Abstract
Objective
To determine whether home-based screening for sexually transmitted infections (STIs) results in a higher STI screening rate compared to clinic-based screening in participants using long-acting reversible contraception.
Methods
We performed a randomized clinical trial of women using long-acting reversible contraception methods in the Contraceptive CHOICE Project (n = 558). Participants were randomly assigned to home-based testing (swabs mailed to the participant's home or clinic-based testing. Self-collected vaginal swabs were tested for Chlamydia trachomatis and Neisseria gonorrhoeae using strand displacement analysis. We estimated the relative risk of screening by group using Poisson regression with robust error variance.
Results
The randomization groups were similar at baseline, except for marital status; the clinic group had more never-married women (62.0% vs. 51.6%), and the home group had more divorced women (12.1% vs. 5.6%, p = 0.007). Women in the home group were more likely to self-report screening compared to women in the clinic group in the multivariable analysis (56.3% vs. 32.9%, RR 1.7, 95% CI 1.4 – 2.0). When analyzed by tests received or documented in medical records, similar results were obtained (56.3% vs. 25.0%, RR 2.2, 95% CI 1.7 – 2.7). Women who completed screening had higher levels of education and were more likely to receive public assistance compared to those who did not complete screening.
Conclusion
Long-acting reversible contraception users randomized to STI screening at home were more likely to complete screening than those randomized to traditional clinic-based screening. Home-based screening may be useful in women using LARC methods who may not present for regular screening.
INTRODUCTION
An estimated 19 million new sexually transmitted infections (STI) occur each year in the United States, making STIs a major public health problem (1). As half of cases occur in young adults, the CDC currently recommends that females age 25 years or younger receive annual STI screening (1, 2). However, only 26-60% of at-risk women in the United States do so (3-6).
Barriers to clinic access can be reduced with the use of self-obtained vaginal swabs in home-based screening. Non-invasive STI screening has been well-accepted by women in a variety of non-clinical settings (7-11). In a prospective cohort study, we found that women preferred home-based screening, and those who chose it were more likely to complete screening (12). Two randomized trials in Denmark (13, 14), and one in the United States (15), which studied high-risk adolescents, have also shown improved screening rates with home tests.
We conducted a trial that randomized women using long-acting reversible contraception (LARC) to home or clinic-based STI screening. LARC, which includes intrauterine devices (IUD) and subdermal implants, was used by 66% of the population in our previous study (12). The National Survey of Family Growth reported that IUD use increased from 2% to 2002 to 5.5% in 2006-2008 (16). As these contraceptive methods are effective for 3-10 years, LARC users may not seek medical care on an annual basis, making STI screening via self-collection a potentially desirable option. We sought to estimate which screening method would produce the highest STI screening rate among LARC users.
MATERIALS AND METHODS
The Contraceptive CHOICE Project is a prospective cohort study seeking to recruit 10,000 women and to promote the use of LARC (17). Participants are recruited from a university-based clinic, two abortion clinics, and several community-based clinics. Women are eligible for the study if they are between the ages of 14 and 45 years, speak English or Spanish, are seeking a new method of reversible contraception or are not currently using a method, have not had a hysterectomy or tubal sterilization, and are currently sexually active with a male partner or planning to become sexually active in the next six months. Informed consent is obtained for all participants. We obtained approval from the Washington University School of Medicine Human Research Protection Office prior to starting recruitment. After receiving comprehensive counseling on contraceptive options, participants choose a reversible contraceptive method that is provided at no cost for the 3-year study period. Telephone follow-up occurs at 3 months, 6 months, and every 6 months thereafter for the duration of the study - a total of seven contacts.
All women enrolled in the study are offered STI screening at baseline then again at 12, 24 , and 36 months postenrollment. At baseline, all women are instructed on proper technique to self-collect a vaginal swab. Swabs were tested for C. trachomatis and N. gonorrhoeae using the BDProbeTec ET (Becton Dickinson, Sparks, MD) instrument through DNA strand displacement amplification (SDA) technology. These specimens can be stored at room temperature and must be received by the testing laboratory within 16 days of collection.
This sub-study randomized 558 women to home- or clinic-based screening for the 12-month scheduled STI screen. Women were randomized using computer based randomization if they completed the baseline survey and consented to the sub-study at enrollment. For each participant a random number was generated using the uniform distribution with a cut-point of 0.50. Those below this cut-point were assigned to one treatment and above to the control. Staff and participants were blinded to the randomization status until the time of testing (12 months), and randomization was performed by an author who did not have contact with study subjects. At the 12 month survey, participants were required to be living in the United States and using a long-acting reversible method of contraception, defined as levonorgestrel intrauterine system (LNG-IUS), Copper T 380A intrauterine device, or the contraceptive subdermal implant. Participants identified as eligible by these criteria were given instructions to screen for STIs by randomization group. Participants could refuse testing for any reason. Recruitment ended August 1, 2009, after the desired sample size was reached.
Participants randomized to the home-based screening group were mailed a collection kit. The kits were packaged in a plain brown box and could be sent to any address the woman provided (e.g. a friend's house) to address potential privacy concerns. A vaginal swab and collection tube were provided, identical to those used at baseline screening. Detailed, step-by-step instructions with photographs explained how to collect the specimen and return it in a prepaid, pre-addressed mailer (Exakt-Pak, Oklahoma City, OK), which complied with Department of Transportation and United States Postal Service regulations.
Women randomized to clinic-based screening were able to test with their regular health care provider or at four local family planning clinics. Women in the clinic arm were mailed written instructions, which stated that participants could be reimbursed for any expenses of STI testing and treatment, and asked participants to provide medical records of such testing. Medical records release forms for the participant's primary provider were signed at enrollment. However, if the participant planned to test with a different provider, a new release form was mailed to the participant with a postage-paid return envelope.
As another clinic-based option, all women in the clinic-based randomization group were given written instructions to obtain testing at one of four family planning clinics. This was designed to accommodate participants without access to a private provider, though all participants were able to test at these clinics. No appointment was necessary and there was no cost for screening. The clinics were provided with self-collected vaginal swab kits as used in the home-based group.
Specimens from home kits were received by mail daily, and specimens from family planning clinics were returned within five days of specimen collection. Specimens that were not in satisfactory condition for testing (e.g. missing swab or low preservative fluid) were rejected, and the participant was asked to come to the study clinic to collect a new sample. Women with positive tests for C. trachomatis or N. gonorrhoeae were contacted by a research nurse and provided with antibiotic treatment for herself and all partners, at no cost to the participant.
Medical records were requested for all participants in the clinic-based group who had not documented screening by providing medical records or a specimen from the family-planning clinics. A records request was sent to the provider with whom the participant had intended to test. If a valid release for this provider was not available, a release was sent to the participant to sign and return, and requests were sent to any other provider for whom we had a valid medical records release. Two attempts were made to contact each medical provider. We did not request records from the family planning clinics where self-collected screening was available if participants listed this facility as their healthcare provider. Participants who presented there could receive testing through our study at no cost and without an appointment, and were thus unlikely to have been tested by other means at these sites.
Baseline and 12-month follow-up interviews collected detailed information on demographic characteristics, contraceptive use, reproductive history, sexually transmitted infection diagnosis and treatment, and sexual behavior with male and female partners. Women were mailed a written survey addressing satisfaction with the STI screening process fifty-six days after testing was offered. A postage-paid return envelope was provided. At the 18-month telephone interview, the next regularly scheduled follow-up after randomized STI screening, women were asked if they had undergone STI screening. If they had not screened, they were asked to provide one or more reasons why not.
The testing period was defined as 56 days following the 12-month interview date (coinciding with mailing of the satisfaction survey), so that the mailed survey did not serve as a reminder to complete screening. A completed test was defined as a received sample from the home kit or family planning clinic during the testing period; medical record documentation of a test for C. trachomatis and N. gonorrhoeae during the testing period; or self-report of screening on the mailed survey or 18-month telephone survey. A second outcome variable, documented completed tests, considered a participant ‘not screened’ if no medical record documentation could be found confirming her self-report of screening during the testing period.
Baseline characteristics of the home and clinic-based testing groups, and screening completers versus non-completers, were compared using chi-square or Fisher's exact test for categorical variables and Student's t-test for continuous variables. Ordinal data in the satisfaction survey were analyzed by the Mann-Whitney-U test. The hypothesis that the home-based screening group would have a higher proportion of completed tests was tested using the chi-squared test and quantified with a simple relative risk calculation. When the outcome of interest (completed tests) is common (>10%) in prospective studies, logistic regression may produce a biased estimate of the relative risk, with the extent of the bias is related to the prevalence of the outcome (18). To correct for this bias, we performed a Poisson regression with robust error variance; this method is one methodologic approach that produces an unbiased estimate of both the relative risk and 95% confidence intervals (19, 20). SPSS 17 (SPSS Inc., Chicago IL) and SAS 9.2 (SAS Institute Inc., Cary, NC) were used for statistical analyses. Based on a testing percentage of 35% in the clinic-based group, a 15% difference testing rates between groups (testing in the home group of 50%), and alpha of 0.05, we needed 242 patients per group (total n = 484) to achieve 90% power.
RESULTS
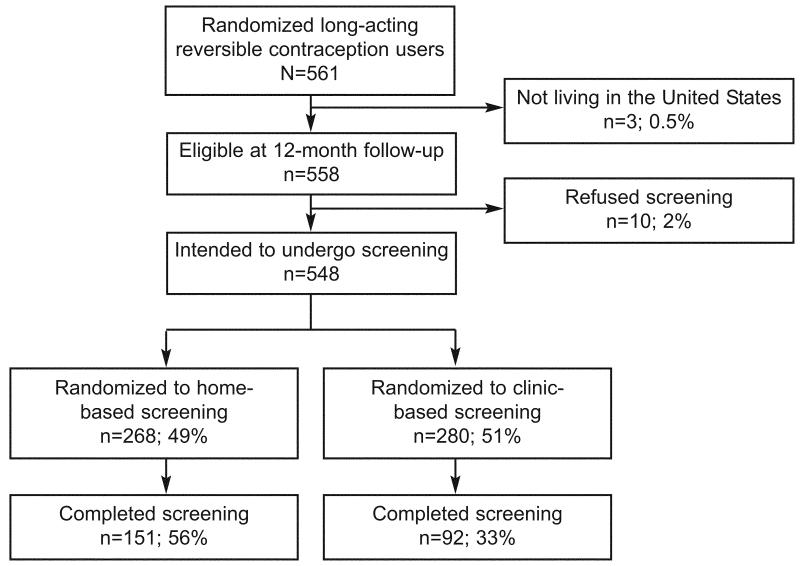
A total of 561 women were eligible for the study (Figure 1). At the 12-month interview, 3 women living outside of the United States were excluded. Ten women (2%) refused STI screening - five women from each randomization group. Five (50%) women refused screening because they considered themselves at low risk, four (40%) because they had recently been tested, and one (10%) woman was not interested in STI screening. There were no differences in refusal reason by randomization group. Of the 548 women intending to screen, 268 were randomized to home-based screening and 280 were randomized to clinic-based screening. Seven women were not offered testing according to their randomization status; two were randomized to home-based screening but offered clinic-based screening, and five in the clinic group were offered home-based screening. These participants were analyzed in the group to which they were randomized. The randomization groups had a different distribution of marital status; the clinic group had more never-married women (62.0% vs. 51.6%), and the home group had more separated, divorced, or widowed women (12.1% vs. 5.6%, p = 0.007). There were no other differences between the randomization groups at baseline in demographic characteristics, sexual behavior, or access to health care (Table 1).
Figure 1.
Illustration of the flow and numbers of study participants.
Table 1.
Baseline Characteristics of Eligible Participants by Randomization Group.
| Home (n = 273) |
Clinic (n = 285) |
p value | |
|---|---|---|---|
| Mean age (SD) | 26.1 (6.2) | 25.5 (5.9) | 0.257 |
| Race/Ethnicity | 0.699 | ||
| Black | 122 (44.7) | 127 (44.9) | |
| White | 133 (48.7) | 142 (50.2) | |
| Other | 18 (6.6) | 14 (4.9) | |
| Education | 0.378 | ||
| Less than high school diploma | 32 (11.7) | 27 (9.5) | |
| High school diploma or GED | 47 (17.2) | 65 (22.8) | |
| Some college | 122 (44.7) | 123 (43.2) | |
| College or graduate degree | 72 (26.4) | 70 (24.6) | |
| Monthly individual income | 0.395 | ||
| $0-800 | 114 (41.8) | 116 (40.8) | |
| $801-1,600 | 87 (31.9) | 84 (29.6) | |
| $1,601+ | 68 (24.9) | 75 926.4) | |
| Refused or missing | 4 (1.5) | 9 (3.2) | |
| Welfare | 10 (3.7) | 4 (1.4) | 0.088 |
| Food stamps | 60 (22.0) | 57 (20.1) | 0.581 |
| WIC | 44 (16.1) | 39 (13.7) | 0.419 |
| Marital Status | 0.007 | ||
| Never Married | 141 (51.6) | 176 (62.0) | |
| Married/Living with a Partner | 96 (36.3) | 92 (32.4) | |
| Separated/Divorced/Widowed | 33 (12.1) | 16 (5.6) | |
| Recruitment Site | 0.067 | ||
| Abortion Clinic | 64 (23.4) | 88 (30.9) | |
| University Clinic | 192 (70.3) | 187 (65.6) | |
| Community Clinic | 17 (6.2) | 10 (3.5) | |
| Contraceptive Method | 0.577 | ||
| LNG IUC | 201 (73.6) | 200 (70.2) | |
| Copper T IUC | 30 (11.0) | 39 (13.7) | |
| Contraceptive implant | 42 (15.4) | 46 (16.1) | |
| Obtained health care in the last 12 months | 252 (92.6) | 257 (90.2) | 0.299 |
| No health insurance | 103 (37.7) | 109 (38.5) | 0.849 |
| History of STI* | 68 (25.0) | 74 (26.0) | 0.794 |
| STI** diagnosed at enrollment | 7 (2.6) | 11 (3.9) | 0.387 |
| Mean number of lifetime male sexual partners (SD) | 5.2 (8.9) | 6.0 (10.9) | 0.266 |
SD, standard deviation; GED, general education development test; LNG, levonorgestrel; IUC, intrauterine contraceptive; STI, sexually transmitted infection; WIC, Women, Infants and Children government assistance program. Data are n (%) unless otherwise indicated.
STI includes chlamydia, gonorrhea, trichomoniasis, syphilis, HIV, and genital herpes.
Includes chlamydia, gonorrhea, trichomoniasis, syphilis, and HIV, for which each participant is screened at baseline.
Fisher's exact test.
Overall, 243 (44.3%) of women completed screening. Of 202 specimens received for processing by the study, three were rejected for quality: two had low liquid and one had no swab. Three additional tests appeared in good condition but were considered unsatisfactory by the laboratory. All women with unsatisfactory results were given the opportunity to retest at the study clinic; two women did and tested negative.
Women randomized to home-based screening were almost 70 percent more likely to complete a screen compared to women randomized to clinic-based screening (56.3% vs. 32.9%, RR 1.7, 95% CI 1.4 – 2.1). Completion status did not differ by age, race, access to healthcare, or history of a prior STI. However, completers were more likely to have higher educational attainment (p = 0.005). Completers were also more likely to be welfare recipients (p = 0.009, RR 1.04, 95% CI 1.01-1.07, Table 2), but there was no difference by any other form of government assistance (WIC, food stamps). Using Poisson regression with robust error variance to adjust for age, race, education, marital status and welfare use, nearly identical results were obtained (RR 1.7, 95% CI 1.4 – 2.0). Seven cases of chlamydia were detected; 3 in the home group and 4 in the clinic group. One case of gonorrhea was detected in the clinic group.
Table 2.
Baseline Characteristics by Screening Status
| Completed Screening ( n = 239) |
Did Not Complete Screening (n = 305) |
p value | |
|---|---|---|---|
| Screening Method | < 0.001 | ||
| Home | 151 (62.1) | 117 (38.4) | |
| Clinic | 92 (37.9) | 188 (61.6) | |
| Mean age (SD) | 25.9 (6.1) | 25.5 (6.0) | 0.43 |
| Race/Ethnicity | 0.89 | ||
| Black | 106 (44.6) | 140 (45.9) | |
| White | 122 (50.6) | 148 (48.5) | |
| Other | 13 (5.4) | 17 (5.6) | |
| Education | 0.005 | ||
| Less than high school diploma | 24 (9.9) | 33 (10.8) | |
| High school diploma or GED | 40 (16.5) | 71 (23.3) | |
| Some college | 101 (41.6) | 142 (46.6) | |
| College or graduate degree | 78 (32.1) | 59 (19.3) | |
| Monthly individual income | 0.91 | ||
| $0-800 | 103 (42.6) | 125 (41.0) | |
| $801-1,600 | 72 (29.8) | 94 (30.8) | |
| $1,601+ | 62 (25.6) | 78 (25.6) | |
| Refused or missing | 5 (2.1) | 8 (2.6) | |
| Welfare | 11 (4.5) | 3 (1.0) | 0.009 |
| Food stamps | 54 (22.2) | 62 (20.4) | 0.60 |
| WIC | 42 (17.3) | 40 (13.1) | 0.17 |
| Marital Status | 0.52 | ||
| Never Married | 134 (55.1) | 180 (59.2) | |
| Married/Living with a Partner | 21 (8.6) | 96 (31.6) | |
| Separated/Divorced/Widowed | 21 (8.6) | 28 (9.2) | |
| Recruitment Site | 0.22 | ||
| Abortion Clinic | 58 (23.9) | 92 (30.2) | |
| University Clinic | 171 (70.4) | 200 (65.6) | |
| Community Clinic | 14 (5.8) | 13 (4.3) | |
| Contraceptive Method | 0.63 | ||
| LNG IUC | 173 (71.2) | 220 (72.1) | |
| Copper T IUC | 34 (14.0) | 35 (11.5) | |
| Contraceptive implant | 36 (14.8) | 50 (16.4) | |
| Obtained health care in the last 12 months | 224 (92.6) | 276 (90.5) | 0.39 |
| No health insurance | 91 (37.4) | 118 (38.9) | 0.72 |
| History of STI* | 67 (27.7) | 86 (28.2) | 0.90 |
| STI** diagnosed at enrollment | 7 (2.9) | 10 (3.3) | 0.79 |
| Mean number of lifetime male sexual partners (SD) | 5.4 (9.4) | 6.1 (10.4) | 0.53 |
SD, standard deviation; GED, general education development test; LNG, levonorgestrel; IUC, intrauterine contraceptive; STI, sexually transmitted infection. Data are n (%) unless otherwise indicated.
Fisher's exact test.
STI includes chlamydia, gonorrhea, trichomoniasis, syphilis, HIV, and genital herpes.
Includes chlamydia, gonorrhea, trichomoniasis, syphilis, and HIV, for which each participant is screened at baseline.
Medical records or a specimen were obtained for 163 (58%) of women in the clinic randomization group. We did not receive specimens from the family planning clinic for 65 women that we assumed were not tested elsewhere. Of these, 42 (15%) listed this clinic as their primary provider, and 23 (8%) did not have access to a primary provider and planned to go to the family planning clinic. For the remaining 52 women for whom we could not obtain medical records, 26 (9%) did not have a valid medical records release for any provider, 10 (4%) providers did not respond, 8 (3%) providers could not be contacted, 7 (2%) providers refused our request, and 1 woman was given a home kit, which was not returned. Of the 94 medical records received, 6 documented a test during the testing period, 13 documented a recent test that was outside of the testing period, and 75 did not record any recent STI tests. Using these documented completed screens, women in the home group were more than twice as likely to complete a test (56.3% vs. 25.0%, RR 2.3, 95% CI 1.8 – 2.8). Poisson regression with robust error variance adjusting for age, race, education, marital status, and welfare use produced similar results (RR 2.2, 95% CI 1.7 – 2.7).
At the next regularly scheduled follow-up (18 months), 207 women who did not complete testing gave reasons for not screening. The most common reason was forgetting to test, reported by 67 (32%) non-completers. Other common reasons included lack of time (n = 34, 16%), not receiving testing materials (n = 31, 15%), testing outside of the study (n = 25, 12%) and losing or damaging testing materials (n = 23, 11%). When analyzed by randomization group, non-completers in the home arm were more likely to have forgotten or not understood how to use the self-swab, and less likely to cite lack of time (Table 3). Financial or transportation concerns, and other clinic barriers, such as appointment availability, were more common in the clinic group, but did not reach statistical significance.
Table 3.
Reasons for not completing screening among 207 women who did not screen.*
| Reason** | Home | Clinic | p value | RR | 95% CI |
|---|---|---|---|---|---|
| Forgot | 34 (49) | 33 (24) | < 0.001 | 2.0 | 1.4-3.0 |
| Did not have time | 3 (4.3) | 31 (22.7) | 0.005 | 0.19 | 0.06-0.60 |
| Did not receive testing materials† | 9 (3.4) | 22 (7.9) | 0.55 | 0.80 | 0.39-1.6 |
| Tested outside of study | 5 (1.9) | 20 (7.1) | 0.13 | 0.49 | 0.19-1.2 |
| Lost or damaged testing materials† | 9 (3.4) | 14 (5.0) | 0.57 | 1.3 | 0.57-2.76 |
| Thought low risk | 2 (2.9) | 5 (3.6) | 0.77 | 0.78 | 0.16-3.9 |
| Other | 4 (5.7) | 4 (2.9) | 0.33 | 2.0 | 0.50-7.6 |
| Don't know | 2 (2.9) | 4 (2.9) | 0.98 | 0.98 | 0.18-5.21 |
| Did not have money for copay, or did not know clinic testing was free |
0 (0) | 5 (3.6) | 0.17 | - | - |
| Did not have transportation | 0 (0) | 5 (3.6) | 0.17 | - | - |
| Other barrier to clinic access | 0 (0) | 4 (2.9) | 0.30 | - | - |
| Did not understand swab instructions | 3 (4.3) | 0 (0) | 0.038 | - | - |
| Delayed test until out of testing window | 0 (0) | 2 (0.7) | 0.55 | - | - |
| Did not want to use randomization method | 1 (0.4) | 0 (0) | 0.34 | - | - |
| Worried about privacy | 0 (0) | 0 (0) | - | - | - |
Data are n (%) unless otherwise indicated. RR, relative risk.
p values calculated by chi-square or Fisher's exact test. Relative risk calculated by Poisson regression with robust error variance.
Multiple reasons were permitted.
Women who did not receive, lost, or damaged testing materials could request new materials.
Twenty-three percent of women returned the mailed satisfaction survey (n = 122), and response rates did not differ by randomization group (p = 0.49). The majority of respondents (n = 98, 81%) were very or somewhat satisfied with the testing process and were not concerned about privacy (n = 92, 95%). All women who used the self-swab thought it was easy to use, with 70% (n = 53) considering the collection step “extremely easy.” By the Mann-Whitney-U test, women in the home group gave higher ratings for convenience (somewhat or very convenient, 89% home group vs. 69% clinic group, p = 0.02). Women randomized to home strongly preferred to test at home again (83%), compared to only 49% of women in the clinic group who would choose clinic-based testing in the future (Mann-Whitney-U, p < 0.001).
DISCUSSION
This randomized trial of home- versus clinic-based screening for chlamydia and gonorrhea demonstrated increased screening rates in women randomized to home-based screening. Home-based screening nearly doubled screening rates compared to clinic-based screening in both univariate and multivariate analysis. Several cases of chlamydia and one case of gonorrhea were detected in both screening arms.
Home-based screening is a viable alternative to clinic-based screening due to the availability of nucleic acid amplification tests (NAAT), such as polymerase chain reaction (PCR), strand displacement analysis (SDA), and transcription-mediated amplification (TMA). These methods have high sensitivity and specificity for detection of both C. trachomatis and N. gonorrhoeae. Self-obtained vaginal swabs produce results that are nearly identical to clinically-obtained endocervical samples (3, 21).
Several studies have demonstrated that women consider self-collected vaginal swabs an acceptable alternative to traditional screening by a clinician (22, 23). Our previous cohort study demonstrated that women strongly preferred home-screening, with 75% of the sample choosing it rather than clinic-based screening (12). Respondents to a mailed satisfaction survey also showed a preference for home-based screening. Most women in the home group would choose to use home-based screening again, while half of the women in the clinic group preferred to switch methods and use home screening in the future.
One strength of this study is its randomized design, which achieved a balanced distribution of clinical and demographic factors. Since assignment of screening method was randomized in this study, we are confident that the improvement in screening rates is due to the screening method itself, rather than characteristics of women choosing home screening. Another strength is the diverse population of contraceptive-seeking women recruited by the Contraceptive CHOICE Project, which has a broad distribution of socioeconomic and STI risk factors. Similar to our previous study, we demonstrated a low rate of unsatisfactory results (3%) among the women using self-obtained vaginal swabs (12). One hundred percent of women responding to the mailed survey considered the swabs “easy” or “extremely easy” to obtain. These results support our conclusion that women can properly obtain and ship vaginal swabs for testing.
We used two different methods to determine the primary outcome measure, the number of completed tests. While all self-obtained vaginal swabs could be accurately counted as they were processed at the study site, women in clinic group may not have reported being tested with a private provider. To reduce bias, we used several methods to determine the screening status for all women in the clinic group, including review of reimbursement forms, self-report by mailed and telephone surveys, and systematic attempts to retrieve outside medical records. Using these strategies, we can estimate that the true screening rate in the clinic testing arm was between the rate of 24% confirmed by records and 32% with the addition of self-report. The rate confirmed by records is a conservative estimate, as we were unable to obtain records for all women, and some women may have tested with a different provider than that listed in her study records. However, self-report is also biased due to poor recall, response bias, and the common misconception that STI screening is performed during all pelvic exams.
This study was limited to women using long-acting methods of contraception. Future research could address whether these conclusions are altered in populations using different contraceptive methods. However, our previous study showed that contraceptive method did not influence completion rates. Use of LARC is increasing, and is likely to continue to rise as patient and provider awareness increase (16). Home-based testing is a promising strategy to screen women, such as women using LARC, who may not present for annual gynecologic exams.
All women, regardless of their choice of contraception, can benefit from improved access to STI screening. Women in the clinic group who did not complete testing were more likely to cite time constraints as a barrier to testing, compared to the home group. Though lack of transportation and other clinic-based barriers were cited by few women, these barriers are eliminated by home-based testing. United States Food and Drug Administration approval of an over-the-counter chlamydia and gonorrhea testing kit and access to affordable home-based testing options would allow private and convenient self-testing to be available outside of the research setting. Screening must be easily available, affordable, and convenient to meet CDC recommendations. Furthermore, the availability of STI screening options outside of the traditional clinic setting for women who may not require or seek annual gynecological care are necessary if we hope to reduce sexually transmitted infections among women.
Acknowledgments
Supported in part by: an anonymous foundation; Midcareer Investigator Award in Women's Health Research (K24 HD01298); Clinical and Translational Science Awards (UL1RR024992); and Award Numbers TL1 RR024995, KL2RR024994, and K3054628 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented as an oral abstract at Reproductive Health 2010, a joint annual meeting of the Association for Reproductive Health Professionals, Planned Parenthood Federation of America, and the Society for Family Planning, September 24, 2010, Atlanta Georgia
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weinstock H, Berman S, Cates W., Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004 Jan-Feb;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006 Aug 4;55(RR-11):1–94. [PubMed] [Google Scholar]
- 3.Cook RL, Hutchison SL, Ostergaard L, Braithwaite RS, Ness RB. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005 Jun 7;142(11):914–25. doi: 10.7326/0003-4819-142-11-200506070-00010. [DOI] [PubMed] [Google Scholar]
- 4.Levine WC, Dicker LW, Devine O, Mosure DJ. Indirect estimation of Chlamydia screening coverage using public health surveillance data. Am J Epidemiol. 2004 Jul 1;160(1):91–6. doi: 10.1093/aje/kwh162. [DOI] [PubMed] [Google Scholar]
- 5.St Lawrence JS, Montano DE, Kasprzyk D, Phillips WR, Armstrong K, Leichliter JS. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002 Nov;92(11):1784–8. doi: 10.2105/ajph.92.11.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlamydia screening among sexually active young female enrollees of health plans--United States, 1999-2001. MMWR Morb Mortal Wkly Rep. 2004 Oct 29;53(42):983–5. [PubMed] [Google Scholar]
- 7.Bauer HM, Chartier M, Kessell E, Packel L, Brammeier M, Little M, et al. Chlamydia screening of youth and young adults in non-clinical settings throughout California. Sexually Transmitted Diseases. 2004 Jul;31(7):409–14. doi: 10.1097/01.olq.0000130456.03464.ea. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield PJ, Kent C, Campbell D, Hanbrook L, Klausner JD. Community-based chlamydia and gonorrhea screening through the United States mail, San Francisco. Sex Transm Dis. 2002 May;29(5):294–7. doi: 10.1097/00007435-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Gaydos CA, Dwyer K, Barnes M, Rizzo-Price PA, Wood BJ, Flemming T, et al. Internet-based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sex Transm Dis. 2006 Jul;33(7):451–7. doi: 10.1097/01.olq.0000200497.14326.fb. [DOI] [PubMed] [Google Scholar]
- 10.Macleod J, Salisbury C, Low N, McCarthy A, Sterne JA, Holloway A, et al. Coverage and uptake of systematic postal screening for genital Chlamydia trachomatis and prevalence of infection in the United Kingdom general population: cross sectional study. BMJ. 2005 Apr 23;330(7497):940. doi: 10.1136/bmj.38413.663137.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak DP, Karlsson RB. Simplifying chlamydia testing: an innovative Chlamydia trachomatis testing approach using the internet and a home sampling strategy: population based study. Sexually Transmitted Infections. 2006 Apr;82(2):142–7. doi: 10.1136/sti.2005.016832. discussion 52-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graseck AS, Secura GM, Allsworth JE, Madden T, Peipert JF. Home screening compared with clinic-based screening for sexually transmitted infections. Obstet Gynecol. 2010 Apr;115(4):745–52. doi: 10.1097/AOG.0b013e3181d4450d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostergaard L, Andersen B, Moller JK, Olesen F. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: a cluster-randomized 1-year follow-up study. Clin Infect Dis. 2000 Oct;31(4):951–7. doi: 10.1086/318139. [DOI] [PubMed] [Google Scholar]
- 14.Ostergaard L, Andersen B, Olesen F, Moller JK. Efficacy of home sampling for screening of Chlamydia trachomatis: randomised study. BMJ. 1998 Jul 4;317(7150):26–7. doi: 10.1136/bmj.317.7150.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook RL, Ostergaard L, Hillier SL, Murray PJ, Chang CC, Comer DM, et al. Home screening for sexually transmitted diseases in high-risk young women: randomised controlled trial. Sex Transm Infect. 2007 Jul;83(4):286–91. doi: 10.1136/sti.2006.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosher W, Jones J. Use of Contraception in the United States: 1982-2008. National Center for Health Statistics Vital Health Stat. 2010;23(29) [PubMed] [Google Scholar]
- 17.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010 Jun 9; doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998 Nov 18;280(19):1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 19.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003 May 15;157(10):940–3. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 20.Robbins AS, Chao SY, Fonseca VP. What's the relative risk? A method to directly estimate risk ratios in cohort studies of common outcomes. Ann Epidemiol. 2002 Oct;12(7):452–4. doi: 10.1016/s1047-2797(01)00278-2. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs MM, van der Pol B, Totten P, Gaydos CA, Wald A, Warren T, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008 Jan;35(1):8–13. doi: 10.1097/OLQ.0b013e31815d968d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiesenfeld HC, Lowry DL, Heine RP, Krohn MA, Bittner H, Kellinger K, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001 Jun;28(6):321–5. doi: 10.1097/00007435-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Chernesky MA, Hook EW, 3rd, Martin DH, Lane J, Johnson R, Jordan JA, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005 Dec;32(12):729–33. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]