Abstract
Purpose
To evaluate the clinical outcomes of percutaneous radiofrequency (RF) ablation of colorectal cancer liver metastases (CLM) recurring after hepatectomy.
Methods
From December 2002 to December 2008 we ablated 71 CLM developing after hepatectomy in 56 patients. We reviewed medical records and imaging to determine: technique effectiveness/complete ablation (ablation defect covering the entire tumor on 4–6 week post-ablation CT), complications and local tumor progression at the site of ablation. Local tumor progression-free and overall survivals were calculated using Kaplan-Meier methodology. A modified clinical risk score (CRS) including nodal status of the primary, time interval from primary to liver metastases, number of tumors and size of the largest tumor was correlated to overall survival and local tumor progression.
Results
Tumor size ranged between 0.5 and 5.7 cm. Complete ablation was documented in 67/71 (94%) CLM. Complications were: liver abscess (1) and pleural effusion (1). Median overall survival was 31 months. One-, 2- and 3-year overall survival rates were 91%, 66% and 41% respectively. CRS was an independent factor for overall survival (74% for CRS 0–2 vs. 42% for CRS 3–4 at 2 years p=0.03) and for local tumor progression-free survival (66% for CRS 0–2 vs 22% for CRS 3–4 at one year after a single ablation p<0.01).
Conclusion
CT-guided RF ablation can be used to treat recurrent CLM after hepatectomy. A low CRS is associated with better clinical outcomes.
Introduction
Colon cancer is the second leading cause of cancer related mortality in the United States (1). Over the past 5 years, more than 145,000 new cases were diagnosed and more than 49,000 people died from this disease each year (1). Colorectal cancer liver metastases (CLM) is one of the most common hepatic malignant tumors(2). Approximately 50% of patients with colorectal cancer develop CLM during the course of their disease(2). Resection is the treatment of choice for CLM, but the majority of patients are not surgical candidates at diagnosis(3). Treatment options, such as radiofrequency (RF) ablation, hepatic arterial chemotherapy(4), chemoembolization(5) and radioembolization(6, 7) have been used for the treatment of non-resectable CLM.
RF ablation delivers high-frequency alternating current in the tumor via an electrode/needle creating ionic agitation, frictional heating and cell death(8). Although safety and efficacy of RF ablation for unresectable CLM have been shown in several recent studies(9–11) incomplete tumor ablation(12), and local tumor progression or recurrence(13–20) remain limitations of the modality. The rates of local tumor progression after ablation vary widely between 2%(21) and 60%(22). Despite the relatively high rates of local tumor progression, overall survival rates after percutaneous RF ablation of non-resectable small CLM may be similar to those reported after surgical resection(15, 20).
In this study, we evaluated the clinical outcomes of percutaneous CT guided RF ablation and their correlation to several risk factors, when treating CLM occurring in the remnant liver in patients who previously underwent hepatic resection. We modified the previously described surgical clinical risk score (CRS) (23, 24) from a 5 point system to 4. This included the nodal status of the primary, time interval from initial diagnosis to liver metastases, number of tumors and size of the largest tumor (over 3 instead of 5 cm). Carcinoembryonic antigen levels were not included because it was not recorded in most patients at the time of ablation.
Material and Methods
From December 2002 to December 2008, all patients that underwent percutaneous RF ablation to treat recurrence after partial hepatectomy for CLM, were identified from our prospectively created HIPAA registered RF ablation database. Institutional Review Board waiver was obtained for retrospective review. Clinical data and all relevant imaging studies were obtained from our database, patients’ medical records and PACS.
Subjects
Fifty-six patients underwent 82 RF ablations for 71 recurrent CLM after hepatectomy. The interval between surgery and RF ablation ranged between 3 and 83, with a median of 16 months. Patients that had recurrence within 6 months from the liver resection as well as patients in which resection did not achieve clear margins were considered at an increased level of suspicion that they may have additional recurrences. Even if these patients technically could undergo repeat hepatectomy they were offered RF ablation instead, as part of a modified “test of time” management approach(18). Patient characteristics including number of previous hepatic resections and previous chemotherapy treatment are listed in Table 1.
Table 1.
Patient Characteristics
| No. of patients (%) | |
|---|---|
| N | 56 (100) |
|
| |
| Sex | |
| Male | 32 (57) |
| Female | 24 (43) |
|
| |
| Timing of liver metastases | |
| Synchronous | 39 (70) |
| Metachronous | 17 (30) |
|
| |
| No. of prior hepatic resections | |
| One | 40 (71) |
| Two | 13 (23) |
| Three | 2 (4) |
| Four | 1 (2) |
|
| |
| Prior chemotherapy | |
| Systemic only | 31 (55) |
| Systemic + HAI pump | 24 (43) |
| None | 1 (2) |
|
| |
| Chemotherapy post RF ablation | |
| Systemic only | 34 (61) |
| Systemic + HAI pump | 9 (16) |
| None | 12 (21) |
| N/A | 1 (2) |
|
| |
| No. of tumors (average, range per patient) | 71 (1.3, 1 – 4) |
| No. of RFA sessions (average, range per patient) | 81 (1.4, 1 – 3) |
|
| |
| RF ablation system used* | |
| LeVeen® Radiotherapeutics | 30 (42) |
| RITA XL or Xli | 14 (20) |
| Radionics/Valleylab | 27 (38) * numbers refer to lesions |
HAI=Hepatic Artery Infusion
The diagnosis of recurrent liver metastases was in general made by radiologic findings in the context of clinical history of metastatic colorectal cancer. In 3 lesions a biopsy was obtained prior to RF ablation. The indication for biopsy was made in the context of previous intraoperative RF ablation with questionable imaging findings of local tumor progression (2) and in a patient with multiple hemangiomas (1). In these 3 cases the colorectal origin was confirmed.
CLM size ranged between 0.5 and 5.7 cm (median1.9 cm). In 64/71 (90%) CLM the largest diameter was ≤ 3 cm and in 7/71 (10%) >3 cm.
Treatments
All ablations were planned after a contrast enhanced CT of the liver within 30 days prior to the ablation(25).
During the procedure, patients were sedated and monitored by an anesthesiologist. Prophylactic antibiotic (cefazolin 1g) was administered intravenously just prior to the procedure. A limited non-contrast CT was performed to localize the lesion. Accurate electrode position to cover the entire CLM was confirmed with CT imaging prior to the initiation of RF ablation. Treatment was performed with the aim of creating a radius of ablation at least 10 mm larger than the largest tumor diameter in order to achieve at least 5 mm margin around the tumor. Post-RF ablation limited CT imaging was obtained immediately after ablation to evaluate for technical success and periprocedural complications. The patients recovered in the post anesthesia recovery unit and were observed overnight.
Imaging follow-up
An ablation defect with lack of enhancement encompassing the ablated tumor at the first follow-up imaging consisting of a contrast enhanced CT scan 4–6 weeks after treatment was considered “complete ablation” or “technique effectiveness” as described in definitions (Table 2). Any evidence of irregular or nodular enhancement of the ablated area at this CT scan was considered treatment failure. This first post-RF ablation CT was used as a baseline to evaluate the area of ablation in subsequent follow-up CT every 2–4 months. Development of new irregular peripheral or nodular enhancement of the ablated area, increase in size or in enhancement of the ablated zone at any time after the first post ablation CT was considered local tumor progression.
Table 2.
Definitions.
| Term | Definition |
|---|---|
| Technical success | Ablation protocol completed and tumor covered (on immediately post-ablation CT) |
| Complete ablation(Technique effectiveness) | Ablation defect covering the entire tumor on the 4–6 week post- ablation CT |
| Local Tumor Progression | Evidence of tumor recurrence (progression) in the previously ablated area by CT criteria
|
| Primary LTP-free survival | Time period between the initial RF Ablation and the first radiologic evidence of LTP |
| Assisted LTP-free survival | Cumulative time interval between the first RF ablation and the latest radiologic follow-up showing evidence of LTP (local tumor recurrence); includes all the ablations performed for the treatment of the same target tumor to treat LTP |
| Overall Survival | Time period between the initial RF ablation and patient’s death from any cause, or most recent follow-up |
| Clinical Risk Score (CRS) modified for Ablation Patients with 0-2 points: low risk Patients with 3–4 points: high risk |
One point for each:
|
Statistical Analysis
Primary and assisted local tumor progression-free and overall survival rates were calculated using Kaplan-Meier methodology. Permutation log-rank test was used to assess the differences between overall survival between the CRS groups and a marginal Cox model was used for primary and assisted local tumor progression-free rates to account for multiple treated tumors per patient. Multivariate analysis (marginal Cox model) was possible for the evaluation of factors affecting local tumor progression but not for overall survival due to the small number of deaths observed during the study period. R (www.r-project.com) software (version 2.10) was used for statistical analysis.
Results
We have adhered to the guidelines regarding terminology and definitions as described by Goldberg et al (26) (Table 2). Risks factors included in the clinical risk score are shown in table 2. Based on our definitions 45 CLM were classified as low clinical risk score (CRS): 0–2 and 26 as high CRS: 3–4.
Technical success was achieved in all 82 sessions. Complete ablation or technique effectiveness was documented in 67/71 (94 %) lesions. The 4 failures were attributed to poor visualization and inadequate tumor targeting (3) and insufficient overlap ablations (1) to provide complete tumor coverage with adequate margin. The median hospital stay after ablation was 1.0 day (range 1 – 5 days).
Local Tumor Progression
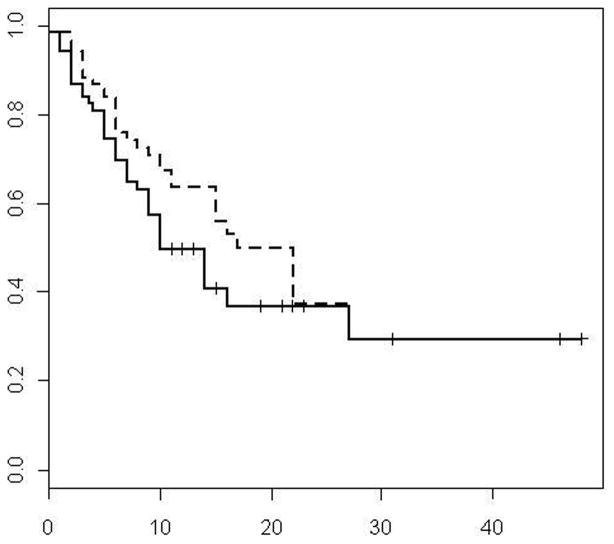
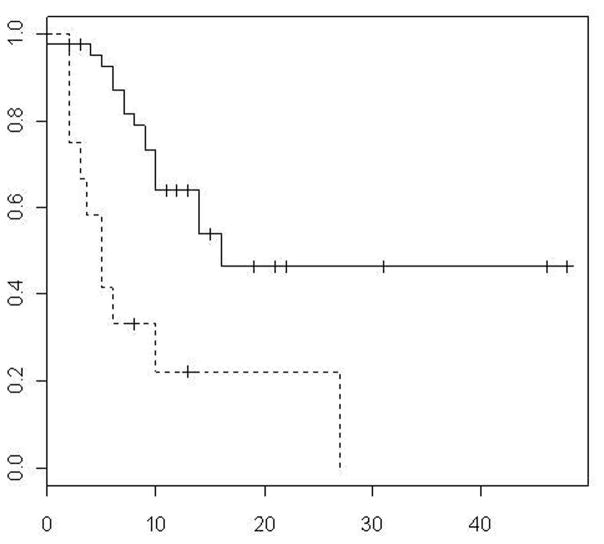
During a median follow-up of 22 months local tumor progression occurred in 36 of 71 CLM. Of these, 17 were observed in the low CRS group (17/45 or 37.7 %) and 19 in the high CRS group (19/26 or 75%). Overall 31/36 (86%) CLM, progressed during the first year and 19/36 (53%) were in patients that developed diffuse disease progression (distant intrahepatic and extrahepatic metastases) and were no longer candidates for local treatment. Median and 1,2, and 3 year primary (after single ablation) local tumor progression-free rates were 10 months and 50% at 1 year, 37% at 2 years and 30% at year 3 respectively (Figure 1). Median time to local tumor progression was 14 months in pump group and 10 months in no pump group. The progression profiles for patients who had previous pump was substantially better at earlier time points than those who did not have pump, however, this was not significant (p=0.19). Median local tumor progression-free survival was 16 months in CRS 0 – 2 group and 5 months in CRS 3 – 4 groups, respectively (p=0.001) (Figure 2). Primary (after single ablation) local tumor progression-free survival rate of 66% was noted at year 1 for CRS 0 – 2 vs 22% for CRS 3 – 4 (p<0.01) respectively.
Figure 1.
Primary and assisted (dotted line) local tumor progression-free survival curves
Figure 2.
TTP by CRS (solid line 0–2, dotted line 3–4)
Secondary treatments
Seventeen of the 36 lesions that progressed were amenable to local treatment and were managed as follows: Nine underwent repeat percutaneous and 2 open RF ablation. Five lesions were treated surgically, and 1 was lost to follow-up. The choice to treat the 5 lesions with local tumor progression with surgery was based on the fact that more than 6 months had elapsed since surgery, there was no other site of intrahepatic or extrahepatic metastasis and the particular lesions were in locations where ablation with clear margin was difficult or not feasible (subcapsular, in close proximity to a blood vessel or organ that could not be protected with technique modifications such as hydrodissection. Local tumor progression after the second RF treatment was observed in 5/8 lesions and was treated by surgical resection (1), third RF ablation (2) and systemic chemotherapy alone (2). Median and 1,2 and 3 year assisted (accounting for all repeated ablations to treat the same tumor) local tumor progression-free rates were 25 months and 64% at 1 year, 38% at 2 years and 30% at year 3 respectively (Figure 1).
Patient Survival
Median overall survival from the time of the initial RF ablation was 31 months (Figure 3). One, 2 and 3-year survival rates were 91%, 66% and 41% respectively. The group that received pump chemotherapy prior to RF ablation (n=24) did not reach the median survival, while the group with no pump treatment had a median survival of 25 months. Median overall survival was 35 months in CRS 0 – 2 group and 21 months in CRS 3 – 4 groups, respectively (p=0.03) (Figure 3). The 1- and 2- year overall survival rate for CRS 0–2 was 98% and 74% vs. 69% and 42% for CRS 3 – 4 respectively (p=0.03). The overall survival was also evaluated between patients that had one versus those that underwent repeated ablations to treat local tumor progression. The patients that underwent repeat ablation for local tumor progression had a significantly prolonged survival with a 3-year survival rate of 89% compared to 23% for patients with one ablation (p=0.03).
Figure 3.
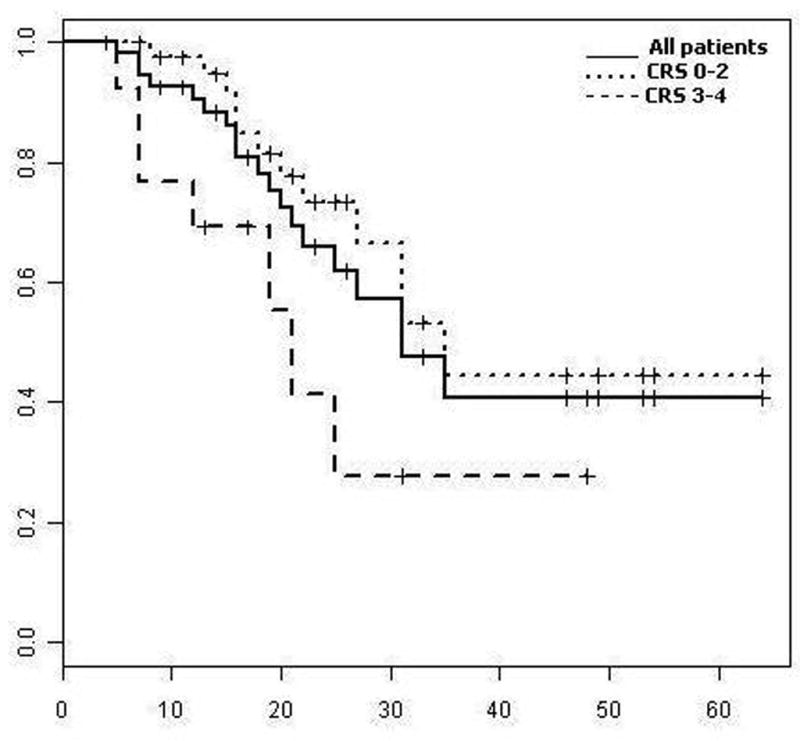
Overall survival curves for entire group (solid line) and by CRS (dotted lines)
Analysis of Factors Affecting Local Tumor Progression-free and Overall survivals
The results of analysis of association of individual covariates with overall survival and local tumor progression-free survival are summarized in Tables 3 and 4. The CRS was an independent factor for both overall survival and local tumor progression free survival. There were 19 deaths in this study, which were not sufficient to perform a multivariate analysis for overall survival. With 36 lesions exhibiting progression, however, we were able to accommodate a multivariate analysis with the two significant factors from the univariate analysis (sex and CRS). Sex was not significant (HR=1.733, p=0.15), leaving CRS as the only significant predictor of progression in this cohort. Patients with CRS of 3 or higher were 3.13 times more likely to progress (95% confidence interval: 1.61–6.06) than those with CRS of 2 or less (p=0.001).
Table 3.
Factors Correlating with Survival.
| Variable | Category | N | 2-yr Surv (%) | 95% CI | P |
|---|---|---|---|---|---|
| Sex | F | 24 | 51 | 30–85 | 0.04 |
| M | 32 | 76 | 61–95 | ||
| Age | <=60 | 31 | 75 | 58–98 | 0.52 |
| >60 | 25 | 58 | 40–85 | ||
| Extrahepatic Disease | No | 39 | 74 | 59–93 | 0.07 |
| Yes | 17 | 49 | 28–87 | ||
| CRS | 0–2 | 43 | 74 | 59–92 | 0.03 |
| 3–4 | 13 | 42 | 19–93 | ||
| Electrode | 1 | 12 | 51 | 27–93 | 0.28 |
| 2 | 28 | 61 | 44–85 | ||
| 3 | 16 | 100 | - | ||
| Prior HAIC | No | 32 | 56 | 39–80 | 0.15 |
| Yes | 24 | 84 | 68–100 | ||
| * Nodal Status of Primary | Negative | 14 | 71 | 48–100 | 0.44 |
| Positive | 42 | 29 | 14–61 | ||
| * Number of Tumors | Solitary | 41 | 46 | 30–70 | 0.05 |
| Multiple | 15 | 37 | 17–78 | ||
| * DFI | >12m | 16 | 62 | 40–95 | 0.94 |
| <12m | 40 | 31 | 16–61 | ||
| * Size | ≤3cm | 51 | 47 | 32–69 | 0.1 |
| >3cm | 5 | 20 | 3–100 |
factor included in CRS; CRS= clinical risk score
Table 4.
Factors Correlating with Progression
| Variable | Category | N | 1-yr PFS (%) | 95% CI | P |
|---|---|---|---|---|---|
| Sex | F | 30 | 65 | 49–87 | 0.056 |
| M | 41 | 39 | 26–60 | ||
| Age | <=60 | 42 | 49 | 35–69 | 0.77 |
| >60 | 29 | 51 | 34–76 | ||
| Extrahepatic Disease | No | 47 | 54 | 41–73 | 0.26 |
| Yes | 24 | 42 | 26–70 | ||
| CRS | 0–2 | 47 | 62 | 48–79 | <0.01 |
| 3–4 | 24 | 26 | 13–54 | ||
| Electrode | 1 | 14 | 61 | 39–95 | 0.15 |
| 2 | 30 | 37 | 22–63 | ||
| 3 | 27 | 57 | 40–82 | ||
| Prior HAIC | No | 41 | 48 | 34–68 | 0.18 |
| Yes | 30 | 53 | 36–77 | ||
| * Nodal Status of Primary | Negative | 16 | 83 | 65–100 | 0.01 |
| Positive | 55 | 47 | 31–65 | ||
| * Number of Tumors | Solitary | 53 | 61 | 47–79 | <0.01 |
| Multiple | 18 | 37 | 17–78 | ||
| * DFI | >12m | 20 | 62 | 40–95 | 0.15 |
| <12m | 51 | 52 | 38–72 | ||
| * Size | ≤3cm | 64 | 54 | 34–64 | 0.02 |
| >3cm | 7 | 17 | 3–100 |
factor included in CRS; CRS= clinical risk score
Adverse Events
There were 2 complications that required hospitalization: one liver abscess treated with percutaneous drainage and IV antibiotics and one pleural effusion managed with thoracostomy. There was no procedure related mortality.
Discussion
Surgery for CLM is possible in approximately 15% of patients resulting in 5 year survival rates between 31–58%(24, 27, 28). RF ablation has been used to control liver metastases successfully in selected non resectable patients with limited number and relatively small tumor size (18, 29–31). In series with good selection criteria median survival of 36 months and 1, 3 and 5 year survival of 86%, 80% and 24% have been reported(30). Similarly a subgroup of patients without extrahepatic disease and with less than 5 liver lesions each under 5 cm in diameter treated with ablation achieved an overall survival rate of 30% at 5 years from diagnosis(31). Although there is no randomized controlled trial between surgery and RF ablation for CLM, retrospective studies comparing the two treatments demonstrated comparable outcomes (32). RF ablation was used in the treatment of CLM in surgical candidates within the concept of “test of time” as described by Livraghi et al (18) in 88 surgical candidates while awaiting surgery. 59% of patients were spared unnecessary surgery either because they were free of disease after RF ablation (44%) or because they developed multiple new sites of disease during the waiting time (56%) deeming them unresectable(18). The local tumor control achieved with RF ablation allowed time to elapse for multifocal disease to develop and these patients were thus spared unnecessary surgery with significant morbidity and not negligible mortality (15, 33). We applied this approach to patients with recurrent colon cancer liver metastases after hepatectomy that were technically re-resectable, but were considered to be at increased risk for additional recurrences in the liver or extrahepatic sites. This was in particular the case for patients that presented with new liver metastasis within 6 months of resection or had prior surgery that did not achieve clear margins. In patients that remained technically and clinically re-resectable, surgery was reconsidered when they developed additional local tumor progression at the site of ablation at a later time (more than 6 months from original surgery) and if they had no other site of disease. In these patients repeat surgery was preferred over repeat ablation when the local tumor progression occurred in a location that would jeopardize ablation with clear margin (subcapsular location; abutting or in close proximity to a hepatic vessel or to an organ that could not be protected).
Complete ablation in this paper was defined as recommended by the Guidelines of the Society of Interventional Radiology (26). Obviously there is a discrepancy between complete ablation and residual microscopic disease that can cause local tumor progression. This is a key limitation of ablation when compared to surgery since tissue sampling or margin analysis is not routinely performed after RF ablation. In a dedicated paper it was demonstrated that the presence of Ki67 tumor cells on ablation electrodes can predict local tumor progression despite an initial technical effectiveness after ablation of liver tumors (29). Similarly, the relatively high rate of local tumor progression reported after RF ablation of CLM when compared to historic surgical data remains a limitation for the widespread use of RF ablation in surgical patients (15, 20). RF ablation can be easily repeated to treat tumor progression and this is one of its advantages when compared to repeat hepatectomy. This point demonstrates a role of the technique as a salvage treatment after surgical failures or recurrences. The effect of repeat ablation in overall survival was significant (p=0.03). It is likely that the added survival benefit for the population that was eligible for repeat ablation is the result of multiple factors related to the biology of disease that could not be addressed in a small sample.
There was an overall high rate of local tumor progression after a single ablation in this series even in the lower CRS 0-2 CLM (37.7%). This may be partially attributed to the aggressive nature of the treated tumors. All patients in this series had recurrence after surgery and more than half of those who had local tumor progression after ablation (19/36, 53%) had multifocal and extrahepatic progression of disease. Repeat ablation was performed to those patients with local tumor progression only and resulted in local tumor progression-free survival of 25 months and 1, 2, and 3 year local tumor progression-free survival rates of 64%, 38% and 30% respectively. Although the local tumor progression-free survival achieved with ablation in this cohort is towards the short end of the spectrum, it is important to remember that this is in addition to the local tumor progression-free survival achieved after hepatectomy (median disease free interval between surgery and hepatic recurrence treated by percutaneous ablation was 16 months, range: 3–83).
The overall survival of 41% at 3 years in this series compares favorable to treatments with chemotherapy only(4). This survival rate, calculated from the day of ablation is achieved in patients that have tumor progression after resection and reflects the additional survival benefit of aggressive locoregional treatment with ablation and pump as well as systemic treatment after hepatectomy(4). As such the population evaluated in this study is highly selected and any direct comparisons to other percutaneous ablation or hepatectomy series are difficult if not impossible. Nevertheless, this survival rate appears similar to several prior series of patients treated with ablation immediately after the diagnosis of liver metastasis (30, 32).
Translating from the surgical knowledge we slightly modified a previously described clinical risk score (CRS) (23, 24) to correlate to our eligibility criteria for CLM RF ablation. We used a 3 instead of 5 cm as a cutoff since tumor size over 3 cm has previously been associated with worse outcomes after ablation (16, 29). The CRS was an independent factor for both overall survival and local tumor progression. These results underscore the importance of appropriate screening when selecting patients with intent to cure their CLM. Our findings are similar to those reported by surgical series showing a strong correlation between clinical risk factors and outcomes (23, 24). The administration of pump therapy was associated with better outcomes but did not reach significance. The only statistically significant factor was the CRS.
This study has several limitations. An important one is the relatively small number of patients and short follow-up period making comparisons between the groups of patients that received pump and those who did not, inconclusive. It is possible that the relatively higher local tumor progression-free and overall survival noted in the pump population would become significant in a larger population over a longer follow-up period. The same limitation did not allow us to complete multivariate analysis regarding all factors affecting survival. We plan to update our analysis when we will have long enough follow-up to estimate the 5 year survival in our population.
The primary objective of the study was to determine outcomes after RF ablation of colon cancer liver recurrences after hepatectomy. Despite the limitations of this small retrospective review we believe that the main goals of the study were met. In addition the introduction of the modified CRS and its significant correlation to local tumor progression and overall survival is described for the first time in the evaluation of this population. We believe that this paper showed that CT-guided percutaneous radiofrequency ablation can be used as a salvage treatment of recurrent CLM after hepatectomy, offering additional local tumor control and prolonging overall survival in a selected heavily pretreated population with limited therapeutic options.
Acknowledgments
This work was supported in part by NIH Grant No. 5R21CA131763.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2008. American Cancer Society; Atlanta, GA: 2008. Aug 10, p. 2008. [Google Scholar]
- 2.Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006;141:181–190. doi: 10.1001/archsurg.141.2.181. [DOI] [PubMed] [Google Scholar]
- 3.Hanna NN. Radiofrequency ablation of primary and metastatic hepatic malignancies. Clin Colorectal Cancer. 2004;4:92–100. doi: 10.3816/ccc.2004.n.012. [DOI] [PubMed] [Google Scholar]
- 4.Kemeny N. Management of liver metastases from colorectal cancer. Oncology (Williston Park) 2006;20:1161–1176. 1179. discussion 1179–1180, 1185–1186. [PubMed] [Google Scholar]
- 5.Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250:281–289. doi: 10.1148/radiol.2501080295. [DOI] [PubMed] [Google Scholar]
- 6.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology. 2008;247:507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–425. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Gillams AR. The use of radiofrequency in cancer. Br J Cancer. 2005;92:1825–1829. doi: 10.1038/sj.bjc.6602582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lencioni R, Crocetti L, Cioni D, Della Pina C, Bartolozzi C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol. 2004;39:689–697. doi: 10.1097/00004424-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 10.White TJ, Roy-Choudhury SH, Breen DJ, et al. Percutaneous radiofrequency ablation of colorectal hepatic metastases - initial experience. An adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Dig Surg. 2004;21:314–320. doi: 10.1159/000080886. [DOI] [PubMed] [Google Scholar]
- 11.Feliberti EC, Wagman LD. Radiofrequency ablation of liver metastases from colorectal carcinoma. Cancer Control. 2006;13:48–51. doi: 10.1177/107327480601300107. [DOI] [PubMed] [Google Scholar]
- 12.Pulvirenti A, Garbagnati F, Regalia E, et al. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516–1517. doi: 10.1016/s0041-1345(00)02577-x. [DOI] [PubMed] [Google Scholar]
- 13.Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol. 2008;190:1544–1551. doi: 10.2214/AJR.07.2798. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 15.Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:144–157. doi: 10.1245/s10434-007-9478-5. [DOI] [PubMed] [Google Scholar]
- 16.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the "test-of-time approach". Cancer. 2003;97:3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- 19.Ng KK, Poon RT. Radiofrequency ablation for malignant liver tumor. Surg Oncol. 2005;14:41–52. doi: 10.1016/j.suronc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.White RR, Avital I, Sofocleous CT, et al. Rates and Patterns of Recurrence for Percutaneous Radiofrequency Ablation and Open Wedge Resection for Solitary Colorectal Liver Metastasis. J Gastrointest Surg. 2007;11:256–263. doi: 10.1007/s11605-007-0100-8. [DOI] [PubMed] [Google Scholar]
- 21.Pawlik TM, Tanabe KK. Radiofrequency ablation for primary and metastatic liver tumors. Cancer Treat Res. 2001;109:247–267. doi: 10.1007/978-1-4757-3371-6_14. [DOI] [PubMed] [Google Scholar]
- 22.Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132:605–611. doi: 10.1067/msy.2002.127545. discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 23.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 24.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pua BB, Sofocleous CT. Imaging to optimize liver tumor ablation. Imaging in Medicine. 2010;2:433–443. doi: 10.2217/IIM.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249:364–374. doi: 10.1148/radiol.2491071752. [DOI] [PubMed] [Google Scholar]
- 30.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 31.Gillams AR, Lees WR. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol. 2004;14:2261–2267. doi: 10.1007/s00330-004-2416-z. [DOI] [PubMed] [Google Scholar]
- 32.Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Are C, Gonen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]