Abstract
Depression is prevalent and undertreated in patients receiving hospice care. Standard antidepressants do not work rapidly or often enough to benefit most of these patients. Here, two cases are reported in which a single oral dose of ketamine provided rapid and moderately sustained symptom relief for both depression and anxiety. In addition, no adverse effects were noted. Further investigation with randomized, controlled clinical trials is necessary to firmly establish the effectiveness of oral ketamine for the treatment of depression and anxiety in patients receiving hospice care. Ketamine may be a promising safe, effective, and cost-effective rapid treatment for depression and anxiety in this population.
Introduction
Psychiatric symptoms are prevalent in patients receiving hospice care. Up to 42% of hospice patients have depression and up to 70% have anxiety.1–3 Depression and anxiety are frequently undertreated in these patients, and untreated psychiatric symptoms are associated with significant morbidity and mortality, even in this population of patients.1,3–7
Current pharmacologic treatments for depression in this population consist of the usual armamentarium of more than 24 antidepressants with at least seven different mechanisms of action.8 Many of these are also indicated for anxiety, as are other medications that have significant associated risks.9 An appropriate standard antidepressant trial is considered 4–6 weeks, and multiple trials may be necessary.10,11 Since the average time patients receive hospice care in the United States is less than 8 weeks and the median is less than 4 weeks,12 current standard antidepressant trials oftentimes do not adequately address the needs of hospice patients suffering from depression.
Methylphenidate, a stimulant with a significantly shorter onset than existing antidepressants, has been studied for depression in cancer (and other medical illnesses) in nonrandomized studies with some success.13,14 There is also a growing body of literature supporting the rapid treatment of depressive symptoms with intravenous (IV) ketamine.15–21 A single case using IV ketamine to treat depression in a patient with advanced cancer has been reported.22 No studies to date have examined ketamine's role in treating depression in the hospice population. To our knowledge, no investigations of depression treatment for any population have been carried out with oral ketamine, nor have any investigations with ketamine assessed symptoms of anxiety.
Overall, ketamine has many properties that make it a good candidate for treating depression and anxiety in the hospice population. It is inexpensive and easy to administer. It also has a rapid onset of action and minimal side effects when used at subanesthetic doses. Effectiveness and safety may theoretically improve further with oral administration. Ketamine's effects on depression have been observed to be relatively long-lasting, although not in every case.22 Significant literature supports its safe use in hospice patients for other symptoms, including pain.23–32
The cases presented here involved subjects in an on-going open label study that is approved by both the Institute for Palliative Medicine Institutional Review Board and the University of California, San Diego Human Research Protection Program.
Case 1
S.B. was a 64-year-old divorced caucasian woman with hospice diagnoses of both respiratory failure and chronic obstructive pulmonary disease. She was oxygen dependent and her prognosis was weeks to months. Over several months prior to psychiatric consultation, she had developed severe depressive symptoms, which included low mood, low energy, hypersomnia, decreased appetite with unintentional weight loss, hopelessness, and excessive feelings of guilt, especially regarding feeling like a burden on her roommate, who was also her close friend and primary caregiver. She was preoccupied with thoughts of wanting to die. She did not plan or intend to end her life, stating “I'm too chicken to die.” Prior to the onset of her depressive symptoms, she greatly enjoyed reading books and socializing with friends; however, she had stopped these activities for at least 2 months. A large pile of bills was noted on the coffee table, which she had been avoiding for weeks.
Additionally, S.B. had severe anxiety symptoms related to shortness of breath. She reported one to three panic attacks per day. She was noticeably anxious during the initial interview, shifting in her seat, picking at her lips, and playing with her toes. She also reported feeling irritable, which was confirmed by her caregiver/roommate. She would shout and appear extremely irritated when the phone rang or the dog would bark. She also exhibited a somatic focus, not only on pain, but her perception of pain and shortness of breath. There was no evidence of cognitive impairment.
Scheduled medications included duloxetine 60 mg daily, morphine sulfate extended release 90 mg daily, prednisone 10 mg daily, aspirin, albuterol sulfate, tiotropium bromide, fluticasone-salmeterol, risedronate sodium, docusate, and a lidoderm patch. As needed medications available (but not necessarily used) included morphine 10 mg hourly, oxycodone/acetaminophen 5/325 mg every 4 hours, diazepam 5 mg every 6 hours, trazodone 50 mg at night, naproxen 220 mg daily, tizanidine up to 4 mg every 8 hours, oxygen, and lactulose. No changes in psychotropic medications (antidepressants, anxiolytics, and opiates) had occurred for at least 8 weeks prior to ketamine dosing. During the study, no scheduled or as needed medication changes occurred until day 14, when her prednisone was increased for worsening shortness of breath.
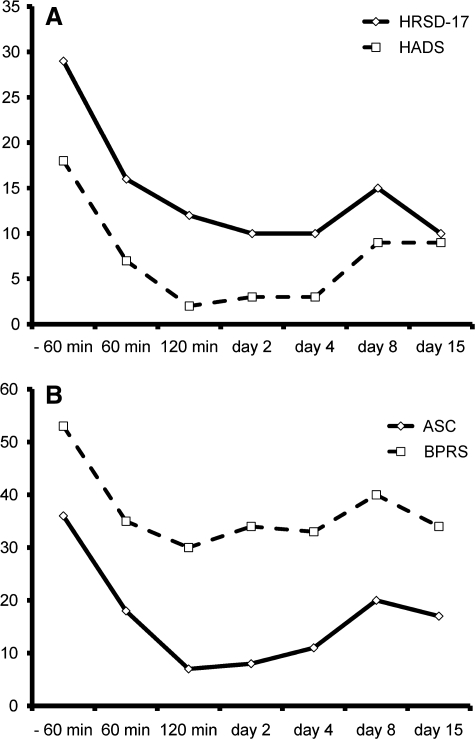
After informed consent and completion of validated scales for evaluating psychiatric symptoms and adverse events (Table 1), S.B. was given a dose of 27.5 mg of ketamine (0.5 mg/kg) orally at home. Subsequently, her mood was greatly improved as indicated by a 45% drop in her 17-item Hamilton Rating Scale for Depression (HRSD17) score just 60 minutes after the dose. By day 15, there was a 66% improvement. The depression subscale of the Hospital Anxiety and Depression Scale (HADS) showed a similar trend, with a 66% reduction in score just 120 minutes after the dose and a 50% reduction at day 15. Surprisingly, the anxiety subscale of the HADS also dramatically improved, with an 83% reduction in score just 120 minutes after the dose and a 50% reduction at day 15. Either improvements or no changes were also found on scales of adverse effects and cognitive status, including the Brief Psychiatric Rating Scale (BPRS), the Young Mania Rating Scale (YMRS), and the Mini-Mental State Exam (MMSE). Fewer somatic symptoms were recorded after dosing with ketamine, and pain scores improved (Table 1, Fig. 1).
Table 1.
Assessment Results for Case 1
| |
Time points relative to ketamine dosing |
|||||||
|---|---|---|---|---|---|---|---|---|
| Assessments | Screen | − 60 min | 60 min | 120 min | Day 2 | Day 4 | Day 8 | Day 15 |
| HRSD17 | 32 | 29 | 16 | 12 | 10 | 10 | 15 | 10 |
| HADS | 25 | 18 | 7 | 2 | 3 | 3 | 9 | 9 |
| HADS-D | 9 | 6 | 5 | 2 | 3 | 1 | 1 | 3 |
| HADS-A | 16 | 12 | 2 | 0 | 0 | 2 | 8 | 6 |
| FIBSER | ∼ | 0 | 0 | 0 | 0 | 7 | 0 | 0 |
| ASC | ∼ | 36 | 18 | 7 | 8 | 11 | 20 | 17 |
| BPRS | ∼ | 53 | 35 | 30 | 34 | 33 | 40 | 34 |
| YMRS | ∼ | 2 | 3 | 1 | 0 | 0 | 0 | 1 |
| MMSE | ∼ | 29 | 30 | 30 | 30 | 30 | 30 | 30 |
| Pain VAS | ∼ | 26 | 17 | 21 | 19 | 6 | 32 | 25 |
HRSD17, hamilton rating scale for depression − 17 item; HADS, hospital anxiety and depression scale; FIBSER, frequency, intensity, and burden of side effects; ASC, adverse symptom checklist; BPRS, brief psychiatric rating scale; YMRS, young mania rating scale; MMSE, mini-mental state exam; Pain VAS, pain visual analogue scale.
FIG. 1.
Rating Scales for Case 1: (A) 17-item Hamilton Rating Scale for Depression and Hospital Anxiety and Depression Scale scores indicating a decrease in depressive and anxiety symptoms and (B) Adverse Symptom Checklist and Brief Psychiatric Rating Scale scores indicating an improvement in what would be considered unwanted symptoms or adverse effects.
Her clinical picture matched her improvement on the scales. By 120 minutes after ketamine dosing, she no longer had suicidal thoughts and noted a significant improvement in her depression and anxiety. S.B. expressed hope for the future and no longer felt irritable. She became much more engaging, desiring to talk about television shows and soap operas. Over the ensuing week, her appetite had improved dramatically. Her affect remained euthymic and she stated “I feel relaxed.” The overt signs of anxiety and irritability she displayed prior to ketamine dosing were absent. She had paid and filed away her entire pile of bills. Her caregiver reported that S.B. was much more alert and no longer “nodded off” throughout the day. Her pain and shortness of breath had also improved. S.B. did report trouble sitting still, but explained “I want to get out and do things now.” She had become less preoccupied with feeling like a burden to her roommate. Additionally, she had begun to read books again, and her caregiver noted that this was a “striking change.” Lastly, she started to call her friends and initiated planning social gatherings. Her mood and anxiety showed further improvement over the subsequent week. Her caregiver commented to the research team “thank you for giving me my friend back.”
S.B. was followed clinically for several months. After a month, significant depressive symptoms returned, but not as severe as prior to ketamine dosing. Both S.B. and her caregiver requested a repeat dose. Her clinical picture at this time was complicated by delirium likely secondary to pain medication adjustments; however, she was given a repeat dose of ketamine to which she did not respond. Her mood and anxiety were eventually stabilized with bupropion XL, gabapentin, and a stable pain medication regimen.
Case 2
K.H. was a 70-year-old married caucasian man with a hospice diagnosis of prostate cancer that was metastatic to the bone, lung, and liver. He had been bed-bound for 8 months and had a prognosis of days to weeks. He had no known previous psychiatric history. K.H. had severe depressive symptoms for 3 months. These included depressed mood, significantly decreased energy, lack of appetite with unintentional weight loss, poor sleep with early morning awakening, and ruminative thoughts of wanting to be dead. He denied a suicidal plan or intent, explaining “even if I wanted to, I could not do anything in this state.” He described significant anhedonia, which contrasted with his prior zest for life. While in a bed-bound state, prior to the onset of his depressive symptoms, he savored visits with friends and family, watching and discussing movies with his wife, reading the newspaper, and eating take-out from his favorite restaurants. However, over the preceding 3 months, he no longer wanted friends to visit, stopped watching movies and reading, and had to force himself to eat. Furthermore, he experienced excessive guilt about feeling like “a burden” to his wife, who was his primary caregiver. His guilt could not be assuaged by his wife's insistence that it was “an honor” to care for him and “not at all a burden.”
Additionally, K.H. reported anxiety symptoms. Over the preceding month, he developed one to two panic attacks per day. Also, he described incessant worries, especially when attempting to fall asleep and upon awakening. He did not exhibit any cognitive impairments.
His scheduled medications included methadone 10 mg four times daily, dexamethasone 4 mg daily, senna, and furosemide. As needed medications included trazodone 50 mg at bedtime and morphine sulfate 20 mg every hour. He consistently took the as needed morphine sulfate three times daily and the trazodone daily. During the study, no scheduled or as needed medication changes occurred until day 4, when ciprofloxacin was added for the treatment of a urinary tract infection.
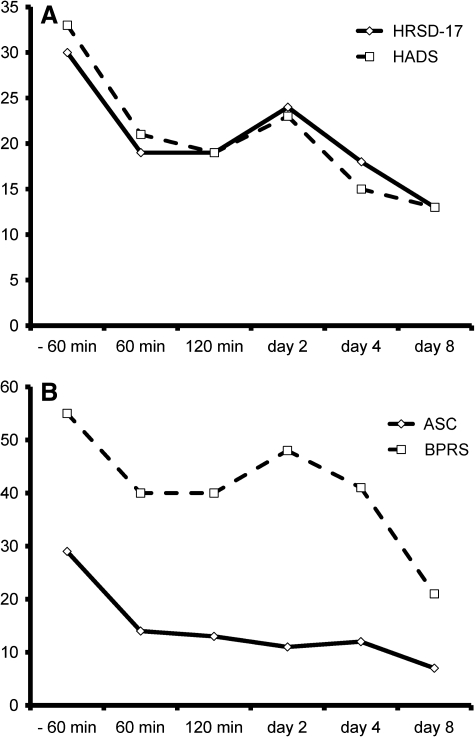
After informed consent and completion of validated scales for evaluating psychiatric symptoms and adverse events (Table 2), K.H., while at home, was given a dose of 32.5 mg of ketamine (0.5 mg/kg) orally. Subsequently, his HRSD17 dropped by 37% 60 minutes after ketamine dosing. By day 8, there was a 57% improvement. The depression subscale of the HADS had a 15% reduction in score at 60 minutes after ketamine dosing and a 45% reduction by day 8. The anxiety subscale of the HADS showed a more striking change with a 69% reduction in score just 60 minutes after the dose and an 85% reduction at day 8. Similarly to the first case, either improvements or essentially no changes were also found on scales of adverse effects or cognitive status. Somatic symptoms decreased and pain scores improved (Table 2, Fig. 2).
Table 2.
Assessment Results for Case 2
| |
Time points relative to ketamine dosing |
||||||
|---|---|---|---|---|---|---|---|
| Assessments | Screen | − 60 min | 60 min | 120 min | Day 2 | Day 4 | Day 8 |
| HRSD17 | 28 | 30 | 19 | 19 | 24 | 18 | 13 |
| HADS | ∼ | 33 | 21 | 19 | 23 | 15 | 13 |
| HADS-D | ∼ | 20 | 17 | 18 | 17 | 13 | 11 |
| HADS-A | ∼ | 13 | 4 | 1 | 6 | 2 | 2 |
| FIBSER | ∼ | 0 | 8 | 0 | 0 | 0 | 0 |
| ASC | ∼ | 29 | 14 | 13 | 11 | 12 | 7 |
| BPRS | ∼ | 55 | 40 | 40 | 48 | 41 | 21 |
| YMRS | ∼ | 1 | 0 | 0 | 0 | 0 | 0 |
| MMSE | ∼ | 27 | 28 | 26 | 27 | 25 | 29 |
| Pain VAS | ∼ | 17 | 8 | 8 | 8 | 3 | 0 |
HRSD17, hamilton rating scale for depression −17 item; HADS, hospital anxiety and depression scale; FIBSER, frequency, intensity, and burden of side effects; ASC, adverse symptom checklist; BPRS, brief psychiatric rating scale; YMRS, young mania rating scale; MMSE, mini-mental state exam; Pain VAS, Pain visual analogue scale.
FIG. 2.
Rating Scales for Case 2: (A) 17-item Hamilton Rating Scale for Depression and Hospital Anxiety and Depression Scale scores indicating a decrease in depressive and anxiety symptoms and (B) Adverse Symptom Checklist and Brief Psychiatric Rating Scale scores indicating an improvement in what would be considered unwanted symptoms or adverse effects.
Similarly to the first case, K.H.'s clinical picture matched the improvement seen with his scales. Within 60 minutes of dosing, he reported an improvement in his anxiety and pain, and his wife observed that he looked “more calm and peaceful.” However, he did not notice any change in his depressive symptoms initially. Per his wife, by day 3, he started to request his favorite foods, and his humor was noted to improve. On day 4, his wife observed “a big difference” explaining he “was more chipper.” He watched an entire movie “without dozing” for the first time in months. His mood continued to dramatically improve over the following week. He began to watch, enjoy, and discuss several movies a day with his wife. His appetite increased and he continued to request his favorite foods. Additionally, he began to have friends visit again and savored their time together. Around day 13, his physical health worsened to the point that he could no longer participate with assessments. His wife articulated that his focus on death qualitatively shifted from wanting to die to “accepting death.” He peacefully died at home within the following 2 weeks.
Discussion
To our knowledge, these are the first case reports wherein ketamine was used to treat depression in hospice patients. It is also the first demonstration of the effectiveness of oral ketamine for the treatment of depression and possibly anxiety. As with other published cases and studies, a significant improvement in depressive symptoms occurred almost immediately after ketamine administration and lasted at least a week. A significant decrease in symptoms of anxiety was also found. Unlike other reports, no adverse events as measured by the YMRS, BPRS, or adverse symptom checklists were found. In fact, both the BPRS scores and the number of complaints listed on the adverse symptom checklist decreased in both cases. Also of note, no significant changes in cognition as measured by the MMSE, nor any evidence of delirium were noted. Some of these alternative findings may be due to the oral dosing, which might provide more effective antidepressant and anxiolytic effects and fewer adverse events, possibly from first pass metabolism to norketamine.33 The effectiveness of repeat dosing needs further exploration.
Caution must be exercised in the interpretation of these results. With a lack of blinding, randomization, and a control group, inherent biases and confounding factors exist. Both patients experienced relief of pain after ketamine dosing. It is not clear if the treatment of the pain accounted for an improvement in depressive or anxiety symptoms or if the ketamine directly reduced their depression and anxiety. As only two cases are presented, these data should be generalized with caution.
Ketamine usage is not without risks. When used at anesthetic levels (much higher than those used here), it has significant neuropsychiatric liability, including hallucinations, derealization, and delirium.33 Also, other adverse effects can be seen, including diplopia, dizziness, nystagmus, tachycardia, and hypertension.33 Ketamine also has abuse potential and is known to be abused recreationally.33
Despite its limitations, these case studies demonstrate that a single oral dose of ketamine significantly decreased both the depressive and anxiety symptoms in two patients receiving hospice care. The mechanism of action is not clear. Ketamine's NMDA antagonism may work more rapidly via alternative pathways rather than the intracellular signaling cascade theorized to explain the antidepressant effect of selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors. Further research with ketamine will add to the growing understanding of the role of glutamate in the pathophysiology of depression and anxiety.
More cases and further investigations with randomized, controlled clinical trials are necessary to firmly establish the comparative effectiveness and safety of oral ketamine for the treatment of depression and/or anxiety in patients receiving hospice care. Repeat and/or continuous dosing by the oral route will also need to be explored. Quick-acting, safe, and effective depression and anxiety treatment is needed in this population if a high-quality end-of-life experience is to be achieved. Oral ketamine may provide such an intervention.
Acknowledgments
We wish to acknowledge the support from the faculty and staff of the Institute for Palliative Medicine at San Diego Hospice, including Charles von Gunten, Helen McNeal, Carlene Gibbons, Connie Car, Debra Pledger-Fonte, Paula Brown, and Tracy Johnson. Support was also provided from the pharmacy at San Diego Hospice, specifically from Mary McDermott and Rosene Pirrello. We also thank Joel Dimsdale, Sid Zisook, and Barry Lebowitz for their helpful comments on the manuscript and ongoing mentorship. Special thanks to Steve Koh for help with data collection and J.S. Tanas. This work was supported, in part, by Dilip Jeste and the John A. Hartford Center of Excellence in Geriatric Psychiatry at the University of California, San Diego, and by donations from the generous benefactors of the education and research programs at The Institute for Palliative Medicine at San Diego Hospice.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wilson KG. Chochinov HM. de Faye BJ. Breitbart W. Diagnosis, management of depression in palliative care. In: Chochinov HM, editor; Breitbart W, editor. Handbook of Psychiatry in Palliative Medicine. New York: Oxford University Press; 2000. pp. 25–50. [Google Scholar]
- 2.National Institutes of Health. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. J Natl Cancer Inst Monographs. 2004;2004:9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 3.Wilson KG. Chochinov HM. Skirko MG. Allard P. Chary S. Gagnon PR. Macmillan K. De Luca M. O'Shea F. Kuhl D. Fainsinger RL. Clinch JJ. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33:118–129. doi: 10.1016/j.jpainsymman.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ. Mor V. A survey of psychotropic use in terminal cancer patients. Psychosomatics. 1985;26:745–748, 751. doi: 10.1016/S0033-3182(85)72803-4. [DOI] [PubMed] [Google Scholar]
- 5.McDaniel JS. Brown FW. Cole SA. Assessment of depression, grief reactions in the medically ill. In: Stoudemire A, editor; Fogel BS, editor; Greenberg DB, editor. Psychiatric Care of the Medical Patient. New York: Oxford University Press; 2000. pp. 149–164. [Google Scholar]
- 6.King DA. Heisel MJ. Lyness JM. Assessment, psychological treatment of depression in older adults with terminal or life threatening illness. Clin Psychol Sci Pract. 2005:339–353. [Google Scholar]
- 7.Spiegel D. Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med. 1983;45:333–339. doi: 10.1097/00006842-198308000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Stahl SM. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge; New York: Cambridge University Press; 2008. p. 1117. [Google Scholar]
- 9.Irwin SA. Ferris FD. The opportunity for psychiatry in palliative care. Can J Psychiatry. 2008;53:713–724. doi: 10.1177/070674370805301103. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi MH. Rush AJ. Wisniewski SR. Nierenberg AA. Warden D. Ritz L. Norquist G. Howland RH. Lebowitz B. McGrath PJ. Shores-Wilson K. Biggs MM. Balasubramani GK. Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME. Friedman ES. Biggs MM. Wisniewski SR. Trivedi MH. Luther JF. Fava M. Nierenberg AA. McGrath PJ. Warden D. Niederehe G. Hollon SD. Rush AJ. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 12.NHPCO; Organization NHaPC. Vol. 2007. National Hospice and Palliative Care Organization; 2006. NHPCO's Facts and Figures—2005 Findings. [Google Scholar]
- 13.Sood A. Barton DL. Loprinzi CL. Use of methylphenidate in patients with cancer. Am J Hosp Palliat Care. 2006;23:35–40. doi: 10.1177/104990910602300106. [DOI] [PubMed] [Google Scholar]
- 14.Rozans M. Dreisbach A. Lertora JJL. Kahn MJ. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20:335–339. doi: 10.1200/JCO.2002.20.1.335. [DOI] [PubMed] [Google Scholar]
- 15.Krystal JH. Ketamine and the potential role for rapid-acting antidepressant medications. Swiss Med Wkly. 2007;137:215–216. doi: 10.4414/smw.2007.11932. [DOI] [PubMed] [Google Scholar]
- 16.Kudoh A. Takahira Y. Katagai H. Takazawa T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg. 2002;95:114–118. doi: 10.1097/00000539-200207000-00020. table of contents. [DOI] [PubMed] [Google Scholar]
- 17.Zarate CA., Jr. Singh JB. Carlson PJ. Brutsche NE. Ameli R. Luckenbaugh DA. Charney DS. Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 18.Berman RM. Cappiello A. Anand A. Oren DA. Heninger GR. Charney DS. Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 19.Correll GE. Futter GE. Two case studies of patients with major depressive disorder given low-dose (subanesthetic) ketamine infusions. Pain Med. 2006;7:92–95. doi: 10.1111/j.1526-4637.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 20.Liebrenz M. Borgeat A. Leisinger R. Stohler R. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137:234–236. doi: 10.4414/smw.2007.11852. [DOI] [PubMed] [Google Scholar]
- 21.Liebrenz M. Stohler R. Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J Biol Psychiatry. 2007:1–4. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- 22.Stefanczyk-Sapieha L. Oneschuk D. Demas M. Intravenous ketamine “burst” for refractory depression in a patient with advanced cancer. J Palliat Med. 2008;11:1268–1271. doi: 10.1089/jpm.2008.9828. [DOI] [PubMed] [Google Scholar]
- 23.Mercadante S. Arcuri E. Ferrera P. Villari P. Mangione S. Alternative treatments of breakthrough pain in patients receiving spinal analgesics for cancer pain. J Pain Symptom Manage. 2005;30:485–491. doi: 10.1016/j.jpainsymman.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Carr DB. Goudas LC. Denman WT. Brookoff D. Staats PS. Brennen L. Green G. Albin R. Hamilton D. Rogers MC. Firestone L. Lavin PT. Mermelstein F. Safety and efficacy of intranasal ketamine for the treatment of breakthrough pain in patients with chronic pain: a randomized, double-blind, placebo-controlled, crossover study. Pain. 2004;108:17–27. doi: 10.1016/j.pain.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Kannan TR. Saxena A. Bhatnagar S. Barry A. Oral ketamine as an adjuvant to oral morphine for neuropathic pain in cancer patients. J Pain Symptom Manage. 2002;23:60–65. doi: 10.1016/s0885-3924(01)00373-6. [DOI] [PubMed] [Google Scholar]
- 26.Lauretti GR. Lima IC. Reis MP. Prado WA. Pereira NL. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology. 1999;90:1528–1533. doi: 10.1097/00000542-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Mercadante S. Arcuri E. Tirelli W. Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage. 2000;20:246–252. doi: 10.1016/s0885-3924(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam K. Subramaniam B. Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004;99:482–495. doi: 10.1213/01.ANE.0000118109.12855.07. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Lossignol DA. Obiols-Portis M. Body JJ. Successful use of ketamine for intractable cancer pain. Support Care Cancer. 2005;13:188–193. doi: 10.1007/s00520-004-0684-4. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgibbon EJ. Hall P. Schroder C. Seely J. Viola R. Low dose ketamine as an analgesic adjuvant in difficult pain syndromes: a strategy for conversion from parenteral to oral ketamine. J Pain Symptom Manage. 2002;23:165–170. doi: 10.1016/s0885-3924(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 31.Fine PG. Low-dose ketamine in the management of opioid nonresponsive terminal cancer pain. J Pain Symptom Manage. 1999;17:296–300. [Google Scholar]
- 32.Clark JL. Kalan GE. Effective treatment of severe cancer pain of the head using low-dose ketamine in an opioid-tolerant patient. J Pain Symptom Manage. 1995;10:310–314. doi: 10.1016/0885-3924(95)00010-V. [DOI] [PubMed] [Google Scholar]
- 33.Evers AS. Crowder CM. Balser JR. Brunton LL, editor; Lazo JS, editor; Parker KL, editor. Chapter 13: General Anesthetics. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11e. http://www.accessmedicine.comcontent.aspx?aID=937527 http://www.accessmedicine.comcontent.aspx?aID=937527