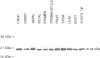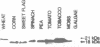Abstract
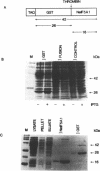
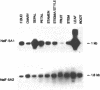
Two Nicotiana plumbaginifolia cDNA clones, NeIF-5A1 and NeIF-5A2, encoding eukaryotic translation initiation factor eIF-5A (formerly called eIF-4D) were cloned by heterologous screening with Dictyostelium and human eIF-5A probes. eIF-5A is the only protein known to contain a unique amino acid modification, hypusine. Comparison of the Nicotiana deduced amino acid sequences with those of other eIF-5A polypeptides reveals conservation throughout the coding sequence, especially in the region of the hypusine residue. Transcript analysis reveals that NeIF-5A1 is preferentially expressed in photosynthetic tissues, while NeIF-5A2 is constitutively expressed in all plant tissues examined. A polyclonal antibody was raised against NeIF-5A1 overexpressed in E. coli. NeIF-5A1 antiserum crossreacts with an 18 kDa polypeptide doublet in all tobacco tissues examined. At least one polypeptide of ca. 18 kDa from a diversity of higher and lower plants crossreacts with NeIF-5A1 antiserum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abath F. G., Simpson A. J. A simple method for the recovery of purified recombinant peptides cleaved from glutathione-S-transferase-fusion proteins. Biotechniques. 1991 Feb;10(2):178–178. [PubMed] [Google Scholar]
- Apelbaum A., Canellakis Z. N., Applewhite P. B., Kaur-Sawhney R., Galston A. W. Binding of spermidine to a unique protein in thin-layer tobacco tissue culture. Plant Physiol. 1988;88:996–998. doi: 10.1104/pp.88.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston A. W., Sawhney R. K. Polyamines in plant physiology. Plant Physiol. 1990 Oct;94(2):406–410. doi: 10.1104/pp.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. Different effects of intron nucleotide composition and secondary structure on pre-mRNA splicing in monocot and dicot plants. EMBO J. 1991 Sep;10(9):2635–2644. doi: 10.1002/j.1460-2075.1991.tb07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. D., Mora R., Meredith S. C., Lee C., Lindquist S. L. Eukaryotic initiation factor 4D, the hypusine-containing protein, is conserved among eukaryotes. J Biol Chem. 1987 Dec 5;262(34):16585–16589. [PubMed] [Google Scholar]
- Hershey J. W., Smit-McBride Z., Schnier J. The role of mammalian initiation factor eIF-4D and its hypusine modification in translation. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):160–162. doi: 10.1016/0167-4781(90)90159-y. [DOI] [PubMed] [Google Scholar]
- Mehta A. M., Saftner R. A., Schaeffer G. W., Mattoo A. K. Translational modification of an 18 kilodalton polypeptide by spermidine in rice cell suspension cultures. Plant Physiol. 1991 Apr;95(4):1294–1297. doi: 10.1104/pp.95.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K. D., Leung D., Lefebvre L., Smith M. The ANB1 locus of Saccharomyces cerevisiae encodes the protein synthesis initiation factor eIF-4D. J Biol Chem. 1990 May 25;265(15):8802–8807. [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- Mizrahi Y., Applewhite P. B., Galston A. W. Polyamine binding to proteins in oat and Petunia protoplasts. Plant Physiol. 1989;91:738–743. doi: 10.1104/pp.91.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owttrim G. W., Hofmann S., Kuhlemeier C. Divergent genes for translation initiation factor eIF-4A are coordinately expressed in tobacco. Nucleic Acids Res. 1991 Oct 25;19(20):5491–5496. doi: 10.1093/nar/19.20.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Chung S. I., Cooper H. L., Folk J. E. The mammalian hypusine-containing protein, eukaryotic initiation factor 4D. Structural homology of this protein from several species. J Biol Chem. 1984 Apr 10;259(7):4563–4565. [PubMed] [Google Scholar]
- Park M. H., Liberato D. J., Yergey A. L., Folk J. E. The biosynthesis of hypusine (N epsilon-(4-amino-2-hydroxybutyl)lysine). Alignment of the butylamine segment and source of the secondary amino nitrogen. J Biol Chem. 1984 Oct 10;259(19):12123–12127. [PubMed] [Google Scholar]
- Park M. H. The essential role of hypusine in eukaryotic translation initiation factor 4D (eIF-4D). Purification of eIF-4D and its precursors and comparison of their activities. J Biol Chem. 1989 Nov 5;264(31):18531–18535. [PubMed] [Google Scholar]
- Pay A., Heberle-Bors E., Hirt H. Isolation and sequence determination of the plant homologue of the eukaryotic initiation factor 4D cDNA from alfalfa, Medicago sativa. Plant Mol Biol. 1991 Oct;17(4):927–929. doi: 10.1007/BF00037075. [DOI] [PubMed] [Google Scholar]
- Sandholzer U., Centea-Intemann M., Noegel A. A., Lottspeich F. cDNA and derived amino acid sequence of the hypusine containing protein from Dictyostelium discoideum. FEBS Lett. 1989 Mar 27;246(1-2):94–100. doi: 10.1016/0014-5793(89)80260-1. [DOI] [PubMed] [Google Scholar]
- Schnier J., Schwelberger H. G., Smit-McBride Z., Kang H. A., Hershey J. W. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jun;11(6):3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit-McBride Z., Dever T. E., Hershey J. W., Merrick W. C. Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein. J Biol Chem. 1989 Jan 25;264(3):1578–1583. [PubMed] [Google Scholar]
- Smit-McBride Z., Schnier J., Kaufman R. J., Hershey J. W. Protein synthesis initiation factor eIF-4D. Functional comparison of native and unhypusinated forms of the protein. J Biol Chem. 1989 Nov 5;264(31):18527–18530. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]