Abstract
Background
Although cognitive flexibility is mediated by different areas of the prefrontal cortex, evidence from patients with Parkinson’s disease suggests an additional involvement of striatal dopamine (DA) signaling. Because both dorsal and ventral striatum receive prefrontal cortex projections, it is unclear whether DA signaling to either one or both of these regions is required for cognitive flexibility.
Methods
Cognitive flexibility was examined with a water U-maze paradigm in which mice had to shift from an initially acquired escape strategy to a new strategy or to reverse the initially learned strategy. We tested mice with conditionally inactive tyrosine hydroxylase genes that can be activated by Cre recombinase. With region-specific viral gene therapy we selectively restricted DA signaling to either dorsal or ventral striatum.
Results
Restricting DA signaling to the ventral striatum did not impair learning of the initial strategy or reversal-learning but strongly disrupted strategy-shifting. In contrast, mice with DA signaling restricted to the dorsal striatum had intact learning of the initial strategy, reversal-learning, and strategy-shifting.
Conclusions
Dopamine signaling in both dorsal and ventral striatum is sufficient for reversal-learning, whereas only DA signaling in the dorsal striatum is sufficient for the more demanding strategy-shifting task.
Keywords: Dopamine-deficient mice, executive function, tyrosine hydroxylase, viral rescue
Introduction
To disengage from previously learned behavior and adopt a new behavioral strategy (cognitive flexibility) is a key element of executive function that declines severely in various neurological and psychiatric conditions (1–3). Lesion experiments have implicated the prefrontal cortex (PFC) in mediating cognitive flexibility (4–6). However, striatum and PFC are anatomically and functionally connected by corticostriatal circuits (7), and degeneration of nigrostriatal dopamine (DA) projections in Parkinson’s disease (PD) or damage to the striatum itself—as in Huntington’s disease—are both associated with deficits in executive function (3,8). Further support for a striatal component in mediating executive function comes from experiments in which lesions or pharmacological inhibition of striatal projection neurons impaired executive function (9–11). These studies provided data implicating the ventral striatum, yet the pathological DA dysfunction in PD results primarily from degeneration of DA projections to the dorsal striatum (3). To investigate contributions of DA projections to either dorsal or ventral striatum, we used flox-stop, DA-deficient (DD) mice in which DA signaling can be restored by Cre recombinase. Injections of canine adenovirus expressing Cre recombinase (CAV2- Cre) into specific brain regions allows selective restoration of DA signaling to those regions (12). Here, we examine two aspects of cognitive flexibility, reversal-learning, and strategy-shifting, in mice that have DA signaling restored to either ventral or dorsal striatum.
Methods and Materials
Mice with DA signaling restricted to only the dorsal (vrDD-Dorsal) or ventral (vrDD-Ventral) striatum were generated by injection of CAV2-Cre in DD mice; sham-control mice received the same viral injections as DD mice (13,14) (see also Methods and Materials in Supplement 1). Success of viral injections was verified by immunohistochemical tyrosine hydroxylase (TH) staining of striatal sections.
Altogether 17 sham, 16 vrDD-Dorsal, and 16 vrDD-Ventral mice were first trained to acquire a turn-based water-escape strategy in a U-maze with one black and one white arm. Then, seven sham, eight vrDD-Dorsal, and seven vrDD-Ventral mice underwent reversal training, in which the escape platform was now located on the opposite side. The remaining mice were subjected to strategy-shift training, during which the position of the escape platform was now linked to the color of the arms (cue-based strategy). As a control procedure, seven sham, six vrDD-Dorsal, and six vrDD-Ventral mice were trained to acquire a cue-based strategy. For all procedures the percentage of correct trials and escape latencies to reach the platform were recorded. If a mouse made a wrong turn, it remained in the maze until it made a correct turn (see Methods and Materials in Supplement 1).
Results
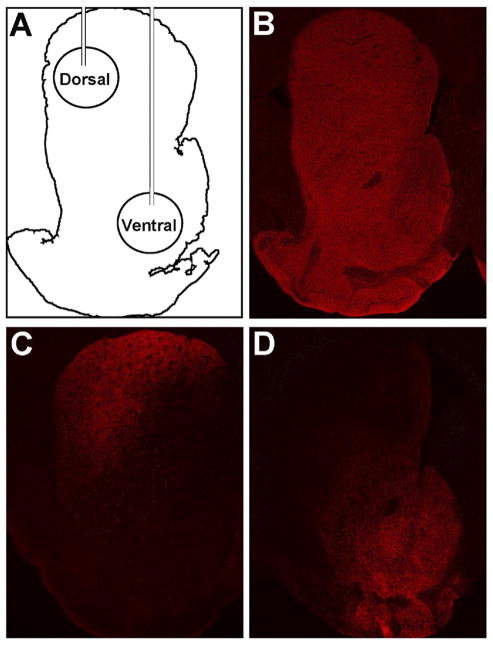
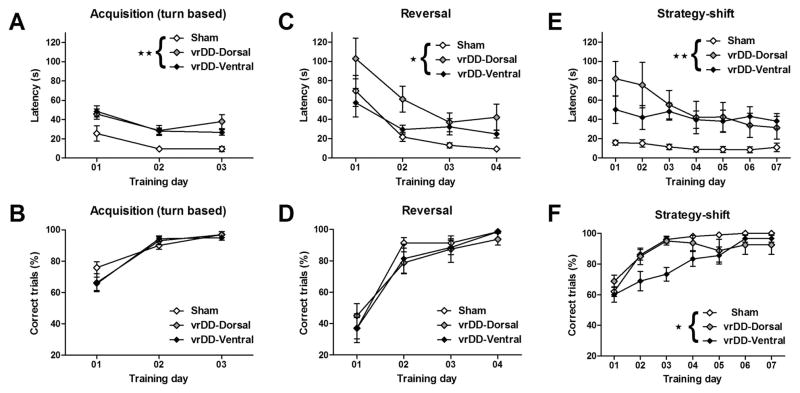
The locations of the viral injection sites are shown schematically in Figure 1A. The TH immunostaining of the striatum in sham-control mice is shown for comparison (Figure 1B); DD mice have no TH staining in the striatum. The CAV2-Cre injections into the dorsal striatum of vrDD-Dorsal mice resulted in strong TH expression in the dorsolateral striatum that also extended into the dorsomedial striatum (Figure 1C). There was no TH expression in the ventral striatum of vrDD-Dorsal mice. In vrDD-Ventral mice, TH expression was restored in the ventral striatum, with intense staining in the nucleus accumbens and olfactory tubercle but also some weaker staining in the most medial part of the dorsal striatum (Figure 1F). No staining was detected in the dorsolateral striatum of vrDDVentral mice. The intensity of TH immunostaining in the injected areas of vrDD-Dorsal and vrDD-Ventral mice was not significantly different (p > .05); and the intensity in the noninjected areas of vrDD mice was not significantly different from background or that in DD mice (p > .05) (Figures S1A and S1B in Supplement 1). Because we did not detect behavioral differences between sham animals that received CAV2-Cre injections into the dorsal versus the ventral striatum, they are grouped together for data presentation. Detailed statistical analyses (two-way repeated-measure analysis of variance) are provided in Table S1 in Supplement 1. Although both vrDD-Dorsal and vrDD-Ventral mice had longer escape latencies than sham-control mice, their acquisition of the initial response-based strategy was intact (Figures 2A and 2B). Analysis of escape latencies demonstrated significant effects of time and group (each p < .01) but not of time X group interaction (p > .05). Analysis of correct trials confirmed only a significant effect of time (p < .01) but not of group or time X group interaction (each p > .05). Sham-control and vrDD mice had intact reversal-learning, with vrDD-Dorsal mice showing a tendency for greater escape latencies (Figures 2C and 2D). Analysis of escape latencies after rule-reversal confirmed significant effects of time (p < .01) and group (p < .05) but not of time X group interaction (p > .05). However, analysis of the percentage of correct trials/day revealed only significant effects of time (p < .01) but not of group or time X group interaction (each p > .05). Although both virally rescued groups performed the strategy-shift task slower than sham-control mice, vrDD-Dorsal mice learned to shift their strategy as effectively as control subjects, but this ability was impaired in vrDD-Ventral mice (Figures 2E and 2F). Analysis of escape latencies after the strategy shift confirmed significant effects of time, group, and time X group interaction (each p < .01). Post hoc comparisons showed that vrDD-Dorsal mice had significantly higher escape latencies than sham mice on Days 1 to 3 (p < .05); vrDD-Ventral mice had only a nonsignificant tendency for higher escape latencies. More important, analysis of the percentage of correct trials/day revealed significant effects of time (p < .01), group (p < .05), and time X group interaction (p < .01). Post hoc comparisons of percentages of correct trials showed that vrDDVentral mice had significantly fewer correct trials than sham mice on days 2 (p < .05) and 3 (p < .01) and fewer correct trials than vrDD-Dorsal mice on day 3 (p < .05). Because all groups learned a cue-based strategy equally well when trained on it first, the differences in strategy-shifting are not due to impaired learning of the cue-based strategy by vrDD-Ventral mice (Figure S2 in Supplement 1). We detected only a significant effect of time (p < .01) but not of group or time X group interaction (each p > .05) on percentage of correct trials/day. We also analyzed trial-by-trial correct choices on each first day of acquisition, reversal, and strategy-shift conditions but did not detect significant effects of group for any of these conditions (p >.05) (see Results in Supplement 1).
Figure 1.
Restriction of dopaminergic signaling to the striatum of vrDDDorsal and vrDD-Ventral mice. Tyrosine hydroxylase (TH) (red) immunostaining was visualized in coronal sections of the striatum from control and vrDD mice. (A) Schematic illustration of the Th-restoration strategy, depicting viral injections into the dorsal and ventral striatum of vrDD and control mice. (B) TH expression pattern in sham control mice. (C) TH expression found in vrDD-Dorsal mice. Expression was restricted to the dorsal striatum with strong immunostaining in both lateral and medial regions. (D) TH expression found in vrDD-Ventral mice. Expression was strongest in the nucleus accumbens with some immunostaining in the dorsomedial striatum and the olfactory tubercle.
Figure 2.
Cognitive flexibility by sham and vrDD mice. (A) Escape latencies and (B) percentage of correct trials/training day during pretraining of the turn-based strategy. Sham control, vrDD-Dorsal, and vrDD-Ventral mice reached > 80% correct trials on the third training day. Escape latencies by vrDD mice were elevated compared with sham control mice. (C) Escape latencies and (D) percentage of correct trials/training day during reversal-learning. Although vrDD mice performed slower than sham control mice, all groups reached > 80% correct trials on the fourth training day. (E) Escape latencies and (F) percentage of correct trials/training day during pretraining of the turn-based strategy. Strategy-shifting was impaired in vrDD-Ventral mice. Sham-control and vrDD-Dorsal mice reached > 80% correct trials on the third training day; vrDD-Ventral mice required 6 training days to reach this criterion. Significant main effects of group (**p < .01; *p < .05). Data are means ± SEM.
Discussion
Where as most pharmacological and lesioning studies have implicated the ventral striatum in mediating cognitive flexibility (9– 11), deficits observed in PD suggest an additional involvement of the dorsal striatum (3,15). Here, we demonstrate that, although restriction of DA-signaling to the ventral striatum supports reversal learning, strategy-shifting is impaired under these conditions. In contrast, both reversal-learning and strategy-shifting are intact when DA signaling is restricted to the dorsal striatum, suggesting that the deficit in strategy-shifting by vrDD-Ventral mice is due to a lack of DA in the dorsal striatum. This interpretation is supported by anatomical studies showing that pyramidal neurons in the PFC project to medium spiny neurons (MSNs) in both dorsal and ventral striatum (16,17), hence potentially allowing DA modulation of cognitive flexibility in both areas. Furthermore, pharmacological inactivation of the dorsomedial striatum can impair cognitive flexibility in rats (18). Although both groups of vrDD mice spent similar amounts of time in the water during strategy-shift trials, we cannot rule out that differences in their ability to respond to experimental stress contribute to the learning-deficit in vrDDVentral mice. Because we have shown that vrDD-Ventral mice swim slower (14) and have similar observations for vrDD-Dorsal mice, their increased escape latencies are predominantly due to reduced swim-speed. Whereas our experiments explore the role of DA signaling, most pharmacological and lesion experiments explore the role of striatal MSNsin integrating dopaminergic and glutamatergic signaling and generating an appropriate output. Hence, inhibiting or ablating MSNs in the ventral striatum probably has wider consequences than just removing DA. Pharmacological manipulations of DA receptors in the ventral striatum impact cognitive flexibility (10), suggesting that the complete absence of DA signaling in the ventral striatum of vrDD-Dorsal mice is not equivalent to an imbalance of DA-receptor–mediated signaling in this region. We used a water-escape task rather than a food-reward task, because mice with DA signaling only in the ventral or dorsolateral striatum had impaired food-reward learning but intact water-escape learning (13,14). We therefore wanted to separate cognitive/strategic components of maze-learning from reward by designing a task that either vrDD-Dorsal or vrDD-Ventral mice could learn as well as control mice—which is important, because the ventral striatum is heavily implicated in motivational aspects of food-reward learning (19). It is possible that strategy-shifting and reversal-learning have different motivational requirements and are therefore differentially affected by DA manipulations. Although both dorsal and ventral striatum receive input from the PFC (16,17), they receive different projections (e.g., from the sensorimotor cortex [dorsal striatum] and the hippocampus [ventral striatum]), which might also contribute to differences in DA mediation of strategy shifting (20). Overall, we show that cognitive flexibility is critically dependent on both activity of MSNs and their DA modulation in the dorsal striatum. However, depending on the motivational drive, cognitive flexibility is probably also modulated by activity in the nucleus accumbens (9).
Supplementary Material
Acknowledgments
This investigation was supported in part by the Pacific Northwest Udall Center NS062684 (MD). We thank Glenda Froelick for preparing histological sections and Dr. Miguel Chillon (Vector Production Unit of Centre de Biotecnologia Animal i Teràpia Gènica at Universitat Autonoma Barcelona) for preparing the CAV2-Cre virus.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leverenz JB, Quinn JF, Zabetian C, Zhang J, Montine KS, Montine TJ. Cognitive impairment and dementia in patients with Parkinson disease. Curr Top Med Chem. 2009;9:903–912. [PMC free article] [PubMed] [Google Scholar]
- 4.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 6.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence AD, Sahakian BJ, Rogers RD, Hodge JR, Robbins TW. Discrimination, reversal, and shift learning in Huntington’s disease: Mechanisms of impaired response selection. Neuropsychologia. 1999;37:1359–1374. doi: 10.1016/s0028-3932(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 9.Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 11.Mair RG, Koch JK, Newman JB, Howard JR, Burk JA. A double dissociation within striatum between serial reaction time and radial maze delayed nonmatching performance in rats. J Neurosci. 2002;22:6756–6765. doi: 10.1523/JNEUROSCI.22-15-06756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc Natl Acad Sci U S A. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darvas M, Palmiter RD. Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J Neurosci. 2010;30:1158–1165. doi: 10.1523/JNEUROSCI.4576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- 16.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins TW, Everitt BJ. Neurobehavioral mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 20.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.