Abstract
Spermatogonial stem cells (SSCs) are increasingly studied for potential use in tissue regeneration due to their ability to dedifferentiate into embryonic stem cell–like cells. For their successful therapeutic use, these cells must first be expanded in vitro using an appropriate culture system. We hypothesized that a hydrogel with proper biochemical and biomechanical properties may mimic the composition and structure of the native basement membrane onto which SSCs reside, thus allowing us to control SSC proliferation. This hypothesis was examined in two-dimensional (2D) and three-dimensional (3D) cultures using hydrogels formed from calcium cross-linked alginate molecules conjugated with synthetic oligopeptides containing the Arg-Gly-Asp sequence (RGD peptides). The RGD peptide density (NRGD) in gel matrices was controlled by mixing alginate molecules modified with RGD peptides and unmodified alginate molecules at varied ratios. The mechanical stiffness was controlled with the cross-linking density of gel matrices. Interestingly, the RGD peptide density modulated cell proliferation in both 2D and 3D cultures as well as the number and size of SSC colonies formed in 3D cultures. In contrast, cell proliferation was minimally influenced by mechanical stiffness in 2D cultures. Overall, the results of this study elucidate an important factor regulating SSC proliferation and also present a bioactive hydrogel that can be used as a 3D synthetic basement membrane. In addition, the results of this study will be broadly useful in controlling the proliferation of various stem cells.
Introduction
The use of stem cells in efforts to repair tissues and organs has become increasingly prevalent in the field of tissue engineering. Stem cells reside in specialized microenvironments, or niches, which regulate their self-renewal and overall maintenance.1,2 In order to best utilize the therapeutic potential of stem cells, it is crucial to understand the interactions between them and their native environment.3,4 Significant progress has been made in characterizing the niche microenvironment of a variety of stem cells; more specifically, recent studies have investigated the hematopoietic, epidermal, intestinal, and neural stem cell niches.5,6 Within each niche, supporting cells and associated blood vessels produce the necessary growth factors and stimuli that will modulate stem cell behavior. In addition, the extracellular matrix (ECM) is responsible for cell attachment and can also regulate cellular response to surrounding stimuli.7,8 Therefore, the composition of the ECM and the interactions between cells and their surrounding matrix are crucial in regulating stem cell fate.
Stem cells belonging to epithelial structures generally reside onto a specialized ECM called the basement membrane. Although composed of a small set of specific proteins, such as collagen IV, laminin, entactin, fibronectin, and perlecan, the basement membrane shows an impressive tissue- and site-specific variability in its composition.9 Maintenance of stemness, proliferation, and migration take place in the basal layer of epithelia, in contact with the basement membrane. These biological activities are regulated in part through interactions between individual components of the basement membrane and cell surface receptors called integrins.10,11 Integrins recognize and bind various ECM proteins, including those that contain the Arg-Gly-Asp (RGD) peptide sequence.12,13
Biomaterials have been increasingly used to simulate and even modulate cell adhesion to the ECM in order to regulate signaling pathways that control cell function and tissue morphogenesis. For this purpose, biomaterials have been designed to mimic the biochemical and biomechanical properties of the natural ECM.14–16 Extensive chemical modifications have been used to incorporate synthetic cell adhesion oligopeptides into biomaterials, and the spatial organization of these cell adhesion cues at the nanometer and micrometer scale has been used to modulate the function of progenitor and precursor cells.17–20 In addition, the mechanical stiffness of biomaterials to which cells are adhered has been shown to significantly regulate the phenotypes of precursor cells.21,22
Mammalian spermatogenesis is a tightly regulated and continuous process in which spermatogonial stem cells (SSCs) self-renew and differentiate to ultimately form spermatozoa. The SSC niche is found within the seminiferous epithelium where nursing Sertoli cells provide structural support and growth factors to the stem cells. Other stimuli are provided by the vascular network and interstitial cells between the seminiferous tubules.23 Another important component of the testicular niche is the basement membrane, to which stem cells and Sertoli cells are attached through integrins. In 2004, the group of T. Shinohara demonstrated that mouse SSCs can dedifferentiate in vitro, reverting into cells that acquire an embryonic stem cell (ES) cell phenotype.24 These ES-like cells can be differentiated into cells belonging to the three embryonic germ layers. More recently, the same potential for pluripotency has been demonstrated for adult SSCs.25 Therefore, SSCs could potentially be used to regenerate and engineer specific tissues in an autologous manner. While progress has been made recently in expanding SSCs in vitro using cocktails of growth factors,26,27 growing and maintaining these cells in vitro can be further optimized. Therefore, other niche parameters that are important for SSC proliferation in vitro, specifically the composition of the ECM, need to be characterized.
In the present study, we hypothesize that a bioactive hydrogel designed to mimic some of the biochemical and biomechanical properties of the basement membrane can be used as part of a synthetic niche; by mimicking the in vivo niche, such a synthetic niche can be used to regulate SSC proliferation. This study presents an initial set of parameters that has been known to significantly regulate the phenotypes of precursor cells, namely cell adhesion ligand density and mechanical properties. Calcium cross-linked alginate hydrogels, which do not naturally facilitate protein adsorption and cell adhesion,28,29 were chemically coupled with synthetic oligopeptides containing the Arg-Gly-Asp (RGD) sequence (RGD peptides) in order to control the number of cell adhesion sites for an SSC line. As a model for SSCs, the C18-4 spermatogonial cell line was used.30 This cell line exhibits many of the characteristics of normal SSCs, including the expression of the GFRα-1/Ret membrane receptor complex that binds the growth factor glial cell line–derived neurotrophic factor (GDNF). Upon stimulation with GDNF, the cells increase their rate of proliferation, which is mediated by Src- and Ras-dependent signaling pathways.31,32 The mechanical stiffness of the hydrogels was varied from 8 to 110 kPa, which is within the stiffness range of the natural basement membrane,33,34 to examine the effects of scaffold stiffness on SSC behavior. The hydrogel properties were first optimized through cell culture on 2D hydrogel surfaces; the cell adhesion and proliferation were then subsequently examined in three-dimensional (3D) hydrogel systems.
Materials and Methods
Material chemistry
Sodium alginate (FMC Biopolymer, Philadelphia, PA) was used as high-molecular-weight alginate (Mw ∼250,000 g/mol). Low-molecular-weight alginate (Mw ∼60,000 g/mol) was obtained by irradiating the sodium alginate with a cobalt-60 source for 4 h at a dose of 5.0 Mrad.35 Alginate molecules were covalently coupled with (Gly)4-Arg-Gly-Asp-Ala-Ser-Ser-Lys (G4RGDASSKY) oligopeptides (Peptides International, Louisville, KY) using a carbodiimide chemistry as previously described.29 The mole ratio between RGD peptides and alginate was kept constant at 2:1. All alginate solutions were filtered, lyophilized, and reconstituted with Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) to obtain a 2% w/v solution prior to gelation.
Cell line and cell culture
The C18-4 cell line was established by stably transfecting undifferentiated type A spermatogonia with the Large T antigen gene driven by the ecdysone promoter. These cells exhibit many features of SSCs, including the expression of Oct-4, Vasa, and the receptor complex for GDNF, a crucial stimulus for self-renewal.36,37 Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 100 units/mL penicillin-streptomycin (PS; Invitrogen) at 37°C in a 5% CO2 incubator. The cell culture medium was changed every other day.
2D cell culture on hydrogels
High-molecular-weight alginate was used for 2D cell culture studies. Gels with three different densities of cell adhesion peptides were made by mixing unmodified high-molecular-weight alginate with G4RGDASSKY-conjugated high-molecular-weight alginate in ratios of 9:1, 1:1, and 1:9. Alginate solutions were mixed with a 20% (w/w) calcium sulfate (CaSO4) slurry at a ratio of 40 μL CaSO4 slurry to 1 mL alginate solution and allowed to gel between glass plates with a 1-mm spacer.
For experiments to examine the effects of hydrogel stiffness on cell proliferation, both high-molecular-weight and low-molecular-weight alginates were used. The ratio between CaSO4 slurry and alginate was varied between 20 and 60 μL CaSO4 slurry to 1 mL high-molecular-weight alginate solution and 20–40 μL CaSO4 slurry to 1 mL low-molecular-weight alginate. Gel disks were punched using a 5-mm-diameter puncher and incubated in DMEM for 36 h, changing media every 12 h. The elastic modulus of the hydrogel was calculated from the slope of the compressive stress versus strain curve acquired using an MTS Insight Electromechanical Testing System. Cells were seeded on the disks at a density of 640 cells/mm2 and incubated in complete growth media. Media were changed after 24 h and every 48 h thereafter.
3D cell culture in hydrogels
Low-molecular-weight alginate was used for 3D cell culture studies. Gels without RGD peptides were made from unmodified low-molecular-weight alginate. Gels with RGD peptides were made by mixing unmodified low-molecular-weight alginate with G4RGDASSKY-conjugated low-molecular-weight alginate in ratios of 9:1, 1:1, and 1:9. Cells were encapsulated in alginate gels at a density of 6 × 105 cells/mL by mixing equal volumes of the cell suspension and an alginate solution. The cell–alginate mixture was cross-linked with CaSO4 slurry at a ratio of 40 μL CaSO4 slurry to 1 mL cell–alginate mixture and allowed to gel between glass plates with a 1-mm spacer. Gel disks were punched using a 10-mm-diameter puncher and incubated in complete growth media. Media were changed after 24 h and every 48 h thereafter.
Analysis of stress fibers for 2D cultures
After culture of cells on the hydrogel surfaces for 5 days, cell–hydrogel constructs were fixed overnight in a solution of 4% formaldehyde, washed with phosphate-buffered saline (PBS), and permeabilized with a solution of 0.5% Triton X-100 and 5% dry milk. Intracellular actin stress fibers were stained using Oregon Green 514 phalloidin (Invitrogen). Oregon Green 514 phalloidin was diluted in PBS to 5 units/mL, and 1 mL was used to stain each gel for 30 min. Gels were rinsed with 0.5% Triton X-100 and 5% dry milk and then washed extensively in DMEM. The stained gels were visualized using a Leica TCS SP2 confocal system.
Cell proliferation assay for 2D cultures
Cell numbers on the two-dimensional (2D) gels were analyzed on days 1, 3, and 5. Four disks for each condition on each day were used for analysis. Four different micrographs were taken from random locations on each gel, and the total number of cells in each set of four micrographs was counted. The cell counts were normalized to those counted on day 1.
Cell proliferation and viability assays for 3D cultures
Cell proliferation in the 3D gels was analyzed with MTS tetrazolium [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] (Promega, Madison, WI). Cells were treated with MTS, and viable cells were evaluated by the formation of a soluble formazan product. The amount of formazan produced was quantified with absorbance at 490 nm, and the quantity of formazan measured was directly proportional to the number of viable cells in culture.
To visualize viable cells after 5 days of culture, the 3D cell–gel constructs were treated with tetrazolium MTT [3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide] (ATCC, Manassas, VA). Viable cells were evaluated by the formation of an insoluble dark purple formazan resulting from the reduction of the MTT reagent in mitochondria of viable cells. The treated cell–gel constructs were visualized using a Leica DMI 4000B microscope.
Immunohistochemistry analysis for 3D cultures
After 5 days of culture, the 3D cell–hydrogel constructs were fixed overnight in either 4% paraformaldehyde in PBS or 10% formaldehyde in PBS, both at pH 7.4. The hydrogels were then dehydrated in ascending percentages of ethanol, cleared in xylene, and embedded in low-temperature paraffin. Sections were cut at a thickness of 5 μm and affixed to pretreated glass slides. Slides were deparaffinized, hydrated, and boiled for 10 min in a microwave for antigen retrieval with 0.01 M citrate buffer, pH 6.0. Sections were incubated in the appropriate serum (10% normal goat or rabbit), followed by incubation with the primary antibody for 1 h at 37°C. Following a rinse with PBS, the sections were incubated with the appropriate biotin-conjugated secondary antibody at room temperature for 1 h, rinsed again, and incubated with ABC solution (Vector Laboratories, Burlingame, CA) for 30 min. Sections were colorized with diaminobenzidine, counterstained with Mayer hematoxylin (Sigma-Aldrich, St. Louis, MO), dehydrated, and coverslipped. Negative control sections were treated in an identical manner but were incubated with PBS instead of the primary antibody. Antibodies used were anti-rabbit DDX4/MVH Primordial Germ Cell Marker (VASA) and anti-rat c-Kit (Caltag Laboratories, Burlingame, CA). Secondary antibodies used were a biotinylated goat anti-rabbit (DAKO USA, Carpinteria, CA) and a biotinylated goat anti-rat (Abcam, Cambridge, MA).
Statistical analysis
Statistical significance between two data populations was evaluated using an unpaired, two-tailed Student's t-test in Microsoft Excel. Differences were considered statistically significant for p < 0.05. Statistical significance among more than two data populations was evaluated using a one-way analysis of variance (ANOVA) in Minitab. Data populations were considered statistically similar for p > 0.1.
Results
Effects of RGD peptide densities and hydrogel stiffness on cell proliferation in 2D cultures
Hydrogels presenting different RGD peptide densities (NRGD) were prepared by altering the ratio between alginate molecules modified with RGD peptides and unmodified alginate molecules from 1:9 to 9:1 to vary NRGD from 6.2 to 56 × 107 RGD/mm2. The RGD concentration on the surface of the hydrogel was calculated from the degree of substitution of RGD peptides and the ratio between modified and unmodified alginates assuming an alginate molecule occupies the same volume as a hard sphere with a radius equal to the radius of gyration of an alginate molecule. Changes in NRGD had minimal effects on the hydrogel stiffness and swelling ratio (results not shown).
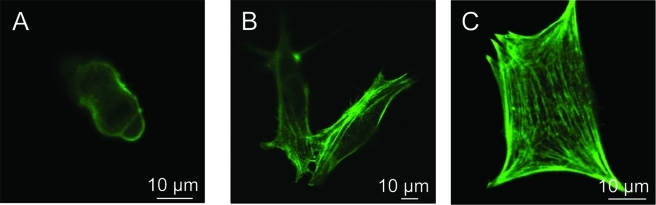
The effects of NRGD on the morphology and cytoskeleton of cells adhered to the gel surface were investigated by evaluating the formation of actin stress fibers. Increasing NRGD enhanced the formation of intracellular actin stress fibers, which were remodeled upon cellular contractility (Fig. 1A–C). SSC colony formation was enhanced with increasing NRGD (Supplemental Fig. 1, available online at www.liebertonline.com/ten), while cell spreading was maximized at an intermediate NRGD; for single cells, the average projected cell area at 6.2, 31, and 56 × 107 RGD/mm2 was 84, 1500, and 510 μm2, respectively.
FIG. 1.
The overall density of RGD peptides immobilized on hydrogel surfaces (NRGD) influenced cellular adhesion and colony formation. Intracellular actin stress fiber formation was visibly enhanced as NRGD was increased. (A), (B), and (C) represent C18-4 cells attached to gels presenting NRGD of 6.2, 31, and 56 × 107 RGD/mm2, respectively. The number of cells per colony also increased as NRGD was increased from 31 × 107 RGD/mm2 (D) to 56 × 107 RGD/mm2 (E). Green fluorescence in microphotographs represents actin fibers stained with Oregon Green 514 phalloidin. Images were captured after 5 days of culture. Color images available online at www.liebertonline.com/ten.
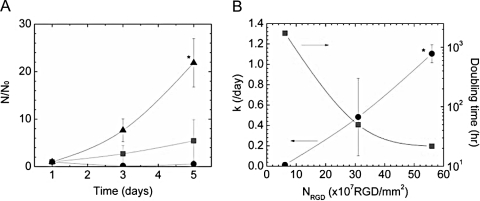
Cell proliferation on 2D hydrogel surfaces was significantly dependent on NRGD. Each SSC seeded formed distinct colonies of daughter cells, and the number of cells in each colony increased with NRGD during 5 days of cell culture, qualitatively indicating an enhancement in cell proliferation with NRGD (Fig. 1D, E). A quantitative analysis of cell number over time also confirmed that increasing cell adhesion sites promoted cell proliferation (Fig. 2A). The lowest NRGD (6.2 × 107 RGD/mm2) showed a minimal increase in cell number, and the difference between normalized cell numbers for the lowest and highest NRGD at day 5 was statistically significant. The cell proliferation rate (k) was further quantified from Eq. (1)
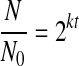 |
(1) |
FIG. 2.
The overall density of RGD peptides influenced C18-4 cell proliferation in 2D cultures. Cell proliferation on 2D hydrogel surfaces was regulated by the overall density of RGD peptides immobilized on hydrogel surfaces (NRGD). The increase in cell number (N) over time was accelerated with NRGD (A). The cell proliferation rate (k) also increased with NRGD (B). In (A), N and N0 represent the cell number and the cell number measured at day 1, respectively. •, ▪, and ▴ represent NRGD of 6.2, 31, and 56 × 107 RGD/mm2, respectively. Differences between N/N0 at day 5 for the highest and lowest NRGD were statistically significant (*p < 0.05). In (B), • and ▪ represent k and doubling time, respectively. Differences between the calculated k values at the highest and lowest NRGD were statistically significant (*p < 0.05). Values represent the mean and standard deviation from four independent measurements.
where N represents the number of cells measured at a given time, N0 represents the initial number of cells measured at day 1, and t represents the cell culture period. The calculated k increased as NRGD increased. The cell doubling time calculated from k decreased significantly as NRGD was increased from 31 to 56 × 108 RGD/mm2 (Fig. 2B).
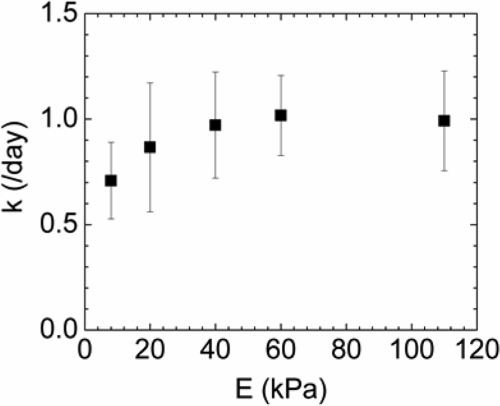
In contrast, the stiffness of hydrogels played a minimal role in regulating cell proliferation. A quantitative analysis of cell number over time showed that at a constant NRGD of 6.2 × 108 RGD/mm2, changes in the elastic modulus did not significantly alter the extent of cell spreading, the number of cells per colony, or the overall cell numbers (Supplemental Fig. 2, available online at www.liebertonline.com/ten). The k values quantified using Eq. (1) were statistically similar on hydrogels of different stiffness, indicating that k was roughly independent of the elastic modulus (Fig. 3).
FIG. 3.
The stiffness of hydrogels did not significantly influence C18-4 cell proliferation. Cell proliferation rate (k) on 2D hydrogel surfaces, calculated from the increase in cell number over time, was roughly independent of the elastic modulus of the hydrogel (E). NRGD was kept constant at 6.2 × 108 RGD/mm2. The calculated k values were statistically similar on gels of different stiffness, as determined with ANOVA. Values represent the mean and standard deviation from four independent measurements.
Effects of RGD peptide densities on cell proliferation in 3D cultures
Based on the results from cell proliferation on 2D gel surfaces, SSCs were encapsulated within four hydrogel formulations: unmodified alginate and alginate modified with RGD peptides at an NRGD of 1.5, 7.3, and 13 × 1012 RGD/mm3 in order to confirm the critical roles of cell adhesion peptides on cell proliferation in 3D cultures. Low-molecular-weight alginate molecules were used for cell encapsulation in order to circumvent a loss of cell viability during the encapsulation process.38 The elastic moduli of the gels were kept constant at 15 kPa since cell proliferation was not dependent on hydrogel stiffness in 2D cell culture.
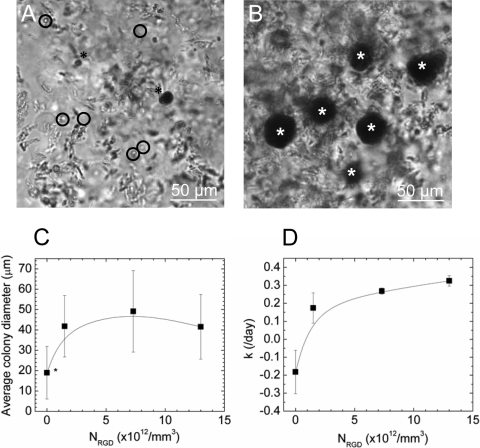
Cell viability was evaluated by identifying viable cells with an MTT assay. The MTT reagent was reduced to an insoluble dark purple formazan in the mitochondria of living cells, thus allowing viable cells to be identified with a dark stain. Increasing NRGD from 0 to 1.3 × 1013 RGD/mm3 increased the number of viable colonies (Fig. 4A, B) and the diameter of viable colonies (Fig. 4C). Further, cells encapsulated within hydrogels modified with RGD were mostly viable after 5 days in culture as compared with cells encapsulated within unmodified hydrogels (Fig. 4A, B).
FIG. 4.
The overall density of RGD peptides influenced C18-4 cell proliferation in 3D cultures. Increasing NRGD from 0 (A) to 1.3 × 1013 RGD/mm3 (B) within a 3D hydrogel matrix roughly doubled the average diameter of cell colonies from 19 to 40 μm. The number of viable cells positively stained with an MTT assay also increased with NRGD. In (A) and (B), viable SSC colonies positively stained with MTT are represented with asterisk (*), while nonviable cells, unstained with MTT, are circled. The average diameter of viable cell colonies increased with increasing NRGD (C). Differences between average cell colony diameters in gels without RGD as compared to gels presenting RGD were statistically significant (*p < 0.05). The cell proliferation rate (k) also increased with increasing NRGD (D). Differences between calculated k values were statistically significant on gels with different NRGD, as determined with ANOVA. The elastic modulus of the hydrogel was kept constant at 15 kPa.
Cell proliferation was quantified with an MTS-based cell proliferation assay. Formazan produced by viable cells in the reduction of MTS was detected with absorbance at 490 nm. The measured absorbance, which was directly proportional to the number of viable cells, increased significantly with NRGD (Supplemental Fig. 3, available online at www.liebertonline.com/ten). The cell proliferation rate (k) quantified using Eq. (1) also increased with NRGD (Fig. 4D). Gels with no RGD peptides resulted in negative k values, likely due to significant cell death.
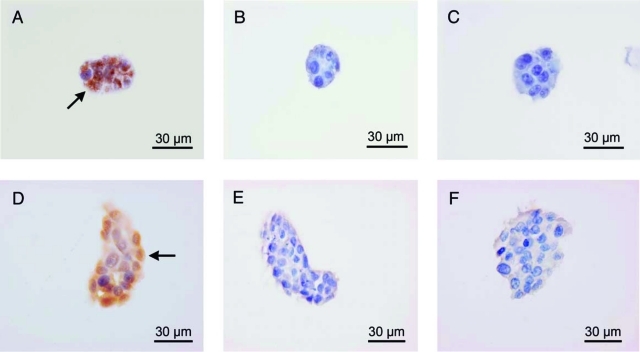
The integrity of the SSCs cultured within hydrogels modified with RGD peptides was further examined. The expression of Vasa, a marker for undifferentiated SSCs, and c-kit, a marker for differentiating SSCs, were probed via immunostaining. Cells cultured within hydrogels modified with RGD peptides were positively stained for Vasa (Fig. 5A, D) and negatively stained for c-kit (Fig. 5B, E), indicating that the cells retained their undifferentiated germ cell properties. In the unmodified hydrogels, which did not contain RGD, cells did not proliferate and died after 5 days in culture; therefore, their phenotype could not be analyzed (data not shown). At an NRGD of 1.5 and 7.3 × 1012 RGD/mm3, cells proliferated but the size of the colonies remained small (Fig. 5A–C). In comparison, colony sizes doubled with cells cultured at an NRGD of 1.3 × 1013 RGD/mm3 (Fig. 5D–F). While NRGD influenced the size of cell colonies, it did not affect Vasa or c-kit expression.
FIG. 5.
C18-4 cell colonies in 3D hydrogels were examined with immunocytochemistry. Cells cultured in 3D hydrogels expressed Vasa but did not express c-Kit. NRGD was varied from 1.5 × 1012 RGD/mm3 (A–C) to 1.3 × 1013 RGD/mm3 (D–F). Cells expressed Vasa at all NRGD, as indicated with arrows (A, D). Cells did not express the c-Kit protein at any NRGD (B, E). (C) and (F) represent negative controls, colonies stained without primary antibodies.
Discussion
This study uses an advanced 3D cell culture system, which mimics some of the biochemical and biomechanical components of the basement membrane, to support SSC proliferation. The culture system used in this study consisted of a bioactive hydrogel presenting cell adhesion molecules (RGD peptides) and was tested with varying cell adhesion sites and stiffness. Interestingly, SSC proliferation could be regulated both in 2D and 3D cell cultures solely by altering hydrogel properties, without the addition of GDNF or other exogenous soluble factors.
The proliferation rates of SSCs and cell adhesion to the gel matrix were significantly dependent on the density of RGD peptides. In 2D cultures, the number of RGD peptides contributed to increasing the number of intracellular stress fibers but did not control the extent of SSC spreading. In 3D cultures, the number of RGD peptides contributed to the formation of viable cell colonies. It is likely that an increase in RGD peptide density led to an increase in the number of bonds between cellular integrins and RGD peptides, as previously examined. This increase in bond number promoted focal adhesion formation at the cell adhesion sites and subsequent intracellular stress fiber formation. It has been reported that this intracellular cytoskeletal reorganization influences the signaling pathways for cell division in both 2D and 3D cell culture.39 SSCs prominently express α6β1 integrins, which are known to bind to laminin in the basement membrane, and laminin is known to be important in the maintenance of stem cells in general.40 The fact that RGD modulates SSC proliferation in vitro implies either that the RGD on the α chain of laminin is important for SSC biology, or/and that other basement membrane proteins containing RGD play a role in SSC biology.41
In contrast, SSC proliferation was not influenced by mechanical stiffness of the hydrogels. Previous studies have demonstrated that the mechanical stiffness of hydrogels plays a critical role in regulating the proliferation of preosteoblast cells but a minimal role in regulating the proliferation of bone marrow–derived stem cells.42 Previous studies have also demonstrated that mechanical stiffness plays a role in regulating the differentiation of both stem and precursor cells.43 The lack of dependency of SSC proliferation on hydrogel stiffness is thus similar to the response of bone marrow–derived stem cells. This result may be due to the lack of intrinsic factors in SSCs that respond to mechanical stress, in comparison to more differentiated cells or cells belonging to other lineages, such as preosteoblasts.
Overall, this study demonstrates that a bioactive hydrogel modified with synthetic oligopeptides can be highly useful in controlling SSC proliferation in both 2D and 3D cell culture by mimicking some of the biochemical properties of the basement membrane underlying the seminiferous epithelium. In addition, the results of this study identified a key ECM variable that might regulate the proliferation of primary SSCs in the natural stem cell niche. The differentiation of primary SSCs into specific lineages may be regulated with the appropriate soluble factors for cells cultured within the hydrogel or for cells isolated from the hydrogel. Thus, the hydrogel system used in this study presents strong potential for use as a primary SSC transplantation vehicle in various cell-based therapies. The crucial role of natural stem cell niches in regulating stem cell activities can also be further clarified using this synthetic hydrogel system. Therefore, the results of this study would be broadly applicable to understanding the interactions between various stem cells and their microenvironments and ultimately controlling the diverse activities of a broad array of stem cells.
Supplementary Material
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the hemopoietic stem cell. Blood Cells. 1978;4:7. [PubMed] [Google Scholar]
- 2.Watt F. Hogan B. Out of Eden: stem cells and their niches. Science. 2000;287:1427. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 3.Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 4.Griffith L.G. Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 5.Li L. Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 6.Moore K. Lemischka I. Stem cells and their niches. Science. 2006;311:1880. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 7.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 8.Geiger B. Bershadksy A. Pankov R. Yamada K.M. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol. 2001;2:793. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 9.McMillan J. Akiyama M. Shimizu H. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J Dermatol Sci. 2003;31:169. doi: 10.1016/s0923-1811(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 10.Giancotti F.G. Ruoslahti E. Integrin signaling. Science. 1999;285:1028. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 11.Erickson A.C. Couchman J.R. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48:1291. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 12.Pierschbacher M. Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 13.Gardner J.M. Hynes R.O. Interaction of fibronectin with its receptor on platelets. Cell. 1985;42:439. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee K.Y. Mooney D.J. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 15.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 16.Stevens M.M. George J.H. Exploring and engineering the cell surface interface. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 17.Rowley J.A. Mooney D.J. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 18.Lee K.Y. Alsberg E. Hsiong S. Comisar W. Linderman J. Ziff R. Mooney D.J. Nanoscale adhesion ligand organization regulates osteoblast proliferation and differentiation. Nano Lett. 2004;4:1501. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong H.J. Polte T.R. Alsberg E. Mooney D.J. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci USA. 2005;102:4300. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong H.J. Hsiong S. Mooney D.J. Nanoscale cell adhesion ligand presentation regulates non-viral gene delivery and expression. Nano Lett. 2007;7:161. doi: 10.1021/nl062485g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong H.J. Boontheekul T. Mooney D.J. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci USA. 2006;103:18534. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boontheekul T. Hill E.E. Kong H.J. Mooney D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13:1431. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida S. Sukeno M. Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 24.Kanatsu-Shinohara M. Inoue K. Lee J. Yoshimoto M. Ogonuki N. Miki H. Baba S. Kato T. Kazuki Y. Toyokuni S. Toyoshima M. Niwa O. Oshimura M. Heike T. Nakahata T. Ishino F. Ogura A. Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Guan K. Nayernia K. Maier L. Wagner S. Dressel R. Lee J. Nolte J. Wolf F. Li M. Engel W. Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 26.Kubota H. Avarbock M.R. Brinster R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanatsu-Shinohara M.H. Inoue K. Ogonuki N. Toyokuni S. Ogura A. Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 28.Smetana K. Cell biology of hydrogels. Biomaterials. 1993;14:1046. doi: 10.1016/0142-9612(93)90203-e. [DOI] [PubMed] [Google Scholar]
- 29.Rowley J.A. Madlambayan G. Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann M. Braydich-Stolle L. Dettin L. Johnson E. Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005;23:200. doi: 10.1634/stemcells.2003-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braydich-Stolle L. Kostereva N. Dym M. Hofmann M.C. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Z. Jiang J. Kokkinaki M. Golestaneh N. Hofmann M.C. Dym M. GDNF up-regulates c-fos transcription via the Ras/ERK1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu X. Pandit A. Kassab G.S. Biaxial incremental homeostatic moduli of coronary artery: two-layer model. Am J Physiol Heart Circ Physiol. 2004;287:H1663. doi: 10.1152/ajpheart.00226.2004. [DOI] [PubMed] [Google Scholar]
- 34.Candiello J. Balasubramani M. Schreiber E.M. Cole G.J. Mayer U. Halfter W. Lin H. Biomechanical properties of native basement membranes. FEBS J. 2007;274:2897. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- 35.Kong H.J. Lee K.Y. Mooney D.J. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer. 2002;43:6239. [Google Scholar]
- 36.Braydich-Stolle L. Kostereva N. Dym M. Hofmann M.C. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z. Jiang J. Kokkinaki M. Golestaneh N. Hofmann M. Dym M. GDNF up-regulates c-fos transcription via the Ras/ERK1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong H.J. Smith M.K. Mooney D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 39.Margadant C. van Opstal A. Boonstra J. Focal adhesion signaling and actin stress fibers and dispensable for progression through the ongoing cell cycle. J Cell Sci. 2007;120:66. doi: 10.1242/jcs.03301. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara T. Avarbock M. Brinster R. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tashiro K.I. Sephel G.C. Greatorex D. Sasaki M. Shirashi N. Martin G.R. Kleinman H.K. Yamada Y. The RGD containing site of the mouse laminin A chain is active for cell attachment, spreading, migration and neurite outgrowth. J Cell Physiol. 1991;146:451. doi: 10.1002/jcp.1041460316. [DOI] [PubMed] [Google Scholar]
- 42.Hsiong S.X. Carampin P. Kong H.J. Lee K. Mooney D.J. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85A:145. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 43.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.