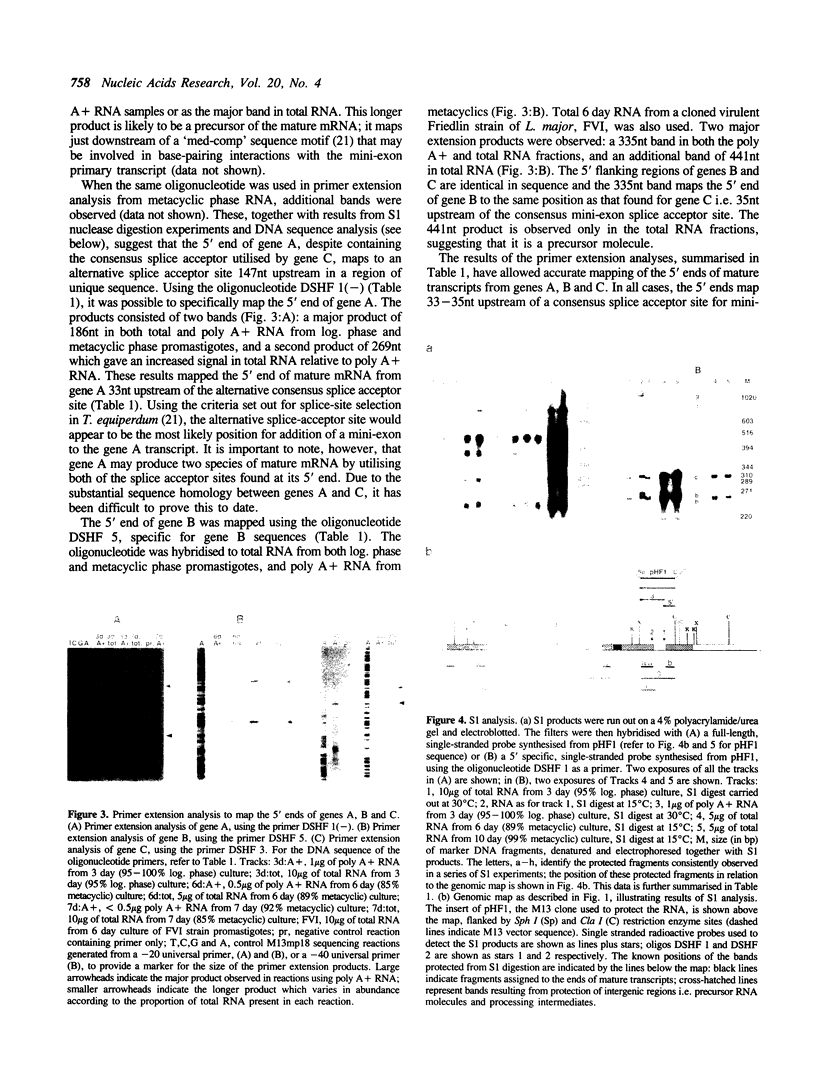
Abstract
We have isolated and characterised a differentially-regulated gene family in the protozoan parasite Leishmania major. The family contains 5 genes linked within a 10Kb region of the genome: three of the genes are closely related in DNA sequence, the other two have only limited homology. Post-transcriptional control of the differential expression pattern is suggested by detection of precursor RNA molecules containing intergenic sequences and evidence that mature mRNA molecules contain a 35nt spliced leader sequence at their 5' ends. These features support a model of polycistronic transcription in which the stability and differential processing of precursor RNA molecules determine the steady state levels of mature mRNA. We have identified several DNA sequence motifs within the gene family that have potential roles in differential processing and/or RNA stability: an alternative 5' splice acceptor site for trans-splicing; a putative polyadenylation site; and a region of potential secondary structure within 3' flanking sequences. The 3' sequence elements are conserved in those genes that share the same pattern of differential regulation. To our knowledge, this is the first example of coordinated differential-regulation of a non-identical gene cluster in Leishmania.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Beatty J. T., Cohen S. N., Belasco J. G. An intercistronic stem-loop structure functions as an mRNA decay terminator necessary but insufficient for puf mRNA stability. Cell. 1988 Feb 26;52(4):609–619. doi: 10.1016/0092-8674(88)90473-4. [DOI] [PubMed] [Google Scholar]
- Coulson R. M., Smith D. F. Isolation of genes showing increased or unique expression in the infective promastigotes of Leishmania major. Mol Biochem Parasitol. 1990 Apr;40(1):63–75. doi: 10.1016/0166-6851(90)90080-6. [DOI] [PubMed] [Google Scholar]
- Evers R., Cornelissen A. W. The Trypanosoma brucei protein phosphatase gene: polycistronic transcription with the RNA polymerase II largest subunit gene. Nucleic Acids Res. 1990 Sep 11;18(17):5089–5095. doi: 10.1093/nar/18.17.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Franke E. D., McGreevy P. B., Katz S. P., Sacks D. L. Growth cycle-dependent generation of complement-resistant Leishmania promastigotes. J Immunol. 1985 Apr;134(4):2713–2718. [PubMed] [Google Scholar]
- Genske J. E., Cairns B. R., Stack S. P., Landfear S. M. Structure and regulation of histone H2B mRNAs from Leishmania enriettii. Mol Cell Biol. 1991 Jan;11(1):240–249. doi: 10.1128/mcb.11.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. C., Swinkels B. W., Borst P. Post-transcriptional control of the differential expression of phosphoglycerate kinase genes in Trypanosoma brucei. J Mol Biol. 1988 May 20;201(2):315–325. doi: 10.1016/0022-2836(88)90140-4. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Polvere R. I., Van der Ploeg L. H. Conserved sequences and transcription of the hsp70 gene family in Trypanosoma brucei. Mol Cell Biol. 1986 Dec;6(12):4657–4666. doi: 10.1128/mcb.6.12.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., van der Ploeg L. H. Maturation of polycistronic pre-mRNA in Trypanosoma brucei: analysis of trans splicing and poly(A) addition at nascent RNA transcripts from the hsp70 locus. Mol Cell Biol. 1991 Jun;11(6):3180–3190. doi: 10.1128/mcb.11.6.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Zhang K., Beverley S. M. Nuclease mapping and DNA sequence analysis of transcripts from the dihydrofolate reductase-thymidylate synthase (R) region of Leishmania major. Nucleic Acids Res. 1990 Nov 11;18(21):6399–6408. doi: 10.1093/nar/18.21.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear S. M., McMahon-Pratt D., Wirth D. F. Tandem arrangement of tubulin genes in the protozoan parasite Leishmania enriettii. Mol Cell Biol. 1983 Jun;3(6):1070–1076. doi: 10.1128/mcb.3.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden R. E., Eisen H. Alternate trans splicing in Trypanosoma equiperdum: implications for splice site selection. Mol Cell Biol. 1988 Mar;8(3):1352–1360. doi: 10.1128/mcb.8.3.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Schümperli D. RNA 3' processing regulates histone mRNA levels in a mammalian cell cycle mutant. A processing factor becomes limiting in G1-arrested cells. EMBO J. 1987 Jun;6(6):1721–1726. doi: 10.1002/j.1460-2075.1987.tb02423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey N. B., Marzluff W. F. The stem-loop structure at the 3' end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4557–4559. doi: 10.1128/mcb.7.12.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J., O'Hare K. Structure and transcription of the singed locus of Drosophila melanogaster. Genetics. 1991 Dec;129(4):1073–1084. doi: 10.1093/genetics/129.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. T., Raibaud A., Florent I. C., Sather S., Gross M. K., Storm D. R., Eisen H. The trypanosome VSG expression site encodes adenylate cyclase and a leucine-rich putative regulatory gene. EMBO J. 1991 Aug;10(8):2047–2053. doi: 10.1002/j.1460-2075.1991.tb07735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Hieny S., Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985 Jul;135(1):564–569. [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am J Trop Med Hyg. 1985 May;34(3):456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Searle S., Campos A. J., Coulson R. M., Spithill T. W., Smith D. F. A family of heat shock protein 70-related genes are expressed in the promastigotes of Leishmania major. Nucleic Acids Res. 1989 Jul 11;17(13):5081–5095. doi: 10.1093/nar/17.13.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Shaw J. RNA editing and the mitochondrial cryptogenes of kinetoplastid protozoa. Cell. 1989 May 5;57(3):355–366. doi: 10.1016/0092-8674(89)90911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber C., Schümperli D. 3' processing of pre-mRNA plays a major role in proliferation-dependent regulation of histone gene expression. Nucleic Acids Res. 1988 Oct 25;16(20):9399–9414. doi: 10.1093/nar/16.20.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri A. F., Hajduk S. L. Posttranscriptional regulation of cytochrome c expression during the developmental cycle of Trypanosoma brucei. Mol Cell Biol. 1988 Nov;8(11):4625–4633. doi: 10.1128/mcb.8.11.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]