Abstract
The development of a suitable neuroprotective agent to treat ischemic stroke has failed when transitioned to the clinical setting. An understanding of the molecular mechanisms involved in neuronal injury during ischemic stroke is important, but must be placed in the clinical context. Current therapeutic targets have focused on the preservation of the ischemic penumbra in the hope of improving clinical outcomes. Unfortunately, most patients in the ultra-early time windows harbor penumbra but have tremendous variability in the size of the core infarct, the ultimate predictor of prognosis. Understanding this variability may allow for proper patient selection that may better correlate to bench models. Reperfusion therapies are rapidly evolving and have been shown to improve clinical outcomes. The use of neuroprotective agents to prolong time windows prior to reperfusion or to prevent reperfusion injury may present future therapeutic targets for the treatment of ischemic stroke. We review the molecular pathways and the clinical context from which future targets may be identified. Antioxid. Redox Signal. 14, 1841–1851.
Stroke remains the most serious and debilitating neurological disorder in the United States, making it the third leading cause of death in this country after cancer and heart disease (42). Moreover, stroke is the leading cause of serious, long-term adult disability, accounting for $68.9 billion in health care costs and loss of economic efficiency in 2009 alone (42). The most recent statistical update by the American Heart Association Statistics Committee and Stroke Statistics Subcommittee states that approximately 795,000 people in the US experience a new or recurrent stroke, of which 610,000 cases are first attacks and the remaining 185,000 cases are recurrent in nature (42). Statistical analyses demonstrate that 87% of all strokes are ischemic in nature (42), indicating that the presence of a thrombosis, an embolism, or a systemic hypoperfusion can all lead to the obstruction of blood flow to the brain, thereby decreasing the amounts of oxygen and glucose reaching this organ (17). Research aimed at understanding and identifying molecular pathways involved in neuronal death following ischemic stroke has uncovered a myriad of cellular processes that lead to neuronal death, rendering it a highly heterogeneous neurological disorder. Processes leading to neuronal death include, amongst others, ionic imbalance, peri-infarct depolarization, glutamate-mediated excitotoxicity, oxidative stress, and apoptosis (17, 48, 51) (Fig. 1).
FIG. 1.
Approximate timeline of molecular pathway onsets following ischemic stroke. Obstruction of blood flow to the brain via the presence of a thrombosis, embolism, or systemic hypoperfusion results in ischemic stroke. The subsequent lack of oxygen and glucose results in the initiation of the depicted ischemic cascade that ultimately results in neuronal death. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Despite our wealth of knowledge regarding the cellular and molecular processes underlying neuronal death following ischemic stroke, we have yet failed to develop a safe and effective neurotherapeutic that can prevent stroke-induced cell death. The only pharmacological agent that has been approved by the Food and Drug Administration for use in acute ischemic stroke is the thrombolytic tissue-plasminogen activator (t-PA), which, if administered intravenously within the first 3 hours of stroke onset, can improve clinical outcome 3 months after treatment (40, 44). More recently, the time window has been expanded to 4.5 hours with intravenous t-PA (30). This strategy is aimed toward clot lysis and ultimately reperfusion of the tissue downstream of the occluded cerebral artery. In spite of the benefits of t-PA, studies have shown that the administration of this thrombolytic agent is associated with a 6% risk of intracranial hemorrhage. Notably, less than 2% of patients in the community receive thrombolysis as they do not arrive at hospitals within the therapeutic window. Exogenously applied t-PA can cross the blood–brain barrier and ultimately reach and destroy the brain parenchyma via its activity as a serine protease (40, 44). More recently, it has become evident that the administration of t-PA may also enhance neurodegeneration due to t-PA's ability to interact with the NR1 subunit of N-methyl-D-aspartate (NMDA) glutamate receptors, thereby promoting calcium influx which can subsequently reach neurotoxic levels (18, 40, 44). Results such as these underline the difficulties and challenges researchers and clinicians are confronted with in the quest of developing effective and safe therapeutics for the treatment of ischemic stroke.
Despite the challenges surrounding t-PA, a recent meta-analysis of clinical trials showed that successful vessel recanalization is the most robust predictor of improved clinical outcomes (54). Due to the challenges of treating patients in a timely manner, an extension of the therapeutic window is necessary to allow for broader appeal to clinicians treating ischemic stroke victims. Advances in radiographic imaging has allowed for a potential opportunity to treat patients based on physiology as opposed to rigid time windows (Figs. 2 and 3). The time concept has been recently challenged by clinical researchers as patients have different cerebral collaterals via the Circle of Willis (21). Quantification of cerebral blood flow (CBF) can be performed using positron emission tomography (PET) imaging. Based on PET quantification methods, the core infarct, which is the territory that is irreversibly injured and can no longer be salvaged, is defined as a CBF of less than 12 cc/100 g/min. The penumbra is tissue that is structurally intact but functionally impaired due to the reduction of blood flow. This is defined as a CBF of 12–20 cc/100 g/min (2). Mismatch defined as a larger penumbra compared to the core has been the focus of clinical research in reperfusion therapy (1, 15) and may be a potential marker of determining patients suited for neuroprotection. Unfortunately, PET imaging is not readily available in the clinical setting and thus is not a practical tool for stroke patients. Xenon CT imaging is the only technology available that can quantify CBF in a clinical setting. CT and MRI perfusion are qualitative estimates and cannot precisely define the penumbra, but can broadly define populations with mismatch (37). Although MRI and CT are not precise tools to quantify penumbra, thresholds are being developed to better identify patients with more potential for tissue salvage during reperfusion treatments and this may be beneficial to future neuroprotection trials.
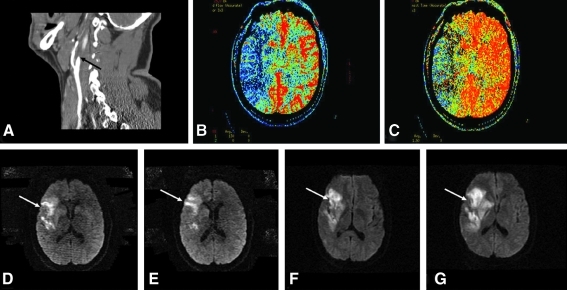
FIG. 2.
Example of clinical progression of ischemic stroke in the setting of a large artery occlusion. A 50-year-old patient presents at 15 h from symptom onset with a left-sided weakness and was found to have (A) occlusion of the right internal carotid artery (black arrow) on CT angiography. A CT perfusion was performed to estimate penumbra and showed (B) reduction of the cerebral blood flow to the right middle cerebral artery territory, and (C) prolongation of the mean transit time to the right hemisphere. MRI of the brain was performed to determine the core infarct size; in (D) and (E) the diffusion weighted sequence reveals a small core (white arrows) relative to the perfusion defect noted on CT perfusion imaging. The patient was admitted to the neuro intensive care unit and his blood pressure was raised with vasopressor agents to attempt to maintain perfusion to the right hemisphere. At 24 h from symptom onset, his weakness worsened and a repeat MRI of the brain revealed (F) and (G) extension of the infarct on diffusion weighted sequences (white arrows). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 3.
Example of reperfusion therapy beyond traditional time window. A 75-year-old patient presented with left sided weakness 3 days earlier and was noted to have (A) and (B) a small diffusion weighted core (white arrows) infarct on MRI. (C) CT perfusion imaging showed prolongation of the mean transit time to the right hemisphere and provided an estimate of penumbra. (D) A cerebral angiogram revealed the presence of a right middle cerebral artery occlusion (black arrow) that was the culprit for the mismatch. (E) A stent was successfully implanted (dashed black arrow) and revealed marked improvement in the (F) mean transit time to the right hemisphere. The patient had marked improvement in his weakness and returned to near baseline function at 30 day follow up. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Patients presenting with a cerebral artery occlusion comprise 70% of ischemic stroke patients. Mismatch is present on CT and MRI in up to 45% of patients at 24 hours from symptom onset (35). A recent meta-analysis (49) of patients treated with thrombolytics in the presence of mismatch was found to have a higher rate of reperfusion, but also higher rates of mortality. The penumbra appears to be consistently present, but it is the size of the core prior to reperfusion treatment that seems to predict hemorrhagic complications (6, 29) and clinical outcomes (34). Thus, clinical trial designs based on rigid time windows or on pre-determined mismatch percentages may poorly select the patients most likely to benefit from neuroprotective therapeutics and moreover test the pathophysiology of the therapy being given. Identifying the core infarct threshold destined to a poor clinical outcome is crucial in determining the patients most likely to profit from reperfusion and neuroprotection trials.
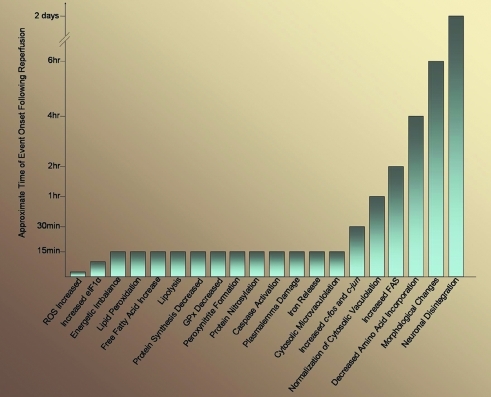
While the use of t-PA is aimed at re-establishing circulation to an ischemic area, a myriad of studies have been conducted with the goal to identify neuroprotective agents that can halt neuronal death in ischemic stroke. Despite the fact that many of these neuroprotective agents were successful in preclinical stroke models involving cell lines and animal models, not a single one has proven to be advantageous for clinical outcome in patients who suffered from ischemic stroke (18). Lack of success may be explained by the fact that many preclinical trials focus on the role of only one molecular pathway involved in cell fate following ischemic stroke, as opposed to the myriad of events elicited in this heterogeneous disease (Fig. 1). Moreover, there is a lack of model systems aside from rodents, and within rodent systems themselves, there is a deficiency in data from aging and diseased animals (56). Data evaluating long-term neurological outcomes as well as the lasting benefits of given treatments following ischemic stroke are also absent from current stroke models (56). Another concern is the insufficient collection of data on the pharmacokinetics of various drugs in preclinical settings involving rodent models, which may account for why failures of these drugs have been observed in human clinical trials (10, 56). Notably, although the use of cell culture models has allowed scientists to identify and understand complex cellular and molecular pathways involved in ischemic stroke and reperfusion (Figs. 1 and 4), they do not represent the intricate physiology of the human brain, thereby posing yet another challenge for the translation of basic science work to the clinical setting (56).
FIG. 4.
Approximate timeline of molecular pathway onsets following reperfusion. The restoration of blood flow to the brain after ischemic stroke allows the reestablishment of glucose and oxygen supply to the damaged area. Despite the benefits of restoring blood flow to the brain, reperfusion injury has become an intensive area of study, as this process results in the activation of many molecular pathways that enhance neuronal death. The approximate timing of these events is shown above. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
For the proper development of effective and safe neuroprotective agents, it is of utmost importance to understand the molecular processes activated upon ischemic stroke (Fig. 1), as well as the appropriate clinical settings to which they may apply. Given the heterogeneity of this neurological disorder, it is currently speculated that a successful neurotherapeutic will not only target one molecular mechanism, but instead several of these highly complex pathways at once. Additionally, it is important to understand that neuroprotection may delay the process of neuronal death, but without definitive reperfusion therapy the tissue will continue to undergo infarction.
During ischemic stroke, the obstruction of CBF to the brain results in a drastic decrease in glucose and oxygen levels to the affected brain region. Prototypic brain damage following ischemic stroke is characterized by two main areas: the inner core and the surrounding penumbra. The core is a brain region of maximum and irreversible damage, where ATP levels are completely depleted and neurons undergo necrotic cell death (48). In contrast, neurons located in the surrounding ischemic penumbra are functionally impaired, unlike those of the ischemic core, and are potentially salvageable if appropriate neurotherapeutics are administered during the therapeutic window of opportunity, which is the critical time period during which the brain surrounding the core is at risk. Within the ischemic penumbra, ATP levels are decreased, but not fully depleted, and neurons within this region are prone to undergo apoptosis (46). Consequently, the penumbra is considered to be an essential target for therapeutic intervention with the ultimate goal of improving functional outcome and recovery following ischemic stroke (48, 51). As discussed earlier, this target must be considered in the context of the size of the core infarct prior to initiation of treatment, as the extent of the core correlates to clinical outcomes.
Under normoxic conditions, the main function of the neuron is to conduct electrical impulses in form of action potentials. In order to process and transmit these impulses successfully, ions such Na+, K+, and Ca2+ have to be maintained at different concentrations across the neuronal plasma membrane. As a result, 50%–60% of the total ATP synthesized in the brain is used for the activity of energy-dependent ionic pumps that are responsible for the maintenance of these electrochemical concentration gradients (19). In the case of ischemic stroke, obstruction of CBF to the brain, and subsequent lack of oxygen and glucose supply to the brain, results in a drastic reduction in ATP production via oxidative phosphorylation in the mitochondria, which consequently leads to decreased total ATP levels within 2 minutes following the insult (17, 19, 51). This lack of ATP affects the ability of the ATP-dependent ionic pumps to function properly, resulting in the inability of neurons to maintain their ionic homeostasis (16). The subsequent cytosolic increase in Na+ and decrease in K+ concentrations leads to the depolarization of neuronal membranes (51). In the ischemic core, this sudden and profound loss of membrane potential is termed anoxic depolarization (AD), an event which is characterized by the death of neurons in this brain region along with the opening of voltage gated Ca2+ channels, allowing rapid influx of Ca2+ into neurons (32, 33, 48, 51). Moreover, the core propagates spontaneous electrical waves known as peri-infarct depolarizations (PID) to the ischemic penumbra, leading to rapid de- and repolarizations of these neurons (20, 48, 51). Studies have demonstrated a direct correlation between the number of PIDs and the extent of infarction in brain regions adjacent to the ischemic core (33, 48). While AD occurs within minutes following the initial insult, recurring PIDs have been shown to arise in the brain region surrounding the ischemic core within the first 3–4 hours following stroke (33, 51). Given the rapidity of ADs, the ischemic core is beyond therapeutic reach. However, the ischemic penumbra serves as a potential target for therapeutic intervention with the goal of reducing the frequency of PIDs in this area, thereby decreasing the number of “at-risk” neurons and preventing a further expansion of the ischemic core into the surrounding tissue (32, 48). Studies have demonstrated that therapeutic agents that block Na+ channels counteract the primary events in stroke. Drugs such as lidocaine, tetrodotoxin, lamotrigine, BW-1003C87, and BW-619C89 have all been shown to provide protection against ischemic stroke via their ability to decrease the rate of ATP depletion and to reduce the ischemia-induced release of glutamate in animal models of stroke (12, 22, 26, 38, 46, 58). Unfortunately, none of these drugs have shown to be beneficial for clinical outcome in human stroke (51). Given the failure of the use of Na+ channel blockers as a means of therapeutic intervention, Chen et al. investigated the potential of hypothermia as a therapeutic target. Using animal models to study acute ischemic stroke, they demonstrate that the number of depolarizations as well as the extent and size of infarction significantly decreased in animals that were kept at hypothermic temperatures (30°C) (11). As a result, the use of hypothermia could prove to be a suitable intervention for the treatment of ischemic stroke and the improvement of clinical outcome.
The loss of ATP and the resulting inability of molecular pumps to maintain ionic gradients across the neuronal membrane ultimately results in the depolarization of neurons, an event that is accompanied by the influx of Ca2+ into neurons via voltage-gated Ca2+ channels (48). The presence of Ca2+ in synaptic boutons triggers the release of neurotransmitters into the synaptic cleft. The neurotransmitter of particular interest for the pathology associated with ischemic stroke is the major excitatory neurotransmitter glutamate. Under physiological conditions, cytosolic glutamate concentrations are approximately 10 mM, while synaptic glutamate concentrations are in the micromolar range (17, 48). The main task of this excitatory neurotransmitter is the initiation of action potentials in the postsynaptic neuron, an event that is governed via the interaction of glutamate with ionotropic and metabotropic glutamate receptors. The presence of sodium-dependent glutamate transporters in pre- and postsynaptic membranes assures the maintenance of appropriate glutamate gradients across the plasma membrane as well as proper clearing of glutamate from the synaptic cleft and is thereby directly involved in the regulation of cellular signaling (17, 29).
The rise in cytosolic Na+ concentrations following ischemic stroke results in the reversal of glutamate transporters, allowing glutamate to flow from the cytosol into the synaptic cleft down its concentration gradient (17). Given the lack of evidence for extracellular metabolism of glutamate, the reversal of the activity of the glutamate transporters prevents the reuptake of glutamate into the cell, thereby allowing extracellular glutamate concentrations to reach neurotoxic levels (55). Specifically, studies have shown that extracellular glutamate levels can rise up to 80 μM and remain at these highly neurotoxic concentrations for several hours (51). The subsequent effect of the presence of high levels of glutamate within the synaptic cleft is the activation of N-methyl-D-aspartate (NMDA) as well as alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, both of which belong to the ionotropic glutamate receptor family (17, 51). Given that NMDA receptors are the prominent route of Ca2+ influx during glutamatergic transmission, their opening results in a drastic increase in cytosolic Ca2+ levels (48). While Ca2+ is involved in the regulation of a variety of cellular processes including cell growth, differentiation, and enzyme function, high cytosolic Ca2+ levels also promote the activation of cellular cascades, leading to cell death (48, 51). In cell culture and animal models of stroke, modifications of glutamatergic signaling via the use of NMDA antagonists as well as the blockage of presynaptic glutamate release have been found to be neuroprotective; however, these results failed to translate to the clinical setting (24, 48, 51).
In contrast to NMDA receptors, AMPA receptors are impermeable to Ca2+ by virtue of their GluR2 subunit. Interestingly, by using the oxygen–glucose deprivation (OGD) paradigm as an in vitro model to study brain ischemia, several studies have shown that both mRNA and protein levels of the GluR2 subunit are substantially reduced following ischemia (25, 41, 50, 52). Moreover, Liu and colleagues demonstrate that neuronal exposure to OGD promotes the internalization of GluR2 containing AMPA receptors, while promoting the insertion of AMPA receptors lacking the GluR2 subunit into the postsynaptic membrane (41). This change in subunit composition of AMPA receptors renders these receptors permeable to Ca2+, thereby further increasing Ca2+ influx. The final outcome of this subunit change is the initiation of delayed neuronal cell death mediated by AMPA receptors (17, 41), suggesting that AMPA receptors which lack GluR2 subunits present another suitable target for the development of neurotherapeutics.
While NMDA and AMPA receptors can directly influence Ca2+ influx into neurons, metabotropic glutamate receptors (mGluRs) have been shown to indirectly govern Ca2+ influx (7). Metabotropic glutamate receptors belong to the family of G-protein coupled receptors, indicating that the binding of glutamate to these receptors results in the initiation of various downstream signaling cascades (7). Nevertheless, it has been shown that metabotropic glutamate receptors can also contribute to excitotoxicity, specifically via the activity of mGluRs belonging to group 1 of the metabotropic glutamate receptor family, including mGluR1 and mGluR5. These receptors are predominantly located at the postsynaptic membrane, and it has been shown that mGluR5 is functionally and physically associated with NMDA receptors, thereby enhancing Ca2+ influx into neurons (7, 17). As a result, decreasing excitotoxicity via the use of mGluR5 antagonist may present another potential target for the development of neurotherapeutics (17, 52).
Recent studies have demonstrated a link between excess intracellular Ca2+ levels and the activation of neuronal nitric oxide synthase (nNOS), resulting in increased levels of intracellular nitric oxide (NO·), which, due to its unpaired electron, is considered a free radical and can therefore ‘steal’ electrons from other molecules (24, 48). NO· enhances the formation of reactive oxygen species (ROS), including superoxide (O2·−), hydroxyl radicals (OH·), and peroxynitrites (ONOO−), all of which can ultimately damage major cellular macromolecules, such as nucleic acids, proteins, and lipids (24). Given the high lipid content of the brain, this organ is particularly susceptible to attack by ROS. Lipid peroxidation has been shown to result in the formation of a variety of by-products, including toxic aldehydes such as 4-hydroxy-2-nonenal (4-HNE) and isoprostanes that can subsequently damage other cellular components (48).
Under physiological conditions, cells are equipped with antioxidants that act as a cellular defense mechanism to aid in the scavenging and removal of ROS, thereby decreasing the damaging effects of ROS on cellular integrity. Antioxidants, including the main intracellular low molecular weight antioxidant glutathione, as well as catalase and superoxide dismutase, aid in the cellular detoxification of ROS (48). During ischemic stroke, however, the balance between ROS and antioxidants becomes disturbed due to the rapid and excessive generation of ROS and the inability of antioxidants to reduce these levels. This imbalance is collectively referred to as oxidative stress, and it has been identified as a common theme in a myriad of acute and chronic neurological diseases (14, 17, 24, 48, 51). Given the damaging effects of ROS on cellular integrity and survival, the removal of ROS by increasing antioxidant levels has been a focus of interest and may present a suitable target for the development of neurotherapeutics (24, 48, 51). While the addition or overexpression of antioxidants has been shown to result in neuroprotection in vitro, the use of antioxidants in human stroke has not provided the same positive results (51). For instance, the use of Bilobalide (EGB 761), an extract from Gingko biloba leaves, is neuroprotective in transient ischemia, yet its use in stroke is not recommended due to increases in intracerebral hemorrhage (51). Studies using the NOS inhibitor Lubeluzole have shown to be ineffective in stroke (16), while recent studies using BN 80933, an agent that affects both nNOS and lipid peroxidation, have shown more promising results (8). Given that ROS are generated under physiological conditions, such as during oxidative phosphorylation in mitochondria or as by-products of enzyme activities such as tyrosine hydroxylase and monoamine oxidase (24, 48), the development of a safe and effective therapeutic targeted at the reduction of ROS poses another challenge for researchers and clinicians.
In order to identify an effective therapeutic agent for ischemic stroke, a precise understanding of the cell death pathways associated with this neurological disorder is required. As a result, an area of intense research has been devoted to cell death pathways activated during ischemic stroke. Due to the rapid loss of ATP in the ischemic core, neurons in this brain region undergo necrosis. In contrast, low levels of ATP present in the ischemic penumbra activate cell death pathways that ultimately lead to programmed cell death (apoptosis) (17, 48). While neurons in the ischemic core are beyond therapeutic reach, research has predominantly focused on identifying agents that can block neuronal death in the ischemic penumbra by interfering with a variety of molecular players involved in apoptosis, including cytochrome c, Bcl-2-related proteins, and caspases (17, 48, 51).
During the first few hours after ischemic stroke, a range of signals such as ionic imbalance, ROS generation, DNA damage, caspase activation, and mitochondrial swelling can trigger apoptosis in the ischemic penumbra, rendering these initial hours a suitable opportunity for intervention (17, 48). From a biochemical perspective, apoptosis is primarily characterized by the activation of cysteine proteases, known as caspases, which cleave substrates specifically after aspartate residues. One way caspases can be activated following ischemic stroke is via the release of cytochrome c from mitochondria in response to ionic imbalance and mitochondrial swelling. Once in the cytosol, cytochrome c associates with Apaf-1 and procaspase-9 to form a structure known as the apoptosome (4). The apoptosome then cleaves and activates executioner caspases, such as caspase-3, -6, and -7. Executioner caspases then continue to cleave their substrates including iCAD, PARP, actin, and p75, resulting in the characteristic morphological changes observed in apoptosis, including membrane blebbing, DNA condensation, and nuclear fragmentation, as well as the redistribution of phosphatidylserine residues to the outer plasma membrane that ultimately prevent the leakage of potentially harmful cellular contents into the surrounding tissue (3, 4, 48). Research in the field of programmed cell death has identified several signaling pathways that can result in cell death, including the extrinsic and intrinsic pathways of apoptosis, death receptor mediated apoptosis, and caspase-independent apoptotic cell death. While a detailed analysis of all pathways is beyond the scope of this article, it is important to know that research aimed at the identification of effective neurotherapeutics has targeted a variety of molecules involved in apoptosis, including caspases, Bcl-2 family members, and poly(ADP-ribose) polymerase (PARP) (48).
Several studies have shown that the administration of caspase inhibitors can reduce infarct volumes in models of stroke (43, 51, 53). Zhao et al. demonstrate that the overexpression of the Bcl-2 protein protects against apoptotic cell death, resulting in increased neuronal survival following the ischemic insult (65). Given that one of the characteristic morphological changes seen in apoptosis is DNA fragmentation, several groups have investigated the effect of inhibiting PARP following ischemic stroke (64, 66). While PARP is involved in mediating poly(ADP-ribosylation) under physiological conditions, ensuring the maintenance of genomic integrity, research has shown that under conditions of cell stress, poly(ADP-ribosylation) actually governs mitochondrial failure and promotes cell death (64,66). Studies investigating the effect of PARP on neuronal death following ischemic stroke demonstrate that the inhibition of PARP activity reduces neuronal death by preventing the translocation of apoptosis inducing factor (AIF) from the mitochondria into the nucleus (64, 66).
Another area of intense research in the field of ischemic stroke is reperfusion injury, which may occur in patients following successful restoration of blood flow to an ischemic brain region (59) (Fig. 5). Although rapid reperfusion of an ischemic brain region is of utmost importance in order to salvage brain tissue, this process also harbors a variety of dangers that can worsen outcome if complications occur. While the initial ischemic stroke leads to the activation of a myriad of cellular and molecular cascades that ultimately result in neuronal death (Fig. 1) (31, 48, 51), reperfusion of the damaged brain area adds to the degree of brain injury due to the activation of additional signaling pathways (Fig. 4). Given that a detailed analysis of all pathways implicated in reperfusion injury is beyond the scope of this review, a focus on oxidative stress, a major and early event elicited following reperfusion injury, is presented here (31, 63).
FIG. 5.
Clinical example of reperfusion injury in the setting of reperfusion therapy. A 65-year-old woman presented with recurring episodes of right sided weakness and speech difficulties. (A) A CT perfusion image showed a large area of reduced perfusion to the left hemisphere (white arrow). (B) Cerebral angiography was performed and showed a severe stenosis of the left internal carotid artery (black arrow). (C) A carotid stent (dashed black arrow) was placed to revascularize the severe narrowing but was complicated with (D) an embolus to the left middle cerebral artery (black arrow). (E) A stent was implanted in the left middle cerebral artery (dashed black arrow) with successful reperfusion achieved at 60 min from the initial time of the embolus. (F) A large hemorrhage (dashed white arrow) was noted within a sizeable infarct despite rapid reperfusion of the iatrogenic embolus. The mechanism was likely reperfusion injury. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Oxidative stress is characterized by a rapid and excessive increase in ROS and a decrease in the detoxifying activities of intracellular antioxidants (31, 48, 63). While ischemia results in the accumulation of free fatty acids, and specifically arachidonic acid, through the breakdown of lipid membranes, reperfusion leads to the generation of ROS via the metabolism of these same fatty acids (60). Specifically, arachidonic acid is metabolized via both the lipoxygenase and cyclooxygenase pathways leading to the generation of prostaglandins and superoxides (60). At this point, an imbalance exists between pro- and antioxidants, posing the brain at a higher risk for subsequent damage. ROS produced during dysregulated antioxidant homeostasis not only injure other cellular macromolecules, thereby enhancing neuronal death, but also activate molecular signaling cascades leading to the recruitment of inflammatory cells to the site of injury (31, 48). As a result, identifying treatments that can scavenge ROS, and thereby decrease the deleterious effects of ROS on other macromolecular structures, would present a potential avenue for the development of safe and effective neurotherapeutics that can decrease neuronal death following reperfusion injury.
Given the heterogeneity of ischemic stroke, one can assume that a successful neurotherapeutic will be able to interfere with several cellular and molecular processes triggered as a result of ischemic stroke at once. Identifying a therapeutic agent, or combination of therapeutic agents, that can block neuronal death while preventing devastating side effects, including excitotoxicity and cerebral hemorrhages proves to be a challenge for researchers and clinicians studying ischemic stroke.
Future Directions
Understanding the clinical context in which these neuroprotective strategies may be incorporated is essential as previously tested agents may have clinical impact if tested in the appropriate set of patients. A neuroprotective agent that impacts multiple molecular pathways administered early in the clinical course in a patient with a small core infarct and a large area of penumbra may be the ideal target. This strategy may afford a delay in the transition of penumbra to core while definitive reperfusion strategies are considered. The combination or multimodal approach may allow for treatment of a larger proportion of patients by extending the time window. A second use of neuroprotection may be to deliver the agent in the hopes of preventing reperfusion hemorrhage (Table 1). Successful reperfusion strategies are hampered by hemorrhagic complications that are partially due to reperfusion injury (Figs. 4 and 5). Removal of hemorrhagic risks may help to benefit a larger population with reperfusion techniques. Treatment paradigms surrounding reperfusion will likely provide the best therapeutic targets going forward.
Table 1.
Methods Used to Reduce Reperfusion Injury
| Agent/Methodology | Proposed Mechanism | Clinical Application and Outcome | References |
|---|---|---|---|
| Cooling for Acute Ischemic Brain Damage (COOL AID); ICTuS Trial | Suppression of generation of reactive oxygen species and inflammatory responses. | Used in clinical settings. Decreased brain damage following ischemia and reperfusion. | 13, 28, 36, 47 |
| Hyperbaric oxygen preconditioning (HBO) | Increased antioxidant activity of catalase and superoxide dismutase results in decreased ischemia-reperfusion injury. | Not used in clinical settings. | 39, 57 |
| Normobaric oxygen therapy (NBO) | NBO results in increased brain oxygen levels, decreased peri-infarct depolarizations, improved brain lactate levels and favorably altered cerebral blood volumes. | Not used in clinical settings. | 23, 57 |
| Ethanol preconditioning (EtOH-PC) | Moderate ethanol ingestion causes mild oxidative stress resulting in decreased postischemic behavioral deficits, oxidative DNA damage, neuronal and dendrite degeneration as well as glia activation in the hippocampus. | Not used in clinical settings. | 61 |
| Caffeine and ethanol (Caffeinol) + hypothermia | Acting as an adenosine receptor antagonist, caffeine increases neural activity, cerebral metabolic rate and stimulates adrenaline release. An increased metabolic rate may promote the continuous functioning of metabolic pathways despite decreased reperfusion. Ethanol acts on GABAA receptors, leading to the inhibition on NMDA receptors, thereby having antiexcitotoxic properties. | Used in clinical settings in combination with hypothermia and t-PA to reduce ischemic brain damage. | 13, 66 |
| Exercise preconditioning | Reduced expression levels of matrix metalloproteinase-9 (MMP-9) after physical exercise decreases neuronal apoptosis. | Not used in clinical settings. | 9 |
Abbreviations Used
- 4-HNE
4-hydroxy-2-nonenal
- AD
anoxic depolarization
- AIF
apoptosis inducing factor
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- Apaf-1
apoptotic protease activating factor 1
- Caffeinol
caffeine and ethanol
- CBF
cerebral blood flow
- COOL AID
cooling for Acute Ischemic Brain Damage
- eIF1α
eukaryotic initiation factor 1 alpha
- ER
endoplasmic reticulum
- EtOH-PC
ethanol preconditioning
- GPx
glutathione peroxidase
- HBO
hyperbaric oxygen preconditioning
- HIF1α
hypoxia-inducible factor 1
- HSP
heat shock protein
- iCAD
inhibitor of caspase-3-activated DNase
- ICTUS
Invasive versus Conservative Treatment in Unstable Coronary Syndromes
- IEG
immediate early genes
- mGluR
metabotropic glutamate receptors
- MMP-9
matrix metalloproteinase-9
- NBO
normobaric oxygen therapy
- NMDA
N-methyl-D-aspartate
- nNOS
neuronal nitric oxide synthase
- NO·
nitric oxide
- O2·−
superoxide
- OGD
oxygen glucose deprivation
- OH·
hydroxyl radical
- ONOO-
peroxinitrate
- PARP
poly(ADP-ribose) polymerase
- PID
peri-infarct depolarization
- ROS
reactive oxygen species
- t-PA
tissue-plasminogen activator
Acknowledgments
The authors wish to thank Dr. BethAnn McLaughlin for the invitation to contribute to the Stroke Forum of Antioxidants & Redox Signaling, as well as the members of the McLaughlin lab for helpful suggestions and discussion. This work was supported by the Vanderbilt Neuroscience predoctoral training Fellowship T32 MH064913 (JS).
References
- 1.Albers GW. Thijs VN. Wechsler L. Kemp S. Schlaug G. Skalabrin E. Bammer R. Kakuda W. Lansberg MG. Shuaib A. Coplin W. Hamilton S. Moseley M. Marks MP. DEFUSE Investigators. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 2.Baron JC. Perfusion thresholds in human cerebral ischemia: Historical perspective and therapeutic implications. Cerbrovasc Dis. 2001;11:2–08. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 3.Beere H. Green DR. Stress management-heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 4.Beere H. The stress of dying: The role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 5.Beere HM. Death versus survival: Functional interaction between apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt A. Vora NA. Thomas AJ. Majid A. Kassab M. Hammer MD. Uchino K. Wechsler L. Jovin TG. Gupta R. Lower pretreatment cerebral blood volume affects hemorrhagic risks after intra-arterial revascularization in acute stroke. Neurosurgery. 2008;63:874–878. doi: 10.1227/01.NEU.0000333259.11739.AD. [DOI] [PubMed] [Google Scholar]
- 7.Bruno V. Battaglia G. Copani A. D'Onofrio A. Di Iorio P. De Blasi A. Melchiorri D. Flor PJ. Nicoletti F. Metabotropic glutmate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Chabrier PE. Auguet M. Spinnewyn B. Auvin S. Cornet S. Demerle–Pallardy C. Guilmard–Favre C. Marin JG. Pignol B. Gillard–Roubert V. Roussillot–Charnet C. Schulz J. Viossat I. Bigg D. Moncada S. BN 80933, a dual inhibitor of neuronal nitric oxide synthase and lipid peroxidation: A promising neuroprotective strategy. Proc Natl Acad Sci USA. 1999;96:10824–10829. doi: 10.1073/pnas.96.19.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry K. Rogers R. Gui M. Lai Q. Goel G. Liebelt B. Ji X. Curry A. Carranza A. Jimenez DF. Ding Y. Matrix metalloproteinase-9 (MMP-9) expression and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation in exercise-reduced neuronal apoptosis after stroke. Neurosci Lett. 2010;474:109–114. doi: 10.1016/j.neulet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Chavez JC. Hurko O. Barone FC. Feuerstein GZ. Pharmacologic interventions for stroke: Looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke. 2009;40:e558–e563. doi: 10.1161/STROKEAHA.109.559914. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q. Chopp M. Bodzin G. Chen H. Temperature modulation of cerebral depolarization during focal cerebral ischemia in rats: Correlation with ischemic injury. J Cereb Blood Flow Metab. 1993;13:389–394. doi: 10.1038/jcbfm.1993.52. [DOI] [PubMed] [Google Scholar]
- 12.Crumrine RC. Bergstrand K. Cooper AT. Faison WL. Cooper BR. Lamotrigine protects hippocampal CA1 neurons from ischemic damage after cardiac arrest. Stroke. 1997;28:2230–2237. doi: 10.1161/01.str.28.11.2230. [DOI] [PubMed] [Google Scholar]
- 13.Damman P. Hirsch A. Windhausen F. Tijssen JGP. de Winter RJ ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus conservative treatment in unstable coronary syndromes) Trial: A randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. JACC. 2010;55:858–864. doi: 10.1016/j.jacc.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Danielson SR. Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis SM. Donnan GA. Parsons MW. Levi C. Butcher KS. Peeters A. Barber PA. Bladin C. De Silva DA. Byrnes G. Chalk JB. Fink JN. Kimber TE. Schultz D. Hand PJ. Frayne J. Hankey G. Muir K. Gerraty R. Tress BM. Desmond PM EPITHET Investigators. Effects of alteplase beyond 3 hours after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): A placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 16.Diener HC. Cortens M. Ford G. Grotta J. Hacke W. Kaste M. Koudstaal PJ. Wessel T for the LUB-INT-13 Investigators. Lubeluzole in acute ischemic stroke treatment. A double-blind study with an 8-hour inclusion window comparing a 10-mg daily dose of lubeluzole with placebo. Stroke. 2000;31:2543–2551. doi: 10.1161/01.str.31.11.2543. [DOI] [PubMed] [Google Scholar]
- 17.Doyle KP. Simon RP. Stenzel–Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducruet AF. Grobelny BT. Zacharia BE. Hickman ZL. Yeh ML. Connolly ES. Pharmacotherapy of cerebral ischemia. Expert Opin Pharmacother. 2009;10:1895–1906. doi: 10.1517/14656560903055095. [DOI] [PubMed] [Google Scholar]
- 19.Erecinska M. Silver IA. Ions and energy in mammalian brain. Prog Neurobiol. 1994;43:37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Fabricius M. Fuhr S. Buatia R. Boutelle M. Hashemi P. Strong AJ. Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- 21.Fink JN. Kumar S. Horkan C. Linfante I. Selim MH. Caplan LR. Schlaug G. The stroke patient who woke up: Clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33:988–993. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 22.Fujitani T. Adachi N. Miyazaki H. Liu K. Nakamura Y. Kataoka K. Arai T. Lidocaine protects hippocampal neurons against ischemic damage by preventing increase of extracellular excitatory amino acids: A microdialysis study in Mongolian gerbils. Neurosci Lett. 1994;179:91–93. doi: 10.1016/0304-3940(94)90942-3. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara N. Murata Y. Arai K. Egi Y. Lu J. Wu O. Singhal AB. Lo EH. Combination therapy with normobaric oxygen (NBO) plus thrombolysis in experimental ischemic stroke. BMC Neurosci. 2009;10:79–87. doi: 10.1186/1471-2202-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilgun–Sherki Y. Rosenbaum Z. Melamed E. Offen D. Antioxidant therapy in acute central nervous system injury: Current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 25.Gorter JA. Petrozzino JJ. Aronica EM. Rosenbaum DM. Opitz T. Bennett MVL. Connor JA. Zukin RS. Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham SH. Chen J. Lan J. Leach MJ. Simon RP. Neuroprotective effects of a use-dependent blocker of voltage-dependent sodium channels, BW619C89 in rat middle cerebral artery occlusion. JPET. 1994;269:854–859. [PubMed] [Google Scholar]
- 27.Gu Y. Shrivastava IH. Amara SG. Bahar I. Molecular simulations elucidate the substrate translocation pathway in a glutamate transporter. Proc Natl Acad Sci USA. 2009;106:2589–2594. doi: 10.1073/pnas.0812299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guluma KZ. Oh H. Yu SW. Meyer BC. Rapp K. Lyden PD. Effect of endovascular hypothermia on acute ischemic edema: Morphometric analysis of the ICTuS trial. Neruocrit Care. 2008;8:42–47. doi: 10.1007/s12028-007-9009-z. [DOI] [PubMed] [Google Scholar]
- 29.Gupta R. Yonas H. Gebel J. Goldstein S. Horowitz M. Grahovac SZ. Wechsler LR. Hammer MD. Uchino K. Jovin TG. Reduced pretreatment ipislateral middle cerebral blood flow is predictive of symptomatic hemorrhage post-intra-arterial thrombolysis in patients with middle cerebral artery occlusion. Stroke. 2006;37:2526–2530. doi: 10.1161/01.STR.0000240687.14265.b4. [DOI] [PubMed] [Google Scholar]
- 30.Hacke W. Kaste M. Bluhmki E. Brozman M. Davalos A. Guidetti D. Larrue V. Lees KR. Medeghri Z. Machnig T. Schneider D. von Kummer R. Wahlgren N. Toni D ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 31.Iida H. Schmeichel AM. Wang Y. Schmelzer JD. Low PA. Orchestration of the inflammatory response in ischemia-reperfusion injury. J Peripher Nerv Syst. 2007;12:131–138. doi: 10.1111/j.1529-8027.2007.00132.x. [DOI] [PubMed] [Google Scholar]
- 32.Jarvis CR. Anderson TR. Andrew D. Anoxic depolarization mediates acute damage independent of glutamate in neocortical brain slices. Cereb Cortex. 2001;11:249–259. doi: 10.1093/cercor/11.3.249. [DOI] [PubMed] [Google Scholar]
- 33.Joshi I. Andrew RD. Imaging anoxic depolarization during ischemia-like conditions in the mouse hemi-brain slice. J Neurophysiol. 2001;85:414–424. doi: 10.1152/jn.2001.85.1.414. [DOI] [PubMed] [Google Scholar]
- 34.Jovin TG. Yonas H. Gebel JM. Kanal E. Chang YF. Gahovac SZ. Goldstein S. Wechsler LR. The cortical ischemic core and the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke. 2003;34:2426–2433. doi: 10.1161/01.STR.0000091232.81947.C9. [DOI] [PubMed] [Google Scholar]
- 35.Kidwell CS. Alger JR. Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2003;35:2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 36.Krieger DW. DeGeorgia MA. Abou–Chebl A. Andrefsky JC. Sila CA. Katzan IL Mayberg MR. Furlan AJ. Cooling for acute ischemic brain damage (COOL AID). An open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32:1847–1854. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- 37.Latchaw RE. Yonas H. Hunter GJ. Yuh WT. Ueda T. Sorensen AG. Sunshine JL. Biller J. Wechsler L. Higashida R, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke. 2003;34:1084–1104. doi: 10.1161/01.STR.0000064840.99271.9E. [DOI] [PubMed] [Google Scholar]
- 38.Lekiefre D. Meldrum BS. The pyrimidine-derivative, BW 1003C87, protects CA1 and striatal neurons following transient severe forebrain ischaemia in rats. A microdialysis and histological study. Neuroscience. 1993;56:93–99. doi: 10.1016/0306-4522(93)90565-w. [DOI] [PubMed] [Google Scholar]
- 39.Li J. Liu W. Ding S. Xu W. Guan Y. Zhang JH. Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Liberatore GT. Samson A. Bladin C. Schleuning WD. Medcalf RL. Vampire bat salivary plasminogen activator (Desmoteplase). A unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke. 2003;34:537–543. doi: 10.1161/01.str.0000049764.49162.76. [DOI] [PubMed] [Google Scholar]
- 41.Liu B. Liao M. Mielke JG. Ning K. Chen Y. Li L. El–Hayek YH. Gomez E. Zukin RS. Fehlings MG. Wan Q. Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J Neurosci. 2006;26:5309–5319. doi: 10.1523/JNEUROSCI.0567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Jones D. Adams R. Carnethon M. De Simone G, et al. Heart disease and stroke statistics-2009 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 43.Loddick SA. MacKenzie A. Rothwell NJ. An ICE inhibitor, z-VAD-DCB attenuates ischaemic brain damage in the rat. Neuroreport. 1996;7:1465–1468. doi: 10.1097/00001756-199606170-00004. [DOI] [PubMed] [Google Scholar]
- 44.Lopez–Atalaya JP. Roussel BD. Levrat D. Parcq J. Nicole O. Hommet Y. Benchenane K. Castel H. Leprince J. To Van D. Bureau R. Rault S. Vaudry H. Petersen KU. Santos JS. Vivien D. Toward safer thrombolytic agents in stroke: Molecular requirements for NMDA receptor-mediated neurotoxicity. J Cereb Blood Flow Metab. 2008;28:1212–1221. doi: 10.1038/jcbfm.2008.14. [DOI] [PubMed] [Google Scholar]
- 45.Lu D. Zhang X. Han YY. Burke NA. Kochanek PM. Watkins SC. Graham SH. Carcillo JA. Szabo C. Clark RSB. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- 46.Lysko PG. Webb CL. Yue TL. Gu JL. Feuerstein G. Neuroprotective effects of tetrodotoxin as a Na+ channel modulator and glutamate release inhibitor in cultured rat cerebellar neurons and in gerbil global brain ischemia. Stroke. 1994;25:2476–2482. doi: 10.1161/01.str.25.12.2476. [DOI] [PubMed] [Google Scholar]
- 47.Martin–Schild S. Hallevi H. Shaltoni H Barreto AD. Gonzales NR. Aronowski J. Savitz S. Combined neuroprotective modalities coupled with thrombolysis in acute ischemic stroke: A pilot study of caffeinol and mild hypothermia. J Stroke Cerebrovasc Dis. 2009;18:85–96. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta SL. Manhas N. Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Mishra NK. Albers GW. Davis SM. Donnan GA. Furlan AJ. Hacke W. Lees KR. Mismatch-based delayed thrombolysis: A meta-analysis. Stroke. 2010;41:e25–e33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- 50.Noh KM. Yokota H. Mashiko T. Castillo PE. Zukin RS. Bennett MVL. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci USA. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onteniente B. Rasika S. Benchoua A. Guegan C. Molecular pathways in cerebral ischemia. Cues to novel therapeutic strategies. Mol Neurobiol. 2003;27:33–72. doi: 10.1385/MN:27:1:33. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrini–Giampietro DE. Zukin RS. Bennet MV. Cho S. Pulsinelli WA. Switch in glutamate receptor subunit gene expression in CA1 subfield of hippocampus following global ischemia in rats. Proc Natl Acad Sci USA. 1992;89:10499–10503. doi: 10.1073/pnas.89.21.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rami A. Agarwal R. Botez G. Winckler J. μ-Calpain activation, DNA fragmentation, and synergistic effects of caspase and calpain inhibitors in protecting hippocampal neurons from ischemic damage. Brain Res. 2000;866:299–312. doi: 10.1016/s0006-8993(00)02301-5. [DOI] [PubMed] [Google Scholar]
- 54.Rha JH. Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 55.Robinson MB. The family of sodium-dependent glutamate transporters: A focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 56.Savitz SI. Fisher M. Future of neuroprotection for acute stroke: In the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 57.Singhal AB. Oxygen therapy in stroke: Past, present and future. Int J Stroke. 2006;1:191–200. doi: 10.1111/j.1747-4949.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 58.Smith SE. Meldrum BS. Cerebroprotective effect of lamotrigine after focal ischemia in rats. Stroke. 1995;26:117–122. doi: 10.1161/01.str.26.1.117. [DOI] [PubMed] [Google Scholar]
- 59.Tapuria N. Kumar Y. Habib MM. Amara MA. Seifalian AM. Davidson BR. Remote ischemic preconditioning: A novel protective method from ischemia reperfusion injury. A review. J Surg Res. 2008;150:304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 60.Traystman RJ. Kirsch JR. Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 61.Wang Q. Sun AY. Simonyi A. Kalogeris TJ. Miller DK Sun GY. Korthius RJ. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: Role of NADPH oxidase derived ROS. Free Radic Biol Med. 2007;43:1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y. Dawson VL. Dawson TM. Poly (ADP-ribose) signals to mitochontrial AIF: A key event in parthanatos. Exp Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White BC. Sullivan JM. Degracia DJ. O'Neil BJ. Neumar RW. Grossman LI. Raflos JA. Krause GS. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 64.Yu SW. Andrabi SA. Wang H. Kim NS. Poirier GG. Dawson TM. Dawson VL. Apoptosis-inducing factors mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Nat Acad Sci USA. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao H. Yenari MA. Cheng D. Sapolsky RM. Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhao X. Strong R. Piriyawa P. Palusinski R. Grotta JC. Aronowski J. Caffeinol at the receptor level. Anti-ischemic effect of N-methyl-D-Aspartate receptor blockade is potentiated by caffeine. Stroke. 2010;41:363–367. doi: 10.1161/STROKEAHA.109.562900. [DOI] [PMC free article] [PubMed] [Google Scholar]