Abstract
An externally regulated delivery model that permits temporal separation of multiple angiogenic factors was used for the delivery of basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF). While bFGF plays a significant role in the sprouting of new capillaries, PDGF plays a role in the recruitment of mural cells, which stabilize neovessels. However, these two factors have been shown to inhibit each other, when presented together. Using the externally regulated model, sequential delivery of bFGF and PDGF led to not only increased endothelial cell migration, but also endothelial cell and vascular pericyte colocalization. More importantly, this delivery strategy was able to induce red blood cell-filled neovessels, suggesting integration of angiogenesis with the existing vasculature.
Introduction
Awide range of biomedical research focuses on biological processes where cell surface-bound and secreted biomolecules control cellular action. It is becoming apparent that combinations of these various biomolecules can form organized sets of instructions that can be accentuated or even inverted depending upon the temporo-spatial organization of the stimuli (see examples from adaptive immunity,1 immunological tolerance,2 pancreatic insulin regulation,3 lipolysis,4 and osteocoupling,5 as just a few emerging examples). Consequently, it is not surprising that the complexity of these biological processes dwarfs the complexity of current treatments intending to direct, accelerate, or repair them. A prominent example of a biological process that is currently over-simplified by existing treatments is angiogenesis (the growth of neovasculature from existing vasculature).
Specifically, angiogenesis is an organized series of events involving multiple angiogenic growth factors that become important at varying stages throughout the process.6 First, endothelial cells residing in existing vessels receive instructions causing their cell–cell and cell–basement membrane contacts to loosen.7–9 This event is followed by the proliferation and migration/invasion of these endothelial cells, ultimately aligning to form a capillary lumen.8,9 Once the vessel is formed, endothelial cells receive signals that cause them to halt proliferation as well as migration and secrete new basement membrane.6,9 Finally, vascular pericytes are recruited to the vessels, where they adhere to endothelial cells.6
Considering the temporal complexity of this process, it is not surprising that strategies focusing on delivery only a single angiogenic factor10–13 or even two angiogenic factors simultaneously14,15 have met limited success. One possible strategy for more effective treatments would involve stage-wise delivery of growth factors known to support cellular action during the corresponding stage of angiogenesis. Yet, only recently has delivery technology (layer-by-layer films,16 rational design of bio-erodible systems17,18) developed to the point where engineers soon will be capable of precisely controlling the temporal separation of these instructions.
To gain information regarding the most relevant time-frames, concentrations, and growth factors to be used in therapeutic sequential delivery strategies, we recently developed a simple and modular, externally regulated delivery model.19 This model consists of a porous hollow fiber that extends into an in vivo acellular site, permitting external control over presence and absence of angiogenic growth factors at any time.19 The fiber wall microstructure is controlled through the fiber fabrication process to ensure that large proteins could be effectively released to the surrounding matrix.20–23 The ends of the hollow fiber remain exposed, providing access to the contents of the lumen of the fiber (and consequently what is delivered) over the course of experimentation. Using this system, we were able to achieve sequential delivery of two different angiogenic instructions: (1) vascular endothelial growth factor (VEGF; involved in vasculature permeability/destabilization24 and endothelial cell recruitment6) and (2) sphingosine-1-phosphate (S1P; promoting vessel stabilization in vivo25,26 and involved in a reduction in endothelial cell migration27,28). In this prior study, when VEGF delivery was followed by delivery of S1P, we observed significantly greater endothelial cell migration as well as substantial increases in vessel maturity, when compared to single or dual delivery of these factors.19 These data suggest that attempting to sequentially stimulate various stages of angiogenesis via the presence and absence of angiogenic factors is a step toward the development of more complex and relevant angiogenic therapies.
Importantly, recent literature suggests that the concept of stage-wise delivery for angiogenesis has broader application than only VEGF and S1P. Specifically, basic fibroblast growth factor (bFGF; 17 kDa) has been shown to play a major role in the initiation (sprouting) of new capillaries in vivo.29 Platelet-derived growth factor (PDGF; 25 kDa) promotes the maturation of blood vessels through the recruitment and support of mural cells, the supporting structure for blood vessels24,30, among other actions.31,32 However, when bFGF and PDGF are presented simultaneously in a modified Boyden chamber assay, bFGF was shown to significantly inhibit PDGF-induced smooth muscle cell migration and proliferation via the PDGF and bFGF receptors.15 Conversely, in a chick chorioallantoic membrane assay, it has been shown that PDGF inhibits bFGF-induced angiogenesis.14 Taken together, these data suggest that not only the presence but also the absence of bFGF and PDGF expression play a role in vascular remodeling.
For this reason, we utilized our model of sequential delivery to explore the sequence and delivery schedule of bFGF and PDGF, delivered alone, in sequence or together. Accordingly, we hypothesized that sequential delivery (bFGF followed by PDGF) would impact the significance and maturity of angiogenesis.
Materials and Methods
Hollow fiber fabrication
Cellulose acetate hollow fibers were prepared using a double injection nozzle as described previously.19 Briefly, 20% cellulose acetate (molecular weight = 30 kDa; Aldrich) was pumped through the outer core of the nozzle and deionized water was pumped through the center core. The cellulose solution and deionized water were extruded into a deionized water bath where the cellulose solution precipitates in the form of a porous hollow fiber. Lyophilized hollow fiber cross sections were sputter coated with 3.5 nm of gold–palladium and imaged at 5 kV using a JEOL 9335 SEM.
In vitro release
In vitro release from cellulose hollow fibers was carried out as described previously.19 Briefly, wells of a six-well cell culture plate were filled with 5 mL Dulbecco's phosphate buffered saline, or phosphate-buffered saline (PBS; Invitrogen). A cellulose hollow fiber was cut to fit the well, injected with 10 μL of rh-bFGF (R&D Systems) and rh-PDGF (R&D Systems) using a 28G½ insulin syringe (1/2 cc Lo-Dose U-100 insulin syringe, Becton Dickinson) and submerged in the PBS bath. Hollow fibers were injected first with bFGF (200 μg/mL, 2 ng bFGF total). Release of bFGF into a PBS bath was measured by sampling the supernatant and measuring using a bFGF ELISA kit (R&D Systems). After 24 h, the fiber was rinsed five times with PBS and lumen contents were replaced with an aqueous solution of PDGF (300 μg/mL, 3 ng PDGF total). Again, release was measured by sampling the supernatant and measuring using a PDGF ELISA kit (R&D Systems).
Murine Matrigel plug assay
A modified murine Matrigel plug assay was utilized as described previously.19 Briefly, growth factor-reduced Matrigel (500 μL) was injected (∼1 cm in diameter) into the subcutaneous space on the dorsal side of C57BL/6 mice (8–10 weeks old; Charles River) on both the left and right flank, after anesthesia with 2%–3% inhaled isoflurane. A 14G catheter was used to thread cellulose hollow fibers through the skin and Matrigel plugs (one fiber per Matrigel plug; two plugs per animal including an internal negative control). Hollow fibers were fixed in place using tissue glue and an Elizabethan collar was used to prevent mice from extracting the hollow fiber. See Supplementary Figure S1 (Supplementary Data are available online at www.liebertonline.com/tea) for a photograph of hollow fiber insertion. On the day of implantation, and every day for the next 6 days, hollow fibers on the left side were injected with sterile saline and hollow fibers on the right side were injected with 10 μL of an angiogenesis promoting factor: 200 μg/mL bFGF (R&D) and/or 500 μg/mL PDGF. The internal negative control (which includes the Matrigel plug, hollow fiber, and saline injection) serves the purpose of controlling for variation between mice (e.g., potentially any variable growth factor secretion due to inflammation caused by the Matrigel injection or hollow fiber implantation). For mice in the sequential delivery groups, factor switching occurred on the third day after implantation, after five rinses with saline. Seven days postimplantation, implants were extracted, fixed in 2% paraformaldehyde for 5 h and 30% sucrose overnight, and snap-frozen in liquid nitrogen. Frozen sections (8 μm) were stained with hematoxylin and eosin (H&E) and analyzed for endothelial cell migration, vessel formation, and red blood cell presence in vessel-like structures where the lumen is >100 μm.
Immunofluorescence
Frozen Matrigel Plug sections (8 μm) were incubated with primary antibodies rabbit anti-CD31 (Abcam) and Cy3-conjugated mouse anti-α-smooth muscle actin (αSMA; Sigma) and secondary antibody goat anti-rabbit Alexa Fluor 488® (Jackson Immuno). Sections were also counterstained with Hoescht (Sigma) to identify all mononuclear cells. CD31-labeled cross-sectional images were taken at 40×. These images were analyzed using threshold analysis on Metamorph to quantify the percent of each image occupied by CD31 staining. These values were averaged to obtain a representative percent for each cross section and normalized to the internal positive control in which only saline was delivered. Negative control plug percent areas (saline injection, left flank) for each mouse was subtracted from the Experimental Group percent areas (right flank) for a normalized percent area for each mouse. CD31 and αSMA-labeled cross-sectional images were taken at 60×. These images were analyzed by counting the number of CD31+ areas (vessel equivalents) and the number of these areas that are colocalized with αSMA labeling.
Statistical analysis
Analysis of variance was performed when assays contained more than one experimental group, as in the tubular formation assay (n = 3) and Murine Matrigel plug assay (n = 3). A power analysis based on a previous, yet similar, experiment was performed to determine N for in vivo experiments. Subsequently, a post hoc multiple comparison test was performed to compare means of different experimental groups (Holm-Bonferroni, α = 0.05, k = 4).
Results
Sequential bFGF and PDGF release from cellulose hollow fibers
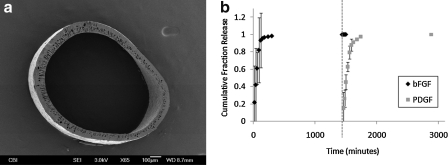
Cellulose hollow fibers were fabricated with an inner diameter of 971 ± 129 μm and wall thickness of 81 ± 18 μm (Fig. 1a). These fibers were used to sequentially release bFGF and PDGF in vitro, via manual injection of the growth factors at the desired time points. Porous fibers were loaded with bFGF for an initial period of release, rinsed, and then subsequently loaded with PDGF. Egress of these molecules through the fibers and into a surrounding saline is represented in Figure 1b. Importantly, when growth factors are exchanged (corresponding with saline flushing before administration of a new factor, depicted by the dotted line), bFGF release is no longer detectable and PDGF is subsequently detectable in the supernatant. These results are in agreement with previous results,19 suggesting that our fibers are capable of detectable release of a growth factor-sized protein over at least 24 h as well as sequential delivery of two factors, as determined empirically.
FIG. 1.
Externally regulated delivery model is capable of producing sequential release of bFGF and PDGF. (a) Scanning electron micrographs of a cellulose hollow fiber fabricated using a double-extrusion process. (b) Sample release profile illustrating how two different growth factors can be sequentially delivered. At time = 0 h, bFGF (200 μg/mL) is injected. At time = 24 h, fiber is rinsed five times with PBS (dotted line), resulting in mo more detectable bFGF. At time = 24 h (following rinse), PDGF (300 μg/mL) is injected into the fiber lumen. bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factor.
Recruitment of endothelial cells to Matrigel plugs in response to various treatment schedules
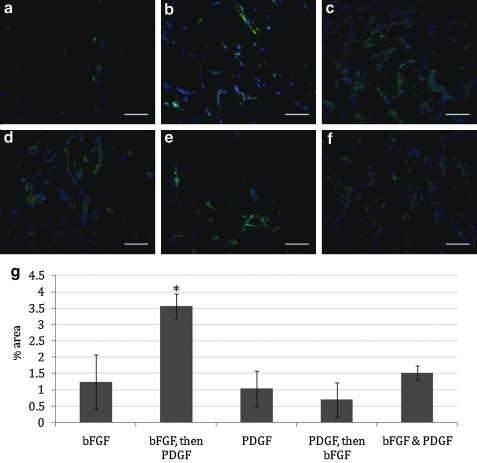
A modified murine Matrigel plug assay was utilized to measure angiogenesis in response to various delivery regimens in vivo. Specifically, a subcutaneous Matrigel plug serves as a cell-free matrix that is amenable to cellular invasion. A fiber is threaded through this plug to create a source for factor release through the Matrigel to the surrounding environment. The ends of the hollow fiber remain exposed, giving access to the contents of the lumen of the fiber (and consequently what is released into the cell-free matrix) over the course of experimentation. We explored delivery of (1) bFGF alone (Fig. 2b), (2) bFGF followed by PDGF (Fig. 2c), (3) PDGF alone (Fig. 2d), (4) PDGF followed by bFGF (Fig. 2e), and (5) dual delivery of bFGF and PDGF (Fig. 2f). Each experimental group contained an internal negative control where saline alone was administered through an implanted fiber (Fig. 2a) over the course of experimentation (7 days). In the sequential delivery groups, factor exchange (when relevant) occurred at 3 days postimplantation. CD31-stained Matrigel plug sections (Fig. 2a–f) reveal endothelial cell infiltration in all plugs where growth factors (bFGF and/or PDGF) are delivered. However, greater amounts of CD31+ staining were observed in plugs where bFGF was followed by PDGF as compared to all other groups (Fig. 2c). A semi-quantitative method for endothelial cell migration was also performed using CD31 staining of Matrigel plug sections. The percent area of images that were labeled with Alexa Fluor 488 (secondary antibody) was used to quantify CD31 expression in each sample. Images representing the entire periphery of the plug were recorded and normalized to the internal negative control, and an average percent area was determined (Fig. 2g). Basic FGF was shown to be active when delivered, demonstrated by an increase in endothelial cell recruitment compared to a saline injection (Fig. 2b, g). It is evident that statistically more CD31+ cells are observed in sections of the Matrigel plug treated with the bFGF-then-PDGF regimen than in any other experimental group.
FIG. 2.
Delivery of bFGF followed by PDGF results in greater recruitment of CD31+ cells in vivo than other delivery schedules. (a–f). Immunofluorescent staining of CD31 (green) and nuclei (blue) in Matrigel plug cross sections (scale bar = 100 μm) treated with (a) saline, (b) bFGF (200 μg/mL), (c) bFGF (200 μg/mL), followed by PDGF (500 μg/mL), (d) PDGF (500 μg/mL), (e) PDGF (500 μg/mL), followed by bFGF (200 μg/mL), (f) bFGF (200 μg/mL) and PDGF (500 μg/mL), and (g) CD31 quantification as normalized to an internal control (saline injected plug) using Metamorph threshold analysis. Percent areas of images covered by CD31 staining are averaged across all plugs. *Significant differences when compared to all other groups (ANOVA, followed by Holm-Bonferroni correction for t-test of multiple comparisons, k = 4, α = 0.05). ANOVA, analysis of variance.
Vessel maturation in response to various treatment schedules
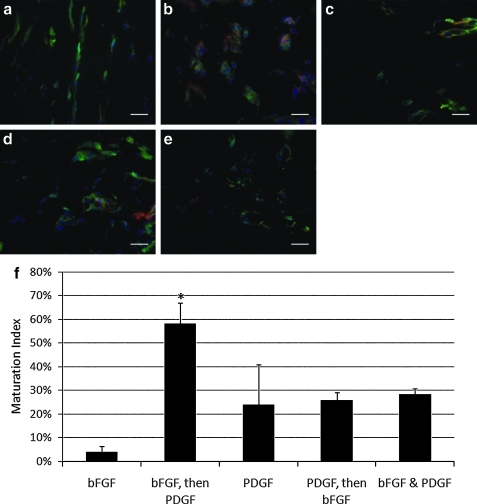
A quantitative method was used for determining the maturation level of a vessel using CD31 and αSMA staining of Matrigel plug explants (CD31 is present on endothelial cells and αSMA is present on mural cells). The colocalization of these two cell types is indicative of mature vessels.33 This method involves immunohistochemical analysis of CD31 and αSMA-stained tissue sections and is a common and validated measure of vessel maturity19,34–36. Five 60× areas in which CD31+ cells have arranged in a capillary-like structure were examined, and the percent of αSMA+ colocalization was recorded as the maturation index.33 In general, fluorescent images illustrate that αSMA colocalization with CD31 can be observed in all plugs where PDGF was delivered (Fig. 3b–e). In the plugs where only bFGF was delivered, we could detect only CD31+ cells and no αSMA+ cells (Fig. 3a). The maturation index (percent of vessels co-localized with αSMA+ cells) associated with the sequential delivery groups was statistically higher than all other groups in our study, specifically when bFGF delivery is followed by PDGF delivery (Fig. 3g), which is in agreement with the overall hypothesis put forth in this article.
FIG. 3.
Delivery of bFGF followed by PDGF results in greater colocalization of CD31 and αSMA in vivo than other delivery schedules. (a–e) Immunoflourscent staining of CD31 (green), αSMA (red), and nuclei (blue) in cross sections of Matrigel plugs (scale bar = 100 μm) treated with (a) bFGF (200 μg/mL), (b) bFGF (500 μg/mL), followed by PDGF (500 μg/mL), (c) PDGF (500 μg/mL), (d) PDGF (500 μg/mL), followed by bFGF (200 μg/mL), (e) bFGF (200 μg/mL) and PDGF (500 μg/mL), and (f) maturation index calculated by the percent of CD31+ cellular structures that are co-localized with αSMA staining. *Significant differences when compared to all other groups (ANOVA, followed by Holm-Bonferroni correction for t-test of multiple comparisons, k = 4, α = 0.05). αSMA, α-smooth muscle actin.
Integration of neovasculature with native vasculature
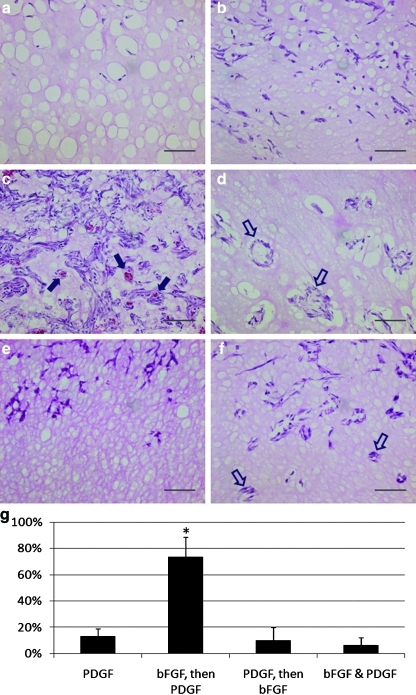
H&E-stained sections (Fig. 4a–f) reveal detectible cellular infiltration in all groups (purple nuclear stain). However, cellular infiltration into the Matrigel is more prevalent in the plugs in which an angiogenic factor has been delivered (Fig. 4b, f). Cells that have infiltrated into the Matrigel plug have arranged in tubular, vessel-like structures in plugs where PDGF is delivered alone (Fig. 4d), following bFGF (Fig. 4c) or at the same time as bFGF (Fig. 4f). However, when bFGF is delivered alone (Fig. 4b) or following PDGF (Fig. 4e), these vessel-like structures are not observed. More importantly, tubular, vessel-like structures are filled with red blood cells only in groups where bFGF delivery is followed by PDGF (indicated by filled in arrow), suggesting integration with native vasculature (Fig. 4c). The presence of red blood cells in the lumen of invading vessels was quantified by random selection of 10 vessel-like structures (lumen exceeding 100 μm in diameter) using multiple wide-field 20× images from each experimental group. The number of these vessel-like structures filled with red blood cells in each group was identified, and statistical analysis was performed to estimate the percent of invading vessels that are functionalized (i.e., integrated with existing vasculature) (Fig. 4g). It was quite obvious from both visual inspection of numerous H&E images (Fig. 4a, f) as well as quantitative data (Fig. 4g) that when bFGF delivery is followed by PDGF delivery, there are dramatically higher numbers of red blood cell-filled lumens than in any other schedules in which vessel-like structures (lumen >100 μm) were observed.
FIG. 4.
Sequential delivery of bFGF and PDGF results in cellular recruitment and functional angiogenesis in vivo. (a–f) Hematoxylin and eosin images of murine Matrigel plugs (scale bar = 500 μm). (a) Saline, (b) bFGF (200 μg/mL), (c) bFGF (200 μg/mL), followed by PDGF (500 μg/mL), (d) PDGF (500 μg/mL), (e) PDGF (500 μg/mL), followed by bFGF (200 μg/mL), and (f) bFGF (200 μg/mL) and PDGF (500 μg/mL).  indicates red blood cell-filled vessels.
indicates red blood cell-filled vessels.  indicates empty vessels. (g) Percent of vessel-like structures (lumen >100 μm) filled with red blood cells. *Significant differences when compared to all other groups (ANOVA, followed by Holm-Bonferroni correction for t-test of multiple comparisons, k = 3, α = 0.05). Color images available online at www.liebertonline.com/tea
indicates empty vessels. (g) Percent of vessel-like structures (lumen >100 μm) filled with red blood cells. *Significant differences when compared to all other groups (ANOVA, followed by Holm-Bonferroni correction for t-test of multiple comparisons, k = 3, α = 0.05). Color images available online at www.liebertonline.com/tea
Discussion
There are many factors, both chemical and physical, involved in the process of angiogenesis. Aside from angiogenic growth factors, there are a number of angiogenesis inhibitors that play a role in the angiogenic switch.37 These factors, which include AGM-1470, angiostatin, BB-94, and endostatin,38 are also being investigated for the treatment of tumors, where the goal is to target the source of nutrient supply to the growing tumor.37 Additionally, there are physical modulators that play a role in vascular function and angiogenesis. Cell growth has been known to activate the endothelial cell nitric oxide synthase pathway, upregulating the production on nitric oxide.39 This pathway also responds to changes in blood pressure, platelet activation, and angiogenesis, where nitric oxide, the product of this pathway, can modulate the production of various growth factors involved in angiogenesis.40 For the purpose of this study, we will focus on the presence of specific growth factors involved in the regulation of angiogenesis.
While the physiological effects of many growth factors are generally known, in some specific tissues, the combination and interactions of the growth factors are only recently being explored. In recent literature, the most cited of these tissues is bone, where researchers are exploring delivery of angiogenesis inducing factors as well as bone morphogenic proteins.41,42 In the context of angiogenesis, it has recently been shown that delivery of an early-stage, endothelial cell recruitment factor, VEGF, before delivery of a late-stage, mural cell recruitment factor, S1P, results in more overall endothelial cell recruitment as well as a higher vessel maturity, than when these factors are delivered together.19 Accordingly, VEGF and S1P may act as a series of instructions to sequentially promote separate stages of the process. Yet, it is likely that other known angiogenic growth factors, as well as the equally important angiogenic inhibitors, are involved in this series of sequential instructions as well. The goal of this study was to extend our sequential delivery model to study two other factors that have been implicated in stage-wise stimulus of blood vessel growth, namely, bFGF and PDGF. Consequently, the knowledge gained from this research could serve as valuable additions to our understanding of the stage-wise process of angiogenesis as well as advancing therapeutic approaches to promoting angiogenesis.
Although the externally regulated delivery model used in this study is not autonomously capable of sequential delivery itself, it provides a flexible format for temporal separation of various growth factors over any desired timeframe. Thus, a primary benefit of the model system discussed here is to inform the design of future systems that are capable of autonomously delivering these growth factors over a successful delivery schedule. Furthermore, the system allows for the introduction of a growth factor in a more gradual method than via bolus injection (a method previously proven to be effective19), where uniform distribution of a growth factor at nontoxic levels would be extremely difficult. A second feature of this model system is the internal negative control. Specifically, a matching Matrigel plug with implanted hollow fiber is present on the left flank of each animal enrolled in this study. This Matrigel plug was analyzed for angiogenesis in the same fashion as the Matrigel plugs in the experimental groups so that the level of angiogenesis cause by the Matrigel injection as well as the hollow fiber implantation can be monitored. Although endothelial cell migration levels in the internal controls are consistently very low and often negligible (Fig. 2A), the level of endothelial migration observed in each mouse was used to normalized all reported results (Fig. 2G).
Basic FGF has been implicated in endothelial cell migration and has also been known to induce a proangiogenic phenotype in endothelial cells.14 This paradigm is consistent with our data suggesting that endothelial cell migration occurs to a greater extent when bFGF is delivered for 7 days as compared to saline controls (Fig. 2a, b), despite the fact that bFGF is found to be unstable in the presence of many proteases expected to be present under inflammatory conditions.43–45 However, it has been shown that bFGF can inhibit PDGF-induced smooth muscle cell migration and proliferation via the PDGF and bFGF receptors,15 events that correspond with late-stage angiogenesis. In agreement with these prior findings, our results suggest that exclusive, persistent delivery of bFGF results in endothelial cell migration (marked by CD31+ cells, Fig. 2b) without colocalization with vascular pericytes (marked by αSMA+ cells, Fig. 3a). These data suggest that delivery of bFGF alone is not sufficient to sustain (and may even inhibit the progress) of growing neovasculature, a theory supported by a recent study in mice.46 It is possible that the role of bFGF may be primarily limited to promotion of early stage angiogenic events.
PDGF, in contrast, is known to promote the maturation of blood vessels through the recruitment and support of mural cells.14,24 Likewise, we observe that in all groups where PDGF is delivered, there is an increased presence of αSMA+ and CD31+ cell colocalization, regardless of the time-frame of delivery (Fig. 3). These data are important given that the process of pericyte coverage is imperative to the stability and, in turn, the fate, of newly forming vessels.46 Importantly, although newly forming vessels can be transient, and often regress,46,47 such blood vessels are known to not contain αSMA+ cells that (when present) interact with, and stabilize endothelial cells and inhibit regression.48 Pericyte coverage begins only once endothelial cells have been recruited and new basement membrane is secreted.6 It is not surprising then that the presence of PDGF, through its binding to PDGF-Rα on endothelial cells, negatively affects the action of bFGF-mediated recruitment.14
For the reasons described above, we hypothesized that the delivery of bFGF should precede delivery of PDGF to best promote the growth of stable and mature neovasculature. Although it is likely that during angiogenesis in vivo there is some degree of overlap between bFGF and PDGF presentation (and correspondingly overlap between the proposed stages of angiogenesis) it is evident in the data presented in this study (as well as other studies in the literature)14,15 that there is at least some conflicting functionality between bFGF and PDGF. Indeed, in the model system described herein, it is clear that the temporal separation of the two factors provides an advantage over dual delivery of the same factors. Specifically, using our simple and flexible hollow fiber model for sequential delivery, a temporally separated delivery schedule (bFGF, followed by PDGF) could be compared to delivery of each factor alone, dual delivery of both factors, as well as the reverse schedule (PDGF, followed by bFGF). In concept, a sequential delivery schedule (in contrast to dual delivery) would support bFGF-induced endothelial cell migration and proliferation without inhibition by PDGF, followed by PDGF-induced vessel maturation, without inhibition by bFGF. Accordingly, this delivery schedule resulted in both greater overall endothelial cell presence in a Matrigel plug after 7 days (Fig. 2) as well as a higher maturation index of vessels formed by these endothelial cells (Fig. 3).
It was observed that delivery of bFGF and PDGF alone, as well as together, induced similar levels of endothelial cell recruitment (Fig. 2g). Although bFGF and PDGF may have conflicting effects on the recruitment and organization of both endothelial cells and vascular pericytes, complete inhibition of angiogenesis is not observed when both factors are added together (Figs. 2f and 3f). This can possibly be explained by the pluripotency of PDGF.49,50 Although PDGF has been shown to be involved in mural cell recruitment and other late stage angiogenesis events,51 it is also the only growth factor involved in FDA-approved treatment for nonhealing wounds, suggesting that its effects may not be limited to one stage of angiogenesis.52 Regardless, this treatment is only 30% effective53 and likely not capable of optimally directing a multi-stage process without the direction of other biomolecules.
The need for additional biomolecular input for functional angiogenesis is evident from our data. Endothelial cells are recruited to the Matrigel plug when PDGF is delivered during early angiogenesis (first 3 days, Fig. 2d, f), but these vessels are not interconnected with existing vessels (as indicated by their lack of red blood cells in the vessels) (Fig. 4d, f, g). Although endothelial cell migration and vessel maturation are important in angiogenesis, vessels do not become functional until they are integrated with the native vasculature and capable of carrying red blood cells, an important sign functional vascular topography.54,55 Only in plugs where PDGF delivery follows bFGF delivery (Fig 4c, g) did we consistently observe red blood cells in the lumen of these structures. This suggests that this growth factor delivery schedule allows for more proper formation of vessels that are integrated with the native vasculature, allowing oxygen and nutrient delivery to newly forming tissue. It is possible that constitutive delivery of PDGF does not allow for destabilization of native vessels to the extent necessary to allow juncture with newly forming vessels. Although PDGF may not be capable of inhibition of endothelial cell migration and proliferation (as seen in Fig. 2d), PDGF might block the act of basement membrane destabilization.14
Because our model is modular and easily tuned, sequential delivery of a wide variety of factors is possible. To date, bFGF and PDGF is now the second set of growth factors that have been shown to be temporally relevant in mature angiogenesis using our sequential delivery model. Additional growth factors can be explored in other wound healing models, as temporal relevance of growth factors is likely not unique to angiogenesis. For example, PDGF and bone morphogenetic protein 2 have been implicated as playing a major role in the osteogenic processes; however, each protein appears to accomplish different tasks during different stages in the regeneration of bone.56–58 For instance, PDGF appears to aid in cellular recruitment, differentiation, and proliferation, as well as angiogenesis, whereas bone morphogenetic proteins seem to play a key role in the development of mature osteoblasts and bone tissue formation.59 Furthermore, PDGF has been shown to actually inhibit mature osteoblast activity in the later stages of bone formation.41 Hence, an ideal delivery strategy would first present early stage factors to induce angiogenesis and recruit osteoprogenitors and then present later stage factors to differentiate cells and induce mineralized tissue formation.
Conclusion
We have created a flexible model for the study of sequentially delivered angiogenic factors. When using this system to explore sequential delivery of bFGF and PDGF, we observed that delivery of bFGF for 3 days followed by delivery of PDGF for 4 days resulted in recruitment of more endothelial cells and a higher maturation index than the reverse sequential delivery schedule, single factor delivery or dual delivery. Additionally, sequential delivery of bFGF followed by PDGF resulted in vasculature that has integrated with the native vasculature, allowing for oxygen delivery to a previously cell-free environment. This approach could be likewise utilized to explore any number of delivery schedules and the resulting therapeutic responses as well as for studying the basic biological signals that accompany stage-wise regeneration of tissues.
Supplementary Material
Acknowledgments
This work was supported by the Commonwealth of Pennsylvania Research Development, the Department of Defense Telemedicine and Advanced Research Center Grant, and The University of Pittsburgh Cardiovascular Bioengineering Training Program (NIH T32 HL-076124).
Disclosure Statement
No competing financial interests exist.
References
- 1.Grakoui A. Bromley S.K. Sumen C. Davis M.M. Shaw A.S. Allen P.M. Dustin M.L. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 2.Steinman R.M. Hawiger D. Nussenzweig M.C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Hamaguchi K. Utsunomiya N. Takaki R. Yoshimatsu H. Sakata T. Cellular interaction between mouse pancreatic alpha-cell and beta-cell lines: possible contact-dependent inhibition of insulin secretion. Exp Biol Med (Maywood) 2003;228:1227. doi: 10.1177/153537020322801020. [DOI] [PubMed] [Google Scholar]
- 4.Bartness T.J. Shrestha Y.B. Vaughan C.H. Schwartz G.J. Song C.K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin T.J. Sims N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Bouïs D. Kusumanto Y. Meijer C. Mulder N.H. Hospers G.A. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res. 2006;53:89. doi: 10.1016/j.phrs.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Bauer S.M. Bauer R.J. Liu Z.J. Chen H. Goldstein L. Velazquez O.C. Vascular endothelial growth factor-C promotes vasculogenesis, angiogenesis, and collagen constriction in three-dimensional collagen gels. J Vasc Surg. 2005;41:699. doi: 10.1016/j.jvs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Przybylski M. A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care. 2009;18:516. doi: 10.12968/jowc.2009.18.12.45609. [DOI] [PubMed] [Google Scholar]
- 9.Raza A. Franklin M.J. Dudek A.Z. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 10.Kawanabe T. Kawakami T. Yatomi Y. Shimada S. Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci. 2007;48:53. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Tabata Y. Miyao M. Ozeki M. Ikada Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J Biomater Sci Polymer Ed. 2000;11:915. doi: 10.1163/156856200744101. [DOI] [PubMed] [Google Scholar]
- 12.Schweigerer L. Neufeld G. Friedman J. Abraham J.A. Fiddes J.C. Gospodarowicz D. Basic fibroblast growth factor: production and growth stimulation in cultured adrenal cortex cells. Endocrinology. 1987;120:796. doi: 10.1210/endo-120-2-796. [DOI] [PubMed] [Google Scholar]
- 13.Simons M. Bonow R.O. Chronos N.A. Cohen D.J. Giordano F.J. Hammond H.K. Laham R.J. Li W. Pike M. Sellke F.W. Stegmann T.J. Udelson J.E. Rosengart T.K. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 14.De Marchis F. Ribatti D. Giampietri C. Lentini A. Faraone D. Scoccianti M. Capogrossi M.C. Facchiano A. Platelet-derived growth factor inhibits basic fibroblast growth factor angiogenic properties in vitro and in vivo through its alpha receptor. Blood. 2002;99:2045. doi: 10.1182/blood.v99.6.2045. [DOI] [PubMed] [Google Scholar]
- 15.Facchiano A. De Marchis F. Turchetti E. Facchiano F. Guglielmi M. Denaro A. Palumbo R. Scoccianti M. Capogrossi M.C. The chemotactic and mitogenic effects of platelet-derived growth factor-BB on rat aorta smooth muscle cells are inhibited by basic fibroblast growth factor. J Cell Sci. 2000;113(Pt 16):2855. doi: 10.1242/jcs.113.16.2855. [DOI] [PubMed] [Google Scholar]
- 16.Hammond P.T. Form and function in multilayer assembly: new applications at the nanoscale. Adv Mater. 2004;16:1271. [Google Scholar]
- 17.Rothstein S.N. Federspiel W.J. Little S.R. A simple model framework for the prediction of controlled release from bulk eroding polymer matrices. J Mater Chem. 2008;18:1873. doi: 10.1016/j.biomaterials.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothstein S.N. Federspiel W.J. Little S.R. A unified mathematical model for the prediction of controlled release from surface and bulk eroding polymer matrices. Biomaterials. 2009;30:1657. doi: 10.1016/j.biomaterials.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tengood J.E. Kovach K.M. Vescovi P.E. Russell A.J. Little S.R. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials. 2010;31:7805. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa M. Sudo A. Komlev V.S. Barinov S.M. Uchida A. High release of antibiotic from a novel hydroxyapatite with bimodal pore size distribution. J Biomed Mater Res B Appl Biomater. 2004;70:332. doi: 10.1002/jbm.b.30047. [DOI] [PubMed] [Google Scholar]
- 21.Nunes S.P. Inoue T. Evidence for spinodal decomposition and nucleation and growth mechanisms during membrane formation. J Membr Sci. 1996;111:93. [Google Scholar]
- 22.Shih C.H. Gryte C.C. Cheng L.P. Morphology of membranes formed by the isothermal precipitation of polyamide solutions from water/formic acid systems. J Appl Polym Sci. 2005;96:944. [Google Scholar]
- 23.van de Witte P. Dijkstra P.J. van den Berg J.W.A. Feijen J. Phase separation processes in polymer solutions in relation to membrane formation. J Membr Sci. 1996;117:1. [Google Scholar]
- 24.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.J. Lee H.J. Song G.Y. Li G. Lee J.H. Lu J. Kim S.H. 6-(1-Oxobutyl)-5,8-dimethoxy-1,4-naphthoquinone inhibits lewis lung cancer by antiangiogenesis and apoptosis. Int J Cancer. 2007;120:2481. doi: 10.1002/ijc.22486. [DOI] [PubMed] [Google Scholar]
- 26.Wacker B.K. Scott E.A. Kaneda M.M. Alford S.K. Elbert D.L. Delivery of sphingosine 1-phosphate from poly(ethylene glycol) hydrogels. Biomacromolecules. 2006;7:1335. doi: 10.1021/bm050948r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendler C.C. Rivkees S.A. Sphingosine-1-phosphate inhibits cell migration and endothelial to mesenchymal cell transformation during cardiac development. Dev Biol. 2006;291:264. doi: 10.1016/j.ydbio.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto H. Takuwa N. Yokomizo T. Sugimoto N. Sakurada S. Shigematsu H. Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges J. Müller M.C. Momeni A. Stark G.B. Torio-Padron N. In vitro analysis of the interactions between preadipocytes and endothelial cells in a 3D fibrin matrix. Minim Invasive Ther Allied Technol. 2007;16:141. doi: 10.1080/13645700600935398. [DOI] [PubMed] [Google Scholar]
- 30.Magnusson P.U. Looman C. Ahgren A. Wu Y. Claesson-Welsh L. Heuchel RL. Platelet-derived growth factor receptor-{beta} constitutive activity promotes angiogenesis in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2142. doi: 10.1161/01.ATV.0000282198.60701.94. [DOI] [PubMed] [Google Scholar]
- 31.Hughes A.D. Clunn G.F. Refson J. Demoliou-Mason C. Platelet-derived growth factor (PDGF): actions and mechanisms in vascular smooth muscle. Gen Pharmacol. 1996;27:1079. doi: 10.1016/s0306-3623(96)00060-2. [DOI] [PubMed] [Google Scholar]
- 32.Chegini N. Rossi M.J. Masterson B.J. Platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and EGF and PDGF beta-receptors in human endometrial tissue: localization and in vitro action. Endocrinology. 1992;130:2373. doi: 10.1210/endo.130.4.1312455. [DOI] [PubMed] [Google Scholar]
- 33.Baudelet C. Cron G.O. Ansiaux R. Crokart N. DeWever J. Feron O. Gallez B. The role of vessel maturation and vessel functionality in spontaneous fluctuations of T2*-weighted GRE signal within tumors. NMR Biomed. 2006;19:69. doi: 10.1002/nbm.1002. [DOI] [PubMed] [Google Scholar]
- 34.Chen R.R. Silva E.A. Yuen W.W. Mooney D.J. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 35.Peters M.C. Polverini P.J. Mooney D.J. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q. Chen R.R. Shen Y. Mooney D.J. Rajagopalan S. Grossman P.M. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22:1110. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 37.Kerbel R. Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 38.Bergers G. Javaherian K. Lo K.M. Folkman J. Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 39.Whitney J.A. German Z. Sherman T.S. Yuhanna I.S. Shaul P.W. Cell growth modulates nitric oxide synthase expression in fetal pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L131. doi: 10.1152/ajplung.2000.278.1.L131. [DOI] [PubMed] [Google Scholar]
- 40.Igarashi J. Michel T. S1P and eNOS regulation. Biochim Biophys Acta. 2008;1781:489. doi: 10.1016/j.bbalip.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Patel Z.S. Young S. Tabata Y. Jansen J.A. Wong M.E. Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto M. Ikada Y. Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 43.Faham S. Hileman R.E. Fromm J.R. Linhardt R.J. Rees D.C. Heparin structure and interactions with basic fibroblast growth factor. Science. 1996;271:1116. doi: 10.1126/science.271.5252.1116. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini L. Burke D.F. von Delft F. Mulloy B. Blundell T.L. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature. 2000;407:1029. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrini L. Role of heparan sulfate in fibroblast growth factor signalling: a structural view. Curr Opin Struct Biol. 2001;11:629. doi: 10.1016/s0959-440x(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 46.Gosain A. Matthies A.M. Dovi J.V. Barbul A. Gamelli R.L. DiPietro L.A. Exogenous pro-angiogenic stimuli cannot prevent physiologic vessel regression. J Surg Res. 2006;135:218. doi: 10.1016/j.jss.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Matthies A.M. Low Q.E. Lingen M.W. DiPietro L.A. Neuropilin-1 participates in wound angiogenesis. Am J Pathol. 2002;160:289. doi: 10.1016/S0002-9440(10)64372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dang D.T. Chun S.Y. Burkitt K. Abe M. Chen S. Havre P. Mabjeesh N.J. Heath E.I. Vogelzang N.J. Cruz-Correa M. Blayney D.W. Ensminger W.D. St Croix B. Dang N.H. Dang L.H. Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition. Cancer Res. 2008;68:1872. doi: 10.1158/0008-5472.CAN-07-1589. [DOI] [PubMed] [Google Scholar]
- 49.Heldin C.H. Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 50.Schollmann C. Grugel R. Tatje D. Hoppe J. Folkman J. Marme D. Weich H. A. Basic fibroblast growth factor modulates the mitogenic potency of the platelet-derived growth factor (PDGF) isoforms by specific upregulation of the PDGF alpha receptor in vascular smooth muscle cells. J Biol Chem. 1992;267:18032. [PubMed] [Google Scholar]
- 51.Darland D.C. D'Amore P. A. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papanas N. Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clin Interv Aging. 2008;3:233. doi: 10.2147/cia.s1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer S.M. Bauer R.J. Velazquez O.C. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovasc Surg. 2005;39:293. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- 54.Giordano F.J. Johnson R.S. Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev. 2001;11:35. doi: 10.1016/s0959-437x(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 55.Hansen-Smith F.M. Capillary network patterning during angiogenesis. Clin Exp Pharmacol Physiol. 2000;27:830. doi: 10.1046/j.1440-1681.2000.03341.x. [DOI] [PubMed] [Google Scholar]
- 56.Sampath T.K. Maliakal J.C. Hauschka P.V. Jones W.K. Sasak H. Tucker R.F. White K.H. Coughlin J.E. Tucker M.M. Pang R.H., et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352. [PubMed] [Google Scholar]
- 57.Wang E.A. Rosen V. D'Alessandro J.S. Bauduy M. Cordes P. Harada T. Israel D.I. Hewick R.M. Kerns K.M. LaPan P., et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wozney J.M. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 1992;32:160. doi: 10.1002/mrd.1080320212. [DOI] [PubMed] [Google Scholar]
- 59.Cheng H. Jiang W. Phillips F.M. Haydon R.C. Peng Y. Zhou L. Luu H.H. An N. Breyer B. Vanichakarn P. Szatkowski J.P. Park J.Y. He T.C. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85A:1544. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.