Abstract
Scaffold-guided vascular tissue engineering has been investigated as a means to generate functional and transplantable vascular tissue grafts that increase the efficacy of cell-based therapeutic strategies in regenerative medicine. In this study, we employed confocal microscopy and three-dimensional reconstruction to assess the engraftment and growth potential of vascular cells within an alginate scaffold with aligned pores. We fabricated honeycomb alginate scaffolds with aligned pores, whose surface was immobilized with fibronectin and subsequently coated with matrigel. Endothelial cells were seeded into aligned pore scaffolds in the presence and absence of human smooth muscle cells. We showed that endothelial cells seeded into alginate scaffolds attach on the surface of aligned pores in vitro, giving rise to stable co-cultures of vascular cells. Moreover, the three-dimensional alginate depots containing the cells were exposed to laminar flow in order to recapitulate physiological shear stress found in the vasculature in vivo. After the flow exposure, the scaffold remained intact and some cells remained adherent to the scaffold and aligned in the flow direction. These studies demonstrate that alginate scaffolds provide a suitable matrix for establishing durable angiogenic modules that may ultimately enhance organ revascularization.
Introduction
Pluripotent stem cells offer considerable clinical potential for the regenerative therapy of injured and diseased tissues. However, cellular regeneration using derivatives of pluripotent stem cells has been confronted with major hurdles. Specifically, early attempts to deliver cellular grafts fabricated from bone marrow-derived stem cells for repair of myocardial infarction have met with little success. It is likely that long-term, functional engraftment of the in vitro generated vascular cells within injured or diseased tissues has failed because of apoptotic cell death and/or inability of the graft to connect with the existing circulatory system. Thus, a synthetic, microfabricated matrix with tubular channels that allow colonization with vascular cells could provide an ideal substrate upon which biologically compatible, vascularized tissue grafts can be built.
Scaffold-guided tissue engineering has emerged as a promising therapeutic strategy to develop functional and transplantable vascular tissue constructs. Scaffolds serve as three-dimensional (3D) templates for cell attachment and subsequent tissue formation,1–9 and unlike conventional cell cultures which involve growing cells in a nonphysiological 2D environment, positioning of the vascular cells within 3D scaffolds may provide cytoskeletal or extrinsic cues involving enhanced interaction with extracellular matrix components essential for formation of tissue grafts that are functional following transplantation.
Several vascular prostheses made from a wide variety of synthetic materials, including nylon, Teflon, Dacron, polyurethane, expanded poly(tetrafluoroethylene), have been previously reported.10 However, as the desirable function of a scaffold is to serve as a temporal substrate for the attachment and proliferation of cells, the design of biodegradable 3D materials with porous structures has been extensively investigated. The biodegradable 3D materials investigated include glycolide–lactide copolymer nonwoven fabrics, collagen sponges, hyaluronic acid sponges, and alginate sponges.11,12
In this study, we fabricated an alginate scaffold with honeycomb aligned pores, which could be suitable for vascular tissue engineering because the dynamic physiological environment of the microvasculature can be simulated. We visualized the attachment of fluorescently labeled live cells within scaffolds and examined the behavior and morphology of vascular cells under both static and dynamic culture conditions. Finally, we showed that alginate scaffolds could serve as a template for intimate co-cultures of vascular endothelial and smooth muscle cells in vitro. This study suggests that a honeycomb alginate scaffold with aligned pores can provide a substrate suitable for establishing durable vascular modules that may ultimately enhance organ revascularization.
Materials and Methods
Fabrication of honeycomb alginate scaffolds with aligned pores
Sodium alginate with a molecular weight of 64,000, 95,000, and 110,000 was purchased from Wako Pure Chemical Industry (Osaka, Japan). Other chemicals were obtained from Nacalai Tesque (Kyoto, Japan), Calbiochem (La Jolla, CA), BD Biosciences (Franklin Lakes, NJ), and Sigma Chemical (St. Louis, MO) and used without further purification.
Alginate gels were prepared according to the ionotropic gelation method described previously.13 Briefly, sodium alginate was dissolved at different concentrations in distilled water as summarized in Table 1. The inner wall of the glass beakers was coated with a thin layer of sodium alginate and dried at 110°C for 0.5 h. An aqueous solution of sodium alginate (20 mL) was poured into the alginate-coated beaker and calcium chloride solution was sprayed until a thin alginate gel layer was formed on top of the sodium alginate solution. Then, 10 mL of calcium chloride solution was added on the thin gel layer, followed by overnight incubation at room temperature to complete gelation. This ionotropic gelation generated parallel channel-like pores in alginate gels. The gels were subsequently washed with deionized water to remove free calcium ions. The resulting gels were frozen at −80°C and lyophilized under vacuum at room temperature for 2 days. The lyophilized alginate gels were cut into disc-shaped units of 5 mm in diameter and 2 mm in thickness and then subjected to further chemical modifications. This lyophilization process allows the gels to form a honeycomb structure.
Table 1.
Effect of Alginate Concentration, Alginate Molecular Weight, and Calcium Concentration on Channel Formation
| Alginate concentration (wt/vol %) | Alginate molecular weight | Calcium concentration (M) | Channel diameter (μm) | Channel density (%) |
|---|---|---|---|---|
| 0.5 | 110,000 | 1.0 | 218 ± 37 | 79.1 |
| 1.0 | 110,000 | 1.0 | 239 ± 43 | 93.3 |
| 2.0 | 110,000 | 1.0 | 352 ± 71 | 93.7 |
| 4.0 | 110,000 | 1.0 | None porous | — |
| 2.0 | 64,000 | 1.0 | 270 ± 45 | 93.8 |
| 2.0 | 95,000 | 1.0 | 286 ± 55 | 91.4 |
| 2.0 | 110,000 | 1.0 | 352 ± 71 | 93.7 |
| 2.0 | 110,000 | 0.5 | 461 ± 110 | 92.9 |
| 2.0 | 110,000 | 1.0 | 352 ± 71 | 93.7 |
| 2.0 | 110,000 | 1.5 | 312 ± 32 | 94.7 |
Alginate gel discs were covalently crosslinked with ethylenediamine (ED) by activating carboxyl group with N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). The alginate discs were immersed in 2-(N-morpholino)ethanesulfonic acid buffer (0.2 M, pH 6.0) containing ethylenediamine (5.17 mM), NHS (114 mM), and EDC (305 mM) and centrifuged at 1500 rpm for 5 min to eliminate air bubbles from channels in the alginate discs. After 24 h of crosslinking, the resulting discs were washed three times with phosphate-buffered saline (PBS).
Characterization of honeycomb alginate scaffolds with aligned pores
Honeycomb alginate scaffolds with aligned pores were prepared at different fabrication conditions as shown in Table 1. After sputter coating with gold/palladium, the samples were viewed on a scanning electron microscope (SEM; S2380N; Hitachi, Tokyo, Japan). The average pore diameter and pore density of the scaffolds were measured by image analysis of the SEM pictures with ImageJ software (version 1.38; National Institutes of Health, Bethesda, MD).
Surface modification of pores with fibronectin and matrigel for cell culture
To facilitate cell attachment onto the channel wall of honeycomb alginate scaffolds with aligned pores, fibronectin was covalently immobilized through NHS and EDC chemistry. Briefly, crosslinked scaffolds were immersed in a 0.1 mg/mL fibronectin/PBS solution containing NHS (114 mM) and EDC (305 mM), followed by overnight incubation at room temperature. Unbound fibronectin was washed out with PBS. The resulting scaffolds were sterilized with 70% ethanol and washed with sterile PBS and serum-free RPMI 1640 medium, respectively. Then the matrigel was coated onto the channel walls by immersing fibronectin-immobilized scaffolds into 10-fold diluted matrigel solution (BD Biosciences) at 4°C.
Cell culture
Primary ECs were maintained in vitro in medium supplemented with serum and proangiogenic factors. Such media may provide confounding variables when ECs are co-cultured with other cells. As a result, E4ORF1-transduced ECs were produced by transducing adenoviral E4ORF1 gene into primary human ECs, to generate a long-lasting angiogenic state. We have recently reported that this approach supports long-term survival of the endothelial cells (ECs) in serum/cytokine-free conditions without inducing transformation.14,15 A lentivirus carrying the pgk-green fluorescent protein (GFP) gene and the pgk-mCherry gene was infected into E4ORF1-transduced human umbilical vein ECs (HUVECs) or wild-type HUVECs and human smooth muscle cells (HuSMCs), respectively. GFP-labeled HUVECs and mCherry-labeled HuSMCs were maintained in medium 199 containing endothelial mitogen, heparin, antibiotics, and 20% fetal bovine serum. To seed these cells into a matrigel-coated scaffold, the scaffold was aseptically placed in an insulin syringe, followed by loading the cell suspension containing GFP-labeled HUVECs and mCherry-labeled HuSMCs at different mixing ratios (total 0.5 × 106 cells/25 μL) into the scaffold-placed syringe. The cell-loaded scaffold in the insulin syringe was put into a Petri dish and shaken at 37°C and 300 rpm for 2 h to achieve a homogeneous cell distribution throughout the scaffolds.
Evaluation of cell number and survival in honeycomb alginate scaffolds with aligned pores
The number of E4ORF1-transduced HUVECs (E4ORF1 + HUVECs) attached to alginate scaffolds was determined by fluorometric quantification of cellular DNA according to the method reported previously.16 Briefly, the cell-seeded scaffolds were washed with PBS and stored at −80°C until assayed. After thawing, the samples were lysed in 30 mM saline-sodium citrate buffer (SSC) (pH 7.4) containing 0.2 mg/mL sodium dodecylsulfate at 37°C for 12 h. The cell lysate (100 μL) was mixed with SSC (400 μL) in a glass tube. After mixing with a dye solution (500 μL; 30 mM SSC, 1 mg/mL Hoechst 33258 dye), the fluorescent intensity of mixed solution was measured in a fluorescence spectrometer (Spectramax M5; Molecular Devices, Sunnyvale, CA) at the excitation and emission wavelengths of 355 and 460 nm, respectively. The calibration curve between the DNA and cell number was prepared using cells of known numbers. The DNA assay was performed three times independently for every experimental sample unless otherwise noted.
E4ORF1 + HUVEC-seeded scaffolds were soaked in trypsin (0.25%)–disodium ethylenediamine tetraacetic acid (53 mM) at 37°C for 5 min to collect the cells from within. The collected cells were washed with fresh media and PBS and then subjected to an apoptosis assay using the Annexin V-FITC apoptosis detection kit (BD Biosciences) according to the manufacturer's instructions. The cells treated with the kit were analyzed by flow cytometry (Cytomics FC-500; Beckman Coulter, Fullerton, CA).
Confocal microscopic observation of cells in honeycomb alginate scaffolds with aligned pores
At different time points, cell attachment was examined by Z-stack-imaging with a confocal microscope (LSM-510 META; Carl Zeiss MicroImaging GmbH, Göttingen, Germany).
Testing of scaffold-vascular integrity in a dynamic shear stress system
A flow chamber with an inner diameter of 3 mm was constructed. Using a biopsy puncher, cell-seeded honeycomb scaffolds were cut into a disc shape with a diameter of 3 mm and aseptically placed into the flow channel. The scaffolds were placed in a flow chamber designed to align the pores of the scaffold along the direction of laminar flow. Then the chamber was connected to a closed-tube circuit with a reservoir of culture media and a buffer dam to avoid the effect of pulsation from a peristaltic pump. The shear stress was changed by flow rate, which was determined experimentally by the volume of media collected per minute.
Results
Honeycomb alginate scaffolds can be engineered into homogenously microfabricated vessel-like structures
Delivery of ECs for vascularization of tissues requires microfabrication of a homogenously aligned capillary-like scaffold. After screening a large number of biomaterials, we have determined that alginate scaffolds can be readily microfabricated into long, parallel pores mimicking the typical structure of blood vessel capillaries (Fig. 1).
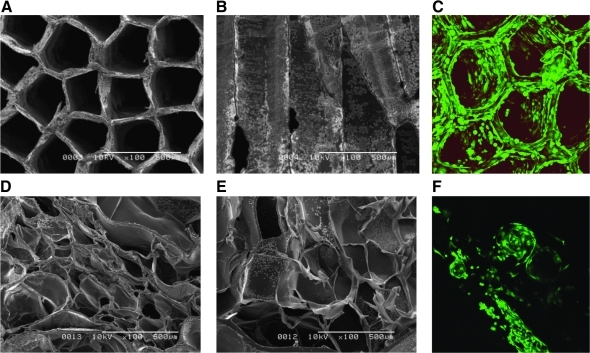
FIG. 1.
A representative image of alginate scaffolds and GFP-labeled E4ORF1 + HUVECs in the scaffolds obtained by scanning electron microscope and confocal microscope. (A–C) Alginate scaffolds with aligned-pore structure. Average pore diameter is 352 μm and porosity is 93.7%. (D–F) Control alginate scaffolds with random-pore structure. (A, D) Horizontal images and (B, E) vertical images of scaffolds. Magnification of each image is 100 ×. In addition, Z-stack images were taken after 1 day of cell seeding at a magnification of 10 × and reconstructed through a projection mode using LSM510 software. GFP, pgk-green fluorescent protein; HUVECs, human umbilical vein endothelial cells.
The alignment of the pores was assessed by SEM. Figure 1 shows a representative SEM image of aligned-pore (Fig. 1A, B) and random-pore (Fig. 1D, E) alginate scaffolds. As a control, the random-pore scaffold was prepared by lyophilizing chemically crosslinked alginate gels for 2 days according to the method described previously.17 Irrespective of the scaffold types, highly porous structures were observed, although their pore arrangements were quite different. Indeed, as described previously,13 the ionotropic gelation generated a massive gel with parallel channel-like pores (data not shown). However, the lyophilization of the resulting gels generated honeycomb-like structure of the aligned-pore alginate scaffolds. Every scaffold had an aligned-pore structure except when prepared from the solution with the highest alginate concentration. Both the diameter and density of the aligned pores varied depending on their fabrication conditions (Table 1). In terms of homogeneity of cell seeding into the scaffolds, cells adhered homogeneously on the walls of aligned-pore scaffolds (Fig. 1C), whereas pores at the surface of random-pore scaffolds were occupied with seeded cells (Fig. 1F). Based on these results, we demonstrate that the structural features of aligned-pore scaffolds allow living cells to be visualized by confocal microscopy even at deep portions within the scaffold. As we used a lens with a short focal depth, there was a limitation of magnification for confocal imaging of thick scaffolds, and we could not visualize living cells in the scaffold at a high magnification capable of single-cell imaging. A 3-D rendering of a Z-stack captured of labeled cells at day 3 of seeding onto aligned pore scaffold is shown under supplemental movie, available online at www.liebertonline.com/ten.
Seeded ECs establish homogenous monolayers within the alginate scaffolds
To quantify the efficiency of the seeding method, mouse hemangioma-derived cells were seeded into the honeycomb alginate scaffolds with an average aligned pore diameter of 352 μm and a porosity of 93.7% by two different methods: dropping the cell suspension onto the honeycomb scaffolds and injecting the cell suspension into the honeycomb scaffolds. After 3 h of cultivation, mouse hemangioma-derived cells were trypsinized for detachment from the scaffolds and were counted with a hemocytometer. Cell-seeding efficiency was calculated by dividing the total number of cells by the number of seeded cells. The injection method showed a sevenfold higher seeding efficiency (12.4%) than the dropping method (1.8%). Based on this result, the injection method was employed for cell seeding in the following studies.
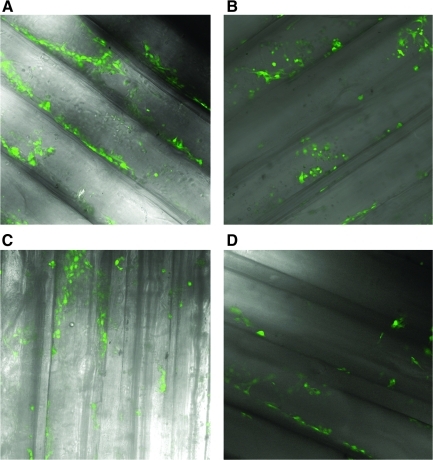
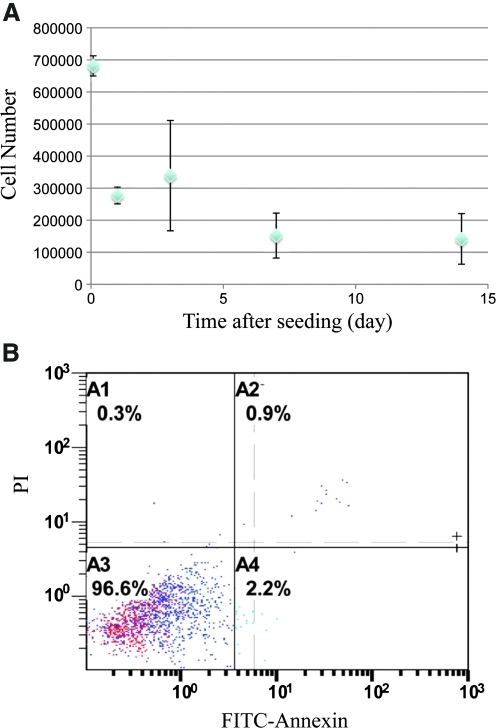
Figure 2 shows the time course of cell attachment in fibronectin-immobilized honeycomb scaffolds without matrigel coating. We found homogeneous cell attachment on the surface of pores in scaffolds at day 1 (Fig. 2A), but the cells on the pore surface detached after 3 days (Fig. 2B) or longer in culture. Therefore, we coated fibronectin-immobilized honeycomb scaffolds with matrigel to improve cell attachment for subsequent studies. After the improvement of cell attachment to the honeycomb scaffolds by matrigel coating, the cells adhered homogeneously to the walls of honeycomb alginate scaffolds (Fig. 1C). These observations are consistent with the quantitative profile of the number of cells in the scaffolds. Even after the initial cell loss from the scaffolds, the remaining cells remained stable over time (Fig. 3A). Flow cytometry analysis for apoptotic and dead cells showed that most of the attached cells (96.6% of the total collected cells) in the alginate scaffolds were alive 1 week after seeding (Fig. 3B).
FIG. 2.
Longitudinal confocal images of GFP-labeled E4ORF1 + HUVECs in alginate scaffolds in the absence of matrigel (A) 1, (B) 3, (C) 5, and (D) 7 days after cell seeding.
FIG. 3.
Cell number and survival of E4ORF1 + HUVECs in honeycomb alginate scaffolds with aligned-pore structure. The mean with the standard deviation of the mean was plotted. (A) Time profile of the number of cells attaching on aligned pores in honeycomb alginate scaffolds and (B) fluorescence activated cell sorting analysis for apoptotic and dead cells in alginate scaffolds 1 week after seeding. The mean with the standard deviation of the mean was plotted. Color images available online at www.liebertonline.com/ten.
Microfabrication of a stable co-culture of vascular cells using E4ORF1 + HUVECs and smooth muscle
Delivery of vascular cell-seeded alginate scaffolds into injured tissue requires that the ECs maintain their angiogenic repertoire for several hours or days. As naive primary ECs require chronic stimulation with serum and angiogenic factors, including fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor A (VEGF-A), microfabrication of alginate scaffolds with such ECs may not be feasible. Therefore, to circumvent the use of primary ECs and optimize the conditions for delivery of scaffolds, we used novel primary ECs that could maintain their angiogenic and structural integrity in the absence of serum or angiogenic factors. To achieve this goal, we used ECs that were transduced with the adenoviral E4ORF1 gene, which allows for long-term survival of the ECs through Akt activation without mitogen-activated protein (MAP)-kinase activation or oncogenic transformation.14
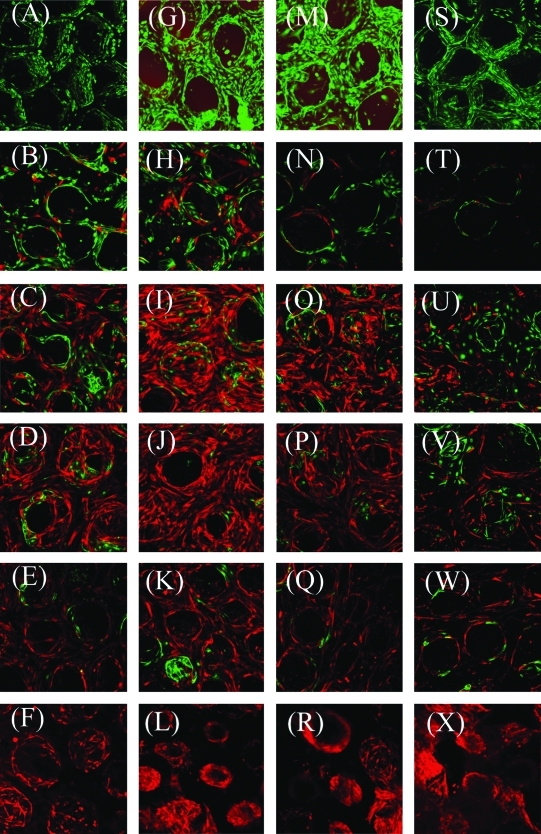
E4ORF1 + HUVECs were seeded into the alginate scaffold under serum-free and cytokine-free conditions. Figure 4 shows a time course of confocal images of cocultured E4ORF1 + HUVECs (green, GFP) with HuSMC (red, mCherry) at different mixing ratios and time points. We could visualize both fluorescent-labeled E4ORF1 + HUVECs and HuSMCs even one millimeter away from the scaffold surfaces. Based on confocal imaging, cell populations varied depending on the initial mixing ratios. Many cells attached to the alginate scaffolds over time, although some cells, especially the E4ORF + HUVECs, seemed to detach from the scaffolds rather than the HuSMC. Moreover, we found similar seeding efficiency for naive primary HUVECs mixed with HuSMCs at different mixing ratios (Supplemental Figs. 1 and 2, available online at www.liebertonline.com/ten).
FIG. 4.
Time course of confocal images for GFP-labeled E4ORF1 + HUVECs co-cultured with mCherry-labeled HuSMCs at different seeding ratios. Z-stack images were taken after (A–F) 3, (G–L) 5, (M–R) 7, and (S–X) 14 days of cell seeding at a magnification of 10 × and reconstructed through a projection mode using LSM510 software. Seeding ratios are (A, G, M, S) 0.50 × 106 HUVECs, (B, H, N, T) 0.25 × 106 HUVECs + 0.25 × 106 HuSMCs, (C, I, O, U) 0.16 × 106 HUVECs + 0.34 × 106 HuSMCs, (D, J, P, V) 0.10 × 106 HUVECs + 0.40 × 106 HuSMCs, (E, K, Q, W) 0.05 × 106 HUVECs + 0.45 × 106 HuSMCs, and (F, L, R, X) 0.50 × 106 HuSMCs, respectively. HuSMCs, human smooth muscle cells.
Testing of scaffold-vascular integrity in a dynamic shear stress system
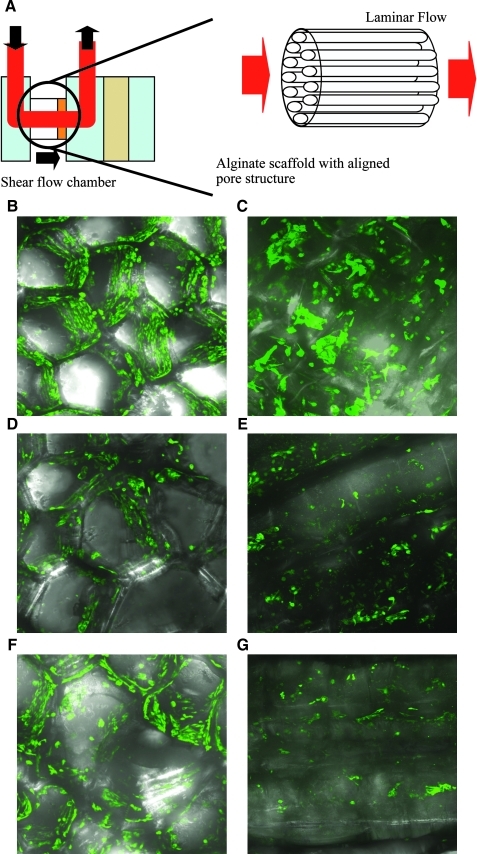
Biomechanical forces, such as shear stress, are known to induce signaling responses that regulate vascular tone. Static conditions commonly used to study vascular cells in culture may not reflect the in-vivo milieu. We have established a protocol to study the influence of shear stress on the behavior of cells seeded onto honeycomb scaffolds. The scaffolds were placed in a flow chamber designed to align the honeycomb scaffold along the direction of the laminar flow. The aligned-pore design enables us to examine the role of fluid shear stress to recapitulate the physiological vasculature.18 To investigate the influence of mechanical forces in 3D environments, the scaffolds were exposed to a shear force of 0 (Fig. 5B, C), 1 (Fig. 5D, E), and 2 dyne/cm2 (Fig. 5F, G). The longitudinal images of cells after the fluid exposure (Fig. 5E, G) show that honeycomb scaffolds remain intact and some cells remain adherent to the honeycomb scaffolds after exposure to shear flow. Despite the limitation of magnification for confocal imaging of the thick scaffolds, we observed that some cells were aligned in the direction of flow (Fig. 5E, G). These confocal images confirm that the cells remain adhered to the scaffold in this dynamic setup, thus making this scaffold system a viable model for studying the role of fluid shear stress on a vascular compartment.
FIG. 5.
Flow shear stimulation for E4ORF1 + HUVECs attaching on aligned pores of alginate scaffolds. (A) Schematic diagram of flow shear stimulation for cells in the scaffolds. (B) Projected confocal image of GFP-labeled E4ORF1 + HUVECs in alginate scaffolds 1 day after cell seeding. (C) Longitudinal confocal image of GFP-labeled E4ORF1 + HUVECs in alginate scaffolds 1 day after cell seeding. (D, F) Projected and (E, G) longitudinal confocal images of GFP-labeled E4ORF1 + HUVECs in alginate scaffolds 1 day after flow stimulation with a shear force of (D, E) 1 and (F, G) 2 dyne/cm2.
Discussion
We have created a 3D honeycomb framework with aligned pores to accommodate cell adhesion and subsequent growth to generate stable co-cultures of vascular cells. This template is composed of sodium alginate and its pore size can be manipulated by changing the fabrication conditions to create different scaffolds for cell seeding. Moreover, the special honeycomb structure allows us to shorten the cell-seeding duration and to establish a homogeneous cell distribution. Finally, the special structure allows for live imaging of cell attachment in the scaffolds over long periods of time. Attempts have been made by other investigators to develop a technique for noninvasive live imaging of cells in scaffolds, using different modalities such as confocal microscopy, magnetic resonance imaging, and X-ray computer tomography.19,20 These noninvasive techniques for live cell imaging could allow for high-resolution monitoring of the development and incorporation of tissue-engineered constructs. Our approach was to combine the fabrication of the well-designed aligned pore structure with confocal microscopy, not only to mimic a vascular structure and expose the cultured cells to laminar shear stress, but also to enable fluorescent-based imaging of attached cells on the inside of the scaffolds.
To generate the honeycomb aligned-pore structure in alginate scaffolds, we employed ionotropic gelation of sodium alginate with calcium, a major divalent cation in the body. When mixed with divalent cations, sodium alginate forms egg-box structures by ionic crosslinking, resulting in gelation. Upon sol–gel transition, alginate chains are condensed and the excess amount of water is separated from the resulting gel. Thus the shrunken gel is formed, although the extent of its shrinkage depends on several fabrication conditions, such as polymer concentration, molecular weight, calcium concentration, and the composition of β-d-mannuronate and its C-5 epimer α-l-guluronate residues. As the reaction vessel is coated with sodium alginate, and an alginate gel membrane is fabricated on the sodium alginate solution by spraying calcium chloride solution, one-directional gelation occurs by the diffusion of calcium ions, followed by isotropic shrinking and condensation in a planar boundary at the gelation front in the sodium alginate solution. This results in the generation of the honeycomb aligned-pore structure in alginate scaffolds. The increased calcium concentration results in an increase in the number of the gelation centers and an eventual decrease in pore size. Moreover, sodium alginate with a higher molecular weight and a higher polymer concentration exhibits stronger shrinkage and condensation, resulting in the generation of a gel with larger pore size. However, only massive gels are formed from the sodium alginate solution with an excessive polymer concentration.
Moreover, alginate is biocompatible and chemically susceptible and has been clinically approved as a wound cover.21 However, as alginate is hydrophilic, which shortens cell-seeding duration, surface modification of the scaffolds is required. Some cell adhesion molecules, such as arginine-glycine-aspartic acid-serine (RGDS) peptide, fibronectin, and laminin, have been utilized for coating and immobilization on cell substrates and scaffolds. Among the cell adhesion molecules listed, fibronectin was employed to be immobilized on the surface of honeycomb aligned-pore alginate scaffolds. Using radioiodinated fibronectin, we quantified the amount of fibronectin immobilized onto alginate scaffolds. Briefly, we used the fibronectin solution containing trace amount of radioiodinated fibronectin for the immobilization reaction and calculated the amount of immobilized fibronectin based on the radioactivity of a scaffold after the reaction and the specific radioactivity of radioiodinated fibronectin against the protein amount. We determined that the density of immobilized fibronectin on the surface of pores in alginate scaffolds was ∼240 ng/cm2. Pompe et al.22 have reported the effect of immobilization properties of fibronectin on the initial phase of EC adhesion and documented cell attachment on a fibronectin-immobilized surface with a surface fibronectin density of 450 ng/cm2. As alginate itself is hydrophilic, it is possible that cells cannot attach firmly on the scaffold pore surface, even with a surface fibronectin density similar to that reported by Pompe et al. Unfortunately, we did not investigate the effect of surface density and molecular orientation of fibronectin on the behavior of HUVECs, but ultimately did observe the detachment of HUVECs from scaffolds. This allowed us to conclude that matrigel coating for fibronectin-immobilized scaffolds could improve cell adhesion, resulting in stable culture or co-culture of HUVECs and HuSMCs in the matrigel-coated scaffolds (Figs. 1 and 4). Taken together, this proves the efficacy of utilizing matrigel-coated fibronectin-immobilized scaffolds in this study.
Various strategies have been utilized to optimize cell seeding and distribution within a scaffold: the pouring of cell suspensions onto scaffolds,23–25 the injection of cell suspensions with a syringe,26 and the seeding of cell suspensions into scaffolds in spinner flasks27–29 and in a perfusion culture system.30,31 Kim et al.32 have reported that the number of cells attached to a scaffold was enhanced with an agitation cell-seeding method, wherein the scaffold is agitated in the cell suspension. Given the strong structural feature of our honeycomb alginate scaffold, we used direct suction of the cell suspension into the scaffolds, followed by agitating cell-loaded scaffolds to allow cells to be well distributed throughout the scaffolds. On the contrary, because of the limited pore surface area available for seeded cells to attach and because we seeded cells in excess of the number needed for efficient cell seeding into honeycomb, we observed some cell aggregates at day 1 (data not shown). The aggregated cells could be washed out by sequential medium change over time. Another possible explanation is that the cells potentially stopped proliferation as a result of contact inhibition, because HUVECs grow as a monolayer culture. The confluent cell density for HUVECs in a 2D model was ∼2 × 104 cells/cm2, and a similar cell density, 3 × 104 cell/cm2, was observed for the 3D culture in alginate scaffolds. These results suggest that HUVECs could be confluent in alginate scaffolds. In addition, if we consider the possibility of a phenotype alteration by activating HUVECs in 3D milieu, resulting in increasing cell number at confluence, an EC density closer to that of native vessels could be demonstrated. This represents a value of ∼1 × 105 cells/cm2—three to four times higher than that of the 2D scaffold cultures.33 Therefore, the number of cells in culture was constant after 1 week of culture. In addition, as shown in Figures 3 and 4, HUVECs are viable and adherent to the surface of pores in scaffolds after 1 week in culture. Although we did not produce additional data that directly could support the viability and growth of the cells seeded onto the scaffold after 2 weeks in culture, taken together, it is conceivable that the viability and the number of HUVECs might remain constant. Further, the structural feature of honeycomb alginate scaffolds most likely enables cells to survive in the scaffolds because of the enhanced media supply from outside of the scaffold.
The objective of this study was to design and fabricate honeycomb alginate scaffolds with aligned pores to recapitulate the process of neovasculature formation by generating stable co-cultures of vascular vessels in vitro. The honeycomb alginate scaffold design allows ECs that are seeded onto the alginate to sprout into tube-like structures. Moreover, this design allows live imaging, to observe the distribution of fluorescently labeled smooth muscle cells and ECs within the 3D microenvironment. Lastly, the aligned pore design enables us to examine the role of physiologic fluid shear stress to recapitulate the physiological vasculature.18 Indeed, we observed by confocal imaging the presence of cell loss postexposure to shear stress (Fig. 5). However, we also observed that some cells in the honeycomb scaffolds remained aligned in the flow direction after exposure to shear flow (Fig. 5E, G), despite presumptions that exposure to shear stress normally changes the phenotype of ECs to an activated phenotype in two dimensions.34 To this end, the scaffolds were used as a 3D template to elucidate the mechanisms of vessel assembly and maturation. Consequently, we devised experiments aimed at establishing vascular morphogenesis.
The EC-loaded scaffolds allow us to guide tissue engineering as a means to grow vascular networks under physiological flow by imparting shear stress in a 3D biocompatible and biodegradable niche. This construct also allows controlled release of both chemokines and cytokines to enhance cell migration and cell proliferation, respectively (unpublished data). Once cells have been successfully seeded, this tissue-engineered platform may be implanted into a recipient to treat vascular disorders. The system has shown to lend itself to co-culture of different cell types (i.e., ECs and smooth muscle cells) to develop an in-vitro model of arteriogenesis. We have performed sequential addition of ECs and smooth muscle cells but observed that the secondary cells adhere poorly (data not shown). Other investigators have studied sequential cell seeding by coating some extracellular matrices on the first cell layer before the second cell seeding.35 This coating between the two layers functions as an adhesive. We did not perform such a coating to improve the adhesion of two layers, or any biological examinations to show the benefit of co-culture. Although challenges inherent to co-culture system remain, we nevertheless have proven that the structural features of honeycomb scaffolds can simulate the vascular beds by having the aligned pores act as parallel capillaries in three dimensions and therefore can serve as a bioreactor to recapitulate the physiological vasculature.
In summary, this approach can be used as a biodegradable scaffold for cell co-culture, a construct to engineer artificial tissue, a system for growth factor delivery, and a system to recapitulate embryonic development.
Supplementary Material
Acknowledgments
The research fellowship for Masaya Yamamoto at the Weill Medical College of Cornell University was supported by a grant from the Kyoto University Foundation for Promotion of Education and Research for a young faculty member.
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 3.Bach A.D. Stem-Straeter J. Beier J.P. Bannasch H. Stark G.B. Engineering of muscle tissue. Clin Plast Surg. 2003;30:589. doi: 10.1016/s0094-1298(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 4.Bannasch H. Fohn M. Unterberg T. Bach A.D. Weyand B. Stark G.B. Skin tissue engineering. Clin Plast Surg. 2003;30:573. doi: 10.1016/s0094-1298(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen R.R. Mooney D.J. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 6.Hubbell J.A. Tissue and cell engineering. Curr Opin Biotechnol. 2004;15:381. doi: 10.1016/j.copbio.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Langer R. Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 8.Saltzman W.M. Olbricht W.L. Building drug delivery into tissue engineering. Nat Rev. 2002;1:177. [Google Scholar]
- 9.Silva E.A. Mooney D.J. Synthetic extracellular matrices for tissue engineering and regeneration. Curr Top Dev Biol. 2004;64:181. doi: 10.1016/S0070-2153(04)64008-7. [DOI] [PubMed] [Google Scholar]
- 10.Jordan S.W. Chaikof E.L. Novel thromboresistant materials. J Vasc Surg. 2007;45(Suppl A):A104. doi: 10.1016/j.jvs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Kakisis J.D. Liapis C.D. Breuer C. Sumpio B.E. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Borenstein J.T. Weinberg E.J. Orrick B.K. Sundback C. Kaazempur-Mofrad M.R. Vacanti J.P. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13:1837. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 13.Dittrich R. Despang F. Bernhardt A. Mannschatz A. Hanke T. Tomandl G. Pompe W. Gelinsky M. Mineralized scaffolds for hard tissue engineering by ionotropic gelation of alginate. Adv Sci Technol. 2006;49:159. [Google Scholar]
- 14.Seandel M. Butler J.M. Kobayashi H. Hooper A.T. White I.A. Zhang F. Vertes E.L. Kobayashi M. Zhang Y. Shmelkov S.V. Hackett N.R. Rabbany S. Boyer J.L. Rafii S. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci USA. 2008;105:19288. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F. Cheng J. Hackett N.R. Lam G. Shido K. Pergolizzi R. Jin D.K. Crystal R.G. Rafii S. Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J Biol Chem. 2004;279:11760. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- 16.Rao J. Otto W.R. Fluorimetric DNA assay for cell growth estimation. Anal Biochem. 1992;207:186. doi: 10.1016/0003-2697(92)90521-8. [DOI] [PubMed] [Google Scholar]
- 17.Kong H.J. Kaigler D. Kim K. Mooney D.J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K. Sokabe T. Watabe T. Miyazono K. Yamashita J.K. Obi S. Ohura N. Matsushita A. Kamiya A. Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol. 2005;288:H1915. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 19.Pancrazio J.J. Wang F. Kelley C.A. Enabling tools for tissue engineering. Biosens Bioelectron. 2007;22:2803. doi: 10.1016/j.bios.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Kino-Oka M. Maeda Y. Sato Y. Khoshfetrat A.B. Yamamoto T. Sugawara K. Taya M. Characterization of spatial growth and distribution of chondrocyte cells embedded in collagen gels through a stereoscopic cell imaging system. Biotechnol Bioeng. 2008;99:1230. doi: 10.1002/bit.21667. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen H. Ubbink D.T. Goossens A. de Vos R. Legemate D.A. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005;92:665. doi: 10.1002/bjs.5055. [DOI] [PubMed] [Google Scholar]
- 22.Pompe T. Kobe F. Salchert K. Jørgensen B. Oswald J. Werner C. Fibronectin anchorage to polymer substrates controls the initial phase of endothelial cell adhesion. J Biomed Mater Res. 2003;67A:647. doi: 10.1002/jbm.a.10130. [DOI] [PubMed] [Google Scholar]
- 23.Ishaug-Riley S.L. Crane-Kruger G.M. Yaszemski M.J. Mikos A.G. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19:1405. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 24.Holy C.E. Shoichet M.S. Davies J.E. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J Biomed Mater Res. 2000;51:376. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Awad H.A. Butler D.L. Harris M.T. Ibrahim R.E. Wu Y. Young R.G. Kadiyala S. Boivin G.P. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res. 2000;51:233. doi: 10.1002/(sici)1097-4636(200008)51:2<233::aid-jbm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno S. Allemann F. Glowacki J. Effects of medium perfusion on matrix production by bovine chondrocytes in three-dimensional collagen sponges. J Biomed Mater Res. 2001;56:368. doi: 10.1002/1097-4636(20010905)56:3<368::aid-jbm1105>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Y.L. Riesle J. Van Blitterswijk C.A. Static and dynamic fibroblast seeding and cultivation in porous PEO/PBT scaffolds. J Mater Sci. 1999;10:773. doi: 10.1023/a:1008946832443. [DOI] [PubMed] [Google Scholar]
- 28.Burg K.J. Holder W.D., Jr. Culberson C.R. Beiler R.J. Greene K.G. Loebsack A.B. Roland W.D. Eiselt P. Mooney D.J. Halberstadt C.R. Comparative study of seeding methods for three-dimensional polymeric scaffolds. J Biomed Mater Res. 2000;52:576. doi: 10.1002/1097-4636(20001205)52:3<576::aid-jbm18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Bruinink A. Siragusano D. Ettel G. Brandsberg T. Brandsberg F. Petitmermet M. Muller B. Mayer J. Wintermantel E. The stiffness of bone marrow cell-knit composites is increased during mechanical load. Biomaterials. 2001;22:3169. doi: 10.1016/s0142-9612(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 30.Walluscheck K.P. Steinhoff G. Haverich A. Endothelial cell seeding of de-endothelialised human arteries: improvement by adhesion molecule induction and flow-seeding technology. Eur J Vasc Endovasc Surg. 1996;12:46. doi: 10.1016/s1078-5884(96)80274-7. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.S. Sundback C.A. Kaihara S. Benvenuto M.S. Kim B.S. Mooney D.J. Vacanti J.P. Dynamic seeding and in vitro culture of hepatocytes in a flow perfusion system. Tissue Eng. 2000;6:39. doi: 10.1089/107632700320874. [DOI] [PubMed] [Google Scholar]
- 32.Kim B.S. Putnam A.J. Kulik T.J. Mooney D.J. Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol Bioeng. 1998;57:46. [PubMed] [Google Scholar]
- 33.Sipehia R. Martucci G. Lipscombe J. Transplantation of human endothelial cell monolayer on artificial vascular prosthesis: the effect of growth-support surface chemistry, cell seeding density, ECM protein coating, and growth factors. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:51. doi: 10.3109/10731199609117431. [DOI] [PubMed] [Google Scholar]
- 34.Remuzzi A. Dewey C.F., Jr. Davies P.F. Gimbrone M.A., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21:617. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- 35.Matsusaki M. Kadowaki K. Nakahara Y. Akashi M. Fabrication of cellular multilayers with nanometer-sized extracellular matrix films. Angew Chem Int Ed Engl. 2007;46:468. doi: 10.1002/anie.200701089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.