Abstract
Background
The prevalence of chronic sleep deprivation is increasing in modern societies with negative health consequences. Recently, an association between short sleep and obesity has been reported.
Purpose
Primary objectives: To assess the feasibility of increasing sleep duration to a healthy length (approximately 7½ h) and to determine the effect of sleep extension on body weight. Secondary objectives: To examine the long-term effects of sleep extension on endocrine (leptin and ghrelin) and immune (cytokines) parameters, the prevalence of metabolic syndrome, body composition, psychomotor vigilance, mood, and quality of life.
Methods
One hundred-fifty obese participants who usually sleep less than 6½ h, are being randomized at a 2:1 ratio to either an Intervention or to a Comparison Group. They are stratified by age (above and below 35) and the presence or absence of metabolic syndrome. During the first 12 months (Efficacy Phase) of the study, participants are evaluated at bi-monthly intervals: the Intervention Group is coached to increase sleep by at least 30–60 min/night, while the Comparison Group maintains baseline sleep duration. In the second (Effectiveness) phase, participants converge into the same group and are asked to increase (Comparison Group) or maintain (Intervention Group) sleep duration and are evaluated at 6-month intervals for an additional 3 years. Non-pharmacological and behavior-based interventions are being utilized to increase sleep duration. Endocrine, metabolic, and psychological effects are monitored. The sleep, energy expenditure, and caloric intake are assessed by activity monitors and food recall questionnaires. At yearly intervals, body composition, abdominal fat, and basal metabolic rate are measured by dual energy X-ray absorptiometry (DXA), computerized tomography (CT), and indirect calorimetry, respectively.
Results
As of January 2010, 109 participants had been randomized, 64 to the Intervention Group and 45 to the Comparison Group (76% women, 62% minorities, average age: 40.8 years; BMI: 38.5 kg/m2). Average sleep duration at screening was less than 6 h/night, 40.3 h/week. A total of 28 Intervention and 22 Comparison participants had completed the Efficacy Phase.
Limitations
The study is not blinded and the sample size is relatively small.
Conclusions
This proof-of-concept study on a randomized sample will assess whether sleep extension is feasible and whether it influences BMI.
Introduction
We report our experiences with designing and conducting the first randomized, controlled, clinical trial to evaluate the efficacy of a behavioral intervention to extend sleep duration in obese (BMI 30–55 kg/m2) 18- to 50-year-old men and premenopausal women who habitually sleep less than 6½ h, as assessed by actigraphy monitors and sleep diaries. This study is funded by the Intramural Program of the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) of the National Institutes of Health (NIH). A number of challenging issues have been encountered during the design and the conduct of this clinical trial. The approaches to meet these challenges and their outcomes are presented herein.
Background
Obesity is a leading cause of morbidity and mortality in the 21st century and its prevalence has been rapidly increasing over the past 30 years [1]. Recently, a similar growth in self-reported sleep deprivation has been noticed, paralleling the obesity epidemic. It is estimated that average sleep time has fallen in the general population by an average of 2 h per night [2,3]. Given their contemporaneous chronological patterns, it has been suggested that chronic sleep deprivation and obesity may be two linked phenomena with similar epidemiology. Both conditions are most prevalent in lower socio-economic classes, women, and minorities [4]. Chronic sleep deprivation has been associated with many chronic conditions, although a causal link remains to be established. Increased body weight, glucose intolerance, cardiovascular disease, hypertension, impaired immune system function, decreased vigilance, motor vehicle accidents, medical errors in residents, and non-accident related mortality have all been associated with chronic sleep deprivation [5–8]. On the other hand, excessive (more than 8 h) sleep duration also has been associated with increased mortality, suggesting a U-shaped curve between sleep duration and morbidity/mortality that is, at least in part, mediated by age [9].
Pre-clinical and clinical evidence
It is a widespread notion that there may be adaptations to chronic sleep deprivation that prevent long-term deleterious effects, but a growing body of scientific evidence suggests otherwise. Mice with faulty body clocks grew to become morbidly obese after only a few weeks of abnormal sleep patterns [10]. In humans, substantial evidence has been generated from recent clinical experiments. A short course of sleep restriction induced insulin resistance, hypercortisolism, leptin deficiency and increase in interleukin-6, and other pro-inflammatory cytokines that may together be conducive to long-term weight gain [11]. Twelve healthy young men were randomized to two nights of sleep restriction or sleep extension in a controlled laboratory setting. Each participant underwent both interventions separated by 6 weeks. When volunteers remained in bed for no more than 4 h, decreased leptin, increased ghrelin, and increased cortisol levels were observed when compared to levels obtained after remaining in bed for at least 10 h. Additionally, hunger rating scores were higher after sleep restriction than sleep extension and were proportional to the changes in the ghrelin to leptin ratio [11].
Studies of young, healthy volunteers suggest that sleep deprivation is both a neurobiological and a physiologic stressor [12]. Chronic sleep deprivation was shown to increase appetite, levels of proinflammatory cytokines, blood pressure, and evening cortisol, insulin and blood glucose levels. Of note, increased interleukin-1, tumor necrosis factor-α, and interleukin-6, have been shown to contribute to a diabetogenic environment [13]. In light of these findings, it is clear that establishing a causal link (and its direction) between sleep and obesity will result in important insights with potential clinical applications.
Starting with the first report by Hasler et al. [14] in 2004, a growing body of epidemiological evidence has been accumulating, mostly in population studies originally designed for different reasons than studying the effects of sleep on weight, on the effects of short sleep on body weight, insulin resistance, hypertension and other ailments. More recently, a meta-analysis has shown that short sleep is associated with an increased risk of obesity both in children (odds ratio 1.89; 1.46–2.43; p <0.0001) and in adults (odds ratio 1.55; 1.43–1.68; p <0.0001) [15]. Although these were large cohort studies, their conclusions were limited by the fact that self-reported sleep and body weight data were collected as a secondary consideration to the original protocol design. It is not clear how much and for how long sleep may be curtailed before an effect on weight may become apparent [16].
Although these studies cannot establish the presence, or determine the direction, of a causal link between short sleep and the development of obesity over time, they make a strong circumstantial case [17]. Age appears to be an important modulator of the interaction between sleep and weight, as collective evidence suggests that weight gain is associated with short sleep in children and in adults only up to the fourth or fifth decade of life. At an older age it is not clear whether sleep duration is associated with changes in body weight. In addition, it remains to be determined whether sleeping longer than 7½ h is beneficial. A very large epidemiological study has reported increased mortality in participants sleeping 8 or 9 h [18]. This study raised the question of inverse causation that remains unanswered. In conclusion, the evidence published so far underscores the need for a prospective, randomized, controlled trial specifically designed to establish the effects of sleep extension on body weight.
Clinical importance of behavioral interventions
It is now commonly acknowledged that lifestyle choices can have dramatic effects on our health and longevity. A poor diet and lack of exercise are accepted causes of cardiovascular disease and Type II diabetes [19]. Smoking is a known cause of many cancers and other medical complications [20]. Although the effects of smoking, poor diet, and lack of exercise are now well documented as a danger to health, it was not until well-controlled clinical trials were performed, as late as the 1960s, that the deleterious effects of these habits became generally accepted by the medical profession and, eventually, the general public. Once sleep hygiene has been given sufficient attention based on the evidence generated from randomized controlled trials such as ours, healthy sleep hygiene also may become one of the pillars of a healthy lifestyle.
Objectives
This proof-of-concept study is aimed at investigating the impact of sleep extension over a long period of time on weight, the endocrine system, body composition, metabolic syndrome, and quality of life in a large population of obese, sleep-deprived participants. According to the US National Cholesterol Education Program Adult Treatment Panel III, metabolic syndrome is defined as having at least three of the following: (1) abdominal obesity: waist circumference greater than 102 cm in males and 88 cm in females; (2) triglycerides greater than 150 mg/dL; (3) HDL-C greater than 40 mg/dL for males and 50 mg/dL for women; (4) blood pressure equal or greater than 130/85 mm Hg or use of medication for hypertension; and (5) fasting glucose equal or greater than 100 mg/dL.
Our primary objective is to explore the extent to which it is possible to implement sleep ‘prescriptions’ and improve habitual sleep duration over time in chronically sleep-deprived participants. To the best of our knowledge, this is the first study that attempts to increase sleep duration outside of the sleep laboratory in a real life setting and for a long period of time. Sleep deprivation is defined here as regularly sleeping less than 6½ h per night. Second, we will observe whether increased habitual sleep duration over 12 months results in significant differences in BMI between the Intervention and the Comparison Groups. The effects of extension of sleep duration on body composition, leptin and ghrelin levels, the prevalence of metabolic syndrome, the immune system, psychomotor vigilance, and quality of life will be assessed. Finally, we will determine whether participation in a 12-month trial prior to sleep education improves sleep hygiene in the long term.
Description of the behavioral interventions
During the first 12 months of the study (Efficacy Phase) we strive to extend sleep duration in participants randomized to the Intervention Group. No modifications of energy intake or energy expenditure are prescribed; rather participants are instructed to do as they wish in terms of diet and exercise. However, all participants are informed of the current standard of care for achieving and maintaining a healthy weight. Special care is given to equalize the quality and quantity of time spent with subjects in each group to avoid any ‘study effect’ bias in participants randomized to the Intervention Group.
During the subsequent 36 months of the study (Effectiveness Phase) a three-component approach that includes individual counseling on sleep, nutrition, and physical activity is offered to every participant, regardless of the original group allocation. We create individualized sleep plans through one-on-one sessions during which the participants are guided to identify obstacles in their daily lives that may be preventing them from improving their sleep hygiene and achieving a healthy body weight. Long-term life style changes to the daily routine are encouraged; whenever possible, the sleep partner and the whole family are involved. The intervention is administered by the principal investigator (endocrinologist), a research nurse, and a dietician in separate sessions. These interventions vary greatly, depending on participant preferences; however, all are nonpharmacological, devoid of potentially negative side-effects, and inexpensive, and could introduce additional positive side-effects (Table 1). Study team and study participants are blinded to the randomization code before assignments are made. The consent for the Efficacy Phase describes the main study hypothesis in a nondirectional fashion – that is, to see whether changes in extension of sleep duration have any effects on body weight during a 12-month study period.
Table 1.
Challenges in study design and conduct
| Type of trials | Challenges |
|---|---|
| Sleep trials | The prevalence of chronic sleep deprivation is not known in the general population. Self-reported sleep duration is at best imprecise. Only few large, long-term studies have been conducted in this field. |
| Obesity trials | Require a very large sample size and long duration. In randomized controlled trials, blinding is difficult as participants can weigh themselves. If pharmacological treatment is effective, greater drop-out from the placebo group is often observed. |
| Common to both type of trials | Both obesity and sleep-deprivation are more prevalent in minorities and participants from lower socio-economic status, but it is difficult to recruit minorities into research studies. Behavioral interventions are difficult to implement, especially in outpatient settings. Behavioral interventional studies are costly, as they have to be long and large. The private sector has little interest in funding behavioral studies. |
Study challenges
There have been many challenges posed by this behavioral study of sleep intervention in obese, sleep-deprived participants (Table 1). When this study was originally planned in 2006, an on-going epidemic of sleep curtailment had been postulated based on a long-term secular trend [21]. However, little objective information on the prevalence of sleep deprivation in the general population was available; it was therefore unclear whether there would be enough candidates to achieve the target sample size. An additional challenge was presented by the fact that trials of extension of sleep duration have never been attempted on a large-scale basis in an outpatient setting; there was no pre-existing blueprint for the design of such a study. Moreover, obesity and sleep deprivation are more common in minorities, a demographic subgroup that is historically difficult to recruit into clinical studies [22].
The implementation and maintenance of long-term life style modifications are common problems of both weight loss and sleep extension trials. It is well established that obesity interventions are more effective when they include all three main components, reduced energy intake, increased energy expenditure, and counseling, compared to any one of these components [23].
Methods
Study design
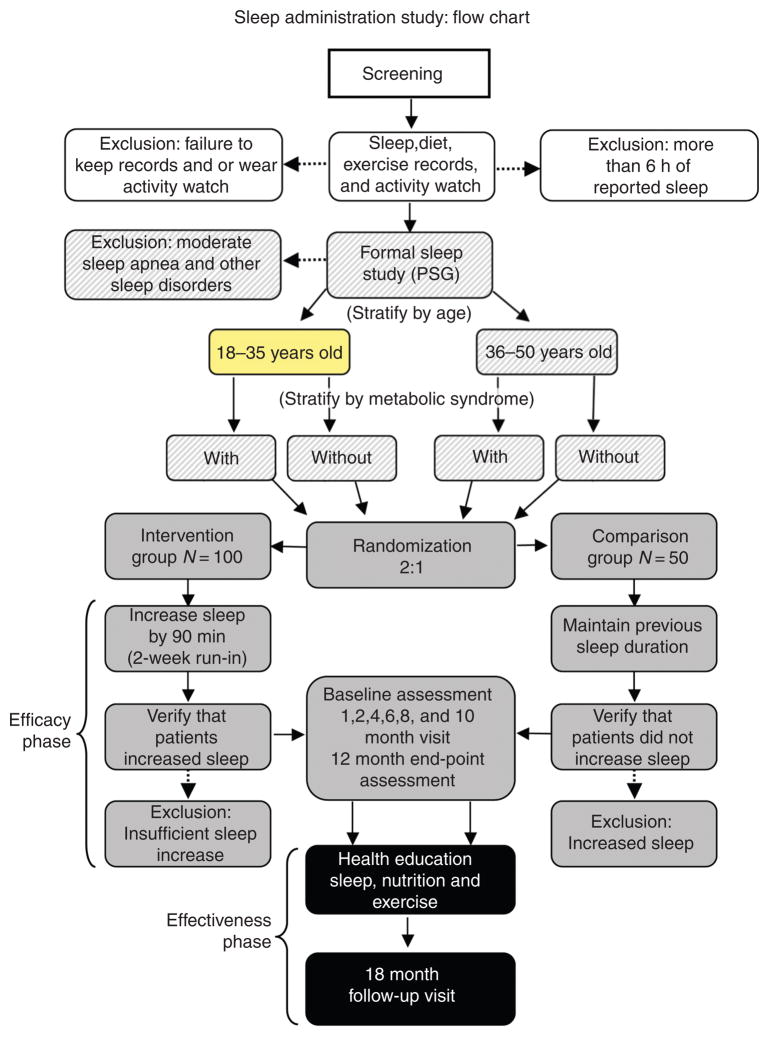
Study participants are 18- to 50-year-old obese (BMI 30–55 kg/m2), sleep-deprived (average sleep less than 6½ h per night) men and pre-menopausal women (Table 2). By history we assess whether body weight has remained stable (within 5%) over the last 6 months. The participants are randomized into either the Intervention or the Comparison Group at a 2:1 ratio. Those in the Intervention Group are coached to increase their sleep gradually by at least 30–60 min, possibly up to a total of 7½ h per night. The Comparison Group is asked to continue its habitual short sleep habits. The study is divided into a 12-month Efficacy Phase followed by a 36-month Effectiveness Phase (Figure 2).
Table 2.
Rationale behind inclusion/exclusion criteria
| Inclusion criteria | Rationale |
|---|---|
| 18–50 years old | Based on biological sleep needs. |
| BMI 30–55 | Obese participants have the potential to benefit the most from sleep intervention. |
| Men and women | Obesity is associated with sleep deprivation in both genders. |
| Sleep <6½ h per night | Threshold established for sleep deprivation based on cross sectional studies. |
| Stable body weight and sleep habits for last 6 months | Recent changes in body weight affect levels of leptin and other hormones. |
|
| |
| Exclusion criteria | Rationale |
|
| |
Diagnosed sleep disorders, including:
|
High likelihood of the inability to comply with study protocol and increase sleep. |
Work non-compatible with sleep changes:
|
Inability to comply with study protocol and increase sleep. |
Medications:
|
Effects on the endocrine system. |
Chronic organ disorders:
|
Effects on endocrine system, sleep habits, as well as energy consumption and expenditure. |
| Current enrollment in weight loss program | Inability to attribute weight loss to sleep therapy. |
Psychological disorders:
|
High likelihood of the inability to comply with study protocol and increase sleep. |
| Substance abuse/excessive caffeine use | High likelihood of the inability to comply with study protocol and increase sleep, and endocrine effects. |
| Planned change in residence | Inability to attend monthly visits. |
| Family | Partner/child/pets that would make compliance difficult. |
| Pregnancy/lactation | Effects on the endocrine system. |
| Menopause | Effects on the endocrine system associated with sleep disturbances and changes in mood. |
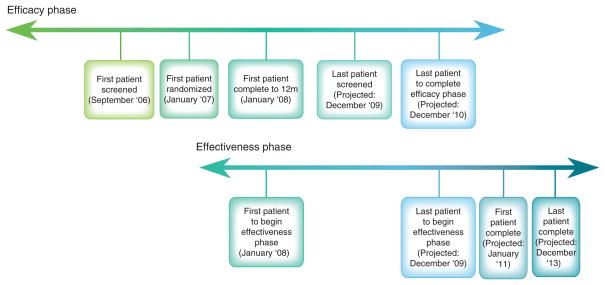
Figure 2.
Study timeline
A 2–3 week run-in period permits assessment of compliance with study requirements (completing questionnaires, wearing devices) and the ability to increase sleep duration (Intervention Group) or maintain current sleep habits (Comparison Group). Table 2 summarizes the inclusion/exclusion criteria.
Participants in both the Intervention and Comparison Groups are encouraged to follow the current standard of care for both exercise (60–90 min of activity daily) and diet. Drastic changes in daily routine in diet and exercise above current standards and/or previous existing individual habits are to be avoided. Participants are monetarily compensated for their time and compliance throughout the course of the study.
Sleep intervention
Table 3 summarizes the non-pharmacological, educational and behaviorally based intervention designed to promote the extension of sleep duration. Each participant in the Intervention and Comparison Groups is given a personalized sleep plan based on an in-depth evaluation of current sleep habits at the beginning of the Efficacy Phase or Effectiveness Phase, respectively. The comprehensive sleep assessment includes several standardized sleep questionnaires. Individualized interventions designed to increase nightly sleep are prescribed, and participants are coached on how to gradually integrate these changes into their daily routine.
Table 3.
Behavioral modifications for extension of sleep duration. The recommendations are individualized to meet each participant’s needs and life-style
| Intervention | Explanation |
|---|---|
| Go to bed at a consistent time each night and get up at a similar time each morning. | Frequent changes in day–night routine promote insomnia. |
| Get exposed to sunlight early in the morning (bright natural or artificial light). Also minimize exposure to bright light later in the day. | Exposure to sunlight early in the day helps the body’s internal clock reset itself each day. In the evening, minimizing exposure assists the ‘body clock’ in getting ready for sleep. |
| No strenuous physical exercise within 2 h of going to bed. | Strenuous exercise may stimulate catecholamines and other stress hormones that may interfere with sleep. |
| Soft, comfortable bed linens, cool temperature, block out outside light, quiet environment, reserve bed for sleep, and sex. | Distractions in the bedroom promote insomnia. |
| Don’t consume caffeine within 6 h of bed time. | It takes caffeine 8 h to be fully metabolized. |
| Don’t consume alcohol in excess within 6 h. of sleep. | Although alcohol may facilitate sleep initiation, it may disrupt sleep in the second half of the night. |
| Avoid heavy, spicy, or sugary food within 4–6 h of bedtime. Do not eat dinner after 8 p.m. | Digestive process may disrupt sleep. |
| A little snack at bedtime is okay. | Milk, bananas, or other foods high in tryptophan may facilitate sleep. |
| Make restful activities a part of bedtime ritual: warm bath, deep breathing, yoga, guided imagery, or reading. | These activities may help relieve anxiety and reduce muscle tension. In general, activities before bedtime should be relaxing. |
| TV in another room or off it before bedtime. Listen to the radio instead. | Watching TV in bed discourages sleep. |
| Set aside time in the evening for relaxation and thinking. Avoid taking the troubles of the day to bed. If you have a lot of things on your mind, make a written list of them and what you plan to do. | Excessive worries and rumination are associated with insomnia. |
Efficacy phase
The study design is depicted in Figure 1. At the time of randomization baseline measurements of resting energy expenditure, glucose tolerance and insulin resistance (oral glucose tolerance test), weight, BMI, quality of life, and vigilance are taken. These measurements are repeated at the end of the Efficacy Phase, the 12-month visit.
Figure 1.
The different phases of the study are outlined in the figure, as they were originally planned. Of note, study duration has been extended to a total of 48 months (12 month, Efficacy, 36 month, Effectiveness). Participants are stratified by age and metabolic syndrome and then randomized in a 2:1 ratio to the Intervention or the Comparison Group, respectively
At the interim visits (1-, 2-, 4-, 6-, 8-, and 10-month visits), study progress is monitored in both groups through sleep evaluations, blood draws, and measurement of vital signs, weight, waist and neck circumferences. The Intervention Group is encouraged to continue individual sleep plans and compliance is evaluated and addressed. Adherence to behavioral recommendations to increase sleep duration is monitored through Actigraphy technology, self-reported sleep diaries, and vigilance testing (see ‘Monitoring Progress and Participant Compliance’ below). Although the Comparison Group is given less coaching, an effort is made to ensure that all participants are given an equal amount of time with the study physician, nurse, and clinicians.
The 12-month Efficacy Phase was designed to facilitate lifestyle change as well as monitor the effects of incremental sleep increase and capture substantial changes that have occurred over the course of a year of altered sleep habits.
Effectiveness phase
All participants who complete the Efficacy Phase are eligible to participate in the Effectiveness Phase. The informed consent for this phase is obtained at the beginning of the Efficacy Phase, immediately after each participant has been randomized to the Intervention or to the Comparison Group. After they have completed the Efficacy Phase, participants from the Comparison Group are invited to increase sleep and are given the same coaching that their counterparts in the Intervention Group received 12 months earlier. The overall effectiveness of sleep education in a ‘real life’ setting is monitored, including the degree to which participants maintain or improve sleep habits. In addition, we will study the difference in the ability to increase sleep between the two groups, and whether regular coaching is a necessity for such a lifestyle change.
During the Effectiveness Phase, participants come to the clinic every 6 months for 3 years, where the same metabolic and vital measurements are assessed along with progress towards extension of sleep duration. Participants also are required to wear activity monitors, record weight and blood pressure, and fill out questionnaires online 1 week/month.
Monitoring progress and participant compliance
The following data elements are closely monitored throughout the course of the study: sleep time (by motion sensors and sleep questionnaires) (Table 4), estimated energy expenditure per day, weight, and hormone/lipid measurements associated with metabolic syndrome, vigilance, and mood. The study employs omni-directional accelerometers, a reliable, practical technology, allowing collection of data in an out-patient, real-life setting [24,25]. The Actiwatch (Mini Mitter Co., Inc., Bend, OR), a small motion-sensing device worn on the wrist, records sleep duration activity over a 14-day period every month. Another device, called Actical, (Mini Mitter Co., Inc., Bend, OR), is worn on the participant’s hip for the same 14 days a month. This device also utilizes motion sensor technology, capturing the participant’s energy expenditure per day. Actical data reveals relative intensity and length of energy expenditure. Together with the participant’s height, weight, age, sex, and basal energy expenditure, this technology enables an acceptable estimate of total calories burned per day.
Table 4.
End-points and parameters assessed
| End-points | Measurement schedule | Monitoring tools |
|---|---|---|
| Body composition | Baseline and annually | CT, DXA, bioelectric impedance |
| Resting energy expenditure | Baseline and annually | Indirect calorimetry |
| Food intake | Performed at randomization, months 4, 12, and annually thereafter | 3-day food record, 24-hour food recall, food frequency questionnaire, nutrient total report. |
| Each visit | Alcohol and caffeine consumption questionnaire, appetite rating. | |
| Daily energy expenditure | Performed at each visit during Year 1 then every 3 months | Actical |
| Performed at each visit | Arizona Activity Frequency Questionnaire | |
| Various sleep-related assessments | Performed at each visit during Year 1 and then every 3 months | Actiwatch, sleep diaries |
| Performed at each visit | Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, Stanford Sleepiness Scale (performed in morning after psychomotor vigilance task), pre-sleep arousal scale. | |
| Psychological assessment | Performed at baseline and every 12 months | Hamilton Anxiety Scale, Hamilton Depression Scale, neuropsychological evaluations, psychomotor vigilance task (performed in morning), structured clinical interview for DSM-IV (SCID). |
| Insulin resistance | Performed at baseline and every 12 months | Oral glucose tolerance test performed in the morning. |
| Full endocrine assessment | Performed at randomization and then annually | Morning fasting blood drawn for: leptin, ghrelin, adiponectin, adrenocorticotropic hormone, catecholamines, cortisol, creatinine, estradiol, follicle stimulating hormone, growth hormone, insulin-like growth factor-1, luteinizing hormone, progesterone, testosterone, TSH. |
| Partial endocrine assessment | Performed at each visit | Morning fasting blood drawn for leptin, ghrelin, adiponectin, estradiol, follicle stimulating hormone, luteinizing hormone, progesterone. |
| Metabolic assessment | Performed at each visit | Blood pressure, pulse, waist circumference, morning fasting insulin and glucose, hemoglobin A1C, complete lipid profile. |
| Personality assessment | Performed at randomization and then annually | Cook–Medley Hostility Scale, NEO Personality Inventory. |
| Quality of life assessment | Performed at randomization and then annually | Brief pain inventory, SF-36 questionnaire, sickness impact profile, visual analog scale for sexual satisfaction. |
The Cook–Medley Hostility Scale is a scale derived by the Minnesota Multiphasic Personality Inventory [27]. Elevated hostility scores on this scale have been associated with cardiovascular events, alcohol intake, smoking greater caloric intake, and sleep apnea [28]. The NEO Personality Inventory Profile, a 240-item, provides a picture of a person’s style. It is divided into five major domains of personality (Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness) as well as six subordinate dimensions called facets that define each domain [29]. Some of these personality traits such as high neuroticism and low conscientiousness have been associated with underweight and obesity, respectively [30].
The sleep assessments are performed in the morning.
Participant vigilance is monitored through the Psychomotor Vigilance Test, a widely accepted technology in the field of sleep research. This procedure, which is administered in the morning, involves the measurement of participant reaction time through a series of signal-response trials over a 10-min period. Completed at each visit, this test provides a measure of participant alertness, corroborating average sleep measurements.
Sample size, recruitment, and progress
The recruitment effort began in September 2006. Figure 2 summarizes the study timelines. The age range was chosen because the benefits of extension of sleep duration are better established in people less than 50 years old. Although studies are in progress to develop non-invasive methods of measuring biomarkers (such as amylase in the saliva [26] that may give an indication of sleep needs on an individual basis), currently it is not possible to determine how much a participant is ‘sleep-deprived’ before the adverse outcomes are evident. Participants whose lifestyle is determined to undermine study compliance or who have medical conditions that may confound results are excluded.
One hundred and fifty participants are being recruited in the Baltimore-Washington metropolitan area. Newspaper and radio ads, flyers located across the NIH campus, and postings on clinical-trials.gov are the major modes of recruitment. In addition, advertising in local university newspapers, TV stations, and radios are used to reach the younger demographic population that has so far been more difficult to recruit. Since January 2007 we have randomized 109 participants; 76% are women, 62% are minorities, with an average age of 40.8 years and BMI of 38.5 kg/m2. Sleep duration at randomization was 40.3 h/week. As of January 2010, 28 Intervention and 22 Comparison participants had completed the Efficacy Phase. Most of the participants enrolled had never participated in a research study.
Sleep apnea is assessed when participants are admitted for randomization. Different from what originally planned (Figure 1), for feasibility reasons so far we have not conducted polysomnography sleep studies. We have screened participants for sleep apnea by the use of an ambulatory device called ARES Unicorder (Advanced Brain Monitoring, Inc., Carlsbad, CA) that is considered a valid and low-cost alternative to traditional polysomnography [31].
Because of a relatively good retention rate of ~70% over time, we have decided to extend the Effectiveness Phase from the original 6 months to a total of 36 months. During this 36-month period participants come for a brief visit every 6 months. During these visits the same measurements collected during the Efficacy Phase are repeated. Imaging studies for body composition, CT and DXA, are repeated every 12 months.
Bioinformatics
The secure database used in this study (created by Esprit Health), provides real-time study data. Management capabilities include a calendar of visits, as well as participants’ payment and contact information. ‘Esphere Online’ serves as a portal for engagement of participants in the study. In addition to serving as a resource for contact information and study procedures, participants also fill out questionnaires and self-reported sleep diaries and enter home measurement data online. Patients are encouraged to enter sleep information in a timely manner; an electronic time stamp provides documentation useful for monitoring. Online entry is believed to increase accuracy of self-reported sleep; use of paper sleep diaries introduces the possibility of retrospective completion. We will test whether maintaining regular contact with study participants improves and facilitates compliance with study requirements.
Design issues and rationale for decisions
Stratification
Participants are randomized according to two factors (age above or below 35, and the presence or absence of metabolic syndrome). Sleep curtailment is known to have a different impact with age, and metabolic syndrome tends to be quite prevalent in obese subjects. Since it was deemed not feasible to stratify for the number of hours slept at study entry, the analysis will be adjusted for this variable.
Allocation ratio
In order to gather more information on the effects of extension of sleep duration, we have specified a 2:1 allocation ratio between the Intervention and Comparison Groups. This ratio customarily is used in proof-of-concept studies to allow collection of more information from the participants in the Intervention Group and to contain costs [27].
Sample size estimation
At the beginning of the study a sample size estimate for unequal randomization was performed. We contemplated two possible scenarios; they were both based on the assumption that subjects in the Comparison Group would experience an increase in BMI over 12 months similar to what was reported by Hasler et al. (i.e., 0.38 kg/m2 of BMI on average with a standard deviation of 0.35). This was a conservative assumption, as the sample studied by Hasler [14] was comprised of mostly nonobese subjects, and the annualized rate of BMI increase would likely to be higher in the current sample of obese subjects. In the first scenario, in which the Intervention Group would experience no increase in BMI over 12 months, only 15 subjects/group were needed. In the second scenario in which annual increase in BMI observed in the Comparison Group would be reduced in the Intervention Group by one half (i.e., 0.19 ± 0.35 kg/m2), 54 subjects/group were needed. In both cases, power was 80% and p = 0.05. Of note is that a 0.19 kg/m2 increase in BMI/year is roughly the equivalent to saving approximately one pound (or 0.45 kg)/yr. Over a 10-year time period this would translate into a ‘savings’ of about 10 pounds (or ~4.5 kg), making this sleep extension intervention still clinically meaningful.
Discussion
Achievements and problems
As of January 2010, we have been able to recruit more than two-thirds of the planned number of participants, including minorities. It has been challenging, however, to recruit people under the age of 25 years. As an outpatient study, a major limitation include monitoring of participant compliance. Although we are able to monitor energy expenditure and sleep length objectively, assessment of energy intake is notoriously less reliable.
Although at the beginning there was skepticism about the possibility of extending sleep duration long-term, we have been successful in increasing sleep duration in the Intervention Group on average by 30 min. In addition, in spite of the original desire of most participants to participate in the Intervention Group, we have succeeded in retaining Comparison Group participants as well. As opposed to pharmacological studies utilizing a placebo, in behavioral studies participants must be told to which group they have been randomized. This introduces the possibility of withdrawal or noncompliance due to dissatisfaction of the group to which the participant was randomized (typically the Comparison Group). The mode of monitoring sleep is indirect; motion sensors (Actiwatch and Actical) determine the likelihood of wake and sleep at any given time during monitoring. Although this technology is remarkably accurate, and we use participant reported sleep to clarify unusual periods of inactivity, this approach cannot be equated to EEG measurements.
Alternative designs considered
Several alternative designs were considered before the final study design was developed. We considered recommending two different ‘doses’ of sleep extension for the Intervention Group, 45 or 90 min. This design eventually was judged to be impractical. We will assess the effect of different amount of sleep on body weight in a secondary analysis.
Because of the dramatic increase in childhood obesity, inclusion of a pediatric population was also considered. Given the challenges encountered while designing this trial, we initially decided to start with an adult population. A future study of extension of sleep duration in children should involve the family and schools as well.
Shorter study duration of about 6 months was also considered. Eventually, a longer duration was chosen in order to allow sufficient time for the participants to make lifestyle changes and to attempt to produce robust results. In addition, the 12-month design accounts for seasonal changes that may have been misinterpreted in a shorter study. A pilot study also was proposed to allow better determination of the ability of participants to comply with the protocol. In the interest of time, the idea of the pilot study eventually was abandoned.
Lessons learned so far
Most of the enrollees are African-Americans (63/109) and/or women (83/109). We have also have been able to recruit moderately to severely obese participants with an average BMI of 38.5 kg/m2. It has been very challenging, however, to recruit participants in the college age range: our youngest participant is 23 years old, and the average age in the study is 40.8 years. In terms of sleep duration the average sleep time at the beginning of the study was less than 6 h, 340 min/night, with a fair number of participants sleeping 5 h or less per night. At the beginning of the study treatment arm allocation was implemented erroneously in reverse. Therefore, initially twice as many participants as originally intended were recruited to the Comparison Group. Since neither study personnel nor participants are blinded to group allocation, this error became soon evident and the study statistician changed the randomization code. The unintended imbalance is being corrected; we have 64 participants randomized to the Intervention Group and 45 to the Comparison Group. As we gained more expertise and confidence, we have widened some of our inclusion criteria in terms of age and BMI. The original BMI upper limit of 40 kg/m2 was eventually raised to 50 and then 55 kg/m2 once it became evident that inevitable co-morbidities associated with obesity did not affect the ability to participate. In an attempt to recruit younger participants, we also have decreased the lower limit of the age range from 21 years to 18 years.
In the Intervention Group, the sleep intervention has been well tolerated; participants who have extended sleep anecdotally report better mood and energy, improved ability to focus, less sleepiness during the day, decreased caffeine intake, more willingness to exercise, and less craving for sweets or salty snacks, especially in the evening. However, few of the participants in the Intervention Group have complained of frequent headaches when sleeping longer. No participant has dropped out from this group due to side effects of extension of sleep duration. In general, participants in the Comparison Group have been able to maintain the baseline sleep duration, while complaining of fatigue, sleepiness, and other consequences of chronic sleep deprivation.
An interim analysis of the Pittsburgh Sleep Quality Index has revealed global scores of 8.37 ± 2.9 N = 107 (mean ± SD) among study participants at randomization (scores as low as 3 are usually observed in healthy controls). Based on the participants who have reached the 12-month visit, the global scores have substantially improved in both groups, though more so in the Intervention Group (Intervention Group: 5.9 ± 2.7 N = 25; Comparison Group 7.3 ± 1.9; N = 21; p = 0.02).
Since the time of randomization 35 participants have withdrawn: 23 from the Intervention and 12 from the Comparison Group. Withdrawal occurred relatively early in the study: 26 of the 35 participants withdrew between randomization and the Month 4 Visit. Participants have withdrawn for the following reasons: unable to be contacted (10), due to travel time and commitment (9), withdrawn consent (7), other reasons (6), and lack of interest (3).
Conclusions/Implications
We currently are testing the hypothesis that increasing sleep duration in sleep-deprived obese participants influences body weight. To this aim, we are conducting a randomized, long-term, controlled, behavioral study of extension of sleep duration. These kinds of studies are challenging to implement and, to be successful, require a multi-disciplinary approach and collaboration with sleep experts, endocrinologists, behavioral scientists, and biostatisticians. The design of controlled randomized studies of behavioral interventions raises specific issues and sets it apart from the classic placebo-controlled trials.
Acknowledgments
This study is supported by the Intramural Program of the National Institute of Diabetes, Digestive, and Kidney Diseases, and the Warren Magnuson, National Institutes of Health Clinical Center. This study is conducted under the NIDDK protocol 06-DK-0036 and is listed in ClinicalTrials.gov (identifier: NCT00261898). Statistical expertise and a central sample-handling and assays facility are provided by the National Institute of Diabetes, Digestive and Kidney Disease Intramural Obesity Initiative of the National Institutes of Health Clinical Center.
We would like to thank the following colleagues for their scientific advice and critical suggestions in the development and conduct of the study protocol: Karim Calis, Janet Gershengorn, Gregor Hasler, Emmanuel Mignot, Susan Redline, Terry Phillips, Duncan Wallace, Bob Wesley, and Elizabeth Wright. We also would like to thank the members of the study team: Peter Bailey, Meredith Coyle, Patrick Michaels, Svetlana Primma, Rebecca Romero, Megan Sabo, Tanner Slayden, Sara Torvik, and Elizabeth Widen. The bioinformatics support of Frank Pierce and Don Dorfman (Esprit Health) is gratefully acknowledged. Finally we are grateful to all of our enthusiastic study participants.
Footnotes
For the Sleep Extension Study Group.
References
- 1.Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 2.Webb WB. Are we chronically sleep deprived? Bull Psychon Soc. 1975;6:47–8. [Google Scholar]
- 3.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;3:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Cauter E, Spiegel K. Sleep as a mediator of the relationship between socioeconomic status and health: a hypothesis. Ann N Y Acad Sci. 1999;896:254–61. doi: 10.1111/j.1749-6632.1999.tb08120.x. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–39. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 6.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;5:503–12. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 7.Arnedt JT, Owens J, Crouch M, et al. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294:1025–33. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 8.Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;3:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliwise DL, Young TB. The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep. 2007;30:1614–15. doi: 10.1093/sleep/30.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–45. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;11:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 14.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;4:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 15.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;5:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horne J. Too weighty a link between short sleep and obesity? Sleep. 2008;5:595–6. doi: 10.1093/sleep/31.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep. 2005;10:1217–20. doi: 10.1093/sleep/28.10.1217. [DOI] [PubMed] [Google Scholar]
- 18.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 19.Bartels DW, Davidson MH, Gong WC. Type 2 diabetes and cardiovascular disease: reducing the risk. J Manag Care Pharm. 2007;13:S2–15. doi: 10.18553/jmcp.2007.13.s2-a.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart SL, Cardinez CJ, Richardson LC, et al. Surveillance for cancers associated with tobacco use —United States, 1999–2004. MMWR Surveill Summ. 2008;8:1–33. [PubMed] [Google Scholar]
- 21.Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 22.El-Khorazaty MN, Johnson AA, Kiely M, et al. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo DC, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Prev Med. 2008;47:573–82. doi: 10.1016/j.ypmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 24.De Vries SI, Van Hirtum HW, Bakker I, Hopman-Rock M, Hirasing RA, Van Mechelen W. Validity and reproducibility of motion sensors in youth: asystematic update. Med Sci Sports Exerc. 2009;41:818–27. doi: 10.1249/MSS.0b013e31818e5819. [DOI] [PubMed] [Google Scholar]
- 25.Paul DR, Kramer M, Moshfegh AJ, et al. Comparison of two different physical activity monitors. BMC Med Res Methodol. 2007;7:26. doi: 10.1186/1471-2288-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seugnet L, Boero J, Gottschalk L, et al. Identification of a biomarker for sleep drive in flies and humans. Proc Natl Acad Sci USA. 2006;103:19913–18. doi: 10.1073/pnas.0609463104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook WW, Medley DM. Proposed hostility and phar-isaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–18. [Google Scholar]
- 28.Iribarren C, Sidney S, Bild DE, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary artery risk development in young adults. JAMA. 2000;283:2546–51. doi: 10.1001/jama.283.19.2546. [DOI] [PubMed] [Google Scholar]
- 29.McCrae RR, Yang J, Costa PT, Jr, et al. Personality profiles and the prediction of categorical personality disorders. J Pers. 2001;69:155–74. doi: 10.1111/1467-6494.00140. [DOI] [PubMed] [Google Scholar]
- 30.Terracciano A, Löckenhoff CE, Crum RM, et al. Five-Factor Model personality profiles of drug users. BMC Psychiatry. 2008;8:22. doi: 10.1186/1471-244X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westbrook PR, Levendowski DJ, Cvetinovic M, et al. Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest. 2005;128:2166–75. doi: 10.1378/chest.128.4.2166. [DOI] [PubMed] [Google Scholar]