Abstract
Putzig (Pzg) was originally identified as being an integral component of the TRF2/DREF complex in Drosophila melanogaster, thereby regulating the transcriptional activation of replication-related genes. In a DREF-independent manner, Pzg was shown to mediate Notch target gene activation. This function of Pzg entails an association with the nucleosome remodeling factor complex NURF, which directly binds the ecdysone receptor EcR and coregulates targets of the EcR via the NURF-specific subunit Nurf-301. In contrast, Nurf-301 acts as a negative regulator of JAK/STAT signaling. Here, we provide evidence to show that Pzg is fundamental for these functions of NURF, apart from the regulation of Notch signaling activity. A jump-out mutagenesis provided us with a pzg null mutant displaying early larval lethality, defects in growth, and molting accompanied by aberrant feeding behavior. We show that Pzg is associated with EcR in vivo and required for the transcriptional induction of EcR target genes, whereas reduced ecdysteroid levels imply a NURF-independent function of Pzg. Moreover, pzg interferes with JAK/STAT-signaling activity by acting as a corepressor of Ken. Lamellocyte differentiation was consistently affected in a JAK/STAT mutant background and the expression level of defense response genes was elevated in pzg mutants, leading to the formation of melanotic tumors. Our results suggest that Pzg acts as an important partner of NURF in the regulation of EcR and JAK/STAT signaling.
THE putzig (pzg) gene is located near the centromere on the left arm of the third chromosome. It encodes a zinc finger protein with a molecular weight of about 160 kDa (Eggert et al. 2004; Kugler and Nagel 2007). Pzg was identified as p160, being an integral component of the TATA-binding protein-related factor 2 (TRF2)/DNA replication-related element binding factor (DREF) multiprotein complex (Hochheimer et al. 2002). This complex activates the transcription of several replication-related genes (Hochheimer et al. 2002).
The downregulation of pzg gene activity by RNA interference (pzg-RNAi) revealed the fact that Pzg is essential for the function of the TRF2/DREF complex, which regulates cell cycling and growth during Drosophila development (Kugler and Nagel 2007). The ubiquitous induction of pzg-RNAi is associated with a developmental delay and leads to loss of tissue due to reduced proliferation (Kugler and Nagel 2007). Pzg was shown to have a dual input on proliferation processes during development. Aside from its role in the TRF2/DREF complex, Pzg positively influences Notch (N) signaling (Kugler and Nagel 2007). The impact of Pzg on N activity is independent of DREF, as only Pzg, and not DREF, can be detected at the promoters of different N target genes. Furthermore, it was demonstrated that Pzg activates N signaling by chromatin activation. In this context, we showed that Pzg is associated with the nucleosome remodeling factor (NURF), thus entailing Notch target gene activation (Kugler and Nagel 2010).
The NURF complex contains four subunits, Iswi, Nurf-38, Nurf-55, and Nurf-301 (Xiao et al. 2001). The Nurf-301 subunit is the only subunit specific to the NURF complex, whereas the other three NURF components also appear in other chromatin remodeling complexes, for example, the TRF2/DREF complex (Vignali et al. 2000; Xiao et al. 2001; Hochheimer et al. 2002; Corona and Tamkun 2004). NURF remodels chromatin by catalyzing energy-dependent nucleosome sliding (Tsukiyama and Wu 1995; Xiao et al. 2001). Nurf-301 contains two AT-hook peptide motifs and an acid domain with high similarity to the high mobility group A (HMGA) proteins (Xiao et al. 2001). Both domains participate in DNA–protein and protein–protein interactions (Reeves and Beckerbauer 2001). It was shown that NURF binds different transcription factors to promote transcriptional activation or repression of target genes, depending on the gene context (e.g., Xiao et al. 2001; Badenhorst et al. 2005; Kwon et al. 2008). Whole-genome expression analyses revealed an important function of NURF for ecdysone receptor (EcR) signaling (Badenhorst et al. 2005). In vitro, NURF binds EcR in the presence of ecdysone, implying that it acts as a coactivator of EcR on ecdysone-responsive promoters (Badenhorst et al. 2005). The Nurf-301 mutants are characterized by a developmental delay and the failure to pupariate (Badenhorst et al. 2005). This phenotype is due to impaired EcR signaling, as most of the known ecdysone targets were significantly downregulated in Nurf-301 mutants (Badenhorst et al. 2005).
In contrast to NURF's function as a coactivator, NURF has been implicated in the transcriptional repression of genes that are downstream of the JAK/STAT pathway (Kwon et al. 2008). The NURF mutants display melanotic tumors, which also occur after dysregulated activation of JAK/STAT signaling (Luo et al. 1995; Badenhorst et al. 2002). NURF physically and genetically interacts with the JAK/STAT repressor Ken and it is localized to promoters containing Ken binding sites. A large proportion of defense response genes contain overlapping Ken and STAT target sequences, suggesting that NURF is recruited by Ken to repress STAT target genes (Kwon et al. 2008). Consistent with this, a common set of defense response genes is significantly upregulated in the NURF loss and JAK/STAT gain-of-function mutants (Kwon et al. 2008).
We recently showed that Pzg forms a complex with NURF and that this association is quintessential for the epigenetic activation of Notch target genes (Kugler and Nagel 2007; Kugler and Nagel 2010). Pzg associates with all four members of NURF and the entire Pzg–NURF complex is found on N target gene promoters (Kugler and Nagel 2010).
In this report, we show that Pzg is also required for metamorphosis and innate immunity in Drosophila melanogaster, apart from its role in Notch target gene activation. We generated a null mutant allele of pzg (pzg66) that displays a range of phenotypes reminiscent of those observed in mutants with an impaired ecdysone-signaling cascade. The pzg66 homozygotes are early larval lethal with defects and delays in larval development, growth, feeding, and molting. Pzg is located at the regulatory region of well-defined EcR target genes with a downregulated expression in pzg66 mutants, suggesting a coregulator function of pzg with respect to EcR nuclear activity. Intriguingly, ecdysteroid levels are perturbed in pzg66/66 larvae, implying an additional NURF-independent influence on EcR-signaling activity. Finally, the pzg66 mutant flies evolve melanotic tumors and show an upregulation of immune response genes. Immunoprecipitation experiments revealed that Pzg can be detected in a complex with the transcriptional repressor Ken, indicating a corepressor activity of Pzg in the JAK/STAT pathway. We suggest that Pzg is an essential cofactor of NURF in the regulation of these pathways, implying a deep interdependence of these two in many developmental processes of Drosophila melanogaster.
MATERIALS AND METHODS
Drosophila strains and fly work:
If not stated otherwise, flies were raised at 25° on standard cornmeal fly food seeded with baker’s yeast. The following stocks were obtained from the Bloomington stock center: the P{SUPor-P}KG04911 (BL13616) line gained in the Berkeley Drosophila Genome Project disruption project (Bellen et al. 2004); the deficiencies Df(3L)Pc/TM3Sb (BL3002), Df(3L)Pc-MK/TM2 (BL4430), and Df(3L)Pc-2q/TM2 (BL3068) all uncovering the pzg locus; the Gal4 lines cg-Gal4.A2 (BL7011; Asha et al. 2003) and Hml-Gal4G.6-4 (BL6396; Goto et al. 2003); the UAS-lines UAS-EcR.A (BL6470), UAS-EcR.B1 (BL6468), UAS-lacZ (BL8529), and the mutant strains y1v1hopTum-l/FM7c (BL8492) and yw; Ki1ry506 Δ2-3 (BL4368). The other stocks used in this study were: da-Gal4 (Wodarz et al. 1995), en-Gal4 (Brand and Perrimon 1993), enGFP-Gal4 (Neufeld and Edgar 1998), phantom-Gal4 (gift from M. O’Connor, University of Minnesota, MN), P0206-Gal4 (Warren et al. 2004) both lines kindly provided from C. Mirth, University of Washington (Seattle, WA); UAS-pzg-RNAi (Kugler and Nagel 2007), Nurf3012/TM6B (Badenhorst et al. 2005), STAT92E-GFP (Bach et al. 2007), and yw; e4tx (Preiss et al. 1988).
pUAST-pzg was cloned by shuttling the pzg cDNA via EcoRI/XhoI into the pUAST vector. The pzg full-length cDNA clone (LD15904) was obtained from Open Biosystems (Heidelberg, Germany). Several transgenic lines were generated by injecting yw67c embryos using established methods (Ashburner 1989) and compared for their expression level. For further experiments, transgenes located on the second chromosome were used.
Generation and verification of the pzg66 mutant allele:
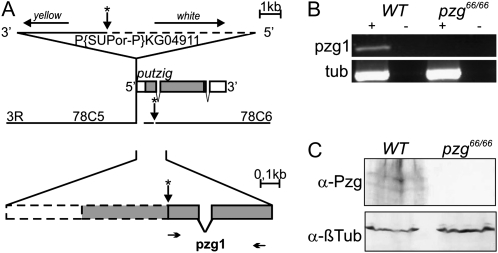
We used imprecise P-element excision to generate pzg mutant alleles. The starting P-element P{SUPor-P}KG04911 was inserted 20 bp upstream of the pzg transcription start site and harbored two marker genes, white (w+) in the 5′ region and yellow (y+) in the 3′ region (see scheme in Figure 1A). Therefore, we were able to perform a site-directed screening for flies that lost the marker w+, located toward the pzg transcription start site, but that still retained the y+ marker. The yw; Ki1ry506Δ2-3 virgin females, providing the transposase, were mated to KG04911 males. The F1 males (yw; Ki1ry506 Δ2-3/KG04911) were then crossed with yw; e4tx virgins, and the F2 progeny was screened for site-directed P-element excision by the loss of the eye color marker w+. 74 white-eyed and y+ flies were collected and individually balanced to establish stocks for further investigations.
Figure 1.—
Schematic of the genomic region of pzg and verification of the pzg66 mutant. (A) Scheme of the pzg genomic region at 78C5–78C6. The 5′ region of the pzg gene is enlarged below. Rectangles indicate the pzg transcript with introns (interrupted rectangles) and the Pzg coding region (shaded). The P element in strain KG04911 (triangle) is oriented in such a way that the white marker gene is adjacent to the pzg gene. The two breakpoints in the pzg66 deletion are indicated by two arrows with asterisks; the deletion is depicted by dashed lines. Small arrows underneath show the position of primer pair pzg1 used for RT–PCR. (B) Semiquantitative RT–PCR analysis of pzg and β-tubulin transcripts in wild-type and pzg66/66 larvae. Note the absence of a pzg PCR product in homozygous pzg66 mutant larvae. For control, reactions were performed with (+) and without (−) reverse transcriptase. (C) Loss of Pzg protein in pzg66/66 mutants compared to the wild type is shown in a Western blot. Anti-β-tubulin was used as the loading control.
Mapping of breakpoints of the pzg66 deletion mutant:
As the y+ body color was still present in the pzg mutant candidates, we designed an upper primer within the y body enhancer and used a set of 3′ lower primers, which bind at different regions within the pzg gene region (see supporting information, Figure S1A and Table S1). The respective PCR products were gel purified and sequenced from both ends (Figure S1B). Most of the potential pzg mutants showed an internal deletion within the P element and did not affect the pzg gene. As pzg66 mutants are homozygous lethal, the allele was maintained in stocks heterozygous for TM6B or for facile selection of homozygous animals balanced over TM6B ubi-GFP (Df(3L)Ly/TM6Bubi GFP; BL4887, obtained from the Bloomington stock center).
Southern blot analysis:
To verify the deletion in the pzg66 allele, we performed Southern blot analysis according to standard protocols (Sambrook et al. 1989). Genomic DNA from the wild-type (Oregon R), KG04911 P-element starter line, as well as pzg66/TM6B, was digested with BglII or NcoI, electrophoresed on a 0.7% agarose gel, blotted on a nitrocellulose membrane, and probed with a radiolabeled genomic probe comprising the deleted region. The predicted restriction fragments and the corresponding bands are shown in Figure S1, A and C, and the details are given in the legend to Figure S1.
Semi-quantitative RT–PCR analysis:
High-purity mRNA was isolated from 100-mg larvae 90–100 hr after egg laying (AEL) of the indicated genotype by using the PolyA Tract magnetic selection kit from Promega (Madison, WI). The mRNA was reversely transcribed using the Photoscript II RT–PCR kit from New England Biolabs (Ipswich, MA) at 42° according to the manufacturer’s protocol. The PCR was performed for 35 cycles. The primer sequences are listed in Table S1.
Immunoprecipitation, cross-linked chromatin immunoprecipitation (XChIP), and Western blot analysis:
Immunoprecipitations were performed according to Nagel et al. (2005) using protein extracts from 100 first-instar larvae. For precipitations we used guinea pig anti-Pzg antibodies at a 1:100 dilution (Kugler and Nagel 2007), and for detection we used rat anti-Pzg (1:500), mouse anti-Ken, and Barbie (1:100, Abcam, Cambridge, UK), mouse anti-EcRA (1:50), mouse anti-EcRB1 (1:50), and mouse anti-EcRcommon (1:50); all three mouse antibodies were developed by C. Thummel and D. Hogness, and were obtained from the Developmental Studies Hybridoma Bank (DSHB), developed under the auspices of the NICHD and maintained by the University of Iowa (Department of Biological Sciences, Iowa City, IA).
For chromatin immunoprecipitation of third-instar larvae (96–112 hr AEL, including the 20-hydroxyecdysone, 20-HE, peak), we used the ChIP Assay Kit according to the manufacturer’s protocol (Upstate Biotechnology, Lake Placid, NY). For precipitation, guinea pig anti-Pzg (1:100) and guinea pig pre-immune sera (1:25, mock control) were used, and 1.5% of the precipitated DNA was used per PCR reaction. There were 35 cycles, and samples were taken every two cycles from the 31st to the 35th cycle to show a linear amplification range. Signals were quantified using the histogram function of ImageJ software (http://rsb.info.nih.gov./ij/). As negative controls, we used primer sets within the open reading frame of the analyzed genes. The primer sets for the amplification process are listed in Table S1.
The Pzg protein levels in pzg66/66 mutants were measured by Western blot experiments. Protein extracts from 100 first instars from either wild-type or homozygous pzg66 mutants (hand picked by the loss of the GFP-balancer marker under a fluorescence microscope) were homogenized in 50 μl RIPAI buffer (50 mm Tris–HCl pH 7.5; 150 mm NaCl; 1% Triton X-100; 0.1% SDS; protease inhibitor cocktail from Roche Diagnostics, Basel, Switzerland) and after 10 min centrifugation 25 μl SDS-loading buffer (250 mm Tris–HCl, pH 7.6, 0.001% bromophenol blue, 5% [vol/vol] SDS, 5% [vol/vol] 2-mercaptoethanol, 40% [vol/vol] glycerol) was added and immediately boiled for 5 min. Then, 15 μl of the supernatant per lane was loaded onto a 10% polyacrylamide gel and separated, followed by electrical blotting on a nitrocellulose membrane. The Pzg protein was detected on the blots by using guinea pig anti-Pzg (1:5000) antibodies and mouse anti-β-Tubulin (1:50; DSHB) antibodies. Secondary antibodies, coupled to alkaline phosphatase (1:500), were obtained from Jackson Laboratories (Dianova, Hamburg, Germany).
Immunofluorescence staining of tissues:
Drosophila hemocytes from 15 third-instar larvae were suspended in 200 µl of Shields and Sang M3 medium with 20% fetal calf serum (FCS) and a protease inhibitor cocktail (Roche Diagnostics). The hemocytes were pelleted after a 10-min centrifugation step at 5000 rpm. The supernatant was discarded and the hemocytes were resuspended in 100 μl of Shields and Sang M3 medium with 20% FCS. Fixation of the hemocytes and antibody staining was performed according to Kwon et al. (2008). The cells were stained with α-Mys-specific antibodies (CF.6G11; DSHB) and rhodamine-coupled phalloidin (Invitrogen molecular probes, Carlsbad, CA); nuclei were stained with DAPI (Roche Diagnostics).
Antibody staining of larval wing disks was performed according to Müller et al. (2005), using guinea pig anti-Pzg antibodies (1:1000). Secondary antibodies coupled to Cy3 were purchased from Jackson Laboratories (Dianova, Hamburg, Germany). The ring-gland-specific induction of UAS-pzg-RNAi was analyzed with the help of phantom-Gal4, UAS mCD::GFP/TM6B Tb, and P0206-Gal4, UAS mCD::GFP, visualizing the prothoracic gland with the help of GFP. Rhodamine-coupled phalloidin was used to stain the boundaries of the cells and guinea pig anti-Pzg antibodies were used to verify the reduction in Pzg activity.
Lethal-phase analysis:
Eggs were collected from pzg66/TM6Bubi-GFP flies during a 1-hr interval on apple juice plates with fresh yeast paste. Homozygous pzg66 first-instar larvae (n > 145) were selected by their lack of GFP expression. These larvae were placed onto fresh plates and the number of living larvae was determined every 5 hr. For comparison, the same procedure was performed with wild-type larvae. All flies were incubated at 25° and larval instars were distinguished by spiracle and mouth-hook development (Bate and Martinez Arias 1993).
Da-Gal4 rescue experiments:
The following strains were established for the rescue experiments: da-Gal4 was recombined with pzg66 mutants to generate the da-Gal4 pzg66/TM6B strain. UAS-pzg was combined with da-Gal4 pzg66/TM6B to generate UAS-pzg/UAS-pzg; da-Gal4 pzg66/TM6B flies or with pzg66/TM6B to establish UAS-pzg/UAS-pzg; pzg66/TM6B strains. The different strains were mated with the aim of obtaining pzg66 homozygous flies with either one or two copies of da-Gal4 and/or UAS-pzg. The rescue was assessed at third-instar larval, pupal, and adult stages by screening for individuals lacking the balancer chromosome. At least 250 animals were analyzed per genotype. The correct genotype of the rescued flies was further verified by PCR (Table S1 and Figure S2).
Ecdysone feeding assay:
To mimic the pulses of ecdysone, staged larvae (pzg66/66 vs. wild type) were periodically transferred between food lacking and food containing the activated form of ecdysone, 20-HE (Sigma, St. Louis, MO). The experiment was performed according to Fluegel et al. (2006), whereby larvae were fed for 8 hr on standard food immediately after a molt and then moved to food with ecdysone until the next molt. The 20-HE was mixed with baker’s yeast (1 mg 20-HE dissolved in 42 μl 100% ethanol, added to 958 μl water and 0.5 g dry yeast). This mixture was evenly spread over apple juice plates. The lethal phase was then noted over the course of development.
Feeding response:
To analyze feeding behavior, a blue-colored yeast paste was offered to first- and second-instar larvae as a food source to follow food uptake within the gut.
Mouth hook contraction studies:
The relative frequency of mouth-hook contraction of the larvae is directly correlated with the ingested amount of food (Gutierrez et al. 2007). Therefore, mouth-hook contractions were counted in 30-sec intervals for first- and second-instar pzg66/66 mutant larvae and were statistically compared with the numbers in wild-type larvae of the same age.
Feeding behavior studies:
First-instar larvae were placed onto the edges of apple juice plates harboring fresh yeast paste as a food source in the middle. According to Gutierrez et al. (2007), wild-type larvae are attracted by the yeast source and wander toward the middle of the dish. Every 15 min we counted how many larvae of the respective genotype (wild type compared with pzg66/66) had reached the source and statistically documented the results.
Documentation of phenotypes:
Pictures of whole larvae were documented using a Wild stereomicroscope equipped with a Pixera camera (Optronics, Goleta, CA) using the Pixera Viewfinder, version 2.0, software. Confocal pictures were taken with a Zeiss Axioskop linked to a Bio-Rad MRC1024 scanhead using Bio-Rad Laser Sharp 3.1 software. The figures were arranged using Corel Photo Paint, GIMP, and Corel Draw software. Hemocyte pictures were taken in the Biosensorik-Department, Institute of Physiology (University of Hohenheim) with the Zeiss ApoTome, using AxioVision LE Rel. 4.5 software. Wing size was determined using ImageJ software for pixel measurements and repeated at least twice under identical conditions. Statistical significance was verified according to Student’s t-test (http://www.physics.csbsju.edu/stats/t-test.html).
RESULTS
Generation and verification of a pzg mutant in D. melanogaster:
Depletion of pzg by RNA interference results in an 80% reduction in Pzg protein levels (Kugler and Nagel 2007). To further study the biological role of pzg during the development of Drosophila, we generated a pzg null mutant by imprecise P-element excision (for a detailed description see materials and methods). As pzg is crucial for cell proliferation and development (Kugler and Nagel 2007), we expected that pzg mutants should be lethal. The P-element jump-out mutagenesis provided us with 74 pzg mutant “candidates” displaying only heterozygous adult viability. From each of these stocks, genomic DNA from about 200 flies was extracted and analyzed by Southern blot and PCR analyses for the presence of pzg sequences (see materials and methods). The boundaries of the pzg66 deletion were mapped by Southern blot analysis and specified by sequence analysis (Figure S1 and data not shown). The pzg66 mutant allele carried a deletion of 7083 bp within the P element and a deletion of 839 bp within the pzg gene, including transcription and translation start sites (Figure 1A and Figure S1A), suggesting that it was a null allele. This is in line with our molecular data, where we did not detect the pzg-specific transcript by RT–PCR analysis or the Pzg protein on Western blots using a Pzg-specific antibody in pzg66 homozygotes (Figure 1, B and C). Finally, the pzg66 mutant chromosome was tested in trans to three deficiencies Df(3L)Pc/TM3Sb, Df(3L)Pc-MK/TM2, and Df(3L)Pc-2q/TM2, all known to uncover the pzg locus: pzg66 failed to complement the lethal phenotype of all three deletions tested.
pzg66 mutants show severe developmental defects:
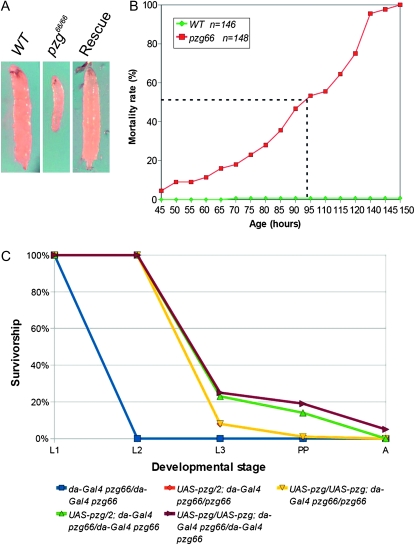
The downregulation of pzg gene activity by RNA interference caused an extensive reduction in tissue size and significantly delayed larval development (Kugler and Nagel 2007). Thus, we expected the pzg66/66 null mutant to be characterized by proliferation and growth defects. The embryonic development of homozygous pzg66 mutants was not affected, presumably due to the large amount of maternal Pzg protein that we detected in pzg66/66 mutant embryos using a Pzg-specific antibody (data not shown). The pzg66/66 larvae displayed a strong developmental delay and early lethality. The pzg66 homozygotes were smaller and thinner than the wild-type larvae (Figure 2A). The pzg66/66 larvae showed an almost linear mortality rate with increasing age, and none of the larvae survived more than 150 hr (Figure 2B). During this time they molted only once, reaching the second larval stage, but then there was no further increase in size. In summary, the pzg66/66 mutants were developmentally delayed and died as tiny larvae in the second larval stage.
Figure 2.—
Developmental delay and lethal phase of pzg66/66 mutants. (A) Comparison of the size of wild-type (WT, left), homozygous pzg66 (pzg66/66, center), and “rescue” larvae (UAS-pzg/UAS-pzg; da-Gal4 pzg66/da-Gal4 pzg66, right). Pictures were taken 97 hr AEL for each genotype. As determined by mouth-hook morphology, the wild-type and rescue larvae have reached the third-instar stage, whereas the pzg66/66 mutant is still in the second larval instar, displaying a small and slim phenotype. (B) The lethal phase of pzg66 homozygous larvae is nearly linear with increasing age. Fifty percent of the analyzed animals died within 93 hr after egg deposition (dashed line) and none of the animals survived for more than 150 hr. Note that they remained in the second larval instar. Wild type n = 146, pzg66/66 n = 148 larvae analyzed. (C) Rescue of pzg mutants. pzg66 homozygotes (da-Gal4 pzg66 recombined line) all died at second larval stage (L2). Ectopic pzg expression pushed the lethal phase beyond the second molt into the third larval stage (L3). The presence of one copy of da-Gal4 allowed a small percentage to form pre-pupae (PP) (genotypes were UAS-pzg/2; da-Gal4 pzg66/pzg66 or UAS-pzg/UAS-pzg; da-Gal4 pzg66/pzg66), whereas even some adults (A) were obtained in the presence of two da-Gal4 copies (UAS-pzg/2; da-Gal4 pzg66/da-Gal4 pzg66). Here, a somewhat stronger rescue is observed with two UAS-pzg copies (UAS-pzg/UAS-pzg; da-Gal4 pzg66/da-Gal4 pzg66). For each genotype at least 250 animals were analyzed.
Rescue of pzg66/66 mutants:
To ensure that the phenotypes observed in pzg66/66 resulted from the loss of pzg gene activity, we performed rescue experiments. We made use of the Gal4/UAS system (Brand and Perrimon 1993) to ectopically express pzg in pzg66/66 mutants with the aim of restoring viability. We built a fly stock that comprised the ubiquitous driver da-Gal4, the UAS-pzg construct containing the pzg full-length cDNA, as well as the heterozygous pzg66 mutant allele. The stock was kept over a TM6B Tb balancer chromosome to easily visualize the genotypes. The correct genotype of rescued pzg66/66 mutants was confirmed by PCR analysis on single animals (Figure S2). We distinguished the endogenous pzg gene copy of the balancer chromosome from the UAS-pzg construct with a primer pair spanning a 60-bp intron (Figure S2). While combining the fly stock, we observed a rescue effect. Some of the pzg66/66 mutants that carried one copy each of da-Gal4 and UAS-pzg survived to the third-instar larval stage, whereas pzg66/66 larvae died as second instars (Figure 2C). By increasing the number of copies of both the Gal4 driver and the UAS-pzg construct, the lifetime of the mutant animals was extended even further, allowing pupariation and even metamorphosis into adults (Figure 2C). The rescued animals showed no apparent phenotype and regained a size comparable to the wild-type control that was already beginning the larval stages (Figure 2A). These data provide definitive evidence that only the pzg gene is affected in the pzg66 mutant and that the pzg66/66 mutant phenotype specifically results from a lack of the Pzg protein.
Pzg acts as a cofactor of NURF in EcR signaling:
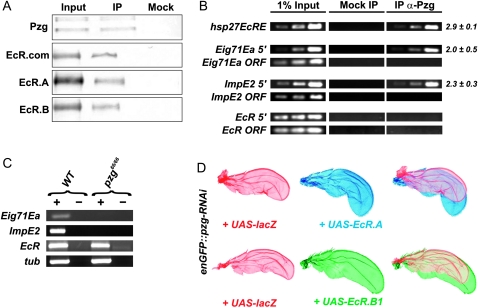
The developmental delay observed in pzg66/66 mutants agrees well with the defects observed in Nurf-301 mutants, the latter playing a well-established role in metamorphosis mediated by ecdysone receptor signaling (Badenhorst et al. 2005). As the NURF complex functions as a direct coactivator of the ecdysone receptor itself (Badenhorst et al. 2005), it is very conceivable that Pzg is also necessary for this function of NURF. In this case, Pzg should be present in a common complex together with NURF and EcR. This was confirmed by co-immunoprecipitation with an anti-Pzg antibody using extracts from wild-type third-instar larvae. Indeed, we detected EcR.A and EcR.B in association with Pzg (Figure 3A).
Figure 3.—
Pzg activity is required for nuclear EcR activity. (A) Pzg associates with EcR in vivo. Proteins immunoprecipitated (IP) from larval protein extracts by anti-Pzg antibodies were probed to detect different EcR forms (EcR.A, EcR.B). The input lane contained 25% of the larval extract used for the IP. Pre-immune serum was used as mock control to demonstrate specificity. (B) Chromatin immunoprecipitation from wild-type larvae using Pzg-specific antibodies; DNA enrichment after the 31st, 33rd, and 35th cycles is shown. Pzg is recruited to the hsp27 promoter that contains ecdysone response elements (EcRE), and binds the regulatory regions of the EcR target genes Eig71Ea and ImpE2, however, not at the EcR itself. Relative enrichment was estimated for the 33rd PCR cycle sample from the ratio between Pzg IP and mock signals. Mean values and standard deviations of at least three independent experiments were calculated. PCR amplification of regions within the ORF of the respective genes was performed as an unrelated control. (C) Semiquantitative RT–PCR analysis of EcR and EcR target gene expression relative to β-tubulin (tub). In pzg66/66 mutants, EcR levels are unchanged, whereas expression of EcR targets Eig71Ea and ImpE2 is strongly reduced in comparison to wild type (WT). As a control, reactions were performed with (+) and without (−) reverse transcriptase. (D) Rescue of pzg-RNAi-induced tissue loss by different EcR isoforms. Adult wings of enGFP-Gal4 UAS-pzg-RNAi/UAS-lacZ (red) are shown superimposed over wings where the EcR isoform EcR.A (blue) or EcR.B1 (green) was co-overexpressed.
Ecdysone-ligated EcR binds to ecdysone response elements (EcRE) in the promoters of EcR-responsive genes (Cherbas et al. 1991). As Pzg was present in a complex with EcR in vivo, we expected Pzg at EcRE as well. Via chromatin immunoprecipitation experiments (ChIPs) we verified the presence of Pzg on the promoters of two EcR target genes, Eig71Ea and ImpE2, as well as on the EcRE of the well-defined hsp27 target gene (Figure 3B). However, Pzg was absent from the regulatory region of the EcR gene itself, which supports the assumption that Pzg acts as a coactivator of EcR rather than influencing EcR gene activity (Figure 3B).
The role of NURF as a cofactor of EcR predicts a positive role for Pzg in the transcriptional activation of EcR target genes. To this end, we examined the transcript levels of Eig71Ea and ImpE2, as well as of EcR itself, in wild-type vs. homozygous pzg66 larvae 90–100 hr AEL by semiquantitative RT–PCR analyses. As shown in Figure 3C, expression of the EcR target genes Eig71Ea and ImpE2 was strongly reduced or even abolished, whereas the transcript levels of EcR and of β-tubulin were not altered. Badenhorst et al. (2005) have already shown that expression levels of the EcR target genes Eig71Ea and ImpE2 are diminished in Nurf-301 mutants whereas the transcript level of EcR itself was not altered.
To address a further functional interplay between EcR signaling and pzg we tested for genetic interactions between pzg and EcR. For technical reasons, we used RNA interference of pzg as the 80% reduction in Pzg protein levels results in distinct phenotypes that can be documented in the adult fly (Kugler and Nagel 2007). Increasing the activity of EcR signaling by overexpressing different isoforms of the receptor significantly suppressed the small wing phenotype caused by the induction of pzg-RNAi (Figure 3D). Altogether, these data strongly indicate that Pzg acts together with NURF in activating EcR target genes.
pzg66/66 mutants show further signs of impaired growth and metamorphosis:
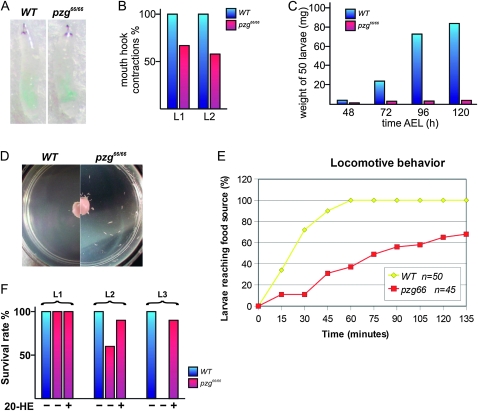
In contrast to the early lethality of pzg66/66 mutants, null alleles of Nurf-301 can develop further and fail to undergo larval to pupal metamorphosis (Badenhorst et al. 2005). The developmental arrest and small body size of pzg66/66 mutants led us to investigate whether or not the animals can take up food at all. A feeding experiment with blue-colored yeast paste as the food source revealed that pzg66/66 mutants were able to grab the offered yeast paste, as visualized by the colored gut; however, this gave no conclusion as to whether the amount of absorbed food was in the wild-type range or not (Figure 4A). The reduced mouth-hook contractions observed in pzg66/66 mutants would rather suggest a reduction in food intake (Figure 4B). Although we observed a slight increase in body weight of the pzg66/66 mutants with increasing age, we must assume that the pzg mutation affected food uptake and/or metabolism as well (Figure 4C). While performing the feeding assay we discovered a defective locomotive behavior in pzg66/66 mutant larvae that stayed dispersed on the plates, whereas the wild type went straight to the yeast (Figure 4, D–E). These defects in locomotive behavior have already been described for larvae with lowered endogenous 20-HE titers and result from a depression in synaptic transmission (Li et al. 2001). In line with the prolonged larval instars and the failure of a second molt, this locomotive problem might originate from a reduced ecdysteroid titer during larval development in pzg66/66 mutants. To test this possibility, we attempted to rescue these defects by feeding ecdysteroids to pzg66/66 first-instar larvae. Such an approach was shown to efficiently rescue phenotypes associated with ecdysone-deficient mutations in Drosophila (Freeman et al. 1999; Bialecki et al. 2002; Fluegel et al. 2006). On food-lacking 20-HE, about 60% of the pzg66/66 mutants passed the first larval instar, but then died in the second instar (Figure 4F). The addition of 20-HE to the diet had a tremendous impact on the survival rate of homozygous pzg66 larvae. Almost 90% of pzg66/66 mutants survived to the second larval instar and nearly all of them reached the third instar (Figure 4F).
Figure 4.—
pzg66/66 mutants show growth disadvantages and signs of reduced ecdysteroid titers. (A) pzg66/66 mutants do uptake food, as visualized by the light-blue-colored yeast in the larval gut. (B) pzg66/66 larvae (red bars) perform significantly fewer mouth-hook contractions per time interval relative to the control larvae (WT, blue bars) at first (L1) and second (L2) instars. (C) Larval weight relative to larval age. In comparison to wild type (WT, blue bars), homozygous pzg66 mutant (red bars) larvae weigh less, but still increase their weight with increasing age. (D–E) Mutant pzg66 larvae display defective locomotive behavior. (D) In contrast to wild-type larvae (left), which quickly moved to the central yeast source, the pzg66/66 animals were more sluggish and remained dispersed. (E) Number of larvae that reached the food source over time. All wild-type larvae reached the yeast within 1 hr (yellow curve), but only 40% of pzg66/66 larvae did (red curve). (F) Ecdysone feeding assay. Relative survival rate of control (wild type was set as 100%, blue bars) and homozygous pzg66 larvae (red bars) on food containing (+) or lacking (−)20-HE.
In Drosophila, ecdysteroids are synthesized in the prothoracic glands (PG) of the larval ring gland and then released in the hemolymph and converted by peripheral tissues to the active form 20-HE (summarized in Gilbert et al. 2002). The obvious failure to achieve correct ecdysteroid titers could reflect problems in ecdysteroid synthesis and/or release or structural defects in the ring gland of pzg66/66 mutants. To analyze these possibilities, we used the Gal4/UAS system to target pzg-RNAi in the PG by using phantom-Gal4 or P0206-Gal4: the latter drives additional expression in the corpora allata. As previously shown, a reduced ecdysteroid titer, induced, for example, by knockdown of the sumoylation gene smt3 in the PG, produces animals arrested in their development at the third instar, followed by additional 3-week persistence at this larval stage (Talamillo et al. 2008). In contrast, no appropriate phenotype was observed when pzg-RNAi was induced in the PG and the progeny hatched without any visible defects (data not shown). The external morphology of the gland in pzg-RNAi-induced larvae did not exhibit obvious changes when compared with the wild type. Finally, no definite changes in size or morphology of PG cells subjected to pzg-RNAi was found, suggesting that pzg has no essential function for their survival and development (data not shown).
Pzg is involved in innate immunity:
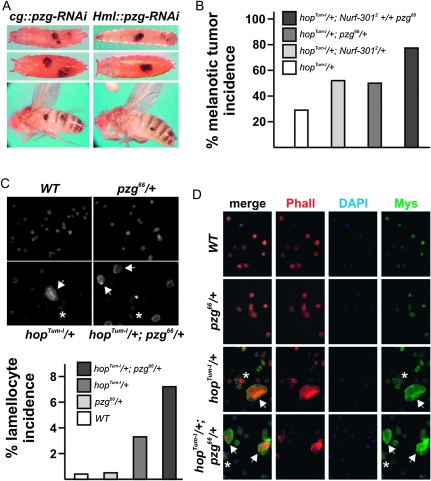
Besides being an activator of gene transcription, NURF antagonizes JAK/STAT signaling by repressing several STAT-dependent genes involved in innate immunity (Kwon et al. 2008). To investigate the requirement of pzg in this process, we first looked for the appearance of melanotic tumors in pzg mutants as a typical indicator of a dysregulated immune system (Minakhina and Steward 2006). In Drosophila, the immune response is sustained by specialized blood cells called hemocytes (plasmatocytes, lamellocytes, crystal cells) and by the fat body that secretes antimicrobial peptides (Lemaitre and Hoffmann 2007). The induction of pzg-RNAi by cgGal4A.2 in hemocytes and the fat body (Asha et al. 2003) induced melanotic tumors in larvae, pupae, and adults, implicating pzg in the innate immune function (Figure 5A). Comparable effects can be observed using the Hml-Gal4 driver line (Goto et al. 2003), which is expressed in a subpopulation of plasmatocytes implying that the melanotic tumor formation after pzg reduction is not exclusively derived from its induction in the fat body (Figure 5A). Melanotic tumors are also found in animals lacking the NURF-specific subunit Nurf-301, and the loss of one copy of Nurf-301 enhanced tumor incidence in the hop gain-of-function mutant hopTum-l. As hop encodes for the Drosophila janus kinase JAK, these findings illustrate the negative role of NURF in JAK/STAT signaling (Badenhorst et al. 2002; Badenhorst et al. 2005; Kwon et al. 2008). We observed a similar enhancement of tumor formation in hopTum-l mutants in the presence of only one pzg gene copy, demonstrating the requirement of Pzg for NURF activity with respect to JAK/STAT regulation (Figure 5B). Tumor frequency was increased in the trans-heterozygous Nurf-3012 +/+ pzg66 combination, reflecting the synergistic impact of the two on tumor formation (Figure 5B).
Figure 5.—
Loss of pzg causes tumor formation. (A) pzg-RNAi induction in the fat body and/or hemocytes induces the formation of melanotic tumors, as seen in larvae, pupae, and adults. Genotypes: cg-Gal4A.2/cg-Gal4A.2; UAS-pzg-RNAi/UAS-pzg-RNAi (left) and Hml-Gal4G.6-4/Hml-Gal4G.6-4; UAS-pzg-RNAi/UAS-pzg-RNAi (right). (B) Loss of one copy of either Nurf-301 or pzg enhances tumor incidence in hopTum-l/+ adult females. A synergistic effect was observed after a concurrent reduction of pzg and Nurf-301 activity. N > 150 females were scored for each genotype. (C–D) Halving the pzg gene dose increases the amount of lamellocytes in hopTum-l/+ larvae. (C) Lamellocyte frequency in the respective genotypes was determined relative to the total number of hemocytes in a constant field. Cells were counterstained with phalloidin; total cell number was at least 350 in each count. (D) Hemolymph from wild type (WT), pzg66/+ (both negative controls), hopTum-l/+ (positive control), and hopTum-l/+; pzg66/+ mutant larvae was isolated and the hemocytes were immunostained with antibodies specific for Mys (green). Cell morphology is revealed by staining with rhodamine-coupled phalloidin (red); nuclei are stained with DAPI (blue). Lamellocytes are recognized by their huge size relative to the smaller plasmatocytes. Whereas nearly no lamellocytes can be observed in the controls (top two rows), the incidence of lamellocytes is significantly increased in hopTum-l/+; pzg66/+ transheterozygotes compared to hopTum-l/+ animals. Arrows indicate large lamellocytes, surrounded by small plasmatocytes, which tend to aggregate in hopTum-l/+ and hopTum-l/+; pzg66/+ mutants (asterisks).
These melanotic tumors result from increased lamellocyte production due to an overactivation of JAK/STAT-signaling activity that triggers lamellocyte differentiation (Meister 2004). In line with reports for Nurf-301 mutants, we expected excess lamellocytes in pzg66/66 mutants (Kwon et al. 2008). Unfortunately, the early larval lethality of pzg66/66 mutants prevented us from isolating circulating hemocytes from third-instar larvae. Instead, we performed antibody staining on hemolymph preparations from hopTum-l/+; pzg66/+ doubly heterozygous larvae compared to the single heterozygous mutant and wild-type animals. Lamellocytes were distinguished by their large size from the smaller plasmatocytes. Wild-type and pzg66 heterozygotes exhibit circulating lamellocytes very rarely: less than 1% of the total hemocytes corresponded to this cell type (Figure 5, C and D). Aggregated plasmatocytes are typically observed in hopTum-l mutants, resulting from increased expression levels of β-integrin subunits (Kwon et al. 2008). As expected, numerous lamellocytes (>3%) were detected in hopTum-l preparations (Rizki 1957; Figure 5, C and D). Lamellocyte incidence in hopTum-l/+; pzg66/+ larvae was significantly increased to >7%, demonstrating the requirement of Pzg for the restriction of JAK activity (Figure 5, C and D). As mutant pzg66 heterozygotes enhance hopTum-l tumor phenotypes, we further analyzed the influence of pzg on JAK/STAT signaling.
Inactivation of pzg leads to precocious activation of JAK/STAT activity:
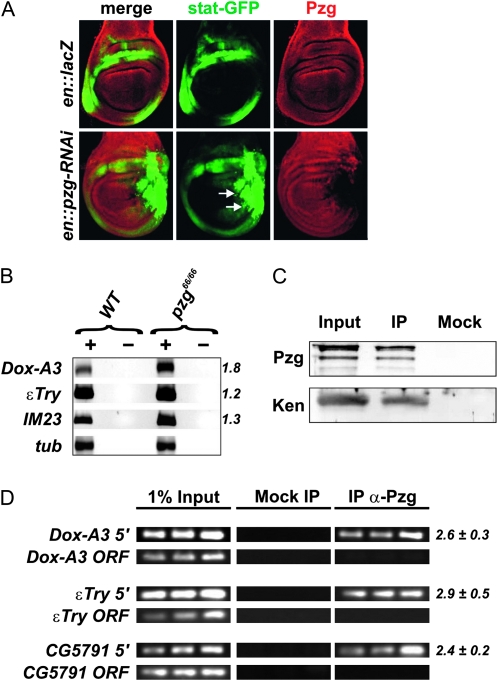
The interaction of loss-of-function pzg66 mutants and gain-of-function hopTum-l mutants supports the idea that Pzg acts together with NURF to prevent ectopic activation of JAK/STAT signaling. Nurf-301 has been shown to repress STAT target gene activation, since Nurf-301 mutants show increased expression of several immune response genes that are also upregulated in hopTum-l mutants (Kwon et al. 2008). If Pzg is involved in the NURF-mediated repression of JAK/STAT targets, loss-of-function of pzg should result in ectopic activation of STAT targets as well. To test this, we first made use of the STAT92E–GFP reporter line. This line contains Stat92E binding sites upstream of the GFP that are derived from the Socs36E gene and reflects activity of the JAK/STAT pathway in vivo (Bach et al. 2007). In control wing imaginal disks, STAT92E–GFP is expressed in a broad ring surrounding the wing pouch as described by Bach et al. (2007) (Figure 6A, top). Downregulation of Pzg activity by means of pzg-RNAi, for example in the posterior half of the wing disk, resulted in a strong ectopic activation of the STAT92E–GFP reporter within the affected cells (Figure 6A, bottom, arrows). This is consistent with our hypothesis that Pzg acts as cofactor of NURF in the repression of STAT target genes. We therefore addressed the expression levels of two different STAT-dependent defense response genes, Dox-A3 and IM23, and of εTry encoding a peptidase that is upregulated in Nurf-301 mutants as well (Kwon et al. 2008). Our semiquantitative RT–PCR analyses revealed an increase in the transcript levels of all three genes in pzg66/66 mutant larval extracts compared to the wild type (Figure 6B).
Figure 6.—
Pzg antagonizes JAK/STAT-signaling activity. (A) pzg-RNAi induction in the posterior compartment of the wing disk increases the expression of a STAT92E-GFP reporter (arrows). Control in top row: en-Gal4::UAS-lacZ/STAT92E-GFP; bottom row: en-Gal4::UAS-pzg-RNAi/STAT92E-GFP. Wing disks were stained with anti-Pzg antibodies (red). Posterior is to the right, dorsal is up. (B) The transcript levels of STAT responsive genes Dox-A3, εTry, and IM23 were determined by semiquantitative RT–PCR. The levels were normalized to β-tubulin (tub). As a control, reactions were performed with (+) and without (−) reverse transcriptase. (C) Pzg can be detected in a complex with the repressor Ken in vivo. Proteins immunoprecipitated (IP) from larval protein extracts by anti-Pzg antibodies were probed to detect Ken proteins. The input lane contained 25% of the larval extract used for the IP. A mock control was performed with preimmune serum to demonstrate specificity. (D) XChIP analyses showing that Pzg can be localized at the promoter regions of Dox-A3, εTry, and CG579, but it is absent from the respective coding regions. Primer sets span regulatory regions that contain predicted Ken and NURF binding sites. Samples of the 31st, 33rd, and 35th PCR amplification cycles are shown. Relative enrichment was estimated for the 33rd PCR cycle sample from the ratio between Pzg IP and mock signals. The mean values and standard deviations of at least three independent experiments were calculated. PCR amplification of regions within the ORF of Dox−A3, εTry, and CG5791 were performed as an unrelated control.
Pzg interacts with Ken in the repression of JAK/STAT signaling:
JAK/STAT signaling is antagonized by a repressor complex consisting of Ken and NURF that competes with STAT for the binding of STAT target genes. In accordance, Nurf-301 interacts with Ken at the genetic and molecular level (Kwon et al. 2008). Our data so far indicate that Pzg is required for NURF repression of JAK/STAT-signaling output as well. In this case, we expected Pzg as an additional component of the Ken–NURF repressor complex. We were able to co-immunoprecipitate Ken with Pzg antibodies from extracts of third-instar wild-type larvae, demonstrating the presence of Pzg in the Ken–NURF repressor complex (Figure 6C).
Finally, we addressed the question of whether Pzg is present on the promoters of genes that are repressed by the Ken–NURF complex. In addition to the immune responsive genes Dox-A3 and εTry, we included CG5791 in our analysis, the function of which is not yet known (Kwon et al. 2008). The CG5791 gene contains overlapping STAT and Ken binding sequences in its promoter region and is transcriptionally upregulated in Nurf-301 mutants, indicating that it is a direct target of NURF as well as of STAT (Kwon et al. 2008). Our ChIP experiments showed the localization of Pzg at the respective promoter regions (Figure 6D). Taken together, our results demonstrate a requirement of Pzg in the Ken–NURF repressor complex, thereby regulating immune responsive genes that are controlled by the JAK/STAT-signaling output.
DISCUSSION
We know from our earlier work that Pzg is involved in the activation of Notch target genes and that this process entails the physical association of Pzg with NURF (Kugler and Nagel 2010). To extend our knowledge of pzg function during the development of Drosophila, we created a loss-of-function mutation in the pzg gene. We found that pzg66/66 null mutants die early in larval development, showing various defects in molting, growth, metamorphosis, and larval immunity. Our work on the pzg66/66 null allele provided evidence to show that Pzg is required for a much broader range of NURF-dependent developmental processes, including the regulation of metamorphosis and innate immunity in the fly.
Pzg and its role in EcR signaling: More than strictly NURF-dependent?:
The observation that a large set of ecdysone responsive target genes is impaired in Nurf-301 mutants was one of the key findings triggering the idea that NURF is a coactivator of the EcR, allowing the progression from larval to pupal development (Badenhorst et al. 2005). Here, we showed that Pzg can physically associate with the EcR and that it is recruited to ecdysone responsive promoters in vivo, the expression of which is lost in a pzg66/66 mutant background. This correlates well with the conception of Pzg being an essential and critical cofactor of NURF-mediated influences on EcR nuclear activity. In contrast to this synergistic effect, we found that pzg null mutants do not exactly phenocopy the defects observed in the Nurf301 mutants, but rather show more severe defects with respect to developmental delay and early larval lethality. This might be due to the fact that Pzg is not just part of the NURF complex but it also coregulates the expression of replication-related genes required for cell survival in a TRF2/DREF-dependent manner (Hochheimer et al. 2002; Kugler and Nagel 2007). The observation that pzg66/66 mutants can molt to the third instar when fed ecdysteroids otherwise suggests that a reduced ecdysteroid level might be an additional consequence of the pzg lesion. The production of ecdysteroids in arthropods is a process that is not yet completely characterized, involving numerous enzymes needed for the stepwise synthesis of 20-HE from cholesterol (Gilbert 2004; Lafont et al. 2004; Gilbert and Warren, 2005). While microarray data showed that the expression level of known ecdysone synthetic enzymes is unchanged in Nurf-301 mutants (Badenhorst et al. 2005), a detailed analyses of their transcript levels in a pzg66/66 background awaits further investigation to decide whether pzg might influence EcR signaling at the level of ecdysteroid biosynthesis as well. Such a “multilevel” control of EcR-signaling activity was recently described for members of the histone acetyltransferase complex dATAC in Drosophila, emphasizing the importance of chromatin modifying factors in the timely and accurate coordination of metamorphosis control (Pankotai et al. 2010).
The apparent reduction in ecdysteroid titers in pzg66/66 larvae could alternatively be caused by impaired growth and/or differentiation of the hormone-producing tissues. So far, only a small number of genes are known to be required for ecdysone production without encoding synthetic enzymes. One example is the molting defective (mld) gene, whose mutants are developmentally arrested in the first-instar larvae harboring enlarged ring glands. This was interpreted as a consequence of their failure to produce enough hormones and a lack of feedback downregulation of their size (Neubueser et al. 2005). Like mld, without children mutants are characterized by an enlargement of the ring gland cells and both genes encode predicted transcription factors with a spectrum of target genes as yet unexplored (Warren et al. 2001; Neubueser et al. 2005). In contrast, pzg-RNAi induction, specifically in the ring gland tissue, had no obvious consequences, neither on the amount nor on the size of the cells studied. However, as the Pzg protein can be detected in the nuclei of wild-type ring gland cells and since a low abundance of pzg activity is still detectable after pzg-RNAi depletion (data not shown; Kugler and Nagel 2007), we cannot completely exclude a subtle function of pzg in this context.
As pzg66/66 mutant larvae display a sluggish and retarded behavior in food uptake we instead favor rather indirect reasons for the impaired ecdysteroid levels. As a sterol auxotrophic organism, Drosophila synthesizes ecdysone from dietary sterols (Gilbert et al. 2002). Therefore, if food or food uptake is limited too much, the initial trigger for the chain reaction leading to ecdysteroid synthesis might be hampered. Notably, it was recently shown that low-nutrition conditions reduce the activity of the target of rapamycin (TOR) in the prothoracic gland. Consequently, lowered TOR signal activity suppresses ecdysone secretion, a defect that can be rescued either by a reinduction of TOR activity or ecdysone-supplied nutrition (Layalle et al. 2008). Therefore, further experiments will be required to clarify whether TOR-signaling activity is lowered in pzg66/66 mutants, which might explain the “hunger-like” phenotype followed by a reduced ecdysteroid titer.
Pzg and NURF: antagonists of JAK/STAT activity and hematopoietic tumor formation:
As a misregulation of JAK/STAT activity is associated with various diseases, including immune disorders and tumorigenesis, the knowledge of its spatial and temporal regulation is of the utmost importance. Consistent with its role in vertebrates, a number of mutant phenotypes in Drosophila that imply a developmental role for the JAK/STAT pathway during cellular proliferation have been described. These include hemocyte overproliferation, which can be observed in the dominant gain-of-function JAK allele hopTum-l (Hanratty and Dearolf 1993). As a consequence, the differentiation of a specialized class of hemocytes, the lamellocytes, is induced and melanotic tumors are formed (Luo et al. 1995). NURF was recently shown to act as an inhibitor of JAK/STAT-signaling activity, thereby antagonizing its tumor-inducing function during hematopoietic development and immune response (Kwon et al. 2008). In a genome-wide RNAi screening aimed at identifying modulators of JAK/STAT activity in cultured Drosophila cells, pzg, formerly known as CG7752, was already mentioned as being a negative regulator verified by a significant increase of JAK/STAT-signaling activity after pzg knockdown (Baeg et al. 2005). Here, we provide the molecular evidence to show that Pzg, with NURF, acts as a corepressor of Ken with respect to STAT responsive genes, thereby preventing an immune-mediated inflammatory syndrome, i.e., melanotic tumor formation. The Pzg protein physically interacts with Ken and is present at STAT responsive promoters, as well as at the promoter of a gene (CG5791) that is bound by both Ken and NURF alike (Kwon et al. 2008). In an attempt to visualize increased JAK/STAT activity, particularly in hemocytes, we tried to monitor the expression of the STAT92E–GFP reporter in a hopTum-l -sensitized background; however, we failed to detect a specific activity, which went beyond the normal background staining in the wild type (data not shown). Although this reporter was demonstrated to accurately reflect JAK/STAT activity in a variety of tissues (Bach et al. 2007), hemocyte-specific induction is obviously more complex to follow. Therefore, we switched our analyses toward the wing disk of third instars, where STAT–GFP expression is known to overlap with the activating ligand unpaired that properly surrounds the wing pouch (Bach et al. 2007). Using this test system, we obtained an ectopic activation of STAT–GFP in the cells where pzg-RNAi was induced (Figure 6A). Although this result is consistent with our idea of pzg being a negative regulator of JAK/STAT-signaling activity, how can we explain that an increase in JAK/STAT activity is, in this context, tantamount to a loss of proliferation rather than causing the more expected pro-proliferative effect? This obvious caveat was nicely resolved by the observation that a functional switch of JAK/STAT activity occurs during wing imaginal disk development. In the early larval stages JAK/STAT activity promotes proliferation, but it also acts as an anti-proliferative factor at later larval stages. This anti-proliferative role is mediated by a yet unidentified noncanonical JAK/STAT pathway (Mukherjee et al. 2005).
Interestingly, but not unexpectedly, the Pzg–NURF complex can function in the activation as well as in the repression of genes. For example, different ISWI-containing complexes have been published as coactivators and corepressors as well (Corona et al. 2007; Burgio et al. 2008), suggesting that the function of a chromatin complex depends on other factors given in the particular developmental context.
Melanotic tumor formation and innate immunity: A consequence of lowered EcR signaling in pzg mutants?:
Ample evidence suggests that hormones and nuclear hormone receptors modulate innate immunity in both vertebrates and invertebrates (reviewed in Webster et al. 2002; Pascual and Glass 2006; Flatt et al. 2008). In insects, most investigations into the hormonal regulation of innate immunity were performed in Drosophila, leading to a quite complex and ambivalent picture of their relationship. In Drosophila Schneider 2 cells EcR-signaling activity promotes humoral immunity by potentiating the production of antimicrobial peptides such as Diptericin and Drosomycin (Flatt et al. 2008). This was further corroborated in the tumorous blood cell line mbn-2 and in larvae where 20-HE renders the cells and tissue competent for the transcriptional induction of diptericin and drosomycin genes (Meister and Richards 1996; Dimarcq et al. 1997; Silverman et al. 2000). EcR-signaling activity plays a further role in the regulation of hematopoiesis and cellular immunity. In genetic backgrounds where ecdysone signaling is compromised, hemocyte proliferation and differentiation is impaired, resulting in a lowered immune capacity of third-instar larvae (Sorrentino et al. 2002). In accordance, injection of 20-HE in third-instar larvae increases the phagocytic activity of plasmatocytes and thereby their cellular immune response (Lanot et al. 2001). In contrast, genome-wide microarray studies performed on Drosophila and the silkworm Bombyx mori revealed that several immune responsive genes were downregulated by 20-HE in an EcR-dependent manner (Beckstead et al. 2005; Tian et al. 2010). These observations led to the conclusion that immunity-related genes are part of the EcR-signaling network, presumably positively regulated at the onset of metamorphosis and coordinately downregulated at the larval to pupal transition (Beckstead et al. 2005). If indeed the increase in JAK/STAT activity is a direct consequence of the reduced EcR signaling observed in the pzg mutant background, we would expect an ectopic activation of ecdysone signaling to reverse this effect. To this end, we concomitantly overexpressed the EcR in a pzg-RNAi mutant during wing disk development; however, this did not change the ectopic induction of the STAT-GFP reporter (Figure S3A). Moreover, feeding hopTum-l/+; pzg66/+ mutant larvae with 20-HE did not change the observed incidence of lamellocytes, as would have been predicted if the reduced EcR-signaling activity is the basic cause of this effect (Figure S3B). Therefore, we have no experimental evidence to show that impaired EcR-signaling activity directly provokes tumor formation in pzg mutants.
Acknowledgments
We thank P. Badenhorst, C. Mirth, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank (DSHB) for flies and antibodies. We are indebted to M. Mezger for the UAS-pzg transgenics and A. Preiss, D. Maier, and C. Protzer for their help during jump-out mutagenesis. We appreciate the excellent technical assistance of H. Mastel, T. Stösser, and I. Wech. We thank A. Huber (Department of Biosensorik) for kindly letting us use the ApoTome microscope. We are grateful to A. Preiss for critical comments on the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft through a grant to A.C.N. (NA 427/2-1).
LITERATURE CITED
- Asha H., Nagy I., Kovacs G., Stetson D., Ando I., et al. , 2003. Analysis of ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Bach E. A., Ekas L. A., Ayala-Camargo A., Flaherty M. S., Lee H., et al. , 2007. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7: 323–331 [DOI] [PubMed] [Google Scholar]
- Badenhorst P., Voas M., Rebay I., Wu C., 2002. Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev. 16: 3186–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenhorst P., Xiao H., Cherbas L., Kwon S. Y., Voas M., et al. , 2005. The Drosophila nucleosome remodeling factor NURF is required for Ecdysteroid signaling and metamorphosis. Genes Dev. 19: 2540–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg G.-H., Zhou R., Perrimon N., 2005. Genome-wise RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 19: 1861–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate M., Martinez Arias A., 1993. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Beckstead R. B., Lam G., Thummel C. S., 2005. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 6: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., Liao G., He Y., Carlson J. W., et al. , 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki M., Shilton A., Fichtenberg C., Segraves W. A., Thummel C. S., 2002. Loss of Ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell 3: 209–220 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Burgio G., La Rocca G., Sala A., Arancio W., Di Gesu D., et al. , 2008. Genetic identification of a network of factors that functionally interact with the nucleosome remodeling ATPase ISWI. PLOS Genet. 4: e1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Lee K., Cherbas P., 1991. Identification of ecdysone response elements by analysis of the Drosophila Eip28/29 gene. Genes Dev. 5: 120–131 [DOI] [PubMed] [Google Scholar]
- Corona D. F., Tamkun J. W., 2004. Multiple roles for ISWI in transcription, chromosome organization and DNA replication. Biochim. Biophys. Acta 1677: 113–119 [DOI] [PubMed] [Google Scholar]
- Corona D. F., Siriaco G., Armstrong J. A., Snarskaya N., Mcclymont S. A., et al. , 2007. ISWI regulates higher-order chromatin structure and histone H1 assembly in vivo. PLOS Biol. 5: e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarcq J. L., Imler J. L., Lanot R., Ezekowitz R. A., Hoffmann J. A., et al. , 1997. Treatment of l(2)mbn Drosophila tumorous blood cells with the steroid hormone ecdysone amplifies the inducibility of antimicrobial peptide gene expression. Insect Biochem. Mol. Biol. 27: 877–886 [DOI] [PubMed] [Google Scholar]
- Eggert H., Gortchkov A., Saumweber H., 2004. Identification of the Drosophila interband-specific protein Z4 as a DNA-binding zinc-finger protein determining chromosomal structure. J. Cell Sci. 117: 4253–4264 [DOI] [PubMed] [Google Scholar]
- Flatt T., Heyland A., Rus F., Porpiglia E., Sherlock C., et al. , 2008. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J. Exp. Biol. 211: 2712–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluegel M. L., Parker T. J., Pallanck L. J., 2006. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics 172: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. R., Dobritsa A., Gaines P., Segraves W. A., Carlson J. R., 1999. The dare gene: steroid hormone production, olfactory behavior, and neural degeneration in Drosophila. Development 126: 4591–4602 [DOI] [PubMed] [Google Scholar]
- Gilbert L. I., 2004. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 215: 1–10 [DOI] [PubMed] [Google Scholar]
- Gilbert L. I., Warren J. T., 2005. A molecular genetic approach to the biosynthesis of the insect steroid molting hormone. Vitam. Horm. 73: 31–57 [DOI] [PubMed] [Google Scholar]
- Gilbert L. I., Rybczynski R., Warren R., 2002. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 47: 883–916 [DOI] [PubMed] [Google Scholar]
- Goto A., Kadowaki T., Kitagawa Y., 2003. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev. Biol. 264: 582–591 [DOI] [PubMed] [Google Scholar]
- Gutierrez E., Wiggins D., Fielding B., Gould A. P., 2007. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445: 275–280 [DOI] [PubMed] [Google Scholar]
- Hanratty W. P., Dearolf C. R., 1993. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol. Gen. Genet. 1–2: 33–37 [DOI] [PubMed] [Google Scholar]
- Hochheimer A., Zhou S., Zheng S., Holmes M. C., Tjian R., 2002. TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature 420: 439–445 [DOI] [PubMed] [Google Scholar]
- Kugler S. J., Nagel A. C., 2007. putzig is required for cell proliferation and regulates Notch activity via an epigenetic mechanism in Drosophila. Mol. Biol. Cell 18: 3733–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler S. J., Nagel A. C., 2010. A novel Pzg-NURF complex regulates Notch target gene activity. Mol. Biol. Cell. 21: 3443–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. Y., Xiao H., Glover B. P., Tjian R., Wu C., et al. , 2008. The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev. Biol. 316: 538–547 [DOI] [PubMed] [Google Scholar]
- Lafont R., Dauphin-Villemant C., Warren J. T., Rees H., 2004. Ecdysteroid chemistry and biochemistry, pp. 125–195 in Comprehensive Molecular Insect Science, edited by Gilbert L. I., Iatrou K., Gill S. Elsevier, Oxford [Google Scholar]
- Layalle S., Arquier N., Léopold P., 2008. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev. Cell 15: 568–577 [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Hoffmann J., 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25: 697–743 [DOI] [PubMed] [Google Scholar]
- Lanot R., Zachary D., Holder F., Meister M., 2001. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 230: 243–257 [DOI] [PubMed] [Google Scholar]
- Li H., Harrison D., Jones G., Jones D., Cooper R. L., 2001. Alterations in development, behavior, and physiology in Drosophila larva that have reduced ecdysone production. J. Neurophysiol. 85: 98–104 [DOI] [PubMed] [Google Scholar]
- Luo H., Hanratty W. P., Dearolf C. R., 1995. An amino acid substitution in the Drosophila hopTum-1 Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 14: 1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., 2004. Blood cells of Drosophila: cell lineages and role in host defence. Curr. Opin. Immunol. 16: 10–15 [DOI] [PubMed] [Google Scholar]
- Meister M., Richards G., 1996. Ecdysone and insect immunity: the maturation of the inducibility of the diptericin gene in Drosophila larvae. Insect. Biochem. Mol. Biol. 26: 155–160 [DOI] [PubMed] [Google Scholar]
- Minakhina S., Steward R., 2006. Melanotic mutants in Drosophila: pathways and phenotypes. Genetics 174: 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T., Schäfer U., Zeidler M. P., 2005. Opposing roles for Drosophila JAK/STAT signalling during cellular proliferation. Oncogene 24: 2503–2511 [DOI] [PubMed] [Google Scholar]
- Müller D., Kugler S. J., Preiss A., Maier D., Nagel A. C., 2005. Genetic modifier screens on Hairless gain-of-function phenotypes reveal genes involved in cell differentiation, cell growth and apoptosis in Drosophila melanogaster. Genetics 171: 1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel A. C., Krejci A., Tenin G., Bravo-Patiño A., Bray S., et al. , 2005. Hairless mediated repression of Notch target genes requires combined activity of Groucho and CtBP corepressors. Mol. Cell. Biol. 25: 10433–10441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubueser D., Warren J. T., Gilbert L. I., Cohen S. M., 2005. molting defective is required for ecdysone biosynthsis. Dev. Biol. 280: 362–372 [DOI] [PubMed] [Google Scholar]
- Neufeld T. P., Edgar B. A., 1998. Connections between growth and the cell cycle. Curr. Opin. Cell Biol. 10: 784–790 [DOI] [PubMed] [Google Scholar]
- Pankotai T., Popescu C., Martín D., Grau B., Zsindely N., et al. , 2010. Genes of the Ecdysone biosynthesis pathway are regulated by the dATAC histone acetyltransferase complex in Drosophila. Mol. Cell Biol. 30: 4254–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G., Glass C. K., 2006. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol. Metabol. 17: 321–327 [DOI] [PubMed] [Google Scholar]
- Preiss A., Hartley D. A., Artavanis-Tsakonas S., 1988. The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J. 7: 3917–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Beckerbauer L., 2001. HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochim. Biophys. Acta 1519: 13–29 [DOI] [PubMed] [Google Scholar]
- Rizki T. M., 1957. Alterations in the haemocyte population of Drosophila melanogaster. J. Morphol. 100: 437–458 [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T., 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Silverman N., Zhou R., Stöven S., Pandey N., Hultmark D., et al. , 2000. Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 14: 2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R. P., Carton Y., Govind S., 2002. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243: 65–80 [DOI] [PubMed] [Google Scholar]
- Talamillo A., Sanchez J., Cantera R., Perez C., Martin D., et al. , 2008. Smt3 is required for Drosophila melanogaster metamorphosis. Development 135: 1659–1668 [DOI] [PubMed] [Google Scholar]
- Tian L., Guo E., Diao Y., Zhou S., Peng Q., et al. , 2010. Genome-wide regulation of innate immunity by juvenile hormone and 20-hydroxyecdysone in the Bombyx fat body. BMC Genomics 11: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Wu C., 1995. Purification and properties of an ATP-dependent nucleosome remodeling factor. Cell 83: 1011–1020 [DOI] [PubMed] [Google Scholar]
- Vignali M., Hassan A. H., Neely K. E., Workman J. L., 2000. ATP-dependent chromatin-remodeling complexes. Mol. Cell. Biol. 20: 1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. T., Wismar J., Subrahmanyam B., Gilbert L. I., 2001. Woc (without children) gene control of ecdysone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 181: 1–14 [DOI] [PubMed] [Google Scholar]
- Warren J. T., Petryk A., Marqués G., Parvy J.-P., Shinoda T., et al. , 2004. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 34: 991–1010 [DOI] [PubMed] [Google Scholar]
- Webster J. I., Tonelli L., Sternberg E. M., 2002. Neuroendocrine regulation of immunity. Ann. Rev. Immunol. 20: 125–163 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M., Knust E., 1995. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82: 67–76 [DOI] [PubMed] [Google Scholar]
- Xiao H., Sandaltzopoulos R., Wang H. M., Hamiche A., Ranallo R., et al. , 2001. Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol. Cell 8: 531–543 [DOI] [PubMed] [Google Scholar]