Abstract
In the context of critical illness, hypotension may be associated with acute kidney injury (AKI). Using the MIMIC II database, we studied the risk of AKI in ICU patients as a function of both the severity and duration of hypotension. Multivariate logistical regression was performed to find correlations between hypotension and AKI. Minimum mean arterial blood pressure (MAP) and the amount of time MAP was below a range of hypotension thresholds in a target 48-hour window (prior to AKI onset) were used as primary predictive variables in the multivariate model. Our results indicate that the risk of AKI was related to the severity of hypotension with an odds ratio (OR) of 1.03, 95% CI 1.02–1.04 (p < 0.0001) per 1 mmHg decrease in minimum MAP ≥ 80 mmHg. For each additional hour MAP was less than 70, 60, 50 mmHg, the risk of AKI increased by 2% (OR 1.02, 95% CI 1.00–1.03, p = 0.0034), 5% (OR 1.05, 95% CI 1.02–1.08, p = 0.0028), and 22% (OR 1.22, 95% CI 1.04–1.43, p = 0.0122) respectively.
1. Introduction
Acute kidney injury (AKI) refers to a rapid decrease in kidney function, occurring within a period of days. The presence of AKI can be detected using well-established definitions based on serum creatinine rise or urine output reduction [1].
Acute kidney injury has been reported to occur in 36% of all patients admitted to ICU [2, 3]. A recent study showed that hospital patients with even very small increases in their serum creatinine (0.3 to 0.4 mg/dL) have 70% greater risk of death than patients without increase [4].
Although the relationship between low blood pressure and kidney function is well documented in an experimental setting based on animal data [5], the association between hypotension and acute kidney injury in an ICU setting is not completely understood. Recent work that studied the relationship between hypotension and AKI or mortality focused on septic shock patients [6, 7]. The present study aims to determine the risk of AKI development in ICU patients as a function of both the severity and the duration of hypotension using the MIMIC II database [8, 9].
2. Methods
Patients were selected from among the 25,328 adult ICU stays in the MIMIC-II [8] database, version 2.4. We examined adult ICU stays (patients ≥ 15 yrs of age) with at least 2 serum creatinine values. ICU stays with evidence of end-stage renal disease (ESRD) were excluded.
Based on the Acute Kidney Injury Network (AKIN) definition [1], AKI was defined as an acute increase in serum creatinine ≥ 0.3 mg/dL, or an increase of ≥ 50% in serum creatinine within 48 hours. The AKI onset time during an ICU stay was defined as the time when the creatinine rise criterion was first met. Creatinine measurements taken 12 hours before or after the ICU stays were included for analysis. The controls were defined as patients who did not develop AKI during any of their ICU stays. A single ICU stay was selected for each patient.
Nurse-verified mean arterial blood pressure samples, recorded on an hourly basis (from either invasive or non-invasive oscillometric method) were used for the analysis. Blood pressure measurements were filtered to remove values outside of reasonable physiological bounds (MAP between 20 to 200 mmHg). To eliminate artifacts due to dampening of the arterial line, measurements where pulse pressures were less than 10% of recorded MAP was rejected. Measurements were excluded if the absolute difference between the estimated and recorded MAPs were greater than 10% of recorded MAP, where MAP was estimated as (2Pdiastolic + Psystolic)/3.
Blood pressure features were extracted during a target window beginning at 48 hours prior to time T in each ICU stay. For the AKI cohort, T was defined as 3 hours prior to the AKI detection time. For the controls, T was defined as 3 hours prior to the last creatinine measurement time.
Multivariate logistical regression was performed to find correlations between blood pressures and development of AKI in the ICU patients. Nine blood pressure features extracted from the target 48-hour window were examined as primary predictors for AKI, including the minimum MAP and maximum number of hours that MAP was continuously less than or equal to 80, 75, 70, 65, 60, 55, 50, and 45 mmHg in the target window. Linear interpolation was performed on nurse-verified blood pressure samples. Hypotensive episodes were considered to begin and end when the interpolated blood pressure values intercepted the target threshold. Hypotensive episodes that were less than one hour apart were merged to form one continuous episode.
We studied the relationship between severity of hypotension and risk of AKI in patients with minimum MAP ≤ 80 mmHg and with at least 40 hours of blood pressure samples in the target 48-hour window. To study the risk of AKI as a function of hypotension duration, we included patients with a minimum of 45 hours of blood pressure samples in the target window. The sampling duration (in hours) in the target 48 hour window for each ICU stay was added in the multivariate model as a control variable. We found no correlation between duration of hypotension and sampling duration for data included for the study.
Age, SAPS-1, admission creatinine, and the presence (based on ICD-9) of chronic renal failure (585.9), hypertension (401.9), diabetes (250.00), coronary atherosclerosis (414.01), congestive heart failure (428.0), and septic shock (785.52) or sepsis (038) were added as potential confounding factors (see [10]). Patients for whom a SAPS-I value could not be determined were excluded from the multivariate analysis.
For each of the nine blood pressure features, we built a separate multivariate logistic regression model, which consisted of 11 variables in total (one blood pressure feature with ten control variables). For each target feature, we reported its p value, odds ratio (OR, with 95% confidence interval) after adjusting for the above mentioned control variables. The overall area under the receiver operating characteristic curve (AUC) of the multivariate model, and the Hosmer-Lemeshow p value were reported to assess the model fit.
3. Results
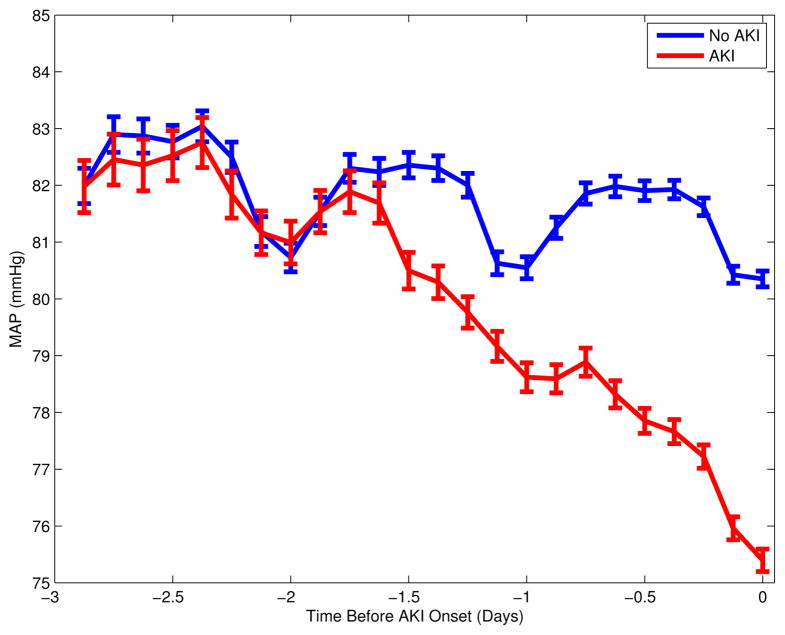
Among the 16,728 adult ICU stays that had at least 2 creatinine measurements without evidence of end-stage renal disease, AKI occurred in 5,207 (31%). The remaining 11,521 cases were identified as the controls. Table 1 summarizes the patient characteristics. As expected, the hospital mortality, SAPS-1 score, and LOS were higher among patients with AKI. The average AKI onset time was 2.34 days after ICU admission. For the controls, the last creatinine sample time was, on average, 2.76 days after ICU admission. Figure 1 plots the population mean of median MAP up to 3 days prior to the AKI onset for the AKI cohort, or prior to the last creatinine measurement time for the controls. Mean arterial blood pressure of the AKI cohort diverged from that of the controls during day two prior to the AKI onset, and both cohorts exhibited prominent diurnal variation.
Table 1.
Patient characteristics. LOS, length of stay. Continuous variables are described as median and interquartile range and are compared with Wilcoxon rank sum test.
| AKI N = 5,207 | No AKI N = 11,521 | P value | |
|---|---|---|---|
| Age | 69 (57, 79) | 63 (49, 77) | < 0.001 |
| Male (n,%) | 3015 (58%) | 6476 (56%) | 0.0414 |
| Weight (kg) | 78.5 (66, 93) | 77.5 (65.2, 91.0) | 0.0130 |
| SAPS-I | 16 (12, 19) | 12 (9, 15) | < 0.001 |
| LOS (days) | 4.8 (2.3, 10.7) | 2.0 (1.1, 3.2) | < 0.001 |
| Mortality | 22% | 7% | < 0.001 |
Figure 1.
Mean arterial blood pressure up to 3 days prior to AKI onset for the AKI cohort, or prior to the last creatinine measurement time for the no AKI group. Plot shows mean and standard error of patients’ median MAP in 3-hour bins. Circadian variations are apparent and reflect the timing of the creatinine measurements, which are usually taken in the early morning.
Table 2 shows the results from multivariate logistic regression using minimum MAP as the primary predictive variable for AKI. A total of 3,658 patients with minimum MAP ≤ 80 mmHg and at least 40 hours of blood pressure samples in the target window were used to fit the model. Among these patients, 1,233 (34%) had AKI. Mean and standard deviation of the minimum MAP in the target 48-hour window for AKI and no AKI groups are shown. Our results indicate that the risk of AKI was related to the severity of hypotension with an odds ratio of 1.03, 95% CI 1.02–1.04, p value < 0.0001, per 1 mmHg decrease in MAP below 80 mmHg.
Table 2.
Analysis of minimum MAP (<= 80 mmHg) as a risk factor in AKI development. N = 3,658. Odds ratio computed as per -1 mmHg below 80 mmHg.
| AKI (N=1,233) | No AKI (N = 2,425) | P val (adj.) | OR (95% CI) | AUC | P val (Hosm.Lem.) | |
|---|---|---|---|---|---|---|
| Minimum MAP (mmHg) | 58.47±10.83 | 61.29±10.33 | < 0.0001 | 1.03 (1.02, 1.04) | 0.78 | 0.0613 |
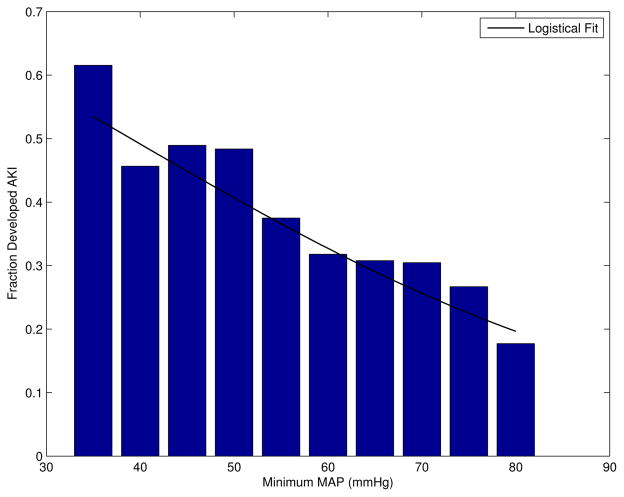
Figure 2 shows the fraction of patients that developed AKI as a function of their minimum MAP ≤ 80 mmHg in the target window. As expected, the fraction of patients that developed AKI increased as the blood pressure decreased below the auto-regulation range (≤ 80 mmHg).
Figure 2.
Fraction of patients who developed AKI as a function of minimum blood pressure in the 48-hour target window. N = 3,658 (34% AKI).
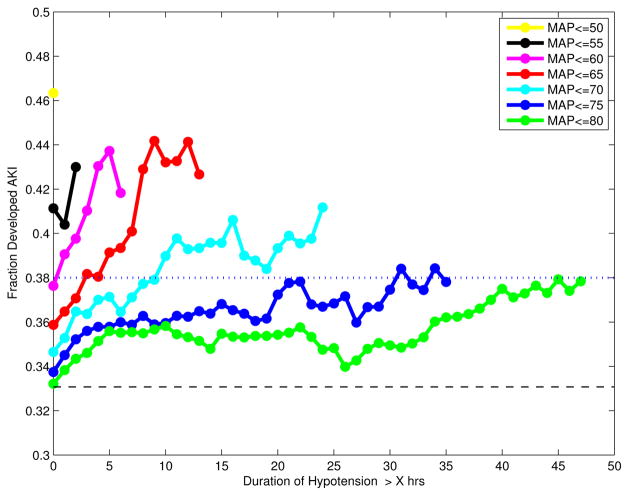
Analysis of hypotension duration (Figure 3) involved a total of 3,613 patients (AKI 1,195, controls 2,418) who had at least 45 hours of blood pressure samples in the target 48-hour window. Figure 3 shows the fraction of patients who developed AKI as a function of hypotension duration using a range of MAP values as hypotension thresholds. For a given threshold of hypotension duration (e.g., > 5 hours of hypotension), the plot suggests that the incidence of AKI increases with severity of hypotension. Further, for a fixed AKI incidence rate, as the degree of hypotension worsened, significantly shorter hypotension duration was observed before AKI arose. Specifically, the duration of hypotension prior to reaching 38% AKI incidence rate (5% above overall AKI incidence rate) decreased roughly exponentially for each additional 5 mmHg drop below 80 mmHg. The fraction of patients who developed AKI when MAP was ≤ 80 mmHg continuously for more than 45 hours was 0.38, which was approximately equivalent to the fraction of patients with MAP ≤ 75, 70, 65, and 60 mmHg continuously for more than 22, 10, 3, and 0.3 hours respectively.
Figure 3.
Fraction of patients who developed AKI as a function of hypotension duration in the target 48-hour window using various MAP values as thresholds. A third of the 3613 patients included in this plot developed AKI, as indicated by the black dashed line. Prolonged hypotension increased the incidence of AKI in these patients. The duration of hypotension associated with a 5% increase in AKI incidence (indicated by the dotted blue line) varies with severity of hypotension (see text).
Multivariate analysis on hypotension duration involved 3,203 patients who had SAPS-1 scores and ≥ 45 hours of blood pressure samples in the target 48-hour window. Table 3 displays the results from the multivariate logistic regression using each of the eight hypotension duration variables as a primary predictive variable. Our results indicate that the duration of time that the patient’s MAP was continuously less than or equal to 70, 65, 60, 55, and 50 mmHg were significant risk factors in AKI development. Further, as the extent of hypotension worsened, the incremental risk for AKI from each additional hour of continuous hypotension more than doubled for each 10 mmHg drop in MAP below 80 mmHg. For each additional hour MAP was continuously less than 70, 60, or 50 mmHg, the risk of AKI increased by 2%, 5% or 22% respectively (see table 3 for odds ratios).
Table 3.
Analysis of hypotension duration as a risk factor in AKI development. N = 3,203. AKI (N = 1,149). No AKI (N = 2,054). Odds ratio (OR) was computed as per 1 hour increase in hypotension duration.
| AKI | No AKI | P val (adj.) | OR (95% CI) (adj.) | AUC | P val (Hosm.Lem.) | |
|---|---|---|---|---|---|---|
| Hrs. MAP ≤ 80 | 17.23±14.58 | 15.58±14.38 | 0.0695 | 1.01 (1.00 1.01) | 0.77 | 0.3449 |
| Hrs. MAP ≤ 75 | 11.42±11.56 | 10.06±11.27 | 0.0583 | 1.01 (1.00 1.01) | 0.77 | 0.3007 |
| Hrs. MAP ≤ 70 | 6.84±8.57 | 5.56±7.40 | 0.0034 | 1.02 (1.00 1.03) | 0.77 | 0.3806 |
| Hrs. MAP ≤ 65 | 3.53±5.45 | 2.69±4.49 | 0.0005 | 1.03 (1.01 1.05) | 0.77 | 0.1458 |
| Hrs. MAP ≤ 60 | 1.45±2.74 | 1.06±2.37 | 0.0028 | 1.05 (1.02 1.08) | 0.77 | 0.4515 |
| Hrs. MAP ≤ 55 | 0.50±1.28 | 0.33±0.99 | 0.0039 | 1.11 (1.03 1.19) | 0.77 | 0.1643 |
| Hrs. MAP ≤ 50 | 0.14±0.67 | 0.08±0.41 | 0.0122 | 1.22 (1.04 1.43) | 0.77 | 0.2503 |
| Hrs. MAP ≤ 45 | 0.04±0.31 | 0.02±0.21 | 0.1339 | 1.26 (0.93 1.70) | 0.77 | 0.4501 |
4. Discussion and conclusions
This study evaluated hypotension severity and duration as risk factors for AKI development using a large ICU database. Results from multivariate logistic regression indicate that the severity and duration of hypotension are both significant risk factors in AKI development in ICU patients. Odds of AKI increased by 3% per 1 mmHg decrease in MAP below 80 mmHg. Further, as the degree of hypotension worsened, the increased risk for AKI from each additional hour of continuous hypotension more than doubled for each 10 mmHg drop in MAP below 80 mmHg.
Our results also suggest that the severity of hypotension significantly reduced the duration of hypotension prior to the onset of AKI. This may motivate future studies to identify the effective “time window of opportunity” for correcting hypotension, and to determine if earlier interventions can reduce the incidence of AKI once hypotension has been observed.
We acknowledge the following limitations in the current study. First, this was a retrospective study, and as such, the incidence of hypotension prior to AKI does not prove a causal mechanism. Second, we did not account for the presence of fluid or pressor treatment in our multivariate analysis. Future work may include analysis that accounts for interventions (contrast agents, NSAIDs, aminoglycosides, ACEI, etc) that may impair renal function.
Acknowledgments
This work was funded in part by the National Institute of Biomedical Imaging and Bio-engineering and by the National Institute of General Medical Sciences, under NIH cooperative agreement U01-EB-008577 and NIH grant R01-EB001659.
References
- 1.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, AKIN Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw S, George C, Dinu I, Bellomo R. A multi-center evaluation of the rifle criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 3.Ostermann M, Chang R. Acute kidney injury in the intensive care unit according to rifle. Critical Care Medicine. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 4.Chertow G, Burdick E, Honour M, Bonventre J, Bates D. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 5.Kirchheim HR, Ehmke H, Hackenthal E, Löwe W, Persson P. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflügers Archiv European Journal of Physiology. 1987;410:441–449. doi: 10.1007/BF00586523. [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw S, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo J, Skrobik Y, Kumar A. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Medicine. 2009;35:871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood K, Light B, Parrillo J, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 8.Saeed M, Lieu C, Raber G, Mark RG. MIMIC II: a massive temporal ICU patient database to support research in intelligent patient monitoring. Computers in Cardiology. 2002;29:641–644. [PubMed] [Google Scholar]
- 9.http://physionet.org/mimic2/
- 10.Abuelo G. Normotensive ischemic acute renal failure. The New England Journal of Medicine. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]