups may be due to the use of different rat strains, antisera, and/or tissue dissection and immunoassay techniques. Third, findings from the present study assessed the tissue content of NPY-LI and CRF-LI and do not provide information on the passive or evoked release of these neuropeptides into the extracellular/synaptic space. Other neurochemical techniques such as microdialysis may be used to address that question. Despite these limitations, this report represents an important step in determining whether the duration of both the chronic ethanol regimen and its withdrawal period differentially affect NPY-LI and CRF-LI levels in brain regions that have been implicated in the development of protracted withdrawal symptoms. Findings from these studies provide the basis for the development of future studies combining neurochemical and electrophysiological in vivo techniques to characterize the neural and cellular mechanisms in implicated in the development of protracted withdrawal symptoms.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH), the National Institute on Alcoholism and Alcohol Abuse grants AA006059 and AA019969 awarded to CLE and by the Swedish Medical Research Council grant 10414 awarded to AAM. The authors thank Greta Berg and Derek Wills for their assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker H. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–13. [PMC free article] [PubMed] [Google Scholar]
- Becker H, Lopez M. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol: Clin Exp Res. 2004;28:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bison S, Crews F. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res. 2003;27:1173–83. doi: 10.1097/01.ALC.0000075827.74538.FE. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob G, Cole M, Zorrilla E, Roberts A. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–21. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado J, Ehlers C. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav Brain Res. 2010;210:164–70. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado J, Wills D, Walker B, Ehlers C. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008a;42:631–9. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado J, Wills D, Walker B, Ehlers C. Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008b;32:1752–62. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- De Soto C, O’Donnell W, De Soto J. Long-term recovery in alcoholics. Alcohol: Clin Exp Res. 1989;13:693–7. doi: 10.1111/j.1530-0277.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol: Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Dunn A, File S. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Bheav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacology (Berl) 1991;104:67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- Ehlers C, Somes C, Seifritz E, Rivier J. CRF/NPY interactions: a potential role in sleep dysregulation in depression and anxiety. Depress Anxiety. 1997;6:1–9. [PubMed] [Google Scholar]
- Ehlers CL, Li T, Lumeng L, Hwang B, Somes C, Jimenez P, Mathe AA. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–82. [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–9. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Finn D, Snelling C, Fretwell A, Tanchuck M, Underwood L, Cole M, Crabbe J, Roberts A. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist d-Phe-CRF(12-41) Alcohol: Clin Exp Res. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in wihtdrawan, ethanol-dependent rats. J Neurosci. 2006;26:11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor I antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin N, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–80. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin W, III, Lopez M, Becker H. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol: Clin Exp Res. 2009;33:1893–900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs S, Menzaghi F, Koob G, Britton K. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–63. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–5. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe J, Becker H. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–84. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Tabakoff B. The role of the NMDA receptor in ethanol withdrawal. EXS. 1994;71:61–70. doi: 10.1007/978-3-0348-7330-7_7. [DOI] [PubMed] [Google Scholar]
- Husum H, Mathe AA. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27:756–64. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- Koob G. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob G, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress; Ciba Found Symp.; 1993; pp. 277–89. [DOI] [PubMed] [Google Scholar]
- Kowalski C, Micheau J, Corder R, Gaillard R, Conte-Devolx B. Age-related changes in cortico-releasing factor, somatostatin, neuropeptide Y, methionine enkephalin and β-endorphin in specific rat brain areas. Brain Res. 1992;582:3–46. doi: 10.1016/0006-8993(92)90314-y. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–64. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Becker H. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez M, Anderson R, Becker H. Repeated cycles of chronic intermitten ethanol exposure increase both self-administration and the reinforcing value of ethanol in C57BL/6J mice. Alcohol: Clin Exp Res. 2008;32:163A. [Google Scholar]
- Mathe AA, Gruber S, Jimenez P, Theodorsson E, Stenfors C. Effects of electroconvulsive stimuli and MK-801 on neuropeptide Y, neurkinin A, and calcitonin gene-related peptide in rat brain. Neurochem Int. 1997;22:629–36. doi: 10.1023/a:1022482322329. [DOI] [PubMed] [Google Scholar]
- Mathe AA, Jimenez PA, Theodorsson E, Stenfors C. Neuropeptide Y, neurokinin A and neurotensin in brain regions of Fawn Hooded “depressed”, Wistar, and Sprague Dawley rats. Effects of electroconvulsive stimuli. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:529–46. doi: 10.1016/s0278-5846(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Miller W, Harris R. A simple scale of Gorski’s warning signs for relapse. J Stud Alcohol. 2000;61:759–65. doi: 10.15288/jsa.2000.61.759. [DOI] [PubMed] [Google Scholar]
- Mossberg D, Liljeberg P, Borg S. Clinical conditions in alcoholics during long-term abstinence: a description, longitudinal treatment study. Alcohol. 1985;2:551–3. doi: 10.1016/0741-8329(85)90133-8. [DOI] [PubMed] [Google Scholar]
- Nellgard B, Mackensen G, Massey G, Pearlstein R, Warner D. The effects of anesthetics on stress responses to forebrain ischemia and reperfusion in the rat. Anesth Analg. 2000;91:145–51. doi: 10.1097/00000539-200007000-00027. [DOI] [PubMed] [Google Scholar]
- O’Dell L, Roberts A, Smith R, Koob G. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol: Clin Exp Res. 2004;28:1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp D, Breese G. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78:459–64. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian J, Criado J, Milner R, Ehlers C. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–54. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–9. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. Effects of tiagabine and diazepam on operant ethanol self-administration in the rat. J Stud Alcohol. 2002;63:100–6. [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol: Clin Exp Res. 1996;20:1289–98. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts A, Heyser C, Cole M, Griffin P, Koob G. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Weiner S, Bloom F. Long-term ethanol administration methods for rats: Advantages of inhalation over intubation of liquid diets. Behav Neural Biol. 1979;27:466–86. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–54. [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–36. doi: 10.1097/01.alc.0000183024.47167.27. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiological responses to corticotropin-releasing factor and neuropeptide Y. Alcohol Alcohol. 1999;34:289–99. doi: 10.1093/alcalc/34.3.289. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of prolonged ethanol exposure on neurophysiological measures during an associative learning paradigm. Drug Alcohol Depend. 2000;58:125–32. doi: 10.1016/s0376-8716(99)00072-1. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Jimenez-Vasquez P, Mathe AA, Ehlers CL. Effect of ethanol on brain neuropeptides in adolescent and adult rats. J Stud Alcohol. 2005;66:46–52. doi: 10.15288/jsa.2005.66.46. [DOI] [PubMed] [Google Scholar]
- Sommer W, Rimondini R, Hansson A, Hipskind P, Gehlert D, Barr C, Heilig M. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amydala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stenfors C, Theodorsson E, Mathe AA. Effect of repeated electroconvulsive treatment on regional concentrations of tachykinins, neurotensin, vasoactive intestinal polypeptide, neuropeptide Y, and galanin in rat brain. J Neurosci Res. 1989;24:445–50. doi: 10.1002/jnr.490240315. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–3. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Thatcher-Britton K, Lee G, Vale W, Rivier J, Koob GF. Corticotropin-releasing factor receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res. 1986;369:303–6. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Neuropeptide Y (NPY) in alcohol intake and dependence. Peptides. 2007;28:480–3. doi: 10.1016/j.peptides.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998;75/76:247–54. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–7. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005a;161:133–40. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki C, Ehlers C. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in Wistar rats with a history of ethanol exposure. Alcohol: Clin Exp Res. 2005b;29:584–90. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol: Clin Exp Res. 2002;26:1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Pich EM, Koob GF, Yee F, Heilig M. Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligonucleotides. Science. 1993;259:528–31. doi: 10.1126/science.8380941. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–93. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse B, Purdy S, Abayasekara D. Inhibition of corticosteroid production by sodium pentobarbitone in rat adrenocortical preparations. J Endocrinol. 1993;136:75–83. doi: 10.1677/joe.0.1360075. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shibasaki T, Tsumori C, Imaki T, Hotta M, Wakabayashi L, Demura H. Neuropeptide Y reverses corticotropin-releasing hormone- and psychological stress-caused shortening of sodium pentobarbidal-induced sleep in rats. Brain Res. 1996;725:272–5. doi: 10.1016/0006-8993(96)00405-2. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla E. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol: Clin Exp Res. 2007;31:1505–15. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]