Abstract
Gap junctional communication between microglia was investigated at rat brain stab wounds and in primary cultures of rat and mouse cells. Under resting conditions, rat microglia (FITC-isolectin-B4-reactive cells) were sparsely distributed in the neocortex, and most (95%) were not immunoreactive for Cx43, a gap junction protein subunit. At brain stab wounds, microglia progressively accumulated over several days and formed aggregates that frequently showed Cx43 immunoreactivity at interfaces between cells. In primary culture, microglia showed low levels of Cx43 determined by Western blotting, diffuse intracellular Cx43 immunoreactivity, and a low incidence of dye coupling. Treatment with the immunostimulant bacterial lipopolysaccharide (LPS) or the cytokines interferon-γ (INF-γ) or tumor necrosis factor-α (TNF-α) one at a time did not increase the incidence of dye coupling. However, microglia treated with INF-γ plus LPS showed a dramatic increase in dye coupling that was prevented by coapplication of an anti-TNF-α antibody, suggesting the release and autocrine action of TNF-α. Treatment with INF-γ plus TNF-α also greatly increased the incidence of dye coupling and the Cx43 levels with translocation of Cx43 to cell–cell contacts. The cytokine-induced dye coupling was reversibly inhibited by 18α-glycyrrhetinic acid, a gap junction blocker. Cultured mouse microglia also expressed Cx43 and developed dye coupling upon treatment with cytokines, but microglia from homozygous Cx43-deficient mice did not develop significant dye coupling after treatment with either INF-γ plus LPS or INF-γ plus TNF-α. This report demonstrates that microglia can communicate with each other through gap junctions that are induced by inflammatory cytokines, a process that may be important in the elaboration of the inflammatory response.
Keywords: inflammation, macrophage, connexin, cytokines, dye coupling
Microglia are ubiquitous in brain and are the main immune effector of the central nervous system (1). Under resting conditions they show a down-regulated immunophenotype but can be activated by diverse signaling molecules; thus, their apparent quiescence is a surveillance state in which they are sensitive to changes in their milieu (2). Consistently, microglia are activated in response to a wide range of injuries that trigger brain inflammatory responses, including head injury and ischemia (2), neurodegenerative diseases (3–6), autoimmune diseases (1, 7), infectious diseases (8), prion diseases (9), and brain tumors (10).
Activation of microglia is characterized by proliferation, recruitment to the site of injury, and expression of proteins associated with immune responses (11). Among these proteins are major histocompatibility complex (MHC) class II molecules and cytokines, such as tumor necrosis factor (TNF-α), interleukin-1, interleukin-6, and transforming growth factor-β1. In addition, cytokines such as interferon-γ (INF-γ), interleukin-1, interleukin-4, and TNF-α induce similar changes in microglia in vitro (11).
It is known that microglia communicate with one another and with other brain cells through specific extracellular signals, such as cytokines (2), neurotransmitters (2, 12), and/or adhesion molecules, including integrins and cadherins (13). In vivo, activated microglia migrate to injured central nervous system areas, where they proliferate and gradually remove cell debris (14). At those places, their proximity to each other may allow for the establishment of physical contacts and formation of intercellular junctions, such as gap junctions. Gap junctions between microglia might provide an additional pathway for communication but, to our knowledge, have not been described. Moreover, the formation of gap junctions or expression of their protein subunits, connexins, by monocytes/macrophages is still a matter of debate (15).
Gap junction channels connect the interiors of contacting cells, allowing the exchange of ions, second messengers, and small molecules of less than about 1 kDa molecular mass and provide a mechanism for coordinating metabolic and electrical activities of multicellular systems (16, 17). Gap junctions are formed by connexins, a family of proteins with more than 15 members (18). A gap junction channel is formed by joining two hemichannels or connexons, one contributed by each of the apposed membranes. A hemichannel comprises six connexin monomers surrounding a central pore (16, 17). Channels formed of different connexins differ in their biophysical properties and permeabilities, and hemichannels differ in their ability to form functional, heterotypic gap junctions with hemichannels formed of other connexins (18). Most gap junctions can be blocked with several compounds, including octanol and 18α-glycyrrhetinic acid (19). The present work was undertaken to examine the possibility that microglia communicate with each other via gap junctions. We found that under resting conditions in vivo, most cortical microglia do not express Cx43 immunoreactivity, but that, at stab wounds, accumulating microglia are immunopositive for this connexin. In primary culture, INF-γ plus the immunostimulant bacterial lipopolysaccharide (LPS) or INF-γ plus TNF-α induced dye coupling and increased levels of Cx43 levels with translocation to cell interfaces. Preliminary findings have been reported.¶
Materials and Methods
Reagents and Antibodies.
F-12 medium, MEM, FBS, horse serum, penicillin, streptomycin, trypsin-EDTA, and bovine pancreas DNase I were from GIBCO/BRL. Lucifer yellow-CH, 18α-glycyrrhetinic acid, probenecid, oxidized ATP, 3,3′-diaminobenzidine, peroxidase-conjugated goat anti-rabbit IgG, monoclonal anti-glial fibrillary acidic protein antibody, FITC-conjugated anti-mouse IgG antibody, alkaline phosphatase-conjugated goat anti-rabbit IgG antibody, FITC-conjugated goat anti-rabbit IgG antibody (Fab), Bandeiraea simplifolia isolectin-B4 labeled with FITC (FITC-isolectin B4), recombinant TNF-α, recombinant IFN-γ, and LPS [E. coli (serotype 0127:B8)] were obtained from Sigma. Rabbit anti-TNF-α human recombinant was obtained from Calbiochem. A monoclonal anti-rat antibody to ED-1, a monocyte/macrophage marker, was obtained from BioSource International (Camarillo, CA). Fab fragments of a previously characterized rabbit polyclonal anti-Cx43 antibody were used (20, 21). Immunoglobulins of the anti-Cx43 serum were isolated with the use of immobilized protein G (Pierce), and then Fab fragments were obtained with the Fab preparation kit purchased from Pierce. The protocol indicated by the provider was followed during each procedure.
Brain Stab Wounds.
Stab wounds were made as described by Amat et al. (22). Adult male Sprague–Dawley rats (180–200 g) were deeply anesthetized with an i.p. injection of ketamine (50 mg/kg). A midline craniotomy 4 mm long and 2 mm wide was made over the forebrain. A unilateral stab wound ≈4 mm long and ≈2 mm deep was made by inserting a scalpel blade through the opening, and the skin incision was sutured.
Microglia Cultures.
Primary cultures of microglia from neocortex of newborn rats or mice were prepared as described by Giulian and Baker (23), with minor modifications. Tissue was minced and incubated at 37°C for 30 min in Ca2+-free PBS that contained trypsin (0.5%) and EDTA (5 mM). After removal of the enzyme solution, tissue was triturated in dissociation medium (MEM medium/1 mg/ml bovine pancreas DNase I/10% horse serum) with the use of a Pasteur pipette. Dissociated cells were pelleted, resuspended in MEM medium supplemented with 10% FBS, 100 units/ml penicillin, and 50 μg/ml streptomycin sulfate and plated on glass coverslips or on 60-mm plastic culture dishes (Nunc) and kept at 37°C in a 5% CO2/95% air atmosphere at nearly 100% relative humidity. Cells were fed every 3 days. About 95% of the cells were positive for glial fibrillary acidic protein, indicating that cultures were highly enriched in astrocytes; presumably most of the remaining 5% were microglia. Because microglia proliferate in astrocyte-conditioned medium (24), feeding of confluent cultures was stopped for the following 12–14 days. Cultures were then rigorously agitated for 30 min in an orbital shaker (Lab-Line Instruments) at 150 rpm and 37°C to detach cells adhering to the astrocyte monolayer. Thereafter, cells suspended in the culture medium were collected and plated [800,000 cells per ml; 3 ml in 60-mm dishes or 1 ml per well of a 24-well plate (Nunclon, Roskilde, Denmark), with each well containing a round glass coverslip at the bottom]. After 15 min nonadherent cells were discarded and adherent cells were maintained in MEM supplemented with 10% FBS, 100 units/ml penicillin, and 50 μg/ml streptomycin sulfate. Close to 99% of the cells obtained after this procedure were immunopositive for the ED-1 antigen but were negative for glial fibrillary acidic protein, indicating a very high enrichment in microglia. All experiments were performed 48 h after microglia were subcultured at 37°C in a 5% CO2/95% air atmosphere at nearly 100% relative humidity.
Dye Coupling.
Coupling between microglia cultured on glass coverslips was evaluated by monitoring the transfer to neighboring cells of a fluorescent dye microinjected into one cell. The dye (5% wt/vol Lucifer yellow in 150 mM LiCl) was microinjected through glass microelectrodes as previously described (25) until the impaled cell was brightly fluorescent. After 1 min of dye injection, surrounding cells were examined to determine whether dye transfer occurred. The incidence of dye coupling as a percentage was calculated by dividing the number of injected cells showing dye transfer to one or more neighboring cells by the total number of cells injected in each experiment, multiplied by 100. In all experiments the incidence of dye coupling was evaluated by injecting a minimum of 10 cells. Dye coupling was observed on an inverted microscope equipped with xenon arc lamp illumination and a Nikon B filter (excitation wavelength 450–490 nm; emission wavelength above 520 nm).
Immunocytochemistry and Immunofluorescence.
Frozen coronal brain sections (10 μm) were fixed in 1% paraformaldehyde for 30 min at room temperature. To label microglia the sections were incubated with FITC-isolectin-B4 diluted 1:100.000 in PBS (stock: 5 μg/ml). Then, endogenous peroxidase activity was inhibited by incubating each section in 20% hydrogen peroxide in methanol for 20 min at room temperature. Thereafter, sections were incubated with Fab fragments of the anti-Cx43 antibody for 1 h, washed several times, incubated with peroxidase-conjugated anti-rabbit IgG antibody for 1 h, washed, and developed for different times up to15 min with 1 mg/ml 3,3′-diaminobenzidine in PBS plus 20 μl of H2O2.
Microglia cultured on no. 1 round glass coverslips were fixed and permeabilized in 70% ethanol for 20 min at −20°C. Cells were incubated in blocking solution containing 5 mM EDTA, 1% fish gelatin, 1% BSA essentially Ig free, and 1% goat serum or 20% rat serum for 30 min at room temperature. Then they were incubated in diluted primary antibody (anti-Cx43, Fab fragments of anti-Cx43, anti-ED1, or anti-glial fibrillary acidic protein) overnight at 4°C, rinsed in PBS (pH 7.4) for 1 h at room temperature, and incubated with diluted FITC-conjugated goat anti-rabbit IgG secondary antibody or FITC-conjugated goat anti-mouse IgG for 1 h at room temperature, followed by another rinse period of 1 h. Coverslips were mounted with Gelvatol-Dabco (Sigma), observed under a Nikon Labophot-2 microscope equipped with epifluorescent illumination, and photographed. The specificity of the immunoreactivity was assessed by replacing the primary antibody or Fab fragments of immunoglobulins with nonimmune serum.
Western Blotting.
Microglia cultures (60-mm dishes) were harvested by scraping with a rubber policeman in ice-cold 2 mM PMSF in PBS (rinse buffer was freshly prepared; pH 7.4), pelleted, and lysed by sonication. Rinse and solubilization buffers contained protease inhibitors (200 μg/ml soybean trypsin inhibitor/1 mg/ml benzamidine/1 mg/ml ɛ-aminocaproic acid/2 mM PMSF) and phosphatase inhibitors (20 mM Na4P2O7/100 mM NaF).
Proteins were measured in aliquots of cell lysates with the Bio-Rad protein assay, and Western blot analyses were performed as described previously (25). An aliquot of rat heart homogenate was used to calibrate the electrophoretic mobility of Cx43 reactive bands.
Cx43-Deficient Mice.
Litters were obtained by interbreeding heterozygous animals of the Cx43del strain, carrying a loxP site and a lacZ gene in place of the Cx43 coding region (26). At birth, separate microglia cultures were prepared from each brain, and each donor animal was genotyped by PCR analysis of tail-tip DNA. Genotyping was performed as described previously (26).
Results
Microglia Accumulating at Stab Wounds Are Cx43 Immunoreactive.
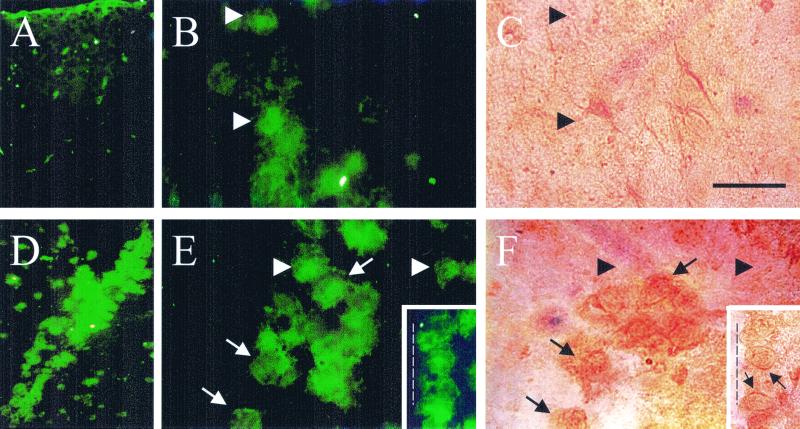
In sections of normal adult rat brain, microglia identified as FITC-isolectin-B4-reactive cells were sparsely distributed throughout the cortex (Fig. 1A), and few of them (<5%) were Cx43 immunoreactive (Fig. 1 B and C). Four days after the stab wound was made, FITC-isolectin-B4-labeled cells were numerous at the edge of and around the wound (Fig. 1D). Many FITC-isolectin-B4-reactive cells (≈60%) were also Cx43 positive, and immunoreactivity was seen as diffuse intracellular labeling with more intense staining at some cell interfaces (Fig. 1 E and F, insets; arrows and arrowheads indicate FITC-isolectin-B4-abeled cells with and without Cx43 immunoreactivity, respectively).
Figure 1.
Stab wounds induce recruitment of microglia that express Cx43 immunoreactivity. Coronal sections were from a control rat brain (A–C) and from a brain 4 days after the stab wound was made (D–F). Microglia were identified by reaction with FITC-isolectin-B4 (A, B, D, E, fluorescence images), and the same sections were subjected to immunoperoxidase detection of Cx43 (C and F). Arrows indicate cells reactive to Cx43 and FITC-isolectin-B4; arrowheads indicate cells reactive only to FITC-isolectin-B4. A and D are low-magnification views, and B, C, E, and F are higher magnification views. Stronger immunoperoxidase labeling at cell interfaces is indicated by arrows in the inset in F (fluorescence image in E Inset). The discontinuous line in the E and F Insets denotes the edge of the stab wound. (Bar, 100 μm for A and D and 400 μm for all other panels.)
INF-γ Plus TNF-α Treatment in Vitro Induces Gap Junctional Communication Between Rat Cortical Microglia.
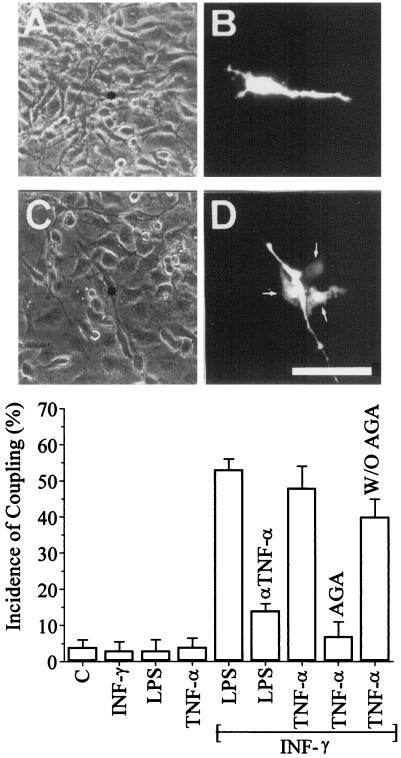
After 48 h of subculture under control conditions, the morphology of rat microglia was rather homogeneous. The predominant cell shape (≈95% of the cells) was polygonal and flat with a few short processes, as was seen most clearly in dye-injected cells (Fig. 2B; see also Fig. 3A). Approximately 5% of the cells showed a more irregular shape or were round. The latter were not adherent and therefore were not included in dye coupling assays. Under control conditions, most cells were not dye coupled; the incidence was ≈5% in the polygonal and flat cells (Fig. 2B and Graph).
Figure 2.
Cytokines induce dye coupling between cultured rat microglia. (A and C) Phase-contrast views of the fluorescence fields shown in B and D, respectively. (B) An example of lack of dye coupling observed under control conditions. (D) A cell that is dye coupled to three neighboring cells in a microglia culture treated with 1 ng/ml INF-γ plus 1 ng/ml TNF-α for 9 h. (Bar, 80 μm.) (Graph) The incidence of dye coupling (Lucifer yellow) was evaluated in cultures of rat microglia under control conditions and after cytokine treatment for 9 h. Treatment with 1 ng/ml INF-γ, 1 μg/ml LPS, or 1 ng/ml TNF-α did not increase coupling above control levels. INF-γ (1 ng/ml) plus LPS (1 μg/ml) caused a large increase in coupling, which was largely prevented by cotreatment with an anti-TNF-α antibody. Treatment with 1 ng/ml INF-γ plus 1 ng/ml TNF-α also caused a large increase in coupling; this coupling was blocked by 35 μM 18α-glycyrrhetinic acid (AGA), and the block was reversed after the blocker was washed out (W/O AGA). Each histogram bar corresponds to the mean ± SD of seven experiments, in each of which a minimum of 10 cells were scored.
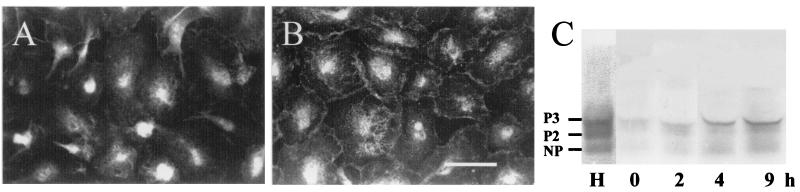
Figure 3.
Treatment with INF-γ plus TNF-α causes an increase in Cx43 immunoreactivity at contacts between rat microglia in culture and an increase abundance of Cx43. Cellular distribution of Cx43 was determined by immunofluorescence. (A) Distribution of Cx43 mmunofluorescence in a control culture at time 0. Most of the labeling was diffuse and localized to the perinuclear region. (B) In a culture treated with 1 ng/ml INF-γ plus 1 ng TNF-α for 9 h, there was marked labeling at intercellular appositions and reduced labeling in perinuclear regions. (Bar, 50 μm.) (C) The relative levels of Cx43 were determined by Western blot analysis of total homogenates of microglia (150 μg of protein per lane). Lane H is from a sample of rat heart to identify the mobility of different phosphorylation states of Cx43. NP is an unphosphorylated form, and P2 and P3 are two phosphorylated forms. The other lanes correspond to cultures of microglia treated with 1 ng/ml INF-γ plus 1 ng/ml TNF-α for 0, 2, 4, and 9 h.
Treatment for up to 24 h with INF-γ (1 ng/ml), LPS (1 μg/ml), or TNF-α (1 ng/ml) one at a time did not increase the incidence of dye coupling measured every 2 h with respect to control microglia (Fig. 2 graph). However, simultaneous treatment with 1 ng/ml INF-γ plus 1 μg/ml LPS induced a marked increase in dye coupling (Fig. 2 Graph). The increase in coupling was largely prevented by coapplication of 2 μg/ml anti-TNF-α antibody known to neutralize TNF-α (Fig. 2 Graph). Microglia treated for 9 h with 1 ng/ml INF-γ plus 1 ng/ml TNF-α also showed a marked increase in incidence of dye coupling (Fig. 2D and Graph). This result and the block by anti-TNF-α of the coupling induced by INF-γ plus LPS suggest that LPS induced secretion of TNF-α, which then had an autocrine action in the induction of dye coupling in the presence of INF-γ.
The intercellular dye transfer observed in rat microglia treated with cytokines was almost completely blocked after the application of 35 μM 18α-glycyrrhetinic acid for 5 min, and dye coupling was restored by washing out the gap junction blocker (Fig. 2, graph). Treatment of coupled cells with 50 μM oxidized ATP (not shown), a nonspecific blocker of P2 receptors (27), or 5 mM probenecid, a blocker of organic ionic transporter (28), did not affect coupling. Thus, the intercellular dye transfer was mediated by gap junction channels and not by leakage and uptake through purinergic receptors (P2X5 or P2X7) known to be permeable to Lucifer yellow (27) or by an ionic membrane transporter known to mediate uptake of Lucifer yellow (28).
INF-γ Plus TNF-α Treatment in Vitro Induces Redistribution of Cx43 to Cell–Cell Contacts.
To analyze further the induction of dye coupling and the increase in Cx43 levels by treatment with INF-γ plus TNF-α, we studied the cellular distribution of Cx43 by immunofluorescence. In microglia maintained in vitro under basal conditions Cx43 was detected as a diffuse cytoplasmic fluorescent label (Fig. 3A). After 9 h of treatment with 1 ng/ml INF-γ plus 1 ng/ml TNF-α, the cytoplasmic Cx43 reactivity was less intense, and much more was present at the interfaces between cells (Fig. 3B).
INF-γ Plus TNF-α Treatment in Vitro Increases Cx43 Levels in Rat Microglia.
To determine if the increases in both dye coupling and Cx43 immunolabeling found in INF-γ/TNF-α-treated cells were associated with changes in total amounts of Cx43, Western blot analyses were performed. In three independent experiments, total homogenates of cells treated for 2, 4, or 9 h with 1 ng/ml INF-γ plus 1 ng/ml TNF-α showed a progressive increase in Cx43 levels relative to microglia under control conditions (Fig. 3C). Under all conditions there were three Cx43 positive bands that comigrated with bands detected in heart homogenates corresponding to the unphosphorylated (NP) and phosphorylated P2 and P3 forms. There were no obvious changes in the relative proportions of the different forms.
INF-γ Plus TNF-α Treatment Induces Dye Coupling Between Normal Mouse Microglia but Not Between Microglia of Homozygous Cx43-Deficient Mice.
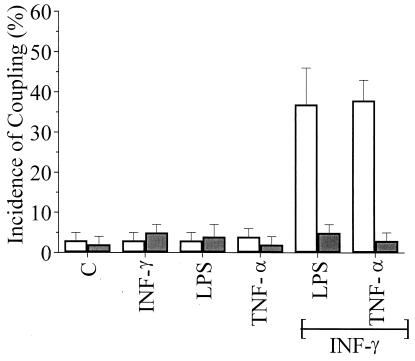
Microglia from wild-type and Cx43del/del mice showed a very low incidence of dye coupling under control culture conditions (Fig. 4). As for rat microglia, treatment with INF-γ (1 ng/ml), LPS (1 μg/ml), or TNF-α (1 ng/ml) one at a time did not increase coupling. However, after 9 h of treatment with 1 ng/ml INF-γ plus 1 μg/ml LPS or 1 ng/ml INF-γ plus 1 ng/ml TNF-α, microglia from wild-type mice showed a much higher incidence of dye coupling. The same treatments did not increase the incidence of dye coupling of microglia from Cx43del/del mice. Microglia from Cx43del/del mice expressed β-galactosidase (not shown), which was under control of the endogenous Cx43 gene regulatory elements (26). This result confirms the expression of Cx43 by microglia.
Figure 4.
Treatment with INF-γ plus LPS or TNF-α enhances dye coupling between microglia from normal mice (open bars), but not between microglia of Cx43del/del mice (filled bars). Cultures were incubated for 9 h under control conditions or with 1 ng/ml INF-α alone, 1 μg/ml LPS alone, 1 ng/ml TNF-α alone, 1 ng/ml INF-α plus 1 μg/ml LPS, or 1 ng/ml INF-γ plus 1 ng/ml TNF-α. There was little coupling under control conditions (C) or in cultures treated with one of the agents, but the two combined treatments resulted in a much higher incidence of dye coupling in cultures from wild-type mice. No cytokine treatment induced dye coupling of microglia from Cx43del/del mice. Each point represents the mean ± SD of three independent experiments in which at least 10 cells were microinjected with Lucifer yellow.
Discussion
Others have shown that microglia interact with each other and other cells of the central nervous system through membrane contacts favored by expression of adhesion molecules, MHC class II molecules, and specific receptors for sugar residues and antibodies (2). Here we report that activation of microglia either in vivo or in vitro induces expression of Cx43, and at least in vitro they are coupled by gap junctions after treatment with inflammatory cytokines. Thus, microglia may use this pathway as an additional or alternative route of homocellular communication that may be an integral part of the activation state induced by specific factors in their microenvironment.
In the normal adult neocortex, microglia are sparse and in an apparent quiescent state, but after a stab wound they migrate to and proliferate at the site of damage (22), where they have the potential to establish homocellular contacts because of their close proximity. Microglial proliferation is maximal 2–4 days after injury and precedes the proliferation of invading blood-borne macrophages (22). We found that under control conditions about 95% of microglia identified as FITC-isolectin-B4-positive were not Cx43 immunoreactive, but that at 4 days after a stab wound, ≈60% of the accumulated microglia were Cx43 positive. Thus, in vivo the induction of Cx43 expression by microglia occurs during the transition from their quiescent to their activated state and presumably is triggered by inflammatory mediators. In adult brain, microglia are not activated and are ED-1 negative, but in primary cultures they express this antigen (29), suggesting that the tissue culture condition induces some degree of activation. Moreover, in primary cultures about 99% of the cells present in our microglia cultures were ED-1 positive and expressed low levels of Cx43, which may have been attributable to a small degree of activation. The expression of connexins and formation of gap junction channels, at least at homocellular contacts, might be a common feature of macrophagic cells. All other macrophagic cells previously studied, such as cultured epidermal Langerhans cells (15), peritoneal macrophages (30, 31), foam cells of atherosclerotic lesions (32), J774 cells (33), polymorphonuclear cells in a tissue subjected to ischemia-reperfusion (31), and Kupffer cells (34), express Cx43. In addition, gap junctional communication has been identified morphologically and/or functionally between cultured macrophages (35–39). Presumably, all of these cell types were activated to some degree, inasmuch as they were either under culture conditions or present at inflammatory foci, supporting the fact that Cx43 expression is induced upon activation. In agreement with the latter, it is known that circulating (nonactivated) human monocytes (32) and hamster polymorphonuclear cells (31) do not express Cx43 mRNA or Cx43 protein, respectively. Nevertheless, Cx43 has been detected in ≈40% of liver Kupffer cells (34), which are activated as indicated by their phagocytic activity (40). Despite the fact that under resting culture conditions microglia appeared to be somewhat activated in that they expressed ED-1 and Cx43, they were rarely dye coupled, suggesting that under the degree of activation induced in culture, microglia do not communicate with each other via gap junctions.
We found that when applied singly, two host cytokines, TNF-α and INF-γ, and a bacteria-derived proinflammatory factor, LPS, did not promote dye coupling between microglia. Similarly, LPS or INF-γ applied singly does not induce dye coupling between mouse peritoneal macrophages or J774 cells, a murine macrophage-derived cell line (30). However, coapplication of LPS or TNF-α with INF-γ induced dye coupling accompanied by an increase in Cx43 levels and translocation to cell–cell contacts. Synergistic action of INF-γ with other inflammatory stimuli, particularly with LPS and TNF-α, is known in diverse macrophage functions, including antitumor, antibacterial, and antiviral activities (41). Moreover, elevation of INF-γ has been found in the brain of animals with autoimmune disorders such as experimental allergic encephalomyelitis (42, 43), suggesting that under these conditions microglia might communicate to each other via Cx43 gap junctions induced by TNF-α plus INF-γ. On the other hand, after acute trauma the brain levels of INF-γ mRNA remain unchanged, and in INF-γ (−/−) mice astrogliosis is still observed (44); thus, the inflammatory response after brain trauma may be mediated by a different set of cytokines. The fact that LPS or TNF-α plus INF-γ did not induce dye coupling in every cultured microglial cell could be due to the known heterogeneity of microglia (45), the need for additional inflammatory mediators, and failure to use an appropriate concentration of the cytokines tested.
Microglia may express other connexins in addition to Cx43, because most cell types that form gap junctions express more than one connexin type (16). Although it might be true that microglia express connexins other than Cx43, the fact that INF-γ plus LPS or TNF-α did not induce dye coupling between microglia of Cx43del/del mice suggests that Cx43 is an essential if not the only protein subunit of gap junctions between microglia. Thus, if microglia express other connexins they might mediate gap junctional communication between microglia and other cell types (heterocellular gap junctions). The inflammatory response is a complex process involving numerous factors. The reaction to an invading pathogen is mediated by factors generated by the host as well as factors coming from the infecting agent. Some cytokines are autocrine in origin, such as TNF-α released by microglia, whereas others are generated by a different type of cell found at inflammatory foci, such as interferons released by T-cells. All of these processes could be modulated and orchestrated by gap junctional communication between inflammatory cells.
Acknowledgments
We thank Ms. Teresa Vergara for the preparation of rat astrocyte cultures. This work was partially supported by Grants 2990004 (to E.A.E.) and 8990008 (to J.C.S.) from the Fondo Nacional de Investigación Científica y Tecnológica, by Grant NS 07512 (to M.V.L.B.) from the National Institutes of Health, by Grant SFB 284 (to K.W.) from the German Research Association, and a travel fellowship from the Consejo Nacional de Investigaciónes Científicas y Tecnicas and the Deutscher Akademischer Austauschdienst (to E.A.E.). The data in this paper are from a thesis submitted in partial fulfillment of the requirements for the degree of Doctor in Sciences (E.A.E.) at the Pontificia Universidad Católica de Chile.
Abbreviations
- LPS
bacterial lipopolysaccharide
- INF-γ
interferon-γ
- TNF-α
tumor necrosis factor-α
Footnotes
Eugenín, E. A. & Sáez, J. C. (2000) Mol. Biol. Cell 11, 227a.
References
- 1.Hofman F M, Hinton D R, Johnson K, Merrill J E. J Exp Med. 1989;170:607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreutzberg G W. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, Itagaki S, Boyes B E, McGeer E G. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 4.McGeer P L, McGeer E G. Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 5.Rogers J, Luber-Narod J, Styren S D, Civin W H. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 6.Shigematsu K, McGeer P L, Walker D G, Ishii T, McGeer E G. J Neurosci Res. 1992;31:443–453. doi: 10.1002/jnr.490310306. [DOI] [PubMed] [Google Scholar]
- 7.Raine C S. J Neuropathol Exp Neurol. 1994;53:328–337. doi: 10.1097/00005072-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Jordan C A, Watkins B A, Kufta C, Dubois-Dalcq M. J Virol. 1991;65:736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki A, Hirato J, Nakazato Y. Acta Neuropathol. 1993;86:337–344. doi: 10.1007/BF00369445. [DOI] [PubMed] [Google Scholar]
- 10.Sawamura Y, De Tribolet N. Adv Tech Stand Neurosurg. 1990;17:3–64. doi: 10.1007/978-3-7091-6925-4_1. [DOI] [PubMed] [Google Scholar]
- 11.Gehrmann J, Matsumoto Y, Kreutzberg G W. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 12.Whittemore E R, Korotzer A R, Etebari R, Cotman C W. Brain Res. 1993;621:59–64. doi: 10.1016/0006-8993(93)90297-z. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin J L, Kehrli M E, Uemura E. Brain Res. 1997;768:279–286. doi: 10.1016/s0006-8993(97)00653-7. [DOI] [PubMed] [Google Scholar]
- 14.Streit W J, Kreutzberg G. J Comp Neurol. 1988;268:248–263. doi: 10.1002/cne.902680209. [DOI] [PubMed] [Google Scholar]
- 15.Sáez J C, Araya R, Brañes M C, Concha J M, Contreras J, Eugenín E A, Martínez A D, Palisson F, Sepúlveda M A. In: Gap Junctions: Molecular Basis of Cell Communication in Health and Disease. Peracchia C, editor. San Diego: Academic; 1999. pp. 555–579. [Google Scholar]
- 16.Bruzzone R, White T H, Paul D L. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 17.Simon A M, Goodenough D A. Trends Cell Biol. 1998;8:477–483. doi: 10.1016/s0962-8924(98)01372-5. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N M. Novartis Found Symp. 1999;219:6–21. [PubMed] [Google Scholar]
- 19.Eugenin E A, Gonzalez H, Saez C G, Sáez J C. Am J Physiol. 1996;27:G1109–G1116. doi: 10.1152/ajpgi.1998.274.6.G1109. [DOI] [PubMed] [Google Scholar]
- 20.Berthoud V M, Rook M B, Traub O, Hertzberg E L, Sáez J C. Eur J Cell Biol. 1993;62:384–396. [PubMed] [Google Scholar]
- 21.Yamamoto T, Ochalski A, Hertzberg E L, Nagy J I. Brain Res. 1990;508:313–319. doi: 10.1016/0006-8993(90)90415-8. [DOI] [PubMed] [Google Scholar]
- 22.Amat J A, Ishiguro H, Nakamura K, Norton W. Glia. 1996;18:368–382. doi: 10.1002/(SICI)1098-1136(199604)16:4<368::AID-GLIA9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Giulian D, Baker T J. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frei K, Bodmer S, Schwerdel C, Fontana A. J Immunol. 1986;137:3521–3527. [PubMed] [Google Scholar]
- 25.Martínez A D, Sáez J C. Brain Res. 1999;816:411–423. doi: 10.1016/s0006-8993(98)01016-6. [DOI] [PubMed] [Google Scholar]
- 26.Theis M, de Wit C, Schlaeger T M, Eckardt D, Krüger O, Döring B, Risau W, Deutsch U, Pohl U, Willecke K. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Chiozzi P, Sanz J M, Ferrari D, Falzoni S, Aleotti A, Buell G N, Collo G, Di Virgilio F. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer F, Bischof S, Röllinghoff M, Lohoff M. J Immunol. 1994;153:3523–3532. [PubMed] [Google Scholar]
- 29.Slepko N, Levi G. Glia. 1996;16:241–246. doi: 10.1002/(SICI)1098-1136(199603)16:3<241::AID-GLIA6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Alves L A, Coutinho-Silva R, Persechini P M, Spray D C, Savino W, Campos de Carvalho A C. Blood. 1996;88:328–334. [PubMed] [Google Scholar]
- 31.Jara PI, Boric M P, Sáez J C. Proc Natl Acad Sci USA. 1995;92:7011–7015. doi: 10.1073/pnas.92.15.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polacek D, Lal R, Volin M V, Davies P F. Am J Pathol. 1993;142:593–606. [PMC free article] [PubMed] [Google Scholar]
- 33.Beyer E C, Steinberg T H. J Biol Chem. 1991;266:7971–7974. [PubMed] [Google Scholar]
- 34.Sáez C G, Eugenín E A, Hertzberg E L, Sáez J C. In: From Ion Channels to Cell-to-Cell Conversations. Latorre R, Sáez J C, editors. New York: Plenum; 1997. pp. 367–380. [Google Scholar]
- 35.Levy J A, Weiss R M, Dirksen E R, Rosen M R. Exp Cell Res. 1976;103:375–385. doi: 10.1016/0014-4827(76)90273-1. [DOI] [PubMed] [Google Scholar]
- 36.Porvaznik M, MacVittie T J. J Cell Biol. 1979;82:555–564. doi: 10.1083/jcb.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell F R. Anat Rec. 1980;196:101–107. doi: 10.1002/ar.1091960110. [DOI] [PubMed] [Google Scholar]
- 38.Martin C A, Homaidan F R, Palaia T, Burakoff R, el-Sabban M E. Cell Adhes Commun. 1998;5:437–449. doi: 10.3109/15419069809005602. [DOI] [PubMed] [Google Scholar]
- 39.Martin C A, el-Sabban M E, Zhao L, Burakoff R, Homaidan F R. Cell Adhes Commun. 1998;5:83–95. doi: 10.3109/15419069809040283. [DOI] [PubMed] [Google Scholar]
- 40.Bouwens L, Baekeland M, De Zanger R, Wisse E. Hepatology. 1986;6:718–722. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- 41.Paludan S R. J Leukocyte Biol. 2000;67:18–25. doi: 10.1002/jlb.67.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy M K, Torrance D S, Picha K S, Mohler K M. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 43.Issazadeh S, Mustafa M, Ljungdahl A, Hojeberg B, Dagerlind A, Elde R, Olson T. J Neurosci Res. 1995;40:579–590. doi: 10.1002/jnr.490400503. [DOI] [PubMed] [Google Scholar]
- 44.Rostworowski M, Balasingam V, Chabot S, Owens T, Wee Yong V. J Neurosci. 1997;17:3664–3674. doi: 10.1523/JNEUROSCI.17-10-03664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streit W J, Graeber M B. Glia. 1993;7:68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]