Abstract
Aging, hypertension and fetal programmed cardiovascular disease are associated with a functional deficiency of angiotensin (Ang)-(1–7) in the brain dorsomedial medulla. The resulting unrestrained activity of Ang II in brainstem regions negatively impacts resting mean arterial pressure, sympathovagal balance and baroreflex sensitivity for control of heart rate. The differential effects of Ang II and Ang-(1–7) may be related to the cellular sources of these peptides as well as different precursor pathways. Long-term alterations of the brain renin-angiotensin system may influence signaling pathways including phosphoinositol-3-kinase and mitogen-activated protein kinase and their downstream mediators, and as a consequence may influence metabolic function. Differential regulation of signaling pathways in aging and hypertension by Ang II versus Ang-(1–7) may contribute to the autonomic dysfunction accompanying these states.
Introduction
Functional deficiency of Ang-(1–7) within the nucleus of the solitary tract (nTS) leads to impairment of baroreceptor reflex sensitivity (BRS) for control of heart rate that may be a predictor of overall autonomic dysfunction, whether or not there is an accompanying increase in resting mean arterial pressure (MAP). Moreover, there is evidence that long-term alterations of the brain renin-angiotensin system (RAS) impact not only MAP and BRS, but also metabolic function in rodents. The focus of this review is on recent insights into the balance between brain Ang II and Ang-(1–7) in the dorsomedial medulla in terms of cardiovascular and metabolic function, addressing three major questions: 1) is a brain Ang II/Ang-(1–7) imbalance in the dorsomedial medulla associated with long-term impairments in sympathovagal and metabolic balance?; 2) are there different cellular sources or precursors for generation of Ang II versus Ang-(1–7) in brain medulla?; and 3) are signaling pathways altered in brain medulla with long-term changes in angiotensin peptides?
Is aging associated with a functional deficiency of Ang-(1–7) in brain medulla?
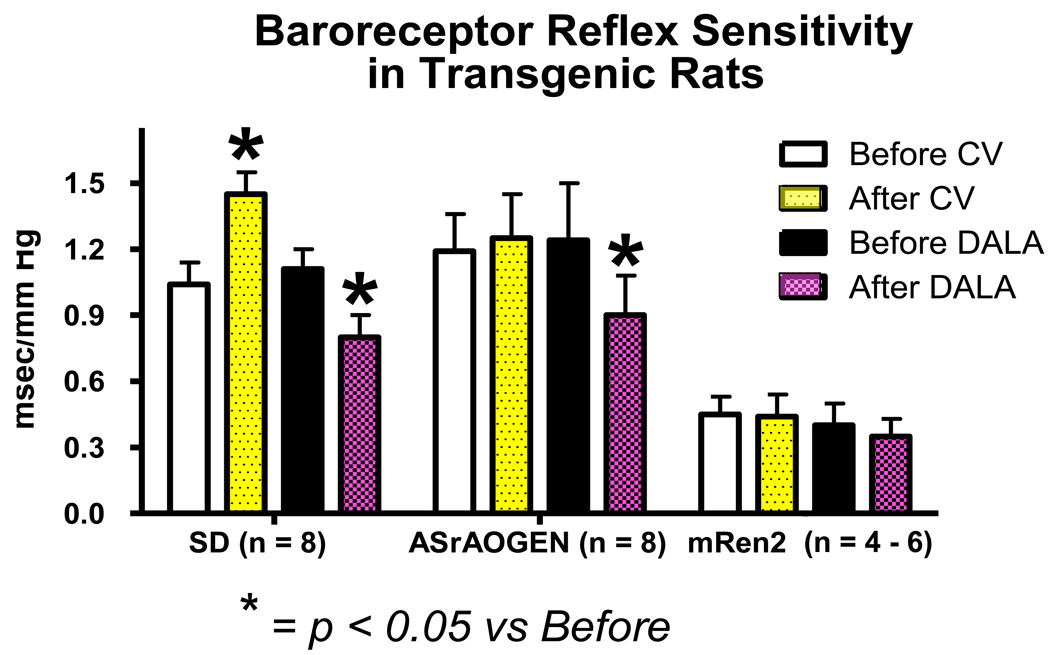
Within the nTS, endogenous Ang II attenuates and Ang-(1–7) facilitates BRS in young Sprague-Dawley (SD) rats as revealed using injection of AT1 or mas receptor antagonists (Figure 1). During aging, MAP increases modestly in SD rats and BRS is impaired in both conscious and anesthetized conditions [1–3]. Functionally, we know that nTS injection of AT1 receptor antagonists increases BRS in old or young SD rats, but Ang-(1–7) receptor blockers decrease BRS only in young SD animals. The inability of Ang-(1–7) blockade to regulate BRS in older rats [2] can be interpreted as a deficiency of Ang-(1–7) in the nTS with increased age. An associated reduction in neprilysin mRNA in dorsal medulla of older SD rats may contribute to lower Ang-(1–7) levels [2], but protein or activity measures are necessary to validate this concept. Functional data show that ACE2 inhibition in the nTS impairs BRS to a similar level as Ang-(1–7) receptor blockade [4], suggesting reliance of Ang-(1–7) formation in this brain region on ACE2,
Figure 1.
BRS for control of heart rate in young SD, ASrAOGEN and (mRen2)27 animals before and after treatment with the AT1 receptor antagonist candesartan (CV) and the Ang-(1–7) receptor antagonist D-Ala7Ang-(1–7) [DALA]. Data replotted from references [6;14] and unpublished data [AT1 receptor blockade in (mRen2)27 rats].
Transgenic ASrAogen rats with targeted disruption of glial angiotensinogen (Aogen) are a model of healthy aging with an extended lifespan [1;3;5]. AT1 receptor blockade in the nTS does not alter the BRS in young ASrAogen rats, clearly demonstrating loss of Ang II tone in the resting condition (Figure 1). Although a decline in BRS is detected in older conscious ASrAogen rats [1], levels are similar in anesthetized younger and older animals [5;6]. In both conscious and anesthetized states, the BRS is similar or higher in older ASrAogen relative to SD rats. ASrAogen rats also maintain lower MAP relative to older SD rats during aging [3]. Ang-(1–7) receptor blockade with D-Ala7-Ang-(1–7) (D-Ala) impairs baroreflex function in both young and old ASrAogen rats to the same extent as young SD rats indicating preservation of BRS modulation by endogenous Ang-(1–7) for in the healthy aging rats [1;3;5;6]. In terms of brain RAS regulation, mas, ACE2 and neprilysin (NEP) mRNA are similar in the dorsal medulla between strains, but mas and ACE2 mRNA are lower in older versus younger ASrAogen rats [2;5;6]. This contrasts with our functional data showing that maintenance of Ang-(1–7) facilitation of BRS in the older ASrAogen rats [5]. However, reductions in mas receptors and Ang-(1–7) synthetic enzymes may be secondary to maintained levels of Ang-(1–7) and a ligand- or substrate-mediated feedback in older ASrAogen rats.
Is genetic hypertension associated with a functional deficiency in brain medullary Ang-(1–7)?
Early studies showed the importance of elevating brain Ang-(1–7) to reverse the impaired BRS in genetic and experimental models of hypertension by demonstrating that the improvement in BRS elicited by ACE inhibitors in spontaneously hypertensive (SHR) and renal hypertensive rats is reversed by central blockade of Ang-(1–7)[7;8]. In contrast, changes in the brain RAS that favor Ang II at the expense of Ang-(1–7) (e.g., increases in AT1 receptor or decreases in ACE2, AT2 or mas expression) may contribute to the decline in cardiovascular function associated with heart failure and hypertension [9;10]. In hypertensive (mRen2)27 rats, overexpression of mouse renin leads to high brain Ang I/Ang II/Ang-(1–7) in hypothalamic tissue, with high Ang II/low Ang-(1–7) in medullary tissue [11]. MAP is highest in young animals and declines with age along with decreases in cardiac function [3;12;13]. The BRS is impaired in young (mRen2)27 rats [14;15], and neither AT1 nor Ang-(1–7) receptor blockade in the nTS alters BRS or MAP (Figure 1). The reduced Ang-(1–7) in the medulla of these animals [11] may leave Ang II actions to attenuate BRS unopposed and explain the lack of response to Ang-(1–7) blockade. However, the lack of responsiveness to AT1 receptor blockade in the nTS is surprising. These effects are not related to the hypertension per se, as SHR show the expected increase in BRS with AT1 receptor blockers and decrease in BRS with Ang-(1–7) receptor blockers injected into the nTS [16]. Recent findings illustrate that ACE inhibition in the nTS of (mRen2)27 rats returns BRS to normotensive levels over approximately two hours, without altering resting MAP [17]. The effect was reversed by local Ang-(1–7) receptor blockade [17]. These observations suggest that the long-term Ang II/Ang-(1–7) imbalance in the medulla of these rats alters signaling pathways that require an increase in Ang-(1–7). However, the data in SHR strongly suggest that a deficit in Ang-(1–7) is not the underlying mechanism in all forms of hypertension.
Is fetal programmed hypertension associated with a deficit in brain Ang-(1–7)?
Antenatal steroids provide great advantages in postnatal survival and current guidelines support their use in threatened premature delivery. Thus, the incidence of steroid exposure in the last trimester of gestation and those who subsequently proceed to full term has increased [18]. However, it is unclear overall whether exposure to antenatal steroids itself mitigates or aggravates the risk factors for early onset cardiovascular problems over the long term. Emerging evidence suggests that antenatal glucocorticoid exposure is associated with development of elevated MAP during adolescence [19] and disturbances of metabolism in young adults [20;21]. Recent findings in a model of in utero exposure to betamethasone (Beta) in sheep illustrate that Beta-exposure causes a shift in components of the RAS favoring Ang II over Ang-(1–7) in the kidney [22;23], without altering circulating peptides. Thus, local tissue effects may underlie the development of cardiometabolic dysfunction at later time points, including elevated MAP, impaired BRS for control of heart rate, and higher insulin, glucose and leptin at ~2 years of age [24]. Acute systemic AT1 antagonist administration lowers MAP and improves BRS, while Ang-(1–7) receptor blockade reduces BRS in control but not Beta-exposed animals [25], suggesting loss of the facilitation of the baroreflex by endogenous Ang-(1–7). Preliminary studies further suggest that loss of brain Ang-(1–7) may contribute to the BRS impairment at 6 wks of age given that nTS injection of D-Ala fails to suppress the BRS in Beta-exposed animals [26]. These findings are similar to observations in (mRen2)27 rats and older SD rats where apparent loss of Ang-(1–7) facilitation of the BRS occurs. We hypothesize that Beta-exposure leads to postnatal disturbances in the balance of Ang II and Ang-(1–7), resulting from changes in receptors, enzymes or peptide levels in the nTS and leading to greater Ang II tone. The imbalance in Ang peptides in the nTS contributes to elevated sympathetic and reduced parasympathetic tone, associated with impairment in BRS, thereby reinforcing a progressive elevation of MAP, impaired autonomic function and cardiac and renal injury as these animals age.
Do Ang II and Ang-(1–7) arise from different cellular sources to influence MAP versus BRS?
ASrAOGEN rats with low glia-derived Aogen have modest hypotension and normal to enhanced BRS. In these animals, there is no effect of nTS AT1 receptor blockade, but Ang-(1–7) blockade still reduces the BRS (Figure 1). Thus, the Ang II - mediated suppression of BRS within the nTS is dependent upon an intact glial source of Aogen, whereas Ang-(1–7) arises from a different cellular source. This phenotype is opposite to transgenic mice with overexpression of renin and Aogen in brain glia [27;28], where impairment of the BRS accompanies modest hypertension. This contrasts with overexpression of the brain RAS in neurons where elevated MAP is not associated with changes in BRS [27;28]. This implies that neuronal elements influence the baroreceptor set point rather than BRS [27;28]. Hemizygous (mRen2)27 rats crossed with ASrAogen rats have significantly lower MAP than animals from the usual cross with SD rats [29]; however, this does not normalize pressure suggesting sources of Ang peptides other than glia contribute to the hypertension. These rats combine the traits of the transgenic mice with over-expression of both the glial and neuronal RAS, having both elevated pressure and an impaired BRS. However, (mRen2)27 rats have normal to high plasma Ang II and normal to low plasma Ang-(1–7) [30]. Therefore, these animals also resemble neurogenic hypertension secondary to systemic ‘low-dose’ Ang II and the actions of Ang II and Ang-(1–7) may result from a circulating source via vascular actions indirectly influencing nTS neurons [31].
Does Ang-(1–12) serve as a precursor to Ang II?
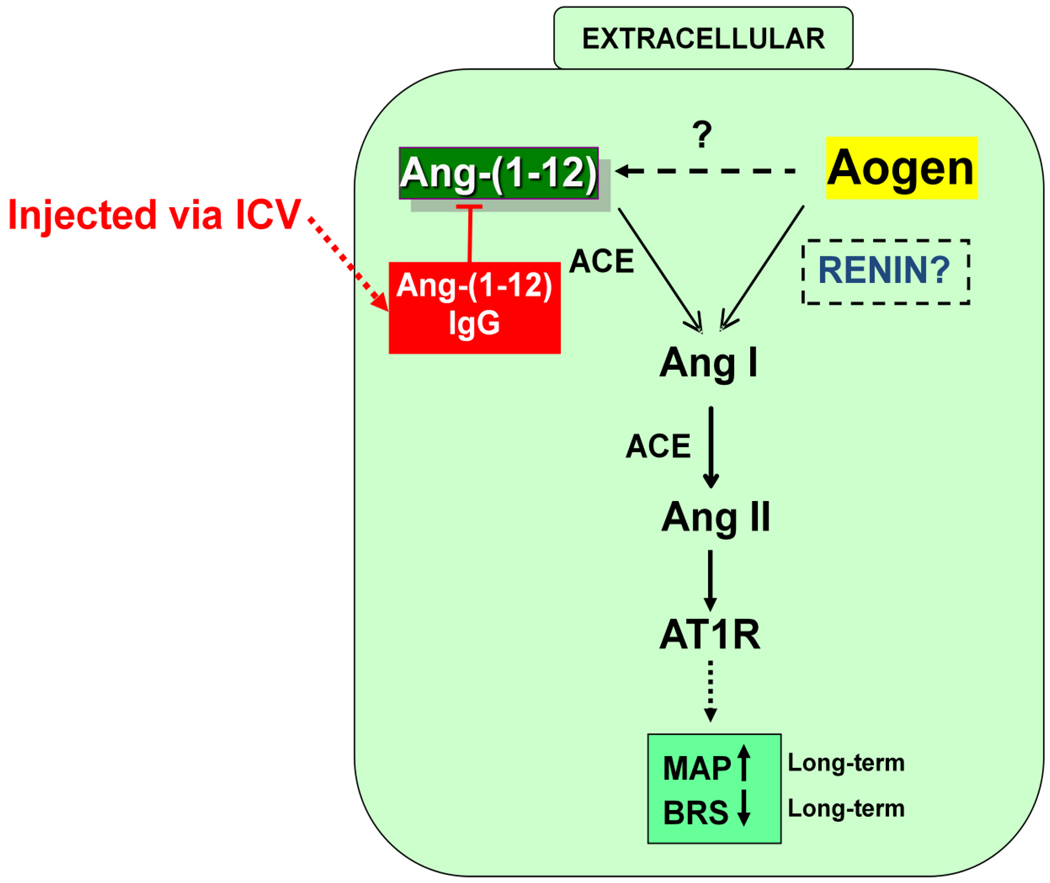
Ang-(1–12) was identified in plasma and tissues in 2007 and is present in brain in equal or higher amounts than Ang II or Ang-(1–7) [32]. Ang-(1–12) would be cleaved from angiotensinogen, the only known precursor for this 12 amino acid sequence, but the enzyme responsible for generation of the peptide is unknown. Several enzymes could convert Ang-(1–12) into either Ang II or Ang-(1–7) as illustrated in Figure 2. In SD rats, nTS injection of Ang-(1–12) reduces BRS, an effect blocked by either an AT1 receptor antagonist or ACE inhibitor [33;34]. These findings suggest that the preferred processing pathway in the nTS is to Ang II. However, immunoneutralization of endogenous Ang-(1–12) in the nTS with a highly selective antibody had no effect on BRS in the SD rats. Thus, while exogenously administered Ang-(1–12) may be converted to Ang II to reduce baroreflex function, Ang-(1–12) does not contribute to BRS in normal animals. In contrast, intracerebroventricular administration of an antibody to Ang-(1–12) reduced MAP in (mRen2)27 transgenic rats, suggesting that Ang-(1–12) serves as an intermediate precursor of Ang II in this model of hypertension [35]. Since it appears that an Ang-(1–12) processing pathway exists in the brain of hypertensive animals for the production of Ang II, further studies to identify the enzyme(s) involved in formation of Ang-(1–12) and regulation of Ang-(1–12) levels in brain are warranted.
Figure 2.
Proposed extracellular renin-independent pathway involving Ang-(1–12) conversion to Ang II. Abbreviations: Aogen= angiotensinogen, ACE= angiotensin converting enzyme, ICV= intracerebroventricular
Does an Ang II /Ang-(1–7) imbalance favoring Ang II in the dorsomedial medulla impact signaling pathways involved in cardiometabolic function?
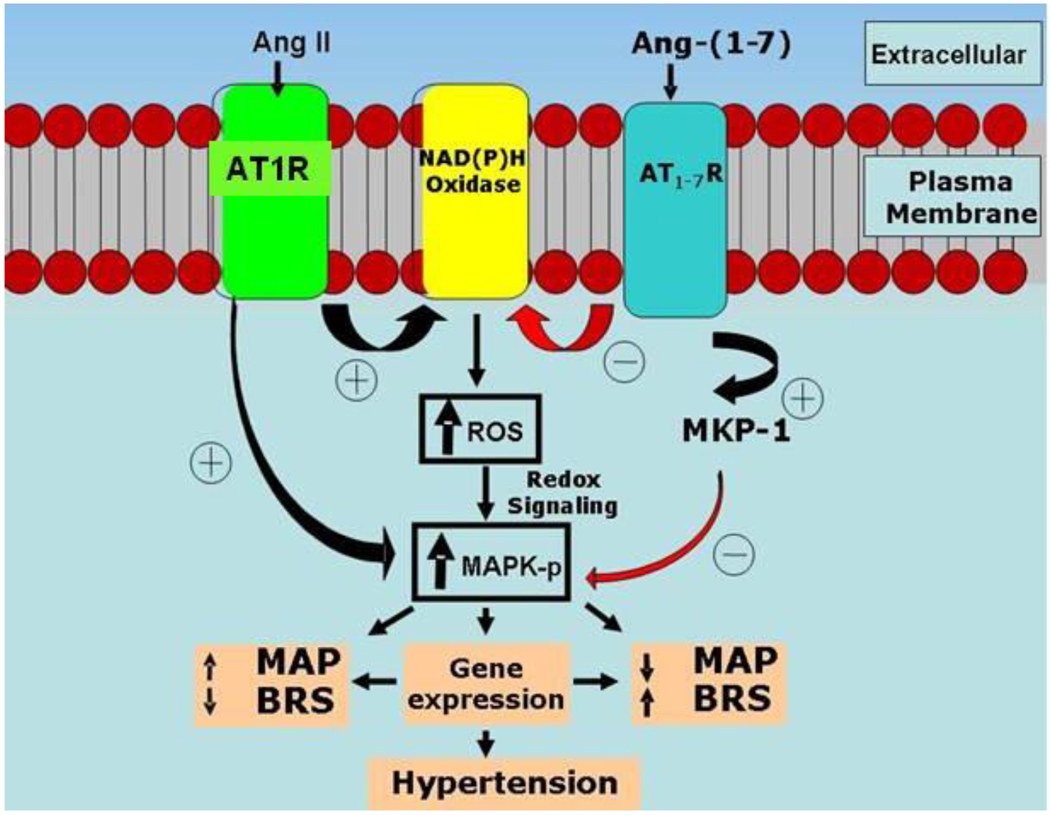
Phosphoinositol 3 kinase (PI3K) and mitogen-activated protein kinase (MAPK) are key intracellular signaling pathways for the cardiovascular actions of insulin, leptin and Ang II. These kinase pathways are negatively modulated by protein tyrosine phosphatase (PTP) 1b and dual specificity phosphatases (DUSP-1 or MKP-1), respectively (see reviews [36;37]). Whether the local brain RAS contributes to regulation of these signaling pathways and how that relates to the alterations in MAP, BRS and metabolic function within the nTS during aging and hypertension is the subject of investigation (Figure 3). The concept is that kinase-phosphatase signaling pathways are altered in the dorsal medulla, in part resulting from imbalances in Ang II and Ang-(1–7) actions.
Figure 3.
Signaling pathways interacting with angiotensin peptides in dorsal medullary cardiovascular nuclei. Proposed model: Ang-(1–7) up-regulates the MAPK phosphatase MKP-1 to attenuate Ang II-stimulated MAPK signaling pathways and ROS (from cytoplasmic, nuclear or mitochondrial sources), resulting in improved BRS and lower MAP. Abbreviations: ROS= reactive oxygen species, MAPK-p= phosphorylated MAPK, MAP= Mean arterial pressure, BRS= Baroreflex sensitivity
Leptin, insulin and Ang II activate PI3K and MAPK pathways to induce rapid signaling responses and these hormones all impair BRS within the nTS [38] [39], [40–42;42]. Not surprisingly, since they share common signaling pathways, cross-desensitization may occur [43;44]. Leptin receptors are found in the nodose ganglion and on vagal afferents in the nTS in normotensive rats [45], similar to the pattern for AT1 receptors, likely mediating suppression of BRS [41]. Leptin increases PI3K and MAPK activity in normotensive rats [46], and the PI3K inhibitors wortmannin and LY294002 prevent the effects of leptin on food intake and renal sympathetic nerve activity [47;48]. The PI3K pathway is also involved in maintenance of Ang II hypertension as PI3K blockade in the nTS of (mRen2)27 rats decreases MAP and increases BRS [49]. However, chronic blockade of PI3K using a mutant receptor expression in the nTS of SHR had the opposite effect [50] suggesting long-term interventions are needed to determine the overall effect of the system. How the brain PI3K pathway contributes to the heavier body weight and development of insulin resistance in the (mRen2)27 rats is also not known. In contrast, ASrAogen rats show high insulin and leptin sensitivity throughout life indicating an important role of the brain RAS in mediating cardiometabolic function during aging [3;41;51;51;52]. ASrAogen rats are at least 3-fold more sensitive to BRS suppression by injections of leptin into the nTS [41]. The increased sensitivity to exogenous leptin is associated with high levels of mRNA for the leptin receptor and the p85 alpha subunit of PI3K, consistent with down-regulation of leptin.
The MAPK pathway is also implicated in the actions of Ang II and leptin as both increase phosphorylation of ERK-1/2 in normotensive rats [46;53]. The MAPK pathway is actively involved in control of resting MAP in the RVLM and mediates Ang II actions in this nucleus in both normotensive and hypertensive rats, unlike recruitment of the PI3K pathway. MKP-1 expression is higher in ASrAogen relative to (mRen2)27 rats [54] supporting the hypothesis that Ang-(1–7) may counteract the actions of Ang II by upregulating MKP-1 expression [55], as outlined in Figure 3.
PTP1b is known to limit the actions of insulin to phosphorylate eNOS via PI3K in the nTS [56;57]. How an increase or decrease in nitric oxide regulates the BRS is, however, controversial. Blockade of nitric oxide in the nTS impairs reflex function under normoxic conditions [58]. A reduction in nitric oxide reportedly precedes the development of hypertension and the BRS impairment in (mRen2)27 rats [59]. In the isolated working heart-brain preparation, release of nitric oxide from the vascular endothelium by Ang II increases GABA causing impairment of BRS through suppression of glutamate transmission in the nTS [60]. Since these experiments were performed under high oxygen conditions of the production of reactive oxygen species may participate in the BRS suppression. Stronger evidence for endothelial derived nitric oxide suppression of BRS is provided in a chronic study in vivo [57]. Ang-(1–7) releases nitric oxide, but whether this mechanism explains the beneficial effect of the peptide on BRS is not known. Alterations in the RAS influence indicators of reactive oxygen species with higher levels in (mRen2)27 versus ASrAogen rats [54]. Ang-(1–7) also plays a role in the NADPH diaphorase – NADPH oxidase balance [61;62]. AT1 receptors are linked to NADPH oxidase in the nTS and these signaling pathways are important to sympathovagal balance [63;64].
Conclusions
A role of the brain RAS in the normal physiologic regulation of the autonomic nervous system involving both cardiovascular and metabolic pathways is clear from comparative studies of wild type and transgenic animals. Insights revealed during the aging process, in hypertension or heart failure illustrate the potential importance of reductions in Ang-(1–7) in dysregulation of autonomic and metabolic function. We concentrated in this review on the role of the Ang peptides in the dorsomedial medulla where they have opposite actions for BRS regulation. It is important to note that actions of Ang II and Ang-(1–7) to increase MAP may be similar in the nTS, paraventricular nucleus and the rostral ventrolateral medulla [5;6;65;66]. Thus, the role of phosphatases, reactive oxygen species and nitric oxide in regulation of MAP and BRS within the nTS and their influence by Ang II versus Ang-(1–7) requires further elucidation and may not extend to other brain sites. While acute regulation of the kinase-phosphatase pathways is likely mediated by rapid phosphorylation and dephosphorylation events, chronic regulation of the expression of protein kinases and phosphatases by the RAS is also possible
Acknowledgements
Funding for the studies described includes HL51952, HD047584, the Lois Groskort Heart Research Fund, the WFUSM Venture Fund and the Farley Hudson Foundation. Dr. Katsunori Isa and Dr. Manisha Nautiyal received support from American Heart Association Mid-Atlantic Affiliate Post-doctoral fellowships (0825482E and 10POST3160006, respectively). Dr. Shaltout is a faculty member in the Department of Pharmacology and Toxicology, School of Pharmacy, University of Alexandria, Egypt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain renin-angiotensin system: insights from studies in transgenic rats. Cleve.Clin J Med. 2007;74 Suppl 1:S95–S98. doi: 10.3949/ccjm.74.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- 2. Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats. Role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. Documents endogenous Ang-(1–7) facilitation of BRS within the nTS of young animals and loss of that facilitation in older animals.
- 3. Kasper SO, Carter CS, Ferrario CM, Ganten D, Ferder LF, Sonntag WE, Gallagher PE, Diz DI. Growth and metabolism disturbances in transgenic rats with altered renin-angiotensin system expression. Physiol Genomics. 2005;23:311–317. doi: 10.1152/physiolgenomics.00163.2005. Characterizes main features of aging in transgenic rats with targeted glial-disruption of Aogen versus overexpression of mouse renin.
- 4.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Tallant EA, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Experimental Physiology. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold AC, Sakima A, Ganten D, Ferrario CM, Diz DI. Modulation of reflex function by endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension. 2008;51:1326–1331. doi: 10.1161/HYPERTENSIONAHA.107.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glial-derived angiotensinogen. Am J Physiol Heart Circ Physiol. 2007;292:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. Demonstration that glial Aogen deficient rats maintain Ang-(1–7) facilitation of the BRS, but lose the Ang II attenuation, revealing that the two peptides arise from different cellular sources. Also, illustrates the acute response to stress in these rats is AT1 - and mas-mediated at the level of the nTS.
- 7. Heringer-Walther S, Batista EN, Walther T, Khosla MC, Santos RAS, Campagnole-Santos MJ. Baroreflex Improvement in SHR After ACE Inhibition Involves Angiotensin-(1–7) Hypertension. 2001;37:1309–1314. doi: 10.1161/01.hyp.37.5.1309. Data show contribution of brain Ang-(1–7) to BRS improvement with ACE inhibition.
- 8. Britto RR, Santos RA, Fagundes-Moura CR, Khosla MC, Campagnole-Santos MJ. Role of angiotensin-(1–7) in the modulation of the baroreflex in renovascular hypertensive rats. Hypertension. 1997;30:549–556. doi: 10.1161/01.hyp.30.3.549. Data show contribution of brain Ang-(1–7) to BRS improvement with ACE inhibition.
- 9.References 9 and 10 illustrate that components of the RAS are shifted towards Ang II in heart failure. Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl.Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009.
- 10.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1216–H1226. doi: 10.1152/ajpheart.00557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 12.Groban L, Ferrario CM, Ganten D, Diz DI. Transgenic rats with low brain renin-angiotensin system activity due to glial deficiency are protected against heart failure late in life. J Card.Fail. 2007 August; [abstract] 2007. [Google Scholar]
- 13.Diz DI, Varagic J, Groban L. Aging and the brain renin-angiotensin system. Relevance to age-related decline in cardiac function. Future Cardiol. 2008;4(3):237–245. doi: 10.2217/14796678.4.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol. 2008;51:542–548. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exp Pharmacol Physiol. 2002;29:473–482. doi: 10.1046/j.1440-1681.2002.03659.x. [DOI] [PubMed] [Google Scholar]
- 16.Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertension. 2000;18:1841–1848. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- 17.Isa K, Arnold AC, Chappell MC, Diz DI. Extracellular angiotensin-converting enzyme inhibition, but not AT1 receptor blockade, in the solitary tract nucleus improves baroreflex sensitivity in anesthetized transgenic hypertensive (mRen2)27 rats. Hypertension. 2009;54:e98. doi: 10.1038/hr.2011.110. [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla BV, Davies J, Hanlon-Lundberg K, Simpson L, Stone J, Wing D, Ogasawara K, Muraskas J. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery: A randomized controlled trial. JAMA. 2001;286:1581–1587. doi: 10.1001/jama.286.13.1581. [DOI] [PubMed] [Google Scholar]
- 19.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin.Sci.(Lond) 2000;98:137–142. [PubMed] [Google Scholar]
- 20.Dalziel SR, Liang A, Parag V, Rodgers A, Harding JE. Blood pressure at 6 years of age after prenatal exposure to betamethasone: follow-up results of a randomized, controlled trial. Pediatrics. 2004;114:e373–e377. doi: 10.1542/peds.2004-0196. [DOI] [PubMed] [Google Scholar]
- 21.Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 22.References 22 – 26 illustrate that glucocorticoid exposure in fetal life chronically alters expression of RAS components favoring a higher Ang II to Ang-(1–7) ratio and promoting elevated blood pressure and impaired BRS. Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339.
- 23.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.164970. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT(1)-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol. 2010;299:H541–H547. doi: 10.1152/ajpheart.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaltout HA, Figueroa J, Rose JC, Chappell MC, Averill DB, Diz DI. Evidence of Ang-(1–7) deficiency in antenatal betamethasone-treated young adult sheep. Hypertension. 2008;52:e107. [abstract] [Google Scholar]
- 26.Shaltout HA, Rose JC, Chappell MC, Diz DI. Antenatal betamethaasone eaxposure attenuates the functional role of angiotensin -(1–7) in the NTS. Hypertension. 2010 [abstract] In Press. [Google Scholar]
- 27.Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem. 2002;277:33235–33241. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- 28.Sakai K, Chapleau MW, Morimoto S, Cassell MD, Sigmund CD. Differential modulation of baroreflex control of heart rate by neuron-vs. glia-derived angiotensin II. Physiol Genomics. 2004;20:66–72. doi: 10.1152/physiolgenomics.00168.2004. [DOI] [PubMed] [Google Scholar]
- 29.Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci U.S.A. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriguchi A, Brosnihan KB, Kumagai H, Ganten D, Ferrario CM. Mechanisms of hypertension in transgenic rats expressing the mouse Ren-2 gene. Am J Physiol. 1994;266:R1273–R1279. doi: 10.1152/ajpregu.1994.266.4.R1273. [DOI] [PubMed] [Google Scholar]
- 31.Tan PSP, Killinger S, Horiuchi J, Dampney RAL. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–R2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
- 32.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 33.References 33 and 34 are the first demonstrations that Ang-(1–12) may serve as a precursor to Ang II in brain nTS through an ACE-dependent pathway. Arnold AC, Isa K, Shaltout HA, Nautiyal M, Ferrario CM, Chappell MC, Diz DI. Angiotensin-(1–12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2010;299:H763–H771. doi: 10.1152/ajpheart.00345.2010.
- 34.Chitravanshi VC, Sapru HN. Cardiovascular responses elicited by a new endogenous angiotensin in the nucleus tractus solitarius of the rat. American Journal of Physiology - Heart and Circulatory Physiology. 2011;300:H230–H240. doi: 10.1152/ajpheart.00861.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am.J Physiol Regul.Integr.Comp Physiol. 2009;297:R111–R115. doi: 10.1152/ajpregu.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robbins ME, Zhao W, Garcia-Espinosa MA, Diz DI. Renin-angiotensin system blockers and modulation of radiation-induced brain injury. Curr.Drug Targets. 2010;11:1413–1422. doi: 10.2174/1389450111009011413. Review highlighting the signaling pathways, including inflammation and oxidative stress responses to Ang peptides, as well as the potential cellular sources for brain Ang peptides.
- 37.Yip SC, Saha S, Chernoff J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem.Sci. 2010;35:442–449. doi: 10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruggeri P, Molinari C, Brunori A, Cogo CE, Mary DA, Picchio V, Vacca G. The direct effect of insulin on barosensitive neurones in the nucleus tractus solitarii of rats. NeuroReport. 2001;12:3719–3722. doi: 10.1097/00001756-200112040-00023. [DOI] [PubMed] [Google Scholar]
- 39.McKernan AM, Calaresu FR. Insulin microinjection into the nucleus tractus solitarii of the rat attenuates the baroreceptor reflex. J Auton.Nerv.Syst. 1996;61:128–138. doi: 10.1016/s0165-1838(96)00074-4. [DOI] [PubMed] [Google Scholar]
- 40.Okada M, Bunag RD. Insulin acts centrally to enhance reflex tachycardia in conscious rats. Am J Physiol. 1994;266:R481–R486. doi: 10.1152/ajpregu.1994.266.2.R481. [DOI] [PubMed] [Google Scholar]
- 41.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs baroreflex sensitivity at the solitary tract nucleus: Evidence for up-regulation of leptin actions in animals with low brain angiotensin. Hypertension. 2009;54:1001–1008. doi: 10.1161/HYPERTENSIONAHA.109.138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casto R, Phillips MI. Angiotensin II attenuates the baroreflexes at nucleus tractus solitarius of rats. Am.J.Physiol. 1986;250:R193–R198. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- 43.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–2169. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folli F, Saad MJ, Velloso L, Hansen H, Carandente O, Feener EP, Kahn CR. Crosstalk between insulin and angiotensin II signalling systems. Exp.Clin.Endocrinol.Diabetes. 1999;107:133–139. doi: 10.1055/s-0029-1212088. [DOI] [PubMed] [Google Scholar]
- 45.Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, Walker F, Lewin MJ, Meister B, Bado A. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur.J Neurosci. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 46.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- 47.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 48.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic overflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 49.Logan E, Diz DI, Ganten D, Ferrario CM, Averill DB. Inhibition of the P13 kinase signal transduction pathway in nucleus tractus solitari of (mRen2)27 transgenic rats improves baroreceptor sensitivity. FASEB J. 2008 [abstract] [Google Scholar]
- 50.Zubcevic J, Waki H, Diez-Freire C, Gampel A, Raizada MK, Paton JFR. Chronic Blockade of Phosphatidylinositol 3-Kinase in the Nucleus Tractus Solitarii Is Prohypertensive in the Spontaneously Hypertensive Rat. Hypertension. 2009;53:97–103. doi: 10.1161/HYPERTENSIONAHA.108.122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasper SO, Ferrario CM, Ganten D, Diz DI. Rats with low brain angiotensinogen do not exhibit insulin resistance during early aging. Endocrine. 2006;30:167–174. doi: 10.1385/ENDO:30:2:167. [DOI] [PubMed] [Google Scholar]
- 52.Arnold AC, Diz DI. Endogenous leptin within the solitary tract nucleus contributes to the suppression of baroreflex sensitivity in older anesthetized Sprague-Dawley rats. Hypertension. 2010;56:e72. [abstract] [Google Scholar]
- 53.Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38:1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- 54.Nautiyal M, katakam p, Busija DW, Gallagher PE, Tallant EA, Chappell MC, Diz DI. Oxidative stress and low expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in dorsal medulla of hypertensive (mRen2)27 transgenic rats. Hypertension. 2010 [abstract] In Press. [Google Scholar]
- 55.Tallant EA, Gallagher PE. Angiotensin-(1–7) upregulates the mitogen-activated phosphatase DUSP1 in vascular smooth muscle cells. Hypertension. 2007;50 [abstract] E77[009] [Google Scholar]
- 56.Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. In Situ Akt Phosphorylation in the Nucleus Tractus Solitarii Is Involved in Central Control of Blood Pressure and Heart Rate. circulation. 2004;110:2476–2483. doi: 10.1161/01.CIR.0000145116.75657.2D. [DOI] [PubMed] [Google Scholar]
- 57. Waki H, Kasparov S, Wong L-F, Murphy D, Shimizu T, Paton JFR. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J Physiol. 2003;546:233–242. doi: 10.1113/jphysiol.2002.030270. Evidence showing role for endothelial NOS in suppression of BRS in the nTS.
- 58.Souza HCD, De Ara·jo JE, Martins-Pinge MC, Cozza IC, Martins-Dias DP. Nitric oxide synthesis blockade reduced the baroreflex sensitivity in trained rats. Autonomic Neuroscience. 2009;150:38–44. doi: 10.1016/j.autneu.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Borgonio A, Pummer S, Witte K, Lemmer B. Reduced baroreflex sensitivity and blunted endogenous nitric oxide synthesis precede the development of hypertension in TGR(mREN2)27 rats. Chronobiol.Int. 2001;18:215–226. doi: 10.1081/cbi-100103187. [DOI] [PubMed] [Google Scholar]
- 60.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol.Med. 2008;86:705–710. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 61.Benter IF, Yousif MHM, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1--7) Prevents Activation of NADPH Oxidase and Renal Vascular Dysfunction in Diabetic Hypertensive Rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 62.Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1–7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur.J Pharmacol. 2010;638:108–114. doi: 10.1016/j.ejphar.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH Oxidase Contributes to Angiotensin II Signaling in the Nucleus Tractus Solitarius. Journal of Neuroscience. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hirooka Y. Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Autonomic Neuroscience. 2008;142:20–24. doi: 10.1016/j.autneu.2008.06.001. Review of the role of reactive oxygen species in regulation of sympathovagal balance.
- 65.Silva AQ, Santos R, Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 66.Fontes MA, Baltatu O, Caligiorne SM, Campagnole-Santos MJ, Ganten D, Bader M, Santos RA. Angiotensin peptides acting at rostral ventrolateral medulla contribute to hypertension of TGR(mREN2)27 rats. Physiol Genomics. 2000;2:137–142. doi: 10.1152/physiolgenomics.2000.2.3.137. [DOI] [PubMed] [Google Scholar]