Abstract
The caudate-putamen (CPu) has been implicated in habit learning and neuroadaptive changes that mediate the compulsive nature of drug-seeking following chronic cocaine self-administration. Recent findings from our laboratory have demonstrated that re-exposure to an operant chamber previously associated with cocaine, but not yoked-saline, increases activity-regulated cytoskeleton-associated (Arc) gene mRNA expression within the dorsolateral (dl) CPu following prolonged abstinence. In this study, we tested the hypothesis that antisense gene knockdown of Arc within the dlCPu would alter cocaine-seeking. Initial studies showed that a single infusion of Arc antisense oligodeoxynucleotide (ODN) into the dlCPu significantly attenuated the induction of Arc mRNA and Arc protein by a single cocaine exposure (20 mg/kg, i.p.) as compared to scrambled-ODN-infused controls. In cocaine self-administering rats, infusion of Arc antisense ODN into the dlCPu 3h prior to a test of context-driven drug-seeking significantly attenuated Arc protein induction, but failed to alter responding during testing, suggesting striatal Arc does not facilitate context-induced drug-seeking following prolonged abstinence. However, Arc antisense ODN infusion increased responding during subsequent 1 h extinction tests 24 and 48 h later. Following re-exposure to a cocaine-paired context, surface expression of the AMPA-type glutamate receptor GluR1 was significantly reduced whereas GluR2 was significantly increased in the dlCPu, independent of Arc antisense ODN infusion. Together, these findings indicate an important role for Arc in neuroadaptations within brain regions responsible for drug-seeking after abstinence and direct attention to changes occurring within striatal circuitry that are necessary to break down the habitual behavior that leads to relapse.
Keywords: self-administration, immediate early gene, AMPA receptors, antisense
Introduction
Central to the nature of addiction, is the high risk of relapse to drug-seeking and -taking even after prolonged periods of abstinence (O’Brien et al., 1998; Robbins and Ehrman, 1998). This relapse is often propelled by exposure to environmental cues that become associated with the drug and its rewarding effects over time (Crombag and Shaham, 2002; Ehrman et al., 1992; Fuchs et al., 2005, 2006). Accumulating evidence suggests a role of the striatum, particularly the dorsolateral caudate-putamen (dlCPu), in the compulsive aspects of addiction (Everitt and Robbins, 2005; Everitt and Wolf, 2002; Ito et al., 2002; Jog et al. 1999). In humans, exposure to drug-related cues increases metabolic activity and dopamine release in the CPu that is highly correlated with self-reported craving measures (Garavan et al., 2000; Volkow et al., 2006). Similarly, prolonged cocaine self-administration produces a progressive recruitment of the dorsal and lateral regions of the caudate and putamen of non-human primates, (Porrino et al., 2004a). More recently, pharmacological inactivation of the dlCPu prior to re-exposure to a previously cocaine-paired environment was found to inhibit drug-seeking (Fuchs et al., 2006; See et al., 2007) in abstinent and extinguished rats. Additionally, re-exposure to an environment previously associated with cocaine self-administration following acute and prolonged abstinence significantly increases activity-regulated gene expression in the CPu (Hearing et al., 2008b).
Arc (activity-regulated cytoskeleton-associated gene), also known as activity-regulated gene 3.1 (arg3.1), is an effector immediate early gene (IEG) that is critical for activity-dependent plasticity underlying learning and memory (Guzowski et al., 2000; Messaoudi et al., 2007; Tzingounis and Nicoll, 2006). Arc mRNA is up-regulated in cortical and striatal regions by cocaine (Fumagalli et al., 2006, 2009) and cocaine-associated stimuli (Hearing et al., 2008a, b ; Zavala et al., 2008). Arc mRNA is rapidly and transiently transcribed in neurons (Guzowski, 2002; Lanahan and Worley, 1998; Rao et al., 2006; Vazdarjanova et al., 2006) and is unique because it is rapidly transported and localized to dendrites that receive active synaptic stimulation (Link et al., 1995; Lyford et al., 1995; Steward and Worley, 2001). Arc is critically involved in activity-induced synaptic plasticity through endocytosis of AMPA-type glutamate receptors (AMPARs) and regulation of the cytoskeleton (Bramham et al., 2008; Chowdhury et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006; Sutton and Schuman, 2006), as well as homeostatic forms of plasticity (Rao et al., 2006). In this study, we used an antisense oligodeoxynucleotide (AS-ODN) to suppress Arc expression to assess the function of Arc in the rat dlCPu in context-elicited drug-seeking and extinction learning following a period of abstinence from cocaine self-administration. Further, we assessed the effects of drug context re-exposure and Arc suppression on alterations in the surface expression of GluR1 and GluR2 AMPAR subunits within the dlCPu.
Methods
Animals
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300-325 g on arrival were individually housed in a temperature- and humidity-controlled vivarium on a reversed light/dark cycle. Rats were, maintained at 90% of free-feeding body weight and were allowed water ad libitum. The housing and treatment of the rats were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996).
Surgery
Rats were anesthetized with ketamine and xylazine (66.6 and 1.3 mg/kg, i.p., respectively) followed by Equithesin (0.5 ml/kg, i.p.), and treated with ketorolac (2.0 mg/kg, i.p.). In self-administering rats, intravenous catheters were implanted into the external jugular vein as described (Hearing et al.2008a,b). Cefazolin (10 mg/0.1 ml, i.v.) was infused at the end of the surgery and stylets inserted into the catheters when the rats were not connected to infusion pumps.
For intracranial surgeries, rats were secured using a stereotaxic device (Stoelting, Wood Dale, IL), and 5 mm stainless steel guide cannulae (Plastics One Inc., Roanoke, VA) were implanted bilaterally into the dlCPu [coordinates (from bregma): +1.2 mm AP, ±3.6 mm ML, −3.4 mm DV]. Guide cannulae were secured to the skull with cranioplastic cement and anchored with three steel machine screws, with stylets (Plastics One, Inc.) inserted following surgery to prevent blockage. Rats were infused i.v. with 0.1 ml of cefazolin (33.3 mg/kg,) and heparanized saline (70 U/ml) daily during a 5-day recovery period.
Oligodeoxynucleotides and intracranial infusions
The 20-mer antisense oligodeoxynucleotide (AS-ODN) encoding the Arc mRNA sequence spanning the translation start site was the reverse complement of bases 209 to 228 (5′-CCGCTCGTGTACCTCGACCG-3′) of the published Arc sequence (Lyford et al., 1995) as described by Guzowski and colleagues (2000). The sequence of the scrambled ODN (SC-ODN) was (5′-GCCGCACTCTCCCTGCTACT-3′). Gel filtration-purified ODNs (Midland Certified Reagent Company, Midland, TX), contained phosphorothioate linkages on the three terminal bases of both the 5′ and 3′ ends.
In acute cocaine and cocaine self-administration studies, all rats were habituated to test rooms (5 days) and the intracranial infusion procedure (2 days) prior to testing. One day prior to infusion, 5 mm-long stylets were replaced with 7.5 mm stylets in order to minimize any new damage the next day (Keefe et al., 1995) when each stylet was replaced with a 33 ga infusion needle (Plastics One), extending 2 mm beyond the tip of the guide cannula. Each infusion needle was connected by tubing to a 10 μl Hamilton syringe that was controlled by a microinfusion pump (Harvard Apparatus, Holliston, MA). After insertion, the injector remained in place for 1 minute before beginning the infusion. One μl (2.0 nmol/μl; in 1×PBS) of AS-ODN or SC-ODN was infused into each hemisphere of an unrestrained rat at a rate of 0.1 μl/min. Following the infusion, the injector remained in place for 2 min to reduce backflow of the solution along the infusion track.
Acute cocaine treatment
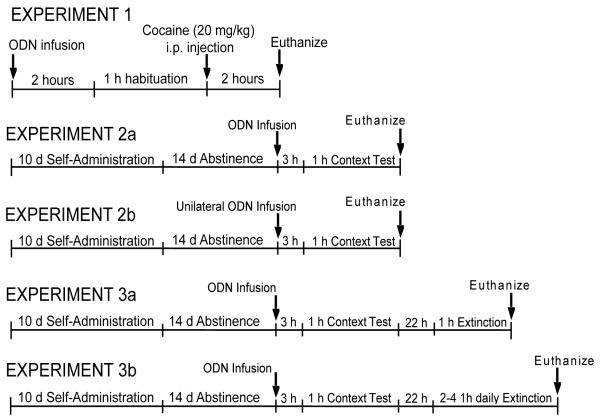
In Experiment 1, the effects of Arc AS-ODN on acute cocaine-induced Arc mRNA and Arc protein expression were evaluated (see Fig. 1 for design of all experiments). Rats were given a single bilateral intra-dlCPu infusion of Arc AS-ODN or SC-ODN 3 h prior to cocaine based on previous findings that infusion of Arc AS-ODN into the hippocampus 3 h before behavioral training disrupted consolidation of spatial learning in a water maze (Guzowski et al., 2000). Two hours after the infusion, rats were habituated in photocell chambers (Accuscan Instruments, Inc., Columbus, OH) for 1 h, followed by the cocaine injection (20 mg/kg, i.p.; National Institute on Drug Abuse, Research Triangle Park, NC) or saline. Total distance traveled was recorded in 5 min bins for 2 h following cocaine by a PC running VersaMax/Digiscan System Software (AccuScan Instruments, Inc.). Immediately after the test, all rats were anesthetized with Equithesin prior to decapitation and brain removal. Brains were bisected with one hemisphere flash frozen in dry ice-cooled isopentane (−40°C) and stored at −80°C for in situ hybridization analysis of arc mRNA. The dlCPu and dorsomedial (dm) CPu were hand-dissected from 2 mm coronal slabs of the contralateral hemisphere cut in a rat brain matrix (Braintree Scientific, Braintree, MA), frozen on dry ice, and stored at −80°C for assessment of Arc immunoreactivity (ir).
Fig 1.
Schematic representation of the experimental paradigms used in Experiments 1-5.
Self-administration
In Experiments 2-5 (Fig 1), rats were trained to press a lever to receive an i.v. cocaine infusion in operant chambers (30 × 24 × 30 cm, Med Associates, St Albans, VT) for 120 min daily during the rats’ dark cycle for a minimum of 10 days as described (Hearing et al., 2008a,b). Cocaine-treated rats received response- (active, right lever presses) contingent infusions of cocaine (0.6 mg/kg in 50 μl of sterile saline) on an FR1 schedule without stimulus cues. Responses on the inactive (left) lever had no programmed consequences but were recorded. Yoked-saline rats received infusions of saline contingent upon the cocaine infusions received by the animal in the adjacent chamber. Data collection and reinforcer delivery were controlled using MedPC software version IV (Med Associates).
Abstinence and drug-seeking
On the last day of self-administration, rats were returned to the colony room for 14 -17 days. During the last 5 days of abstinence, rats were habituated for 2 h/day to a different procedure room (alternate environment) where intracranial infusions were performed on the test day.
In Experiment 2a-b, rats underwent cocaine or yoked-saline sessions daily followed by 15 d of homecage abstinence. In Experiment 2a, 3 h after rats received a single bilateral intra-dlCPu infusion of Arc AS-ODN or SC-ODN on the 15th day, they were placed into the operant chambers for a 1 h drug-seeking test during which lever presses were recorded but had no programmed consequences. Immediately following the 1 h test, rats were anesthetized with Equithesin (1 ml/kg i.v. or 5 ml/kg i.p., depending on catheter patency) 5 min before decapitation. Brains were bisected with one hemisphere flash-frozen for analysis of cannula placement. The dm and dlCPu were dissected from the contralateral hemisphere for analysis of Arc-ir. In Experiment 2b, rats received a unilateral infusion of Arc AS-ODN and a contralateral infusion of SC-ODN in the dlCPu 3 h before a 1 h drug-seeking test. Immediately following testing, rats were decapitated, brains removed, and CPu tissue processed for measurement of GluR1 and GluR2 surface expression.
In Experiment 3a-b, following self-administration and abstinence as described above, rats received a single bilateral intra-dlCPu AS or SC ODN infusion 3 h prior to a 1 h drug-seeking test. Twenty-two h later, they underwent a 1 h extinction test in the operant chamber (Experiment 3a) after which they were anesthetized with Equithesin, and decapitated. The brains were bisected for cannula placement analysis and immunoblotting as described above. In Experiment 3b, rats underwent 2-4 daily 1 h extinction sessions to assess the duration of any AS-ODN effects on extinction learning. Rats were anesthetized and decapitated within 24 h of the final extinction test for analysis of cannula placements.
Biotinylation
Surface biotinylation procedures were adapted from Fourgeaud and colleagues (2004). The centrolateral CPu was punched from a 2 mm section and cut into 200 μm slices using a McIlwain Tissue Chopper (Wood Dale, IL). Slices were immediately transferred into 1 ml of EZlink NHS-SS-Biotin (1 mg/ml; Pierce, Rockford, IL) in artificial cerebrospinal fluid (ACSF) and incubated for 1 h at 4°C with gentle agitation. Slices were washed in 100 mM glycine/ACSF followed by two washes in ACSF for 20 min at 4°C. The washed slices were centrifuged (10,000g for 1 min) and sonicated in 100 μl of lysis buffer [25 mM HEPES, 150 mM NaCl, 1% Triton X-100, 0.1% SDS] containing Complete Mini Protease Inhibitor (Roche, Indianapolis, IN) and Halt phosphatase inhibitor (Pierce, Rockford, IL). Homogenates were then centrifuged at 1000g at 4°C for 7 min, the supernatant was collected, and protein concentrations were measured using a BCA assay kit (Pierce, Rockford, IL). Lysates (250 μg) were incubated overnight at 4°C with 50 μl streptavidin agarose beads (Pierce, Rockford, IL), saving the remaining homogenate as a total protein fraction. The next day, samples were centrifuged at 10,000g for 5 min and the supernatant was collected (non-biotinylated fraction). Beads were then washed three times in ice-cold lysis buffer followed by ice-cold 50 mM Tris-HCl, pH 7.4. All protein bound to beads was then extracted by heating samples to 90°C for 7 min in 6× SDS-sample buffer containing 100 mM DTT. Samples were then centrifuged for 1 min at 10,000g and the supernatant containing biotinylated proteins was collected for immunoblotting.
Immunoblotting
Tissue from the dl and dmCPu was sonicated in 1% SDS lysis buffer, boiled, and centrifuged at 4°C for 20 min at 10,000g. Protein concentrations were determined by BCA assay (Pierce, Rockford, IL). Protein aliquots [5 μg-Arc; 20 μg total GluR1 or GluR2] were separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes using an iBlot dry transfer system (Invitrogen, Carlsbad, CA). Membranes were incubated for 1 h in 5% milk/Tris buffered saline (TBS) then in primary antibody diluted in 5% milk/TBS /0.1% Tween-20 overnight at 4°C. Each membrane was washed in TBS/0.1% Tween-20, followed by incubation in goat anti-rabbit horseradish peroxidase-conjugated secondary antiserum (Millipore) for 1 h at room temperature. The membranes were again washed and developed for 1-5 min using enhanced chemiluminescence (ECL plus-Amersham Biosciences Piscataway, NJ,). The signals were exposed on Hyperfilm (Amersham Biosciences) for variable durations to optimize signal to noise ratios. The integrated density of each band was measured using Image J software (NIH, Bethesda, MD). For Arc, the same membranes were probed for calnexin and a ratio of Arc:Calnexin was used to express changes in Arc-ir. The following primary rabbit antisera were used in the study: Arc (Synaptic Systems, Goettingen, Germany; 1:80,000), AGS1 (1:3000, a gift from Dr. Stephen Lanier, MUSC), GluR1 (Abcam; Cambridge, MA; 1:5000), GluR2 (Millipore; 1:1000), Calnexin (Cell Signaling; Danvers, MA; 1:20,000). The secondary antiserum (Cell Signaling) was used at the following dilutions for each primary antiserum: Arc (1:12,000), GluR1 (1:10,000), GluR2 (1:5000), Calnexin (1:20,000).
In situ hybridization histochemistry
In situ hybridization histochemistry was performed as described previously using antisense 35S-labelled oligodeoxynucleotide probes complementary to rat Arc mRNA (Hearing et al. 2008a,b). The 48-mer oligodeoxynucleotide probe (Integrated DNA Technologies (IDT), Coralville, IN) was labeled at the 3′ end using alpha-[35S]-dATP (Perkin Elmer, Waltham, MA) and terminal deoxynucleotidyl transferase (Roche Diagnostics Corp, Indianapolis, IN). Twelve μm slide-mounted tissue sections were pretreated and then hybridized at 37°C for 16-20 h. After a series of stringent washes in saline-sodium phosphate-EDTA (SSPE), slides were dried and placed in an X-ray film cassette with 14C standards (American Radiolabeled Chemicals, Inc., St Louis, MO) and Biomax MR film (Eastman Kodak, Rochester, NY). The films were developed after 6-9 d to establish linearity and an optimal signal:noise ratio.
Image analysis
Quantitation of film autoradiograms was performed using the Macintosh-based NIH Image program as previously described (Hearing et al., 2008b). The 14C standards were measured and plotted against known dpm/mg to generate a calibration curve. The mean density and number of pixels per area were measured in the striatum from three adjacent sections (AP levels: +1.08 - +1.32 mm from bregma - based on Paxinos and Watson, 2007) per brain. The measurements are expressed as integrated density (# of pixels per area × mean density).
Statistical analysis
In all experimental data analyses, a p-value <0.05 was accepted in order to reject the null hypothesis. Acute cocaine-induced locomotor activity was analyzed by calculating the area under the curve (AUC) for total distance traveled over time, followed by a two-way ANOVA with drug and ODN treatment as main factors. In Experiment 2a, a one-way ANOVA was used to individually evaluate active and inactive lever responses, in all four treatment groups, averaged over the final 3 d of self-administration. Active and inactive lever responses during the 1 h context test were analyzed individually using two-way ANOVAs (drug × ODN treatment). In Experiment 3a, active and inactive lever responses in all four treatment groups were averaged over the final 3 d of self-administration and individually analyzed by one-way ANOVAs. Active and inactive lever responses during the 1 h context test and extinction day 2 were individually assessed using a two-way ANOVA to evaluate the effects of drug and ODN treatment within each test day. A two-way repeated measures ANOVA was also performed to analyze the effects of ODN treatment and extinction day on active lever responding only in cocaine-treated groups. Additionally, active lever responses in all four treatment groups were assessed in 15 min bins during the 1 h context test and extinction day 2 by two-way repeated measures ANOVA (treatment group × time). In Experiment 3b, self-administration active and inactive lever responses in cocaine-treated groups were analyzed with ANOVA in cocaine groups. In addition, a two-way repeated measures ANOVA was used to analyze the effect of ODN treatment across test days (extinction) on active lever responding. A Student Neuman Keuls (SNK) multiple comparison test was performed following all ANOVAs when a significant interaction or more than one main effect was found using SNK with Sigma Plot 11.0 (Systat Software Inc.; San Jose, CA). Mean protein ratios were analyzed using a two-way ANOVA, followed by SNK multiple comparisons to analyze more than one main effect or interaction.
Measurements of the hybridization signals (Integrated Density, ID) are strongly correlated and cannot be treated independently. Therefore, the data were fit with a hierarchical linear model using ID as the response variable with drug treatment and infusion (as well as a drug treatment by infusion interaction) as fixed effects explanatory variables and rat as a random effect (mixed model ANOVA SAS 9.2) followed by Tukey-Kramer tests when an interaction was found or to further analyze the source of main effects.
Results
Histology
Schematic diagrams indicating the placement of the infusion cannula tips and representative Nissl-stained sections illustrating S-ODN and AS-ODN cannula tracts in Experiments 1, 2a, 3a and 3b are illustrated in Supplementary Fig S1. Rats for Experiment 2b were decapitated and cannula placements in the dlCPu were verified during dissection. Animals that did not have proper bilateral placements (n=8) were not used in the data analysis. The final number of rats per group was as follows: Experiment 1, Saline-scrambled ODN (SAL-SC); n=5, Saline-antisense ODN (SAL-AS); n=5, Cocaine-scrambled ODN (COC-SC); n=5, Cocaine-antisense ODN (COC-AS); n=5. Experiment 2a, SAL-SC; n=5, SAL-AS; n=7, COC-SC; n=6, COC-AS; n=8; Experiment 3a, SAL-SC; n=7, SAL-AS; n=7, COC-SC; n=5, COC-AS; n=6. Experiment 3b, COC-SC; n=9, COC-AS; n=11.
Arc AS-ODN infusion attenuates acute cocaine-induced Arc mRNA and protein expression in the dlCPu
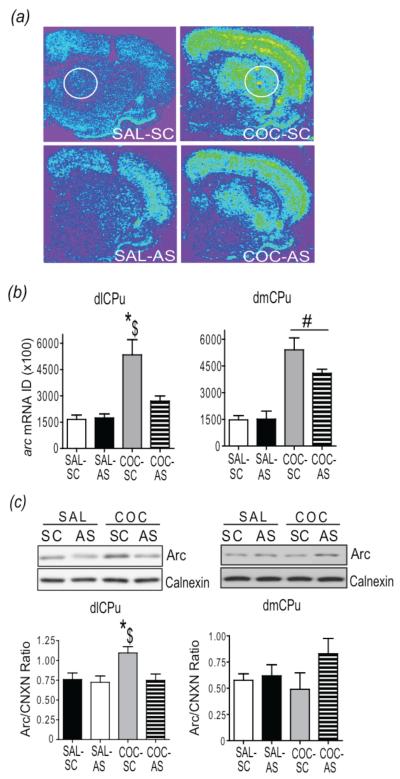
Infusion of Arc AS-ODN into the dlCPu blocked acute cocaine-induced increases in Arc mRNA and Arc protein in the dlCPu, but not in the dmCPu, 2 h following the cocaine injection (Fig 2). In Fig 2a, Arc hybridization signals at the level of the ODN infusion from each group are illustrated. Two-way ANOVA revealed a significant interaction of drug (saline, cocaine) × ODN (SC-ODN, AS-ODN;F(1,15) = 7.81, p<0.05) on Arc mRNA in the dlCPu. Tukey-Kramer multiple comparison tests revealed significantly greater Arc mRNA expression in COC-SC-treated rats than in COC-AS and SAL-SC-treated rats (Fig 2b-left). In contrast, there was a significant main effect of drug treatment (F(1,15)=55.49, p<0.05) in the dmCPu where Arc mRNA was significantly greater in both COC-SC and COC-AS treated rats than in SAL-SC rats controls (Fig 2b-right).
Fig 2.
Intra-dlCPu infusion of AS-ODN suppresses acute cocaine-induced Arc mRNA and protein. (a) Representative coronal hemi-sections and (b) quantitative analysis of the integrated density of the arc mRNA hybridization signal in the dlCPu (left) and dmCPu (right). (c) Top: Representative subsections of immunoblots illustrating Arc and calnexin bands in the dlCPu (left) and dmCPu (right) from each group. Bottom: Quantitative analysis of Arc protein levels in the dlCPu (left) and dmCPu (right) 2 h following acute cocaine injection. $p<.51 vs. SAL-SC; *p<.05 vs COC-AS; #p<.05 cocaine vs. saline. ID = Integrated Density; CNCX= Calnexin. SAL = saline; COC = cocaine; SC = S-ODN; AS = AS-ODN.
Two-way ANOVA of Arc protein in the dlCPu also revealed a significant drug × ODN interaction (F(1,16)=6.044, p<0.05). Student Neuman Keuls (SNK) multiple comparison tests revealed that Arc-ir was significantly higher in the dlCPu of COC-SC rats than in COC-AS and SAL-SC rats. Importantly, Arc–ir in COC-AS rats was not significantly greater than in SAL-AS-treated rats (Fig. 2c-left). No significant main effect of drug (F(1,18) = 0.74; p<0.05) or infusion (F(1,18) = 3.45; p<0.05) was found on Arc-ir in the dmCPu (Fig. 2c-right). Analysis of locomotor activity revealed a significant main effect of cocaine (F(1,19) = 16.15, p<.05) on total distance traveled; however, there was no significant difference between COC-SC and COC-AS groups (Supplementary Fig. S2). The ability of a single infusion of Arc AS-ODN into the dlCPu to block acute cocaine-induction of Arc mRNA and Arc protein provided support for the feasibility of suppressing cocaine context-induced Arc expression by antisense gene knockdown in order to investigate the role of striatal Arc in drug-seeking in the following experiments.
Intra-dlCPu AS-ODN infusion suppresses Arc protein induced by re-exposure to a cocaine-paired context following abstinence, but fails to alter drug-seeking behavior
Experiment 2 sought to determine if induction of Arc within the dlCPu following re-exposure to a cocaine-paired environment is necessary for the expression of context-driven drug-seeking after abstinence. To test this hypothesis, rats with a history of cocaine or yoked-saline received a single bilateral intra-dlCPu infusion of Arc AS- or SC-ODN 3 h prior to a 1 h test session. Daily cocaine intake collapsed across cocaine groups was 30.81 ± 2.19 infusions (~15.4 mg/kg per session). ANOVA of lever responding in all four treatment groups (SAL-SC, SAL-AS, COC-SC, COC-AS) averaged over the last three days of self-administration found significant differences in active (F(1,24)= 10.84, p<0.05) and inactive (F(1, 24) = 14.67, p<0.05) lever responses. SNK multiple comparisons revealed significantly greater responding on the active lever in COC-SC and COC-AS groups compared to SAL-SC and SAL-AS respectively (p<0.05); however, no differences were found within either cocaine or saline-treated groups. Additionally, inactive lever responding was significantly lower in COC-SC and COC-AS rats compared to SAL-SC (p<0.05) and SAL-AS (p<0.05) groups compared to COC-SC and COC-AS, but were not significantly different from each other. However, responding in SAL-SC was greater than SAL-AS (p<0.05; Fig. 3a).
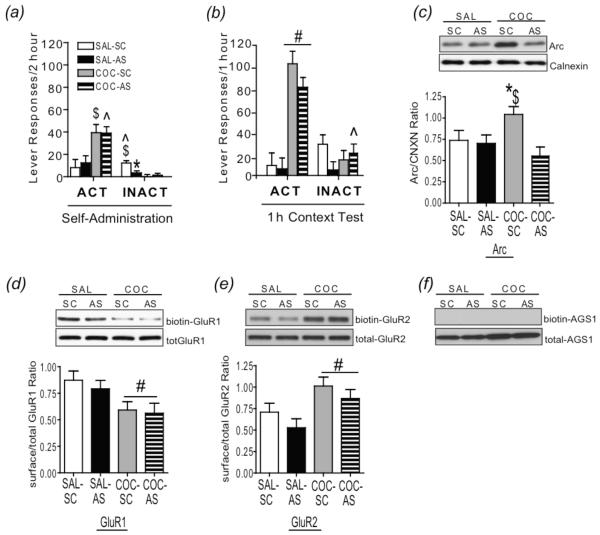
Fig 3.
Intra-dlCPu AS-ODN infusion suppresses Arc protein induced by re-exposure to a cocaine-paired context following abstinence, but fails to alter drug-seeking behavior. (a) Active and inactive lever responding averaged over the last 3 d of 2 h self-administration sessions and (b) during a 1 h context re-exposure test following prolonged abstinence. (c) Top: Representative subsection of an immunoblot illustrating Arc and calnexin bands in the dlCPu from each group. Bottom: Quantitative analysis of Arc protein in the dlCPu (Experiment 3) following a 1 h context test. (d) Top: Representative subsection of an immunoblot illustrating biotinylated GluR1 and total GluR1 bands in the dlCPu from each group. Bottom: Ratio of surface/total GluR1 in the dlCPu following a 1 h context test. (e) Top: Representative immunoblot illustrating biotinylated GluR2 and total GluR2 bands in the dlCPu from each group. Bottom: Ratio of surface/total GluR2 expression in the dCPu following a 1 h context test. (f) A control image shows the specificity of the biotinylation assay. No band can be detected for the cytoplasmic-rich protein AGS1 in the biotinylated fraction in representative immunoblot of each treatment group. $p<.05 vs. SAL-SC; ^p<.05 vs. SAL-AS; *p<.05, vs. COC-AS;. #p<.05, cocaine vs. saline; SAL = saline; COC = cocaine; SC = SC-ODN; AS = AS-ODN; AGS1= activator of g-protein signaling 1; CNCX= Calnexin.
Two-way ANOVA of total active lever responses during the 1 h drug-seeking test revealed a significant main effect of cocaine (F(1,21) = 74.78, p<0.05) but not AS-ODN (F(1, 21) = 0.87, p>0.05), indicating that AS-ODN infusion prior to context re-exposure did not significantly alter drug-seeking induced by re-exposure to a previously cocaine-paired context. (Fig 3b). Comparison of inactive lever responding during the 1 h context re-exposure revealed a significant drug × ODN interaction (F(1,26) = 7.83, p<0.05). SNK multiple comparisons revealed significantly greater responding in COC-AS rats than in SAL-AS controls.
Infusion of Arc AS-ODN 3 h prior to a 1 h test of context-induced drug-seeking blocked induction of Arc protein expression in the dlCPu (Fig. 3c). Two-way ANOVA revealed a significant interaction of drug × ODN treatment (F(1,25) = 4.80, p<0.05). SNK multiple comparison tests showed that Arc was significantly greater in the COC-SC rats than in COC-AS and SAL-SC rats, and that Arc-ir was not significantly greater in the COC-AS rats than in SAL-AS rats.
Given that Arc protein functions to regulate trafficking of GluR1- and GluR2-containing AMPARs (Chowdhury et al., 2006; Rial Verde et al., 2006; McCurry et al., 2010) and that subunit composition plays a critical role in determining the biophysical properties of AMPARs (Dingledine et al. 1999; Palmer et al. 2005), we evaluated alterations in the surface expression GluR1 and GluR2 immediately following a 1 h context test. Comparison of biotinylated- versus total-ir in rats that received unilateral AS- and SC-ODN infusions revealed that surface expression of GluR1 was decreased, whereas GluR2 was increased, following re-exposure to a cocaine-paired context. A main effect of drug treatment was found for both GluR1 (F(1,21) = 5.95, p<0.05; Fig 3d) and GluR2 (F(1,19)=9.53, p<0.05; Fig 3e) surface expression. A brain-enriched cytosolic protein termed activator of G-protein signaling 1 (AGS1, Fang et al., 2000), was used as an intracellular control; a lack of AGS1 signal in the biotinylated fraction demonstrates the specificity of the biotinylation assay (Fig. 3f).
Intra-dlCPu Arc antisense ODN infusion prior to context-driven relapse attenuates extinction of cocaine-seeking behavior
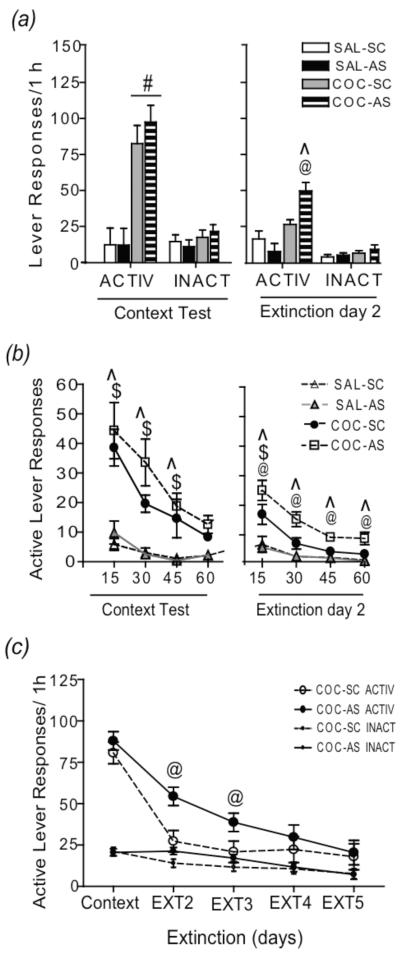
Suppressing Arc expression in the dlCPu prior to a test of context-driven relapse did not alter drug-seeking during the initial 1 h test; however, it is possible that the induction of Arc within the dlCPu following re-exposure to a previously drug-paired context under extinction conditions may reflect neuroadaptive processes necessary for extinction learning. To test this hypothesis, Experiment 4a examined drug-seeking in rats receiving a bilateral intra-dlCPu infusion of Arc AS- or SC-ODN prior to initial re-exposure to a cocaine-paired context during a 1 h extinction session 22 h later. Collapsed across cocaine groups, daily cocaine intake was 28.53 ± 2.53 infusions (~14.27 mg/kg per session). ANOVA of lever responding averaged over the last three days of self-administration in all four treatment groups found significant differences in active (F(1,24)= 11.09, p<0.05) and inactive (F(1, 24) = 8.78, p<0.05) lever responses. SNK multiple comparisons revealed significantly greater responding on the active lever in COC-SC (38.88 ± 4.02) and COC-AS (35.88 ± 5.54) groups compared to SAL-SC (11.03 ± 4.7, (p<0.05) and SAL-AS (9.04 ± 2.47) respectively (p<0.05). No differences were found within either cocaine or saline-treated groups. Additionally, inactive lever responding was significantly lower in COC-SC (0.06 + 0.06) and COC-AS (0.22 ± 0.22) rats compared to SAL-SC (5.91 ± 1.29; p=0.03) and SAL-AS (9.2 ± 2.33; p=0.001) groups, however, no difference was found between either cocaine or saline-treated groups. Two-way ANOVA of total active lever responses during a 1 h context test following abstinence revealed a significant main effect of cocaine treatment (F(1,24) = 36.48, p<0.05). Responding was significantly greater in cocaine-treated than in saline-treated rats, confirming findings in Experiment 2 that prior infusion of Arc AS-ODN does not alter drug-seeking behavior (Fig. 4a-left). There was no significant effect of drug (F(1,24) = 0.95, p>0.05) or ODN (F(1,24) = 0.11, p>0.05) on inactive lever presses. Twenty-two hours following the 1 h context test, rats were returned to the operant chamber for an additional 1 h extinction session (Extinction day 2). Two-way ANVOA of active lever responding during extinction day 2 revealed a significant interaction of drug × ODN effect (F(1,24) = 8.82, p<0.05). SNK multiple comparison tests showed significantly higher responding on the active lever in COC-AS than in COC-SC and SAL-AS rats; however, active lever responding in COC-SC rats was not significantly greater than in SAL-SC rats (Fig. 4a-right).
Fig 4.
Intra-dlCPu Arc AS-ODN infusion prior to context-driven relapse attenuates extinction of cocaine-seeking behavior during subsequent context exposures. (a) Active and inactive lever pressing during a 1 h context test (left) and extinction day 2 (right; Experiment 4). (b) Active lever responses with the 1 h context test (left) and extinction day 2 (right) represented in 15 min time bins. (c) Active and inactive lever responding during a 1 h context test and subsequent extinction days (Experiment 5). #p<.05, cocaine vs saline; ^p<.05, COC-AS vs. SAL-AS; @p<.05, COC-AS vs. COC-SC; $p<.05 COC-SC vs. SAL-SC. SAL = saline; COC = cocaine; SC = S-ODN; AS = AS-ODN.
Figure 4b (left) illustrates active lever responding for cocaine and yoked-saline-treated rats in four separate 15 min time bins during the 1 h context test and subsequent 1 h context exposure on extinction day 2 (right). Two-way repeated measure ANOVA of drug-seeking during the context test revealed a significant treatment (SAL-SC, SAL-AS, COC-SC, COC-AS) × time (15, 30, 45, 60 m) interaction (F(9,99) = 3.08, p<0.05). SNK multiple comparison tests of treatment group within each time point revealed that COC-SC and COC-AS rats displayed significantly greater active lever responding during the first 45 min than SAL-SC and SAL-AS rats. However, active lever pressing of COC-SC rats was not significantly different from that of COC-AS rats. Drug-seeking of COC-SC and COC-AS rats returned to yoked-saline control levels by 60 min of the context re-exposure. Analysis of active lever responding during extinction day 2 also revealed a significant interaction of treatment (drug/ODN) × time (F(9,99)=2.08, p<0.05). SNK multiple comparison tests of treatment group within each time point revealed that COC-AS rats displayed significantly higher responding compared to SAL-AS rats during each 15 min bin whereas COC-SC responses were significantly greater than SAL-SC responses only during the first 15 min (Fig 4b-right). Additionally, COC-AS active lever pressing was significantly greater than COC-SC pressing during each 15 min time bin. Further analysis of drug-seeking within cocaine groups across test days revealed a significant main effect of time (extinction day) on active lever responding (F(1,21) = 11.37, p<0.05), but not ODN treatment (F(1,21)=2.63, p>0.05). Analysis of Arc protein in the dlCPu immediately following the extinction day 2 test did not reveal any significant differences between groups (Supplementary Fig. S3).
In order to determine if the attenuating effects of AS-ODN on extinction learning extended beyond day 2, in Experiment 3b, additional cohorts of rats underwent cocaine self-administration and received a single infusion of AS-ODN or SC-ODN prior to a 1 h context test, followed by 2 or 4 subsequent daily 1 h extinction tests. Total active (ANOVA; F(1,18) = 0.09, p>05) and inactive (ANOVA; F(1,18) = 0.00, p>0.05) lever responses and infusions [COC-SC (30.91 ± 3.26); COC-AS (31.73 ± 1.67); ANOVA F(1,18) = 0.03, p>0.05] averaged over the last 3 d of self-administration was not significantly different between COC-SC and COC-AS groups. Two-way repeated measures ANOVA of active lever responding over extinction days revealed a significant main effect of treatment (drug/ODN; F(1,82)=4.67, p<0.05) and time (F(1,82)=32.53, p<.05). SNK multiple comparison tests revealed that COC-SC and COC-AS active lever responding was not significantly different during the context test; however, COC-AS rats displayed significantly greater responding during extinction day 2 and extinction day 3 than COC-SC rats (Fig. 4c).
Discussion
This study demonstrated that infusion of Arc AS-ODN into the dlCPu 3h prior to a test of context-induced drug-seeking significantly attenuated context-induced arc mRNA and Arc protein expression in the dlCPu, but failed to alter responding during testing, suggesting striatal Arc does not facilitate context-induced drug-seeking following prolonged abstinence. However, Arc AS-ODN-infused rats responded more than SC-ODN-infused rats with a cocaine history during extinction tests 24 and 48 h later, indicating an important role for Arc in striatal molecular changes necessary to extinguish drug-seeking. Additionally, AMPA-type glutamate receptor GluR1 surface expression was significantly decreased, whereas GluR2 was increased, in the dlCPu following re-exposure to a cocaine-paired context.
Previous findings from this lab demonstrated that drug-seeking upon re-exposure to a cocaine-paired context following abstinence without extinction increased cortical and striatal activity-regulated gene expression (Hearing et al., 2008a,b), including Arc. However, it remained unclear whether these changes reflected activation of the circuitry underlying drug-seeking or, alternatively, neuroadaptive changes involved in extinction learning, since drug was not available during testing. In the present study, the delayed extinction of conditioned responding across the first three days of extinction testing is not likely due to a disruption in acquiring new contextual information during the relapse event because active lever responding was reduced to yoked-saline control levels after 45 min of the initial context exposure in both AS- and SC-ODN-treated cocaine rats. Therefore, all rats with a cocaine history learned at the same rate that the context was no longer indicative of cocaine availability. However, during extinction day 2, only COC-AS rats displayed significantly greater responding throughout testing, indicating delayed extinction of drug-seeking. In contrast, COC-SC rats’ responding was significantly greater than yoked-saline rats’ only during the first 15 min, suggesting retention of extinction learning from the previous day. Interestingly, the attenuating effect of Arc suppression during extinction learning was maintained until day 3 of extinction testing, after Arc levels had returned to normal, suggesting that initial suppression of Arc in the dlCPu had a prolonged effect on extinction learning. Therefore, given that Arc is suppressed throughout the initial 1 h context test and that basal Arc protein is suppressed up to 6 h following a single AS-ODN infusion (Guzowski et al., 2000), it is possible that Arc induction within the dlCPu is involved in maintaining or consolidating extinction learning-induced plasticity. This is consistent with the proposed role of Arc in maintaining synaptic plasticity (Lu, 2003; Rial Verde et al., 2006) and consolidating long-term memory (Guzowski et al., 2000; McIntyre et al., 2005).
Following re-exposure to a previously cocaine-paired context, GluR1 surface expression was significantly decreased independent of Arc knockdown. Previous studies have shown that induction of long-term depression (LTD) corresponds to an internalization of GluR1-containing AMPARs (Beattie et al., 2000; Ehlers, 2000); therefore, it is plausible that internalization of GluR1 following cocaine context extinction may represent a weakening of dlCPu synapses previously responsible for driving cocaine-seeking behavior. It is possible that these decreases reflect adaptations that occurred during abstinence prior to testing; however, cell surface GluR1 AMPARs increase in the nucleus accumbens (Boudreau et al., 2007; Conrad et al., 2008) and CPu (Tang et al., 2004; Hemby et al., 2005) during cocaine withdrawal but internalize following cocaine challenge (Boudreau et al., 2007). Interestingly, decreases in GluR1 surface expression were paralleled by significant increases in GluR2-containing AMPARs. Because no significant differences in total GluR1-ir or GluR2-ir were found between cocaine and yoked-saline groups, it is likely that following re-exposure to a cocaine-paired context, there is a redistribution of pre-existing GluR1 and GluR2 subunits, whereby GluR1-containing receptors may be replaced by GluR2-containing receptors. This idea is consistent with findings that high frequency stimulation induces rapid replacement of calcium-permeable GluR2-lacking receptors with calcium impermeable GluR2-containing AMPA receptors (Liu and Cull-Candy, 2000). Given that the presence or absence of GluR2 can regulate synaptic strength (Cull-Candy et al. 2006; Liu and Zukin et al. 2007), it is possible that incorporation of GluR2-containing AMPARs decreases the responsiveness of striatal neurons to glutamate inputs from cortical regions previously responsible for drug-seeking, and may reflect mechanisms by which extinction-induced changes in synaptic strength are stabilized. In vitro investigations of Arc’s function at the synapse have demonstrated a role for Arc in AMPAR endocytosis (Chowdhury et al., 2006; Rial Verde et al., 2006; Shepard et al., 2006). However, decreases in GluR1 surface expression following the context test were not altered by prior suppression of Arc protein in this study, suggesting that suppression of Arc may not be critical for facilitating GluR1 or GluR2 receptor trafficking in the dlCPu under these behavioral conditions. Alternatively, it is possible that the context-induced changes in GluR1 and GluR2 may occur in a subpopulation of striatal medium spiny neurons not affected by Arc, a possibility that this study could not distinguish. However, a more likely explanation is that the partial knockdown exerted by intra-striatal Arc AS-ODN may not have been sufficient to affect GluR surface expression, as evident in a recent study in which complete loss of Arc in vivo prevented removal of GluR1 AMPARs from the surface in mouse visual cortex (McCurry et al., 2010).
The current study demonstrates that Arc induction in the dlCPu during re-exposure to a cocaine-paired context plays an important role in extinguishing drug-seeking following abstinence and that altered GluR surface expression following drug-context exposure may be indicative of extinction-related plasticity occurring within the dlCPu. Importantly, Arc suppression and alterations in GluR surface expression were largely confined to the dlCPu, consistent with the claim that a lateral corticostriatal circuit mediates the transition to habitual decision processes (Jog et al., 1999). Together, these neuroadaptations may promote a weakening of synapses within the striatal network that were previously responsible for promoting compulsive drug-seeking.
Supplementary Material
Acknowledgements
The authors would like to thank Phong Do and Shannon Ghee for excellent technical support. This research was supported by NIH P50 DA15369, NIH T32 DA07288, and NIH CO6 RR015455.
Footnotes
Statement of interest: None
References
- Beattie EC, Carroll RC, Yu X, Morishita W, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neuroscience. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. Journal of Neuroscience. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. Journal of Neuroscience. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behavioral Neuroscience. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Current Opinion in Neurobiology. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacology Review. 1999;51:7–61. [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. Journal of Neuroscience. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, et al. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. Journal of Neuroscience. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Differential neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. Journal of Neuroscience. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Frasca A, Di Pasquale L, et al. Corticostriatal upregulation of activity-regulated cytoskeletal-associated protein expression after repeated exposure to cocaine. Molecular Pharmacology. 2006;70:1726–1734. doi: 10.1124/mol.106.026302. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Franchi C, Caffino L, Racagni G, et al. Single session of cocaine intravenous self-administration shapes goal-directed behaviors and up-regulates Arc mRNA levels in rat medial prefrontal cortex. International Journal of Neuropsychopharmacology. 2009;12:423–429. doi: 10.1017/S1461145708009681. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. Journal of Neuroscience. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology. 2008b;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Structure and Function. 2008a;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Research. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. Journal of Neuroscience. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, et al. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1-D2 dopamine receptors synergy in striatum: Effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience. 1995;66:903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiology of Learning and Memory. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, et al. Phosphorylation of the AMPA receptor subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Kaiwen H, Lihua S, et al. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. Journal of Neurophysiology. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proceedings of the National Academy of Sciences USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends in Neurosciences. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learning and Memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Alzawa Y, Huganir RL. Regulation of alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proceedings of the National Academy of Sciences USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry CL, Shepherd JD, Tropea D, Wang KH, et al. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nature Neuroscience. 2010;13:450–458. doi: 10.1038/nn.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, et al. Memory-influencing intra-basolateral amygdale drug-infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences USA. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. Journal of Neuroscience. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Lau LF, Huganir RL. Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacology Review. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, et al. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. Journal of Neuroscience. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, et al. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nature Neuroscience. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, et al. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Cocaine use is associated with increased craving in outpatient cocaine abusers. Experimental and Clinical Psychopharmacology. 1998;6:217–224. doi: 10.1037//1064-1297.6.2.217. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proceedings of the National Academy of Sciences USA. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, et al. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. Journal of Neurochemistry. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52:403–407. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramierez-Amaya V, Insel N, Plummer TK, et al. Spatial exploration induces arc, a plasticity-reglated immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. Journal of Comparative Neurology. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6583. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.