Abstract
Vitamin D is a multifunctional hormone that can affect many essential biological functions, ranging from the immune regulation to mineral ion metabolism. A close association between altered activity of vitamin D and vascular calcification has been reported in various human diseases, including in patients with atherosclerosis, osteoporosis, and chronic kidney disease (CKD). Vascular calcification is a progressive disorder and is a major determinant of morbidity and mortality of the affected patients. Experimental studies have shown that excessive vitamin D activities can induce vascular calcification, and such vascular pathology can be reversed by reducing vitamin D activities. The human relevance of these experimental studies is not clear, as vitamin D toxicity is relatively rare in the general population. Contrary to the relationship between vitamin D and vascular calcification, in experimental uremic models, low levels of vitamin D were shown to be associated with extensive vascular calcification, a phenomenon that is very similar to the vascular pathology seen in patients with CKD. The current treatment approach of providing vitamin D analogs to patients with CKD often poses a dilemma, as studies linked vitamin D treatment to subsequent vascular calcification. Recent genetic studies, however, have shown that vascular calcification can be prevented by reducing serum phosphate levels, even in the presence of extremely high serum 1,25-dihydroxyvitamin D and calcium levels. This article will briefly summarize the dual effects of vitamin D in vascular calcification and will provide evidence of vitamin D-dependent and -independent vascular calcification.
Keywords: calcium, CKD, klotho, phosphate
VITAMIN D
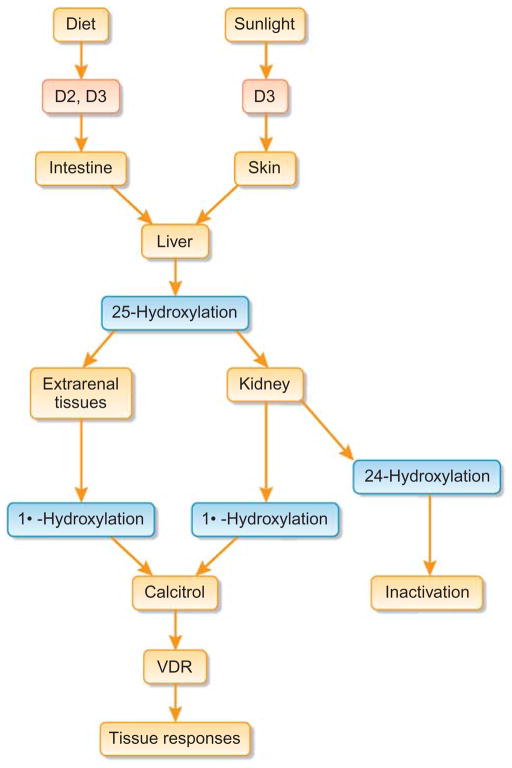
Vitamin D is a multifunctional hormone that exerts effects on most of the organ systems.1–7 Calcitriol or 1,25-dihydroxyvitamin D is the active form of vitamin D, formed by dual hydroxylation in the liver and kidney (Figure 1). Active vitamin D metabolites exert biological activities mainly through the vitamin D receptor (VDR).8 There are numerous factors that can influence vitamin D production. For instance, parathyroid hormone induces the synthesis of 1-α hydroxylase in proximal tubular epithelial cells to produce 1,25-dihydroxyvitamin D. Similarly, low serum levels of phosphate can also induce the biosynthesis of 1,25-dihydroxyvitamin D in the proximal tubular epithelial cells. Circulating 1,25-dihydroxyvitamin D, acting through VDR, can enhance the intestinal absorption of calcium and phosphate, while can also suppress the biosynthesis of parathyroid hormone.9 In bones, high levels of 1,25-dihydroxyvitamin D usually increases osteoclastic bone resorption to mobilize calcium and phosphate into the circulation to ensure optimal serum balance.
Figure 1. Simplified diagram of the different stages of vitamin D synthesis.
VDR, vitamin D receptor.
In contrast to the endocrine effects of VDR, the autocrine functions of VDR require local generation of the active ligand (calcitriol). VDRs are present in most of the human tissues, including endothelial and vascular smooth muscle cells (VSMCs).10,11 More importantly, VSMCs also possess an enzymatically active 25-hydroxyvitamin D-1α-hydroxylase system12 and can synthesize active vitamin D metabolites locally. Therefore, the circulating levels of calcitriol may not always reflect vascular activities of vitamin D. Moreover, 1α-hydroxylase activity was also detected in endothelial cells.13 Experimental studies have shown that calcitriol can increase the expression of the VDR and decrease the proliferation of VSMCs.11,14 Furthermore, calcitriol can accelerate cell migration and promote the transition of contractile VSCMs into the osteoblast-like phenotype.15,16 It appears likely that autocrine, paracrine, and endocrine functions of vitamin D can influence vascular structure, functions, and remodeling. Importantly, the above mentioned effects of vitamin D on blood vessels are in addition to the most commonly appreciated functions of vitamin D in active regulation of mineral ion metabolism, such as facilitating intestinal calcium absorption, as well as renal, and skeletal mobilization of phosphate.8,17,18 More importantly, vitamin D is a strong inducer of fibroblast growth factor 23 (FGF23) and klotho, two important factors that maintain physiologic calcium and phosphate balance.19,20 Of particular interest, loss-of-function mutations of either FGF23 or klotho can induce ectopic calcification in the affected patients.21–23 Because survival can be negatively influenced by vascular calcification, minimizing the risk of such vascular pathology requires that the molecular mechanisms involved in the tightly controlled regulatory pathways and cross talk between organ systems be understood.
VASCULAR CALCIFICATION
Vascular calcification is a complex process that induces stiffening of the vessel wall and reduces vascular compliance.24 The exact molecular mechanisms of vascular calcification is not yet clear; it seems that an initial insult to the cellular components of the vessels can function as a triggering event, which is further facilitated by the presence of calcifying-promoting factors such as hypervitaminosis D, hyperphosphatemia, and hypercalcemia.25–28 Studies also suggested that reduction of the calcification repressor factors, such as ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP-1), ANK (transmembrane protein), and matrix Gla protein, can also propagate vascular calcification.29–31 For instance, matrix Gla protein deficiency in humans (Keutel syndrome) and in genetically modified mice (matrix Gla protein knockout) is associated with ectopic calcification.32,33 Matrix Gla protein is thought to suppress vascular calcification partly through its Gla residues, which have a calcium/hydroxyapatite-chelating ability. In a similar study, in vivo ablation of the ANK gene from mice resulted in ectopic calcification.34 Interestingly, blocking the pyrophosphate degrading action of alkaline phosphatase can reduce the calcification in VSMCs obtained from genetically altered NPP-1- and ANK-deficient mice.35 Vascular calcification was also detected in patients with loss-of-function mutations in the human NPP-1 gene.36 Studies have shown that in the absence of calcification repressor factors, calcification can occur at normal calcium and phosphate levels.
Vascular calcification was previously considered as a passive process related to non-specific responses and had little clinical significance. However, availability of electron beam and multidetector row computed tomography imaging helped clinicians to monitor the in vivo active progression of vascular calcification and predict consequences of acute and chronic coronary events. Furthermore, recent identification of calcification-promoting and -suppressing factors (Table 1) provided convincing molecular evidence that vascular calcification is not only an active process, but one that may be reversible if manipulated in early stages. For instance, hyperphosphatemia in dialysis patients correlates with vascular calcification, and reducing phosphate levels can attenuate progression of vascular calcification in these patients.37 Prevention of vascular calcification is of great clinical importance, as it can induce fatal complications such as thrombosis, arterial rupture, and myocardial infarction.
Table 1.
Partial list of known inducers and inhibitors of vascular calcification
| Inducers |
| BMP-2/4 |
| Calcium |
| Hyperparathyroidism |
| Inflammatory cytokines |
| LDL |
| Leptin |
| MSX2 |
| Oncostatin |
| Osteocalcin |
| Phosphate |
| Pit-1 |
| RUNX2 |
| TGF-β1 |
| TNAP |
| Uremic toxins |
| Vitamin D |
| Inhibitors |
| Adiponectin |
| ANK |
| BMP-7 |
| Fetuin-A |
| HDL |
| IGF-1 |
| Insulin |
| Klotho |
| Magnesium |
| Matrix Gla protein |
| NPP-1 |
| Omega-3 fatty acid |
| Osteopontin |
| Osteoprotogerin |
| PTH |
| PTHrP |
| Pyrophosphate |
Abbreviations: ANK, transmembrane protein; BMP, bone morphogenetic protein; LDL, low-density lipoprotein; IGF-1, insulin-like growth factor-1; NPP-1, pyrophosphatase/phosphodiesterase; PTH, parathyroid hormone; PTHrP, PTH-related peptide; TGF-β1, transforming growth factor-β1; TNAP, tissue-non-specific alkaline phosphatase.
Vascular calcification often mimics certain molecular events including those seen in skeletal mineralization.38 Alteration of mineral ion balance can induce both endothelial and VSMC apoptosis, and facilitate phenotypic transformation of VSMCs into skeletal cells. Studies have linked bone morphogenetic proteins, RUNX2, MSX2, and activation of wingless-type MMTV integration site family member signaling to such cellular transformation processes.27,39 Of relevance, MSX2 can not only promote osteogenesis but can also suppress adipogenic differentiation of multipotent mesenchymal progenitors.40 Some researchers believe that once vascular cells are transdifferentiated into skeletal cells, subsequent mineralization processes lead to the development of vascular calcification. As mentioned, vitamin D can induce transdifferentiation of VSCMs into the osteoblast-like phenotype promoting the complex process of vascular calcification.15,16,41
VITAMIN D-DEPENDENT VASCULAR CALCIFICATION
Studies have linked vitamin D therapy to higher incidences of vascular calcification; in a retrospective study, the severity and progression of vascular calcification correlated with the plasma levels of vitamin D in chronic kidney disease (CKD) patients undergoing prolonged dialysis treatment.42 A similar observation was also reported in young adults suffering from CKD since childhood; the use of active vitamin D preparations has shown to be independently associated with cardiovascular anomalies, including intima–media thickness and coronary artery calcification.43 In accordance with the human observations, high doses of vitamin D metabolites were shown to be associated with the experimental vascular calcification in rats and other animals.44,45 Exogenous treatment of vitamin D and nicotine can markedly increase the aortic calcium content, causing medial elastic fiber calcification and arterial stiffness in experimental animals.46 A time-dependent aortic calcification was also shown in calcitriol-treated rats with relatively normal renal function.44 In a separate study, diffuse calcification involving the intimal and medial layers of the aorta was noted in uremic rats that received relatively low doses of oral calcitriol (0.25 mg/kg per day).47 More importantly, calcitriol-induced vascular calcification has been shown to be a reversible process, by reducing vitamin D activities.44 Although the in vivo optimal levels are difficult to estimate, it seems that a delicate balance of vitamin D is important for normal vascular functions, as studies have shown that both low and high vitamin D levels are associated with vascular calcification (a U-curve relationship) in pediatric patients undergoing dialysis treatment.48
Vitamin D is a strong inducer of both FGF23 and klotho. 1,25-Dihydroxyvitamin D has been shown to increase FGF23 promoter activity in osteoblasts through a vitamin D-responsive element.49 In contrast, FGF23 can suppress 1,25-dihydroxyvitamin D activity by reducing renal expression of 1α-hydroxylase, and increasing 24-hydroxylase.19 Recent studies have elegantly demonstrated the importance of the FGF23–klotho system in the regulation of calcium and phosphate balance.50,51
Vitamin D-associated vascular calcification was also noted in various genetically modified mouse models.51–55 For instance, genetic inactivation of either Fgf23 or klotho leads to increased serum calcium, phosphate and 1,25-dihydroxyvitamin D levels in these mutant mice; such abnormal mineral ion and vitamin D homeostasis in Fgf23- and klotho-knockout mice are associated with widespread soft tissue and vascular calcifications.53,55 More importantly, genetically reducing active vitamin D synthesis in either Fgf23- or klotho-knockout mice can completely eliminate soft tissue and vascular calcifications in Fgf23/1a(OH)ase and klotho/1a(OH)ase-double-knockout mice.20,28,51,52,54,56 However, it should be mentioned that the loss of vitamin D-related processes, either from Fgf23 or klotho mice resulted in a change from severe hyperphosphatemia to hypophosphatemia. Whether biomineralization of the vascular wall through excessive deposition of hydroxyapatite can occur without vitamin D-dependent processes is not yet clear, as dissociating the in vivo effects of vitamin D from mineral ion dysregulation is difficult to estimate. For instance, experimental calcification induced by extremely low doses of vitamin D (0.04 μg/kg of calcitriol intraperitoneally three times/week for 1 month) resulted in a marked increase in serum calcium, phosphate, and the calcium–phosphate (Ca × P) product.57 Thus, it is difficult to determine in vivo whether calcitriol can directly induce vascular calcification or if such vascular pathology is related to the altered mineral ion balance and increased Ca × P product levels, as seen in these low vitamin D-treated animals.57 Moreover, neither calcitriol nor its analogs (paricalcitol or doxercalciferol) (1–100 nM) were able to induce mineralization in human VSMCs, whereas increasing extracellular phosphate concentration induced mineralization in human VSMCs.58 Consistent with the in vitro studies, recent in vivo mouse genetic studies have provided insights into vitamin D-independent vascular calcification.
VITAMIN D-INDEPENDENT VASCULAR CALCIFICATION
Studies have shown a negative correlation between serum 25-hydroxyvitamin D levels and vascular calcifications,59 and that both serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels were also negatively correlated with aortic pulse wave velocity.60 The endothelial functions (determined by flow-mediated vasodilatation) of type 2 diabetic patients with low levels of vitamin D were shown to improve with vitamin D treatment.61 Similarly, vitamin D deficiency has shown to increase the hazard ratio of incident cardiovascular diseases.62 Shoji et al.63 have shown lower risk for cardiovascular mortality in CKD patients using oral 1α-hydroxy vitamin D3 (alfacalcidol) in a hemodialysis population compared to non-users. In a similar study, Shoben et al.64 found improved survival in non-dialyzed CKD patients receiving oral calcitriol therapy. Collectively, the above mentioned studies clearly suggest that cardiovascular functions are impaired in vitamin D-deficient states. Reduced serum levels of 1,25-dihydroxyvitamin D is one of the major biochemical changes detected in patients with CKD. The current treatment approach of providing vitamin D analogs in patients with CKD often poses a dilemma because studies have linked vitamin D treatment to subsequent vascular calcification.65,66 Of particular importance, 50% of mortalities in patients with CKD undergoing dialysis treatment were shown to be related to vascular calcification.38,66
Vitamin D-independent vascular calcification was shown recently in genetically altered mouse models. For instance, klotho-knockout mice have increased renal expression of sodium/phosphate co-transporters (NaPi2a), associated with severe hyperphosphatemia (Figure 2).20,53–55,67 Such serum biochemical changes in klotho-knockout mice led to extensive soft tissue anomalies and vascular calcification. Interestingly, lowering the serum phosphate levels in klotho-knockout mice reduced vascular calcification, despite the presence of significantly higher serum calcium and 1,25-dihydroxyvitamin D levels.20,54 This study provides in vivo evidence of beneficial effects of reducing serum phosphate levels on preventing vascular calcification, even in the presence of extremely high serum 1,25-dihydroxyvitamin D levels (Figure 3).20,54 Such in vivo information may be of particular importance in designing the therapeutic strategies for patients with CKD, in whom low serum levels of vitamin D and phosphate toxicity have been shown to be associated with high mortality. Phosphate retention in patients with CKD and dialysis-associated inflammatory responses can accelerate VSMC apoptosis, as well as induce vascular calcification and calcifying uremic arteriolopathy or calciphylaxis. In the experimental animals, inflammation has shown to promote vascular calcification, as overexpression of tumor necrosis factor-α in the vessels resulted in calcification, possibly by activating wingless-type MMTV integration site family member activities in the VSMCs.27,39 Recently, other signaling pathways are also implicated in vascular calcification process. For instance, phosphatidylinositol 3-kinase/Akt signaling pathway has shown to be involved in VSMC calcification, induced by inflammatory mediators, such as interferon-γ and tumor necrosis factor-α. Suppression of phosphatidylinositol 3-kinase pathway with wortmannin (inhibitor of phosphatidylinositol 3-kinase) can increase inflammatory mediator-induced VSMC calcification, along with the expression of alkaline phosphatase.68 In patients with CKD, an inverse association is reported between serum levels of proinflammatory cytokines (tumor necrosis factor-α, interleukin-1β, interleukin-6) and Fetuin-A (an inhibitor of extraskeletal calcification).69 Fetuin-A can inhibit de novo precipitation of calcium–phosphate, without exerting effects on already formed hydroxyapatite.70 Of relevance, Fetuin-A-knockout mice develop severe ectopic calcifications in the heart, lung, kidney and skin.71
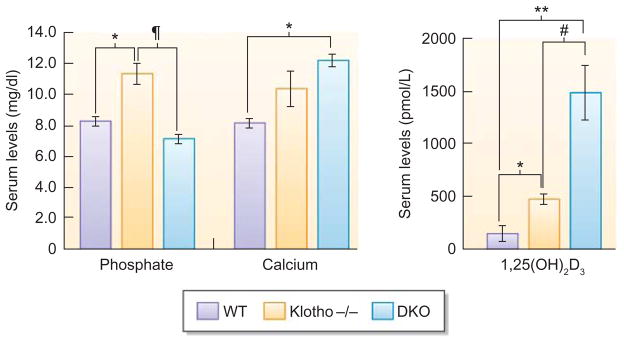
Figure 2. Serum phosphate, calcium, and 1,25 dehydroxyvitamin D levels.
Phosphate and calcium levels in wild-type (WT), klotho−/−, and NaPi2a−/−/klotho−/− double knockout (DKO) mice. Serum phosphate, calcium, and 1,25 dehydroxyvitamin D levels were higher in klotho−/ − mice compared with WT mice at 9 weeks of age. In contrast to klotho−/ − mice, serum phosphate levels were markedly reduced in DKO mice. Note that in contrast to the serum phosphate levels, serum calcium and 1,25 dehydroxyvitamin D levels remained higher in DKO mice (*P<0.01, versus WT; **P<0.005, versus WT; ¶P<0.001, versus klotho−/−; #P<0.05, versus DKO). 1,25 dehydroxyvitamin D3, 1,25(OH)2D3.
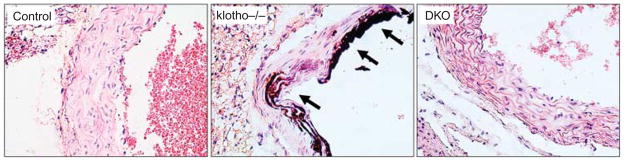
Figure 3. Sections prepared from aortae of wild-type mice, klotho−/− mice, NaPi2a−/−/klotho−/−double-knockout (DKO) mice, showing extensive calcifications in the aortic wall of klotho−/− mice.
Aortic calcification of klotho−/− mice is associated with increased serum calcium, phosphate, and 1,25-dihydroxyvitamin D levels. No such aortic calcification was detected in aortae obtained from DKO mice with reduced serum phosphate levels. Note that compared with the klotho−/− mice, DKO mice had increased serum calcium, and 1,25-dihydroxyvitamin D levels (von Kossa staining; × 60).
Furthermore, hyperphosphatemia and hypercalcemia can induce cardiovascular calcification, affecting the myocardium, and cardiac valves.72 Hyperphosphatemia (>6.5 mg/dl) and elevated Ca × P product (>72) are known to be related to cardiovascular anomalies associated with increased mortality in patients with CKD undergoing hemodialysis treatment.73 Comparable results were also noted in the experimental model of CKD, in which controlling serum phosphate levels prevented the development of vascular calcification.74 Similarly, the administration of calcium carbonate delayed the progression of aortic calcification in CKD-induced apolipoprotein-E-knockout mice, possibly by reducing serum phosphate levels.75 Of relevance, high serum levels of phosphate and low serum vitamin D levels have been identified as independent risk factors not only for rapid deterioration in renal function but also for high mortality of the pre-dialysis phase in CKD patients.62,63,76
CONCLUSIONS
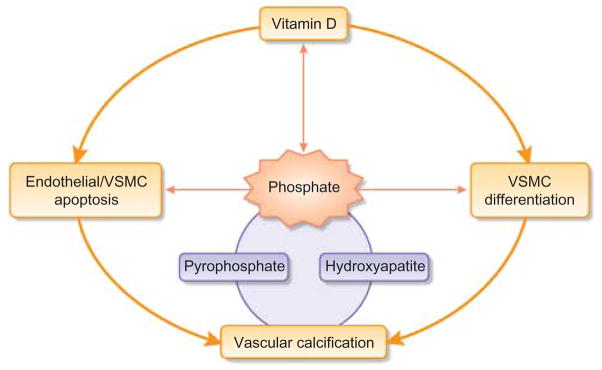
Vascular calcification is a complex, ectopic biomineralization process, involving autocrine, paracrine and endocrine interactions of numerous factors (Table 1). There is increasing evidence suggesting that phosphate toxicity plays an important role in vascular calcification. Phosphate toxicity can not only facilitate essential hydroxyapatite deposition in the calcifying vessels but also initiate the early events by inflicting apoptotic cell death on both endothelial cells and VSMCs (Figure 4). Moreover, reducing or eliminating vascular calcification in klotho-knockout mice—by lowering serum phosphate levels—provides compelling genetic evidence of vitamin-D-independent calcification, even in the presence of extremely high serum calcium and 1,25-dihydroxyvitamin D levels.20,54,77 More importantly, these results suggest that reducing ‘phosphate toxicity’ should be a critical therapeutic priority for minimizing the risk of vascular calcification and disease progression,78 Although there is no disagreement about the harmful effects of phosphate toxicity on the survival of CKD patients, the survival advantage for hemodialysis patients taking vitamin D was questioned in the recently published Dialysis Outcomes and Practice Patterns Study.79 Analyzed data from 38,066 Dialysis Outcomes and Practice Patterns Study participants from 12 countries between 1996 and 2007 did not show any differences in mortality in patients with or without vitamin D treatment in the adjusted baseline standard regression models.79
Figure 4. Simplified schematic diagram outlining the possible pathological events that are associated with vascular calcification.
VSMC, vascular smooth muscle cell.
Delineating the kidney-vascular axis in the context of regulating calcium, phosphate and vitamin D metabolism in health and disease will help us not only to determine the molecular mechanisms of vascular calcification but also in achieving therapeutic modulation of the involved pathways and prevent/delay this deadly vascular disorder.
Acknowledgments
I am grateful for the technical support of Razzaque lab members, particularly, Drs Teruyo Nakatani, Kazuyoshi Uchihashi and Mutsuko Ohnishi of the Department of Oral Medicine, Infection and Immunity at the Harvard School of Dental Medicine, Boston, MA. Some of the original research that formed the basis of this review was supported by a grant (R01-DK077276 to MSR) from the National Institute of Diabetes and Digestive and Kidney Diseases, and institutional support from Nagasaki University School of Biomedical Science and the Harvard School of Dental Medicine.
Footnotes
DISCLOSURE
The author declared no competing interests.
References
- 1.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 2.Valdivielso JM, Cannata-Andia J, Coll B, et al. A new role for vitamin D receptor activation in chronic kidney disease. Am J Physiol Renal Physiol. 2009;297:F1502–F1509. doi: 10.1152/ajprenal.00130.2009. [DOI] [PubMed] [Google Scholar]
- 3.De Broe ME. Phosphate: despite advances in research, the benefits to patients remain limited. Kidney Int. 2009;75:880–881. doi: 10.1038/ki.2008.692. [DOI] [PubMed] [Google Scholar]
- 4.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deb DK, Chen Y, Zhang Z, et al. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-\{kappa\}B pathway. Am J Physiol Renal Physiol. 2009;296:F1212–F1218. doi: 10.1152/ajprenal.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstuyf A, Carmeliet G, Bouillon R, et al. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 7.Razzaque MS. The FGF23 Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 10.Merke J, Hofmann W, Goldschmidt D, et al. Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int. 1987;41:112–114. doi: 10.1007/BF02555253. [DOI] [PubMed] [Google Scholar]
- 11.Rajasree S, Umashankar PR, Lal AV, et al. 1,25-dihydroxyvitamin D3 receptor is upregulated in aortic smooth muscle cells during hypervitaminosis D. Life Sci. 2002;70:1777–1788. doi: 10.1016/s0024-3205(02)01473-x. [DOI] [PubMed] [Google Scholar]
- 12.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 13.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 14.Carthy EP, Yamashita W, Hsu A, et al. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 15.Tukaj C, Kubasik-Juraniec J, Kraszpulski M. Morphological changes of aortal smooth muscle cells exposed to calcitriol in culture. Med Sci Monit. 2000;6:668–674. [PubMed] [Google Scholar]
- 16.Rebsamen MC, Sun J, Norman AW, et al. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res. 2002;91:17–24. doi: 10.1161/01.res.0000025269.60668.0f. [DOI] [PubMed] [Google Scholar]
- 17.Chau TS, Lai WP, Cheung PY, et al. Age-related alteration of vitamin D metabolism in response to low-phosphate diet in rats. Br J Nutr. 2005;93:299–307. doi: 10.1079/bjn20041325. [DOI] [PubMed] [Google Scholar]
- 18.Lanske B, Razzaque MS. Vitamin D and aging: old concepts and new insights. J Nutr Biochem. 2007;18:771–777. doi: 10.1016/j.jnutbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 20.Ohnishi M, Nakatani T, Lanske B, et al. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chefetz I, Heller R, Galli-Tsinopoulou A, et al. A novel homozygous missense mutation in FGF23 causes Familial Tumoral Calcinosis associated with disseminated visceral calcification. Hum Genet. 2005;118:261–266. doi: 10.1007/s00439-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 23.Benet-Pages A, Orlik P, Strom TM, et al. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 24.Guerin AP, London GM, Marchais SJ, et al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 25.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 26.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Towler DA, Shao JS, Cheng SL, et al. Osteogenic regulation of vascular calcification. Ann N Y Acad Sci. 2006;1068:327–333. doi: 10.1196/annals.1346.036. [DOI] [PubMed] [Google Scholar]
- 28.Razzaque MS, St-Arnaud R, Taguchi T, et al. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, Shioi A, Jono S, et al. Expression of matrix Gla protein (MGP) in an in vitro model of vascular calcification. FEBS Lett. 1998;433:19–22. doi: 10.1016/s0014-5793(98)00870-9. [DOI] [PubMed] [Google Scholar]
- 30.Jono S, Ikari Y, Vermeer C, et al. Matrix Gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb Haemost. 2004;91:790–794. doi: 10.1160/TH03-08-0572. [DOI] [PubMed] [Google Scholar]
- 31.Gheduzzi D, Boraldi F, Annovi G, et al. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest. 2007;87:998–1008. doi: 10.1038/labinvest.3700667. [DOI] [PubMed] [Google Scholar]
- 32.Hur DJ, Raymond GV, Kahler SG, et al. A novel MGP mutation in a consanguineous family: review of the clinical and molecular characteristics of Keutel syndrome. Am J Med Genet A. 2005;135:36–40. doi: 10.1002/ajmg.a.30680. [DOI] [PubMed] [Google Scholar]
- 33.El-Maadawy S, Kaartinen MT, Schinke T, et al. Cartilage formation and calcification in arteries of mice lacking matrix Gla protein. Connect Tissue Res. 2003;44(Suppl 1):272–278. [PubMed] [Google Scholar]
- 34.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 35.Narisawa S, Harmey D, Yadav MC, et al. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 36.Babij P, Roudier M, Graves T, et al. New variants in the Enpp1 and Ptpn6 genes cause low BMD, crystal-related arthropathy, and vascular calcification. J Bone Miner Res. 2009;24:1552–1564. doi: 10.1359/jbmr.090417. [DOI] [PubMed] [Google Scholar]
- 37.Raggi P, Bellasi A. Clinical assessment of vascular calcification. Adv Chronic Kidney Dis. 2007;14:37–43. doi: 10.1053/j.ackd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 39.Al-Aly Z, Shao JS, Lai CF, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 40.Cheng SL, Shao JS, Charlton-Kachigian N, et al. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 41.Tukaj C. Enhanced proliferation of aortal smooth muscle cells treated by 1,25(OH)2D3 in vitro coincides with impaired formation of elastic fibres. Int J Exp Pathol. 2008;89:117–124. doi: 10.1111/j.1365-2613.2008.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldsmith DJ, Covic A, Sambrook PA, et al. Vascular calcification in long-term haemodialysis patients in a single unit: a retrospective analysis. Nephron. 1997;77:37–43. doi: 10.1159/000190244. [DOI] [PubMed] [Google Scholar]
- 43.Briese S, Wiesner S, Will JC, et al. Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease-impact of calcium and vitamin D therapy. Nephrol Dial Transplant. 2006;21:1906–1914. doi: 10.1093/ndt/gfl098. [DOI] [PubMed] [Google Scholar]
- 44.Bas A, Lopez I, Perez J, et al. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res. 2006;21:484–490. doi: 10.1359/JBMR.051211. [DOI] [PubMed] [Google Scholar]
- 45.Henley C, Colloton M, Cattley RC, et al. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol Dial Transplant. 2005;20:1370–1377. doi: 10.1093/ndt/gfh834. [DOI] [PubMed] [Google Scholar]
- 46.Niederhoffer N, Bobryshev YV, Lartaud-Idjouadiene I, et al. Aortic calcification produced by vitamin D3 plus nicotine. J Vasc Res. 1997;34:386–398. doi: 10.1159/000159247. [DOI] [PubMed] [Google Scholar]
- 47.Haffner D, Hocher B, Muller D, et al. Systemic cardiovascular disease in uremic rats induced by 1,25(OH)2D3. J Hypertens. 2005;23:1067–1075. doi: 10.1097/01.hjh.0000166849.72721.1c. [DOI] [PubMed] [Google Scholar]
- 48.Shroff R, Egerton M, Bridel M, et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19:1239–1246. doi: 10.1681/ASN.2007090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 50.Razzaque MS. Therapeutic potential of klotho-FGF23 fusion polypeptides: WO2009095372. Expert Opin Ther Pat. 2010;20:981–985. doi: 10.1517/13543771003774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohnishi M, Nakatani T, Lanske B, et al. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ Cardiovasc Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakatani T, Sarraj B, Ohnishi M, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Memon F, El-Abbadi M, Nakatani T, et al. Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int. 2008;74:566–570. doi: 10.1038/ki.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizobuchi M, Finch JL, Martin DR, et al. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- 58.Wu-Wong JR, Noonan W, Ma J, et al. Role of phosphorus and vitamin D analogs in the pathogenesis of vascular calcification. J Pharmacol Exp Ther. 2006;318:90–98. doi: 10.1124/jpet.106.101261. [DOI] [PubMed] [Google Scholar]
- 59.Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, et al. Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol. 2006;17:S267–S273. doi: 10.1681/ASN.2006080925. [DOI] [PubMed] [Google Scholar]
- 60.London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 61.Sugden JA, Davies JI, Witham MD, et al. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 64.Shoben AB, Rudser KD, de Boer IH, et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1529–1539. doi: 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 66.Mizobuchi M, Ogata H, Koiwa F, et al. Vitamin D and vascular calcification in chronic kidney disease. Bone. 2009;45(Suppl 1):S26–S29. doi: 10.1016/j.bone.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Nabeshima Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- 68.Okazaki H, Shioi A, Hirowatari K, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates inflammatory mediators-induced calcification of human vascular smooth muscle cells. Osaka City Med J. 2009;55:71–80. [PubMed] [Google Scholar]
- 69.Dervisoglu E, Kir HM, Kalender B, et al. Serum fetuin–a concentrations are inversely related to cytokine concentrations in patients with chronic renal failure. Cytokine. 2008;44:323–327. doi: 10.1016/j.cyto.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 70.Heiss A, DuChesne A, Denecke B, et al. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 71.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joy MS, Karagiannis PC, Peyerl FW. Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm. 2007;13:397–411. doi: 10.18553/jmcp.2007.13.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 74.Mathew S, Tustison KS, Sugatani T, et al. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phan O, Ivanovski O, Nikolov IG, et al. Effect of oral calcium carbonate on aortic calcification in apolipoprotein E-deficient (apoE−/−) mice with chronic renal failure. Nephrol Dial Transplant. 2008;23:82–90. doi: 10.1093/ndt/gfm699. [DOI] [PubMed] [Google Scholar]
- 76.Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 77.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Razzaque MS. Phosphate toxicity: new insights into an old problem. Clin Sci. doi: 10.1042/CS20100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tentori F, Albert JM, Young EW, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009;24:963–972. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]