Abstract
Aims
Cardiac resynchronization therapy (CRT) has dramatically improved the symptoms and prognosis of patients with heart failure in large randomized clinical trials. Optimization of device settings may maximize benefit on an individual basis, although the best method for this is not yet established. We evaluated the use of cardiogenic impedance measurements (derived from intracardiac impedance signals) in CRT device optimization, using invasive left ventricular (LV) dP/dtmax as the reference.
Methods and results
Seventeen patients underwent invasive haemodynamic assessment using a pressure wire placed in the LV cavity at the time of CRT device implantation. Intracardiac impedance measurements were made at different atrioventricular (AV) and interventricular (VV) delays and compared with LV dP/dtmax. We assessed the performance of patient-specific and generic impedance-based models in predicting acute haemodynamic response to CRT. In two patients, LV catheterization with the pressure wire was unsuccessful and in two patients LV lead delivery was unsuccessful; therefore, data were acquired for 13 out of 17 patients. Left ventricular dP/dtmax was 919 ± 182 mmHg/s at baseline and this increased acutely (by 24%) to 1121 ± 226 mmHg/s as a result of CRT. The patient-specific impedance-based model correctly predicted the optimal haemodynamic response (to within 5% points) for AV and VV delays in 90 and 92% of patients, respectively.
Conclusion
Cardiogenic impedance measurements are capable of correctly identifying the maximum achievable LV dP/dtmax as measured by invasive haemodynamic assessment. This study suggests that cardiogenic impedance can potentially be used for CRT optimization and may have a role in ambulatory assessment of haemodynamics.
Keywords: Cardiac resynchronization therapy, Cardiogenic impedance, Device optimization, Haemodynamics
Introduction
Cardiac resynchronization therapy (CRT) has dramatically improved both symptoms and prognosis in patients with heart failure,1–5 although a significant proportion of patients do not derive clear clinical benefit.6 Given that the principal mechanism of CRT is to restore cardiac synchrony, an important approach to increase clinical benefit from CRT is optimization of device programming, so as to establish the atrioventricular (AV) and interventricular (VV) delays that give maximal improvement in cardiac function. Acute improvement in systolic function has been demonstrated as a result of optimization of AV and VV delays at the time of CRT implantation.7,8 Several small studies have shown improvements in acute haemodynamics or myocardial efficiency as a result of device optimization.9–11 Moreover, optimization of device settings in CRT is an inherent part of evidence-based practice, given that it was performed in the landmark clinical trials that demonstrated the clinical benefit of CRT.1,3,4,6 With large numbers of CRT implantation procedures being performed12 and the potential for device optimization to be performed at various intervals during clinical follow-up,13 CRT optimization has major financial implications. However, the best method for device optimization is not yet established. The optimal method should be clinically relevant, non-invasive, reproducible, and quick to perform in the outpatient setting. Echocardiographic optimization is commonly used, but is time consuming and operator dependent. Invasive haemodynamic assessment remains the gold standard, but this carries procedural risks and is not ideally suited for the outpatient setting, or for repeated optimizations to be performed at different time intervals during clinical follow-up.
To date, the majority of published data regarding the use of impedance measurements for device optimization relate to impedance cardiography. This technique is based on measurements of trans-thoracic impedance. This approach for CRT device optimization had a good correlation with echo-derived optimal AV delays14 but was less accurate for VV optimization when compared with invasive haemodynamics as the gold standard.15 Inaccuracy may arise as this technique is affected by changes in flow in the great vessels as well as variations in electrode positions, thoracic anatomy, respiration, and external factors such as humidity and skin temperature.16 This brings into question the reliability of impedance cardiography for CRT device optimization. In contrast, one might expect cardiogenic impedance measurements, derived from recordings taken at endocardial or epicardial sites, to be a more specific reflection of volumetric changes in the heart.
We assessed a novel approach based on the fact that cardiogenic impedance can be derived from intracardiac impedance signals. Cardiogenic impedance reflects the volumetric fluctuations within the heart during the cardiac cycle and has been used to monitor cardiac contraction in an animal model.17 This method therefore has the potential to be used for device optimization and may be performed using the device alone, without other specific equipment. Furthermore, as the impedance data are acquired from leads positioned within and on the surface of the heart, this technique should be more specific than the use of trans-thoracic impedance. We evaluated the feasibility of using cardiogenic impedance as a marker for acute haemodynamic response to CRT. We also evaluated its use for CRT device optimization with invasive left ventricular maximum pressure gradient (LV dP/dtmax) as a reference.
Methods
Patients
The study was a prospective, non-randomized, multi-centre feasibility study. Patients were enrolled in two centres: St Thomas’ Hospital, London, and Uppsala University Hospital, Sweden. The local hospital ethics committees approved the study, which complied with the Declaration of Helsinki.
Patients were enrolled if they fulfilled criteria for CRT-D device implantation and gave written informed consent. Exclusion criteria were contra-indication for left heart catheterization or anticoagulation, pregnancy, third-degree AV block, or indication for cardiac transplantation. The patients were observed at enrolment, at implantation, and on one additional occasion prior to hospital discharge.
Hypothesis
The study was designed to evaluate the following hypotheses: (i) recording cardiogenic impedance measurements through the CRT-D device at the time of the implantation procedure is feasible, (ii) there is a correlation between cardiogenic impedance parameters and invasive haemodynamic measurements (LV dP/dtmax) acquired during the time of the implantation procedure, and (iii) cardiogenic impedance could be used to perform AV and VV optimizations at the time of CRT implant.
Implant protocol
Venous access was established using either the cephalic or the sub-clavian vein, and the pocket for the device was made. Following this, arterial access was obtained from either the radial or the femoral artery using a 5F sheath. A long exchange wire was passed retrogradely across the aortic valve into the LV cavity using a pigtail catheter. This was then exchanged for a multi-purpose catheter, which was used to pass a pressure wire (Radi™ wire, Radi Medical Systems, Uppsala, Sweden) into the LV cavity. In order to minimize the potential risk of any thrombo-embolic complications from the pressure wire in the systemic circulation, a 2500 U heparin bolus was administered, followed by a slow infusion of heparinized saline (500 U per 500 mL). A Promote™ CRT-D device (St Jude Medical, Sylmar, CA, USA), LV and right ventricular (RV) leads were implanted in all patients. An atrial lead was implanted in patients in sinus rhythm at baseline.
Data acquisition
The pressure wire is a fine-calibre (0.014-inch) high-fidelity wire that has a torque similar to an angioplasty wire. There is a pressure sensor located 30 mm from its tip. This was used to record intraventricular pressure with a frequency response of 400 Hz. The output from the pressure wire was connected to a Radi analyzer® and connected to a computer with PhysioMon® software to give curves showing real-time blood pressure and LV dP/dtmax.
The Promote device includes an impedance sensor. The device is able to measure the impedance between several electrode surfaces (device case, tip electrodes, ring electrodes, and coils of the high-voltage lead) within the pulse generator system. To obtain an impedance measurement, the device delivers a continuous sequence of sub-threshold current impulses between two selected electrodes. It is a tri-phasic, charge-balanced pulse with +850/−125µA and a pulse duration of 19 µs. These pulses are emitted at 128 Hz. The impedance was calculated by Z= u/i (where Z is impedance, u is voltage, and i is current).
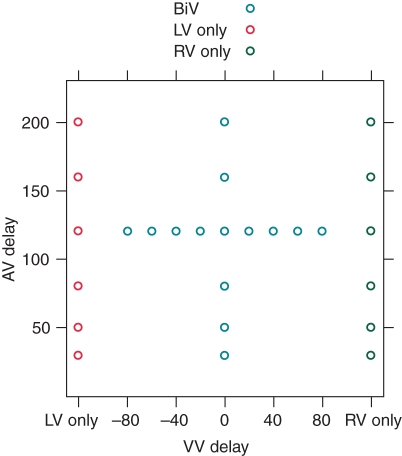
Once the pacing electrodes were in satisfactory positions and with good pacing and sensing parameters, the AV (in patients implanted with an atrial lead) and VV delays were varied according to the protocol shown in Figure 1.
Figure 1.
Protocol for atrioventricular and interventricular optimization.
This protocol was designed to cause variation in haemodynamics rather than to reflect a clinical optimization procedure (which would have a narrower range of AV and VV delay values). This was done so that comparison could be made across a larger spectrum of invasive haemodynamic data along with the cardiogenic impedance data acquired.
At each device setting, recordings were made after 1 min of pacing in atrial and ventricular pacing, atrial and ventricular sensing and dual response (DDD) mode at a rate of 10 bpm above intrinsic, once steady state pacing was achieved. Left ventricular dP/dtmax was recorded beat by beat for 1 min and two separate sets of cardiogenic impedance parameter data were recorded beat by beat for ≥20 s each. These sequences were each summarized with a median value.
Cardiogenic impedance vectors
Two different impedance vectors were used in the protocol: V1 (with injection of the measurement current from RVring to LVring and voltage sensing from RVtip to LVtip) and V3 (with injection of the measurement current from RVring to RVtip and voltage sensing from RVring to RVtip). It is important to note that the use of impedance monitoring does not affect the choice of pacing vectors available or the pacing functions of the device.
Cardiogenic impedance characteristics and development of impedance-based models
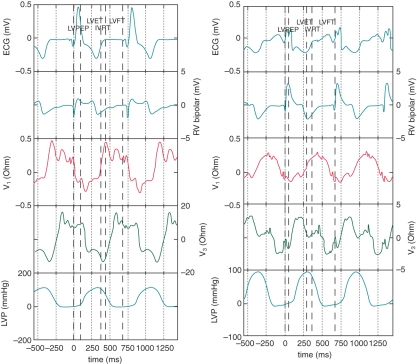
Example data are shown inFigure 2.
Figure 2.
Simultaneous data sets are shown for Patients 2 (left) and 4 (right). These include (from top to bottom in the figure) the surface electrocardiogram, right ventricular bipolar electrogram, V1 impedance waveform, V3 impedance waveform, and left ventricular pressure trace.
The main characteristics inherent in the impedance waveform were peak-to-peak, slope (dZ/dt), fractionation, diastolic dispersion, and the average value of the impedance containing the DC component, Z_0.
The impedance waveform was therefore quantified using these characteristics. The impedance waveform—and the resulting characteristics—varied substantially between patients. This is exemplified by comparison of Patients 2 and 4. In Patient 2, the maximum rate of change of V3 occurred during ventricular diastole [decline of left ventricular pressure (LVP)] whereas for Patient 4, the maximum rate of change of V3 occurred during ventricular systole (rise in LVP). However within each patient, the impedance characteristics were remarkably reproducible.
For this reason, a patient-specific model was developed for each subject. This incorporated a different weighting of the various impedance characteristics for each patient, according to their relative contributions to explain the variation of LV dP/dtmax. This was calculated using a partial least-squares regression model in which each impedance characteristic was assigned a coefficient so that the sum of the impedance characteristics was fitted to describe the measured LV dPdtmax. In this way, the patient-specific model was calibrated to a haemodynamic reference.
For the patient-specific models, the contribution of different impedance characteristics in predicting LV dP/dtmax varied; therefore, the regression coefficient values differed between patients.
A generic impedance-based model was also developed, in which data were centred patient by patient in order to normalize for interpatient variability, and then the same coefficient values were used for all patients. The generic model did not require patient-specific calibration to a haemodynamic reference.
In order to compare measurements taken during different device settings and at different time points and to suppress variation due to respiratory motion, the recordings were averaged into waveform templates that are representative of one cardiac cycle. The template waveforms were normalized for time and amplitude. This was done because of intra- and interpatient variation in the R–R interval.
Impedance-based models used to predict haemodynamic response
The patient-specific and generic models were applied to predict a value for LV dP/dtmax at each step in the AV and VV optimization. The LV dP/dtmax was predicted on the basis of impedance values and waveform characteristics and this was compared with the LV dP/dtmax measured invasively.
We compared the following outcomes for predicted vs. measured LV dP/dtmax values: (i) programming difference in milliseconds and (ii) difference in percentage increase in LV dP/dtmax from intrinsic rhythm.
Data analysis and statistical analysis
Data are presented as mean ± standard deviation (SD). In analyses at patient level, these are mean and SD of beat-by-beat values. In analyses at group level, these are mean and SD of patient values. Statistical analysis was performed using paired t-tests. A P value of <0.05 was considered statistically significant.
Results
Patient demographics are summarized in Table 1.
Table 1.
Patient demographics
| Age | 66 ± 12 years |
| Male/female | 14/3 (82/18%) |
| Cardiomyopathy | |
| Ischaemic | 12 (71%) |
| Dilated (non-ischaemic) | 5 (29%) |
| Hypertrophic | 0 (0%) |
| LV ejection fraction | 23 ± 6% |
| NYHA functional class | 2.8 ± 0.4 |
| Heart failure medications | |
| Beta-blockers | 12 (71%) |
| ACE/ARB | 17 (100%) |
| Diuretics | 14 (82%) |
| Digitalis | 7 (50%) |
| ECG characteristics | |
| Intrinsic heart rate | 72 ± 19 bpm |
| Atrial fibrillation | 5 (29%) |
| QRS duration | 150 ± 27 ms |
| Baseline LV dP/dtmax | 919 ± 182 mmHg/s |
NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Procedural success
Seventeen patients were enrolled. In two of the patients (7 and 17), LV catheterization with the pressure wire was unsuccessful and therefore no LV pressure data were recorded. In Patients 3 and 13, LV lead implantation was unsuccessful, and these patients were scheduled for a redo procedure for LV lead implantation and were excluded from the analysis.
Haemodynamic response to cardiac resynchronization therapy
The mean LV dP/dtmax in intrinsic rhythm for the study group of 13 patients was 919 ± 182 mmHg/s and increased with CRT to 1136 ± 247 mmHg/s (P< 0.01). The average acute increase in LV dP/dtmax by optimized CRT when compared with intrinsic rhythm was 24 ± 22%.
Using a cut-off value of 10% increase in dP/dtmax from baseline as a marker of acute response to CRT, 10 out of 13 patients (77%) acutely responded. The magnitude of increase in LV dP/dtmax from baseline in the responder group ranged from 11 to 62% (mean 31%). In the three non-responders the mean increase was 5%.
Effect of optimizing atrioventricular and interventricular delays
Three patients had atrial fibrillation and were therefore not included in the AV delay protocol. The optimal paced AV delay in 10 patients included in the analysis was 141 ± 41 ms (range 50–200 ms). Biventricular pacing with optimization of the paced AV delay improved haemodynamics from 935 ± 186 mmHg/s at baseline to 1150 ± 248 mmHg/s (P< 0.05).
The optimal VV delay in 13 patients analysed was LV first by 35 ± 33 ms (range LV first by 80 to 0, also one RV only and one LV only). Haemodynamics improved further from 1106 ± 236 mmHg/s with zero VV delay to 1121 ± 226 mmHg/s as a result of VV delay optimization. However, this slight improvement was not statistically significant.
Patient-specific cardiogenic impedance model for atrioventricular and interventricular delay optimization
The results of the haemodynamic response to AV delay optimization are shown in Table 2. Data are shown for 10 patients.
Table 2.
Effect of optimization of atrioventricular delay
| Patient | LV dPdtmax (clinical benefit) |
AV delay |
||||
|---|---|---|---|---|---|---|
| Reference (mmHg/s) | Impedance (mmHg/s) | Difference (mmHg/s) | Reference (ms) | Impedance (ms) | Difference (ms) | |
| Pat01 | 1077 (4%) | 1077 (4%) | 0 (0%) | 160 | 160 | 0 |
| Pat04 | 1206 (3%) | 1204 (3%) | 2 (0%) | 200 | 200 | 0 |
| Pat05 | 1434 (25%) | 1431 (25%) | 3 (0%) | 160 | 160 | 0 |
| Pat06 | 928 (16%) | 928 (16%) | 0 (0%) | 120 | 120 | 0 |
| Pat09 | 628 (−1%) | 628 (−1%) | 0 (0%) | 160 | 160 | 0 |
| Pat10 | 1051 (24%) | 1051 (24%) | 0 (0%) | 120 | 120 | 0 |
| Pat11 | 1103 (2%) | 1102 (2%) | 1 (0%) | 160 | 160 | 0 |
| Pat12 | 1355 (14%) | 1355 (14%) | 0 (0%) | 120 | 120 | 0 |
| Pat14 | 1352 (58%) | 1296 (51%) | 56 (7%) | 160 | 200 | −40 |
| Pat15 | 1368 (66%) | 1368 (66%) | 0 (0%) | 50 | 50 | 0 |
There are two numbers in each cell on the left side of the table: (i) the LV dP/dtmax value achieved with the AV setting suggested by the method (reference or impedance) and (ii) the corresponding ‘clinical benefit’ value in parentheses. The clinical benefit is defined as the percentage change in LV dP/dtmax from intrinsic rhythm. For the patient with second-degree AV block, the change from the minimum LV dP/dtmax was used.
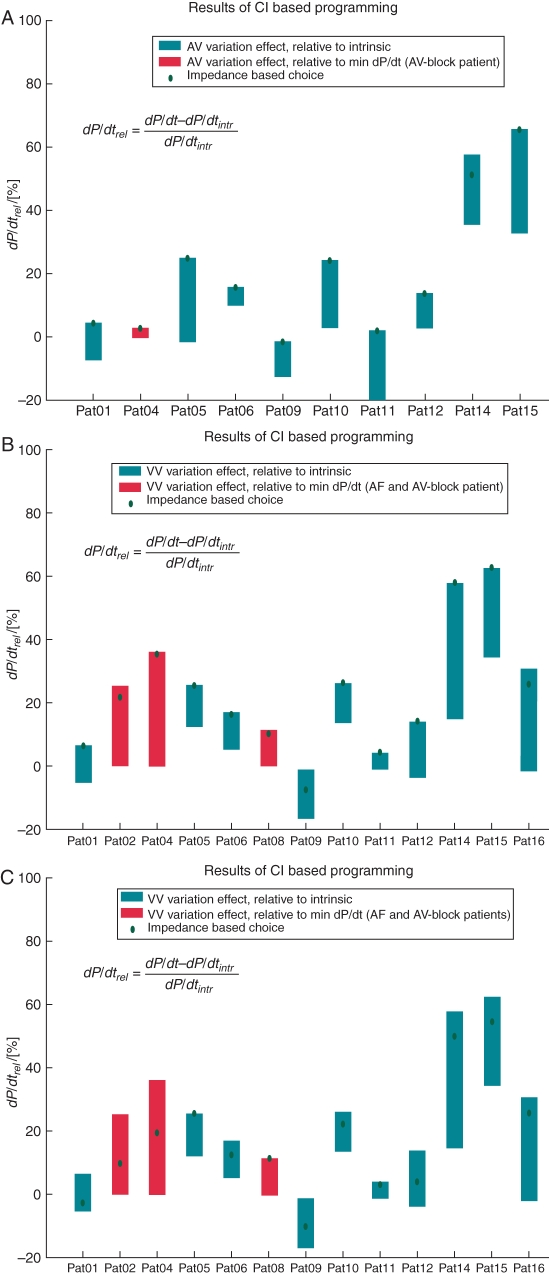
These results are shown in Figure 3A.
The patient-specific impedance-based model identified the optimal setting for the AV delay in 9 of 10 patients to within 5% points (of change in dP/dtmax from intrinsic rhythm). In Patient 14, the difference between impedance-predicted LV dP/dtmax and the measured LV dP/dtmax was 56 mmHg/s (7%) and the discrepancy from optimal AV delay was one interval in the protocol.
The results for the VV delay optimization are shown in Table 3.
Table 3.
Effect of optimization of interventricular delay
| Patient | LV dPdtmax (clinical benefit) |
VV delay |
||||
|---|---|---|---|---|---|---|
| Reference (mmHg/s) | Impedance (mmHg/s) | Difference (mmHg/s) | Reference (ms) | Impedance (ms) | Difference (ms) | |
| Pat01 | 1098 (6%) | 1098 (6%) | 0 (0%) | LV only | LV only | 0 |
| Pat02 | 1109 (25%) | 1075 (22%) | 34 (4%) | −40 | −80 | 40 |
| Pat04 | 1214 (36%) | 1206 (35%) | 8 (1%) | −20 | 0 | −20 |
| Pat05 | 1440 (26%) | 1437 (25%) | 3 (0%) | −40 | −20 | −20 |
| Pat06 | 938 (17%) | 932 (16%) | 6 (1%) | −60 | −40 | −20 |
| Pat08 | 888 (11%) | 878 (10%) | 10 (1%) | −80 | −40 | −40 |
| Pat09 | 628 (−1%) | 588 (−8%) | 40 (6%) | 0 | 20 | −20 |
| Pat10 | 1066 (26%) | 1066 (26%) | 0 (0%) | RV only | RV only | 0 |
| Pat11 | 1126 (4%) | 1126 (4%) | 0 (0%) | −60 | −60 | 0 |
| Pat12 | 1355 (14%) | 1355 (14%) | 0 (0%) | 0 | 0 | 0 |
| Pat14 | 1352 (58%) | 1352 (58%) | 0 (0%) | 0 | 0 | 0 |
| Pat15 | 1340 (62%) | 1340 (62%) | 0 (0%) | 0 | 0 | 0 |
| Pat16 | 1015 (31%) | 976 (26%) | 39 (5%) | −80 | −60 | −20 |
These data are displayed in Figure 3B. The variation in acute clinical benefit, that is the percentage increase in reference LV dP/dtmax, is shown as a bar, and the red diamond displays the value obtained with the setting selected by the impedance-based LV dP/dtmax optimization.
Figure 3.
(A) Graph of the atrioventricular delay optimization based on patient-specific impedance-based prediction models (red diamonds) and comparison with the range of measured values across all settings (blue bars). (B) Graph of the interventricular delay optimization based on patient-specific impedance-based prediction models (red diamonds) and comparison with measured values (blue bars). (C) Graph of the interventricular delay optimization based on the generic impedance-based prediction models and comparison with measured values.
For the VV optimization, the impedance-based model correctly identified the optimal VV delay in 12 of 13 patients to within 5% points. In the remaining patient (Patient 9), the discrepancy between the impedance-predicted LV dP/dtmax and the measured value was 40 mmHg/s (6%) and the difference between the optimal value and that predicted by the model was 20 ms, one increment in the VV optimization protocol.
Generic cardiogenic impedance model for atrioventricular and interventricular delay optimization
Results for the application of the generic impedance-based prediction model for the whole patient group are shown in Figure 3C.
The generic model yielded predictions that were far less accurate than the patient-specific model. This approach identified the optimal VV delay in only 2 of 13 patients.
Discussion
The main findings of this study are that: (i) it is feasible to derive cardiogenic impedance measurements from the lead impedance measurements using a CRT-D device; (ii) the patient-specific model was accurate in predicting the optimal AV and VV delays during device optimization, using the invasive LV dP/dtmax as a reference; and (iii) the generic impedance-based model was relatively poor in predicting the optimal AV and VV delays.
This study suggests that impedance parameters that can be readily obtained from a CRT-D device may be used for prediction of optimal AV and VV delays. However, it seems that a patient-specific model is needed for accurate prediction of optimal device settings. The patient-specific model was made using several characteristics of the impedance waveform. In 9 of 10 cases for the AV delay and 12 of 13 patients for the VV delay, the patient-specific model predicted acute haemodynamic response to within 5% points of the optimal response based on invasive haemodynamics alone. The patient-specific model may therefore represent a clinically effective tool for device optimization. In contrast, the generic impedance-based model was difficult to construct, owing to substantial variability in impedance waveforms between different individuals. Furthermore, the accuracy of the generic impedance-based model in predicting optimal AV and VV intervals was poor.
Preliminary work using cardiogenic impedance has previously been performed in animal models of heart failure. Stahl et al.17 showed a strong correlation between intracardiac impedance parameters and stroke volume in a porcine model. Panescu et al.18 examined multiple impedance vectors derived from implanted biventricular implantable cardioverter-defibrillator devices in a canine/ovine model of heart failure, and demonstrated good correlation between cardiogenic impedance and progression of heart failure.
One recent study in humans has shown the feasibility of cardiogenic impedance measurements in patients with non-ischaemic cardiomyopathy, either in the setting of an electrophysiologic study or at CRT device implantation.19 This study used an external purpose-built device for impedance measurements and the findings suggested that cardiogenic impedance signals correlate well with invasive haemodynamics.
In our study using impedance measurements via the CRT device itself, the mean improvement in acute haemodynamics resulting from CRT compared with baseline was 24 ± 22%. This is in line with findings from several other studies that used invasive LV dP/dtmax as an outcome measure.15,19–21
A limitation of this study is that it involved a small number of patients. The principal aim of the study was to establish the feasibility of cardiogenic impedance measurement using the CRT-D device, and we therefore used a wider range of AV delays than would be applied in clinical practice. We used invasive LV dP/dtmax as this is widely regarded as the gold standard for reference purposes, and is a reproducible and operator-independent marker of LV contractility. One limitation of the patient-specific impedance-based model we developed is that it relies on an invasive reference value, and it has not been established whether a non-invasive reference may be used as a surrogate for this in the patient-specific model. However, there is a good correlation between invasively and non-invasively derived LV dP/dt,22 so one would anticipate that the impedance model can be calibrated in this way.
In view of the fact that LV dP/dtmax is affected by heart rate, we attempted to minimize the impact of heart rate on haemodynamic response by pacing just above the intrinsic rate. However, any increase in heart rate may alter the optimal AV and VV delays. Furthermore, our optimization protocol was performed with the patient supine, as necessitated by invasive haemodynamic monitoring. This did not allow us to assess the effect of exercise on optimal device settings, but our intention was primarily to assess the validity of cardiogenic impedance as an approach to optimization. Cardiogenic impedance monitoring itself may then be evaluated in the ambulatory setting in a subsequent study.
Suggestions for further research
Further studies are needed to assess the clinical utility of cardiogenic impedance-based device optimization. This should be in the form of a large randomized trial evaluating the performance of the patient-specific impedance-based model against the gold standard in the outpatient setting of echocardiographic optimization. Key questions are: does the impedance-based approach identify the optimal AV and VV delays in large numbers of patients? Is the outcome of patients whose devices are optimized using cardiogenic impedance equivalent to the outcome of those optimized using echocardiography? If that is found to be the case, there may be a cost benefit in device-based optimization, which requires fewer resources.
Conclusions
We have demonstrated the feasibility of recording cardiogenic impedance measurements through the CRT-D device at the time of implantation.
The development of a generic impedance-based model was challenging, and did not give a clinically useful platform for device optimization.
A patient-specific impedance-based model was capable of accurate prediction of the optimal AV and VV delays during device optimization and may have a future role in clinical practice.
Conflict of interest: C.A.R. is on an advisory board for St Jude Medical and a consultant for Medtronic. A.K., J.G., and M.S. are employees of St Jude Medical.
Funding
This work was supported by St Jude Medical, UK. M.G. received an educational grant from St Jude Medical. Funding to pay the Open Access publication charges for this article was provided by St Jude Medical, UK.
References
- 1.Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 4.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 5.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–43. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 7.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 8.Kass DA, Chen C-H, Curry C, Talbot M, Berger R, Fetics B, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–73. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 9.León AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, Gurley JC, et al. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol. 2005;46:2298–304. doi: 10.1016/j.jacc.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Kurzidim K, Reinke H, Sperzel J, Schneider HJ, Danilovic D, Siemon G, et al. Invasive optimization of cardiac resynchronization therapy: role of sequential biventricular and left ventricular pacing. Pacing Clin Electrophysiol. 2005;28:754–61. doi: 10.1111/j.1540-8159.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 11.Christenson SD, Chareonthaitawee P, Burnes JE, Hill MRS, Kemp BJ, Khandheria BK, et al. Effects of simultaneous and optimized sequential cardiac resynchronization therapy on myocardial oxidative metabolism and efficiency. J Cardiovasc Electrophysiol. 2008;19:125–32. doi: 10.1111/j.1540-8167.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 12.Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, et al. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–60. doi: 10.1093/eurheartj/ehp359. [DOI] [PubMed] [Google Scholar]
- 13.Qing Z, Jeffrey Wing-Hong F, Yat-Sun C, Hamish Chi-Kin C, Hong L, Skiva C, et al. The role of repeating optimization of atrioventricular interval during interim and long-term follow-up after cardiac resynchronization therapy. Int J Cardiol. 2008;124:211–7. doi: 10.1016/j.ijcard.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 14.Braun MU, Schnabel A, Rauwolf T, Schulze M, Strasser RH. Impedance cardiography as a noninvasive technique for atrioventricular interval optimization in cardiac resynchronization therapy. J Interv Card Electrophysiol. 2005;13:223–9. doi: 10.1007/s10840-005-2361-z. [DOI] [PubMed] [Google Scholar]
- 15.Sciaraffia E, Malmborg H, Lonnerholm S, Blomstrom P, Blomstrom Lundqvist C. The use of impedance cardiography for optimizing the interventricular stimulation interval in cardiac resynchronization therapy—a comparison with left ventricular contractility. J Interv Card Electrophysiol. 2009;25:223–8. doi: 10.1007/s10840-009-9367-x. [DOI] [PubMed] [Google Scholar]
- 16.Engoren M, Barbee D. Comparison of cardiac output determined by bioimpedance, thermodilution, and the fick method. Am J Crit Care. 2005;14:40–5. [PubMed] [Google Scholar]
- 17.Stahl C, Walker T, Straub A, Kettering K, Knubben K, Greiner TO, et al. Assessing acute ventricular volume changes by intracardiac impedance in a chronic heart failure animal model. Pacing Clin Electrophysiol. 2009;32:1395–401. doi: 10.1111/j.1540-8159.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 18.Panescu D, Naware M, Siou J, Nabutovsky Y, Holmstrom N, Blomqvist A, et al. Usefulness of monitoring congestive heart failure by multiple impedance vectors. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5668–70. doi: 10.1109/IEMBS.2008.4650500. [DOI] [PubMed] [Google Scholar]
- 19.Bocchiardo M, Meyer zu Vilsendorf D, Militello C, Lippert M, Czygan G, Schauerte P, et al. Resynchronization therapy optimization by intracardiac impedance. Europace. 2010;12:1589–95. doi: 10.1093/europace/euq273. [DOI] [PubMed] [Google Scholar]
- 20.Perego GB, Chianca R, Facchini M, Frattola A, Balla E, Zucchi S, et al. Simultaneous vs. sequential biventricular pacing in dilated cardiomyopathy: an acute hemodynamic study. Eur J Heart Fail. 2003;5:305–13. doi: 10.1016/s1388-9842(02)00204-0. [DOI] [PubMed] [Google Scholar]
- 21.van Gelder BM, Bracke FA, Meijer A, Lakerveld LJM, Pijls NHJ. Effect of optimizing the VV interval on left ventricular contractility in cardiac resynchronization therapy. Am J Cardiol. 2004;93:1500–3. doi: 10.1016/j.amjcard.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Pai R, Bansal R, Shah P. Doppler-derived rate of left ventricular pressure rise. Its correlation with the postoperative left ventricular function in mitral regurgitation. Circulation. 1990;82:514–20. doi: 10.1161/01.cir.82.2.514. [DOI] [PubMed] [Google Scholar]