Abstract
We have shown previously that mutations in the apico-basal cell polarity regulators cooperate with oncogenic Ras (RasACT) to promote tumorigenesis in Drosophila melanogaster and mammalian cells. To identify novel genes that cooperate with RasACT in tumorigenesis, we carried out a genome-wide screen for genes that when overexpressed throughout the developing Drosophila eye enhance RasACT-driven hyperplasia. RasACT-cooperating genes identified were Rac1 Rho1, RhoGEF2, pbl, rib, and east, which encode cell morphology regulators. In a clonal setting, which reveals genes conferring a competitive advantage over wild-type cells, only Rac1, an activated allele of Rho1 (Rho1ACT), RhoGEF2, and pbl cooperated with RasACT, resulting in reduced differentiation and large invasive tumors. Expression of RhoGEF2 or Rac1 with RasACT upregulated Jun kinase (JNK) activity, and JNK upregulation was essential for cooperation. However, in the whole-tissue system, upregulation of JNK alone was not sufficient for cooperation with RasACT, while in the clonal setting, JNK upregulation was sufficient for RasACT-mediated tumorigenesis. JNK upregulation was also sufficient to confer invasive growth of RasV12-expressing mammalian MCF10A breast epithelial cells. Consistent with this, HER2+ human breast cancers (where human epidermal growth factor 2 is overexpressed and Ras signaling upregulated) show a significant correlation with a signature representing JNK pathway activation. Moreover, our genetic analysis in Drosophila revealed that Rho1 and Rac are important for the cooperation of RhoGEF2 or Pbl overexpression and of mutants in polarity regulators, Dlg and aPKC, with RasACT in the whole-tissue context. Collectively our analysis reveals the importance of the RhoGEF/Rho-family/JNK pathway in cooperative tumorigenesis with RasACT.
CANCER is a multistep process, and transformation from a normal cell to an invasive/metastatic cancer has been considered to involve in six steps: (1) activation of mitogen-signaling pathways to allow growth factor independence; (2) elimination of cell-cycle inhibitors, (3) prevention of apoptosis, (4) promotion of invasion and metastasis, (5) acquiring unlimited replicative ability (upregulation of Telomerase), and (6) activation of angiogenesis (reviewed by Hanahan and Weinberg 2000). In addition, there is strong evidence that normal cells surrounding tumor cells (tumor microenvironment) can significantly affect the growth and development of the tumor and that the tumor and stroma (surrounding normal cells) evolve together in the development of the tumor (reviewed by Bissell and Radisky 2001). The vinegar fly, Drosophila melanogaster, presents an excellent genetically amenable system with which to model the first four of these cancer hallmarks, as well as the interaction of tumor cells with their microenvironment (reviewed by Brumby and Richardson 2005).
Genetic analyses in Drosophila have revealed many genes that when deregulated induce or contribute to tumorigenesis. Drosophila tumor suppressors have been classed as hyperplastic (such as those of the Salvador/Warts/Hippo, SWH, pathway), which result in increased proliferation or survival but do not disrupt tissue structure or differentiation, or neoplastic (such as Dlg, Scrib, and Lgl), which lead to loss of tissue structure, differentiation defects, and failure to exit the cell cycle (reviewed by Brumby and Richardson 2005; Hariharan and Bilder 2006). Dlg, Scrib, and Lgl act antagonistically to the apical polarity modules, the atypical protein kinase C (aPKC), and Crumbs (Crb) complexes, to regulate epithelial apico-basal cell polarity and limit proliferation (reviewed by Humbert et al. 2008). We have recently shown that deregulation of Lgl, aPKC, or Crb promotes tissue growth without affecting cell polarity by deregulation of the SWH pathway (Grzeschik et al. 2010). However, homozygous mutant epithelial tissue from scrib, lgl, or dlg mutant larvae that has lost apico-basal cell polarity shows all four hallmarks of cancer that can be modeled in the fly; the tissue continues to proliferate, does not die, fails to differentiate, and is capable of invasive behavior (Gateff and Schneiderman 1969; Gateff 1978; Woodhouse et al. 1998; Bilder and Perrimon 2000; Bilder et al. 2000).
By contrast, when scrib or lgl mutant tissue is generated in the context of wild-type tissue in the developing Drosophila eye using clonal analysis, it exhibits only some of the hallmarks of cancer. While both lgl and scrib mutant clones are unable to cease proliferation, showing increased expression of the key G1-S-phase cell-cycle regulator cyclin E (Richardson et al. 1993, 1995; Knoblich et al. 1994) and ectopic cell cycles, they are still capable of differentiation, thereby preventing overgrowth (Brumby and Richardson 2003; Grzeschik et al. 2007). In addition, scrib mutant cells are eliminated by Jun kinase (JNK)-mediated cell death that is induced by the surrounding wild-type tissue (Brumby and Richardson 2003). However, when activated Ras or Notch oncogenes are expressed in scrib mutant clones, cell survival is dramatically increased and invasive/metastatic behavior is observed (Brumby and Richardson 2003; Pagliarini and Xu 2003). This includes the breakdown of the basement membrane and invasion/migration of mutant cells to distant sites. Thus scrib loss-of-function shows many hallmarks of cancer and exhibits the ability to cooperate with oncogenic Ras or Notch in tumor progression.
The cooperation of scrib loss-of-function with RasACT and activated Notch (Notchintra or NotchACT) in tumorigenesis most likely depends on the loss of cell polarity, as mutations in other apico-basal cell polarity regulators of the Scrib, aPKC, or Crb complexes can also cooperate with oncogenic Ras in tumorigenesis in Drosophila eye epithelial tissues (Pagliarini and Xu 2003). Furthermore, overexpression of Crb, which results in a loss of apico-basal cell polarity, cooperates with RasACT in tumorigenesis (Leong et al. 2009). One important factor that contributes to RasACT-mediated cooperative tumorigenesis with scrib-, revealed by our and other studies, is the JNK signaling pathway (Brumby and Richardson 2003; Igaki et al. 2006; Uhlirova and Bohmann 2006; Leong et al. 2009). Blocking JNK function in scrib- RasACT tumors reestablishes differentiation and reduces the tumor’s invasive properties. Downregulation of the E-cadherin–β-catenin complex in apico-basal polarity mutants also contributes to tumorigenesis (Igaki et al. 2006). Whether JNK activation and E-cadherin–β-catenin downregulation are the only events downstream of apico-basal polarity mutants contributing to RasACT-cooperative tumorigenesis is unclear. We envisioned that insight might be gained on the nature of other critical functions that are affected by loss of cell polarity for RasACT-cooperative tumorigenesis, by identifying other genes that cooperate with oncogenic Ras. In this study, we present the results of a genetic screen to identify genes that when overexpressed enhance a RasACT-induced hyperplastic eye phenotype. We identified key regulators of the actin cytoskeleton and cell morphology, including Rho1-family GTPases and RhoGEFs as RasACT-cooperating proteins. We show that JNK pathway activation underlies the cooperation of these actin cytoskeletal regulators with RasACT. Moreover, we show that JNK and Ras signaling cooperate to promote invasive growth in normal human mammary epithelial cells and reveal by bioinformatics analysis that JNK signaling correlates with upregulation of Ras in human breast cancer. Our studies reveal a RhoGEF/Rho-family/JNK pathway as an important factor in oncogenic Ras mediated tumorigenesis.
MATERIALS AND METHODS
Fly stocks, conditions of culture, overexpression, and clonal analysis:
For the screening of GS lines, a recombinant of ey-GAL4 and UAS-Ras85DV12 (ey>RasACT) was generated. Potential interacting GS lines were retested against ey>RasACT and also to ey-GAL4 to assess the effect of expression of the gene alone on the adult eye. At least 50 progeny were analyzed for each cross, and representative images are shown. All flies were raised on a standard cornmeal agar food at 25°.
Validating transgenes used were: UAS-rib (Deborah Andrew), UAS-Rho1CFP2a (Greco et al. 2001), UAS-Rho1ACT (Billuart et al. 2001), UAS-RhoGEF2 (Udo Hacker, Widmann and Dahmann 2009), UAS-east (Wasser and Chia 2000), UAS-pbl-GFP#3, and UAS-pbl-GFP#8 (Robert Saint, Somers and Saint 2003), UAS-Rac1 (Luo et al. 1994).
The MARCM (mosaic analysis with repressible cell marker) system (Lee and Luo 2001) with FRT82B, ey-FLP, and UAS-GFP (ey-FLP1, UASmCD8-GFP;;Tub-GAL4 FRT82B Tub-GAL80; Lee and Treisman 2001) was used to induce GFP positively marked clones.
Other stocks used were: dlg-RNAi 4689 C2V (gift from B. Dickson, Dietzl et al. 2007), validated for knockdown of Dlg and specificity (Grzeschik et al. 2010), msn06946 (msn-lacZ) (Mattila et al. 2005); UAS-P35 (Hay et al. 1994); UAS-bskK53R (UAS-bskDN) (Weber et al. 2000), UAS-aPKCΔN (Betschinger et al. 2003); UAS-aPKCCAAX-DN (Sotillos et al. 2004); UAS-Ras85DV12 (UAS-RasACT) (Karim and Rubin 1998); UAS-Rac1N17 (UAS-Rac1DN) (Luo et al. 1994); UAS-Rho1RNAi #12734 [Vienna Drosophila Resource Center (VDRC), Dietzl et al. 2007] and scrib1 (D. Bilder).
Immunocytochemistry for analysis of Drosophila tissues:
For analysis of third-instar larval eye-antenna discs, the discs were dissected in PBS, fixed in 4% PFA, washed in PBT (0.1% TritonX-100), and blocked in PBT + 2% normal goat serum. BrdU labeling for the detection of S phase cells was carried out as previously described (Leong et al. 2009). Antibodies used were mouse Elav (Developmental Studies Hybridoma Bank, DSHB, 1:20), mouse β-galactosidase (Rockland, 1:500), and mouse anti-BrdU (Becton-Dickinson, 1:50). Secondary antibodies were: anti-mouse Alexa647 (Invitrogen; 1:400) or anti-mouse Alexa488 (Invitrogen; 1:400). F-actin was detected with phalloidin-tetramethylrhodamine isothiocyanate (Rhodamine; Sigma, 0.3 mm).
Matrigel invasion assay for mammalian MCF10A cells:
Parental MCF10A cell lines were retrovirally co-infected with JNK1a1, MKK4, and MKK7 overexpression constructs and H-RasV12cherry selected with puromycin, sorted for GFP/cherry on a FACSVantage SE-DiVa flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and maintained as previously described (Dow et al. 2008). MCF10A derivative cell lines stably expressing candidate genes were quantified for invasive phenotypes in 3D organotypic cultures as previously described (Dow et al. 2008) using growth factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ) and the standard overlay method (Debnath et al. 2003; Dow et al. 2008). After 7 days in culture, individual acini were classified as “normal” acini, defined as those with a contiguous acini boundary with no cellular extensions, or “invasive,” defined as acini with disorganized boundary structures showing cellular protrusions or cellular spikes invading into the surrounding matrix. Cell lines were plated in duplicate wells for each experiment and 100 acini were scored per well.
Constructs and antibodies for mammalian cell analysis:
JNK1a1, MKK4, and MKK7 overexpression constructs were PCR amplified from Addgene plasmids 13798 (pcDNA3-Flag-JNK1a1), 14615 (pcDNA3-Flag-MKK4), and 14538 (pcDNA3-Flag-MKK7a1) and inserted into pMSCVpuro (clontech, PT3303-5) or MSCV-IRES-GFP. MSCV-cherry-IRES-H-RasV12 and MSCV control vectors were already available (Dow et al. 2008).
The following primary antibodies were used for probing Western blots: α-tubulin (no. T5168; Sigma-Aldrich), Mouse anti-MKK7 (no. 32-7000, Invitrogen), monoclonal mouse Anti-Flag (F3165, Sigma), rabbit anti-SEK1/MKK4 (no. 9152, cell signaling), rabbit anti-Ras (cloneMC57, no. 05-775, Upstate). Peroxidase-labeled horse anti-mouse IgG (S1018, vector laboratories, Burlingame, CA) and goat anti-rabbit IgG (no. 170-6515, Bio-Rad laboratories, Hercules, CA) secondary antibodies were used for LiCor Western blotting.
Scanning electron microscopy and imaging:
Fluorescent labeled samples were mounted in 80% glycerol and analyzed by Confocal microscopy (Bio-Rad MRC1000 or Olympus FV1000) and images were processed using Confocal AssistantR and Fluorview software and assembled using Adobe Photoshop CS2 and Adobe Illustrator CS2. Adult eyes were imaged with a Scitec Infinity1 camera. Scanning electron micrographs of adult eyes were carried out as previously described (Richardson et al. 1995), except that the samples were gold coated before imaging and were imaged on a Philips XL30 FEG field-emission scanning electron microscope, at 2 V, and working distance 10 mm.
Breast Cancer Gene expression data sets:
Breast cancer data sets used are publicly available and were downloaded from the authors’ websites (see supporting information, File S1). We used normalized data (log2 intensity in single-channel platforms or log2 ratio in dual-channel platforms) and probe sets were mapped to Entrez-Gene IDs to merge data across the various datasets. Breast cancer subtypes were defined using a two-dimensional clustering model previously described on the basis of two module scores, ESR1 and HER2, representing ER and HER2 phenotypes, respectively. Gene sets representing JNK and RAS signaling were combined to compute a gene signature score defined as the weighted linear combination of the log2 expression values of the genes in the signature. Kruskal–Wallis tests were used to determine differences in expression between classes and Spearman’s Rho correlations were used to assess correlations between the signatures. For further information see File S1.
RESULTS
A screen for RasACT-cooperating genes in the developing Drosophila eye reveals cell morphology regulators:
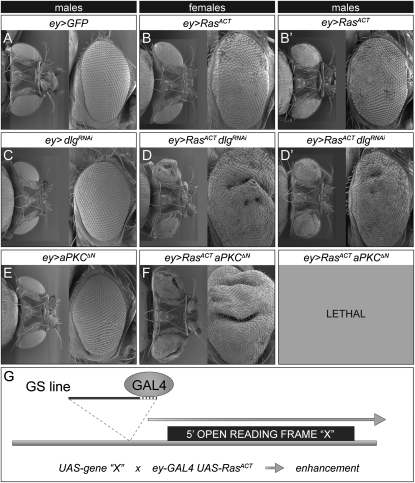
To identify novel genes able to cooperate with activated Ras85D (RasACT), we first sought to generate a hyperplastic phenotype mediated by RasACT that could be used in an F1 screen. Expression of RasACT via the eyeless-GAL4 (ey-GAL4) driver in the developing eye has been previously shown to result in hyperplasia during larval development and generates an overgrown adult eye phenotype (Karim and Rubin 1998). Therefore, we generated a stock containing ey-GAL4 and UAS-RasACT (ey>RasACT), which resulted in overgrown adult eyes that was more obvious in males than females (Figure 1, A and B, and Figure S1). At the larval stage, the expression of RasACT led to enlarged eye discs with enlarged ommatidia and greater spacing between ommatidial clusters (Figure S3 and Figure S4), consistent with the documented role of RasACT in cell growth and proliferation (Karim and Rubin 1998; Prober and Edgar 2000). To validate that the phenotype of ey>RasACT was responsive to genes known to cooperate with RasACT in tumorigenesis, we tested if knocking down the junctional neoplastic tumor suppressors, dlg, scrib, or lgl, by RNAi, could enhance the phenotype. Indeed, dlg knockdown enhanced the ey>RasACT hyperplastic eye phenotype at the adult stage and resulted in subtly larger eye discs than RasACT alone, with greater spacing between ommatidial clusters (Figure 1D, Figure S3, and Figure S4), but had no obvious defects when expressed alone (Figure 1C, see Figure S3, and Figure S4). lgl or scrib knockdown had only mild effects on the ey>RasACT phenotype (data not shown), perhaps due to a lower level of knockdown achieved with these RNAi lines. To determine whether the ey>RasACT adult eye phenotype was sensitive to increased activity of polarity regulators, we then tested whether overexpression of an activated version of the apical cell polarity regulator aPKC (aPKCΔN), which alone does not affect the adult eye, could enhance the ey>RasACT phenotype. Indeed ey>RasACT aPKCΔN adult females exhibited strongly enhanced hyperplastic eyes (Figure 1, E and F, and Figure S1), whereas no males eclosed (presumably reflecting the stronger phenotype of RasACT expression exhibited in males). Furthermore, overexpression of the apical cell polarity regulator Crb, via the ey driver, resulted in an ablated eye phenotype alone, but was pupal lethal with RasACT (data not shown). Thus, these data show that deregulation of polarity regulators can enhance the RasACT phenotype and validate the use of the ey>RasACT adult eye phenotype as a system suitable for screening for genes that when overexpressed can cooperate with oncogenic Ras, to increase hyperplasia or result in pupal lethality.
Figure 1.—
- Strategy for RasACT-cooperating gene screen: scanning electron micrographs of adult eyes (dorsal and lateral views) from female or males flies expressing oncogenic Ras (RasACT) and activated aPKC (aPKCΔN) or knockdown of Dlg (dlgRNAi), compared with RasACT alone and controls. Posterior is to the left and dorsal is to the top in the lateral views, in this and all other adult eye figures. (A) ey-GAL4 UAS-GFP (ey>GFP), (B) ey-GAL4 UAS-RasACT (ey>RasACT), (C) ey-GAL4 UAS-dlgRNAi (ey>dlgRNAi), (D) ey-GAL4 UAS-RasACT UAS-dlgRNAi (ey>RasACT dlgRNAi), (E) ey-GAL4 UAS-aPKCΔN (ey>aPKCΔN), (F) ey-GAL4 UAS-RasACT aPKCΔN (ey>RasACT aPKCΔN). RasACT expression via the ey-GAL4 driver results in hyperplastic eyes. Expression of aPKCΔN or dlgRNAi via ey-GAL4 results in slight roughening. Expression of aPKCΔN or dlgRNAi with RasACT via ey-GAL4 results in enhanced overgrowth of the RasACT hyperplastic adult eye. Males expressing RasACT and aPKCΔN die at the pupal stage. (G) Diagram of ey>RasACT screening strategy. ey-GAL4 UAS-RasACT flies were crossed to a library of enhancer P (GS) lines, expressing via UAS(GAL4) adjacent genes (UAS-gene “X”), and those which enhanced the ey>RasACT phenotype were selected for further analysis.
To identify novel genes that when overexpressed cooperate with RasACT, we screened the GS line collection of enhancer P lines (Toba et al. 1999; Aigaki et al. 2001). The map position of these lines in the genome, as well as the tagged gene (the gene that is overexpressed by the line) has, in most cases, been determined and a database established to enable ready access to this information (http://gsdb.biol.metro-u.ac.jp/%7Edclust/). This enhancer P transgenic set has been successfully used in several screens to identify interacting genes (Aigaki et al. 2001; Kanuka et al. 2005; Laviolette et al. 2005; Zhang et al. 2006). To identify enhancers of RasACT, we carried out an F1 screen, scoring for lines that enhanced the mild hyperplastic phenotype of ey>RasACT. Approximately 5000 GS lines were screened and lines that scored as moderate or strong enhancers were retested against ey>RasACT. Confirmed interacting GS lines (Table 1 and see Table S1) were then validated by testing whether independent enhancer P lines or transgenes could also enhance the ey>RasACT phenotype. The interactors were also analyzed for whether they resulted in hyperplasia alone by crossing to ey-GAL4. Those that were unable to be confirmed by independent enhancer P lines or transgenes, or produced only mild enhancement with an independent line, were not pursued (see Table S1). A possible reason why some of these could not be validated by an independent transgene or enhancer P line is that the GS line was inserted in the open reading frame of the gene, and therefore a truncated neomorphic protein may be produced (see Table S1). Alternatively, the level of expression of the gene may be critical for cooperation with RasACT and the GS line may express the protein at a different level than the independent lines. An example of this is Src, which has previously been described as promoting hyperplasia at a lower level of expression, but inducing cell death and tissue ablation when expressed at a higher level (Vidal et al. 2007).
TABLE 1.
Validated ey>RasACT-cooperating genes, function, and human homologs
| GS line | Potential overexpressed genes, position of GS insertion | Validating transgene | Validated gene Function | Closest human homolog |
| 13019 (GSV6) | CG2248-RA,−2385 CG9149-RA, +2119 (antisense) | UAS-Rac1 | CG2248 (Rac1) Rho-family GTPase. Actin cytoskeleton regulation. | Rac1(e–101) |
| 12503 (GSV6) | CG8416-RA, −125 | UAS-Rho1-CFP2a UAS-Rho1ACT | CG8416 (Rho1) Rho-family GTPase. Actin cytoskeleton regulation. | RhoA (e–94) |
| 45 (GSV1) | CG9635-RD, −122 CG6829-RB, +7054 (anti-sense) | UAS-RhoGEF2 | CG9635 (RhoGEF2) Rho family activator. Actin cytoskeleton regulation. | p115-RhoGEF (e–47) LARG (e–50) PDZ-RhoGEF (e–53) |
| 9792 (GSV6) | CG8114-RA, +344 (5′-UTR) | UAS-pbl-GFP#8 | CG8114 (pbl) Rho family activator (RhoGEF) | Ect2 (e–144) |
| 14458 (GSV6) | CG8114-RA, −484 | UAS-pbl-GFP#8 | Actin cytoskeleton regulation, cytokinesis, migration | Ect2 (e–144) |
| 9641 (GSV6) | CG7230-RA, −6758 | UAS-rib | CG7230 (rib) BTB/POZ transcription factor. | ZFP161 (e–8) |
| 1106 (GSV1) | CG4399-RB, −163 | UAS-east | CG4399 (east) metallopeptidase M14 | None |
| 1211 (GSV1) | CG4399-RB, −706 | UAS-east | Carboxypeptidase A domain and putative proteolysis activity, nuclear endoskeleton regulation | None |
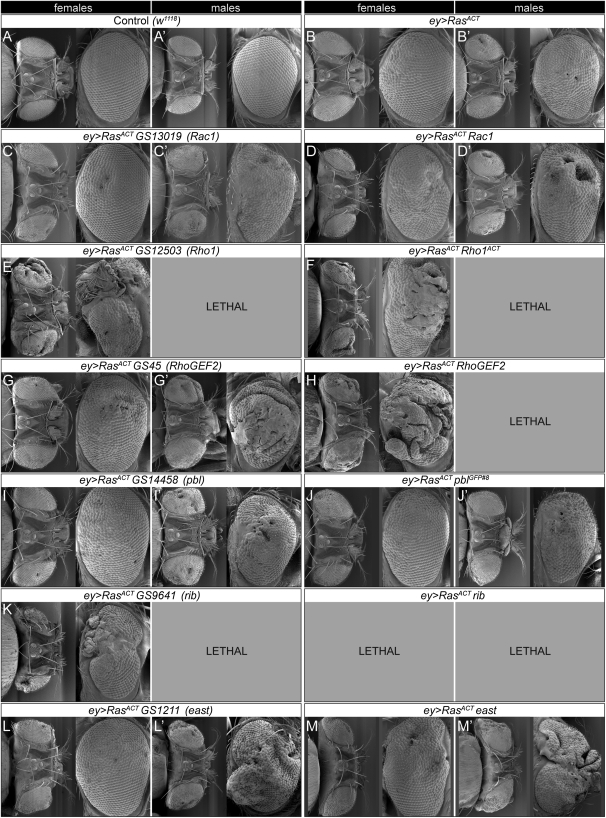
One validated enhancer was Delta, which is a ligand for Notch; however, as has been previously described (Baonza and Freeman 2005; Ferres-Marco et al. 2006), it also showed a hyperplastic eye phenotype when expressed alone (data not shown) and was not further analyzed. Validated enhancers exhibited phenotypes ranging from eyes with regions of aberrant differentiation (cuticle and bristles within the eye field), morphological defects, and male lethality at the pupal stage to enlarged, overgrown adult eyes (Table 1 and Figure 2). Strikingly, the majority of the cooperating proteins fell into the category of Rho-family GTPases, Rho1 and Rac1 (Settleman 2001), and Rho1 regulators, RhoGEF2 (Barrett et al. 1997; Hacker and Perrimon 1998) and Pbl (Prokopenko et al. 1999) (see below). The other two cooperating proteins were the BTB/POZ and Psq domain nuclear localized protein, Ribbon (Rib), required for cell shape changes and epithelial morphogenesis (Bradley and Andrew 2001; Shim et al. 2001), and the nuclear cytoskeletal regulator, East (Wasser and Chia 2000) (Figure 2, see Figure S1, and Figure S2).
Figure 2.—
- Interaction of cooperating GS lines with ey>RasACT and validation: scanning electron micrographs of adult eyes (dorsal and lateral views) from female or male flies expressing RasACT and cooperating GS lines or UAS transgenes via the ey driver, compared with ey>RasACT alone and wild-type control eyes. (A) Control (w1118), (B) ey>RasACT, (C) ey>RasACT GS13019 (Rac1), (D) ey>RasACT Rac1, (E) ey>RasACT GS12503 (Rho1), (F) ey>RasACT Rho1ACT, (G) ey>RasACT GS45 (RhoGEF2), (H) ey>RasACT RhoGEF2, (I) ey>RasACT GS14458 (pbl), (J) ey>RasACT pblGFP#8, (K) ey>RasACT GS9641 (rib), (L) ey>RasACT GS1211 (east), (M) ey>RasACT east. Expression of ey>RasACT with GS9641 (rib), GS12503 (Rho1), or RhoGEF2 was male lethal, and with rib was male and female lethal.
The effects of the RasACT-cooperating genes on cell survival, proliferation, differentiation, and morphology:
Expression of the RasACT-cooperating genes alone did not result in hyperplasia, and indeed rib, Rho1, and RhoGEF2 resulted in small eyes with morphological defects, suggesting that they were inducing cell death (see Figure S1 and Figure S2). Since activation of Ras inhibits apoptosis (Bergmann et al. 1998; Kurada and White 1998), it was possible that RasACT was cooperating with these genes by preventing cell death. However, expression of the cell-death inhibitor, P35 (a caspase inhibitor, Hay et al. 1994), with the RasACT-cooperating genes did not lead to increased hyperplasia (see Figure S1 and Figure S2), although the male lethality of Rho1 was rescued by expressing p35 (see Figure S2). Thus, RasACT does not cooperate by simply blocking cell death, although it is possible that its cell survival function could contribute to the cooperative effects. Therefore, RasACT must be providing other functions, such as promoting cell growth and proliferation or affecting cell–cell adhesion, as has been previously reported (Prober and Edgar 2000; Prober and Edgar 2002; O'keefe et al. 2007), and we have observed in cooperative tumorigenesis with scrib mutants (Brumby and Richardson 2003).
As detailed below, while all of the RasACT-cooperating genes enhanced RasACT tissue growth, a spectrum of cooperative effects were observed: pbl, Rac1, and east enhanced RasACT tissue growth, RhoGEF2 enhanced the effect of RasACT on tissue growth, as well as affected cell morphology and differentiation, and Rho1 or rib (which alone had strong morphology defects, differentiation defects, and disorganized proliferation patterns) cooperated with RasACT by enhancing tissue growth, as well as affecting cell morphology and differentiation (see Figure S1, Figure S2, Figure S3, and Figure S4).
Rac1:
The GS line and an independent transgene for Rac1 (Luo et al. 1994) showed similar hyperplastic phenotypes with RasACT (Figure 2, C and D). In the larval eye disc, expression of Rac1 alone did not affect eye development; however, with RasACT it resulted in an increased tissue growth and morphological defects, although differentiation still occurred, albeit aberrantly patterned (see Figures S3 and Figure S4).
Rho1:
The Rho1 GS line showed a strong effect with ey>RasACT resulting in male lethality (Figure 2E); however, expression of several Rho1 transgenes did not enhance the ey>RasACT phenotype to the same extent as the GS line, although UAS-Rho1CFP2a (Greco et al. 2001) showed slight to moderately increased hyperplasia (see Figure S2). Expression of the Rho1 GS line alone via the ey driver led to male lethality and females had very reduced eyes with differentiation defects, but ey>Rho1CFP2a did not noticeably affect the adult eye (see Figure S2). It is possible that the wild-type Rho1 transgenes tested did not express Rho1 to the same level as the GS line, and therefore could not accumulate sufficient levels of active GTP-bound Rho to show cooperation with RasACT. Therefore, we tested an activated allele of Rho1, Rho1V14 (Rho1ACT) (Lee et al. 2000). Rho1ACT alone was male lethal, but female eyes were not as severely affected as with Rho1GS12503 (see Figure S2). Expression of Rho1ACT with RasACT strongly enhanced the ey>RasACT phenotype (Figure 2F, and see Figure S2), indicating that activated Rho was required for cooperation with RasACT. Consistent with the effect on the adult eyes, Rho1 or Rho1ACT alone resulted in very small eye discs, although S phases were observed throughout the eye disc, and exhibited altered cell morphology and reduced differentiation (see Figure S3 and Figure S4). Coexpression of RasACT with Rho1 or Rho1ACT resulted in larger eye discs relative to these genes alone; however, proliferation and differentiation were similarly affected (see Figure S3 and Figure S4).
RhoGEF2:
The GS line targeting RhoGEF2 and an independent RhoGEF2 transgene (Mulinari et al. 2008) cooperated with ey>RasACT (Figure 2, G and H, and see Figure S2). However, the RhoGEF2 transgene showed more severe effects than the GS line, resulting in greater hyperplasia in females and male lethality at the pupal stage (Figure 2H; see Figure S2; and data not shown). When expressed alone the RhoGEF2 transgene was also more severe than the GS line, resulting in ablation of eye tissue (Figure S2). Consistent with these effects on the adult eye, in the larval eye discs, RhoGEF2 alone resulted in aberrant proliferation patterns, tissue morphology (see Figure S3), and partially blocked differentiation (see Figure S4), and when expressed with RasACT they strongly affected tissue morphology and blocked differentiation (see Figure S4).
Pbl:
GS lines targeting pbl, and two out of five independent pbl transgenes (Somers and Saint 2003), enhanced the ey>RasACT phenotype (Figure 2, I and J, and see Figure S2; and data not shown). Of the independent transgenes, UAS-pblGFP#8 showed a stronger effect than UAS-pblGFP#3 (data not shown). Although we have not tested it directly, it is possible that the level of pbl expression is critical for the cooperative effects with RasACT. In the larval eye disc, expression of pbl alone did not prevent differentiation nor did it substantially affect the pattern of S phases or tissue morphology (see Figure S3 and Figure S4). Coexpression of RasACT with pbl resulted in an enhancement of the tissue growth effect of RasACT, as well as morphological defects, although differentiation still occurred, albeit in an aberrant pattern (see Figure S3 and Figure S4).
Rib:
An independent transgene of rib (Bradley and Andrew 2001) resulted in a more extreme phenotype than the GS line with ey>RasACT, since it was lethal in both males and females (data not shown). Expression of rib GS line alone via the ey driver resulted in reduced adult eyes with differentiation defects in both males and females (see Figure S1), while the rib transgene was male and female lethal when expressed with ey-GAL4. Consistent with the adult phenotypes, expression of rib alone resulted in very small eye discs, although S phases were observed throughout the eye disc, that had altered cell morphology and reduced differentiation (see Figure S3 and Figure S4). Coexpression of RasACT with rib resulted in larger eye discs relative to rib expression alone; however, proliferation and differentiation were similarly affected (see Figure S3 and Figure S4).
East:
The cooperation of east with RasACT was confirmed by expression of a UAS-east transgene (Wasser and Chia 2000) (Figure 2, L and M). In larval eye discs, expression of east alone did not prevent differentiation (see Figure S4) or obviously affect the pattern of S phases or tissue morphology (see Figure S3), but with RasACT it enhanced the tissue growth effect of RasACT and led to morphological and differentiation defects (see Figure S3 and Figure S4).
The requirement of Rac or Rho1 activity for cooperation with RasACT:
Since Pbl and RhoGEF2 are known actin cytoskeletal regulators that function through the Rho-family GTPase, Rho1 (Hacker and Perrimon 1998; O'keefe et al. 2001; Padash Barmchi et al. 2005; Patch et al. 2009), we reasoned that other RasACT-cooperating genes may work in a common pathway via Rho1 or Rac1 in their cooperation with RasACT. To address this, we assessed the requirement of Rho1 or Rac1 on the ability of the RasACT-cooperating genes for the cooperation with RasACT in a whole-tissue setting. To block Rac1 function we expressed a dominant negative allele, blocked in the inactive GDP-bound state, Rac1N17 (Rac1DN) (Luo et al. 1994). Three Rac genes in Drosophila have overlapping functions and it is likely that the Rac1 dominant-negative allele interferes with the function of all Rac genes (Hakeda-Suzuki et al. 2002). To reduce Rho1 function, we used a RNAi transgene (Rho1RNAi), which has been shown to effectively knockdown Rho1 protein levels and function (Massarwa et al. 2009; Yan et al. 2009). While expression of Rac1DN or Rho1RNAi showed no discernable effects alone (data not shown) or on the ey>RasACT phenotype (see Figure S5), Rac1DN suppressed the cooperation with Rac1 and RasACT, and Rho1RNAi suppressed Rho1GS12503 and Rho1ACT cooperation with RasACT, as expected (Table 2; see Figure S6). Both Rac1DN and Rho1RNAi showed suppression of the ey>RasACT dlgRNAi and ey>RasACT aPKCΔN phenotypes (Table 2; see Figure S5). RhoRNAi suppressed RhoGEF2 and pbl cooperation with RasACT (Table 2; see Figure S7), as expected. Interestingly, Rac1DN suppressed pbl and showed partial suppression of RhoGEF2 cooperation with ey>RasACT, but did not alter the ability of Rho1GS12503, Rho1ACT, rib, or east to cooperate with RasACT (Table 2; see Figure S6 and Figure S7). RhoRNAi partially suppressed ribGS9641 (although not the stronger rib transgene; data not shown), but not Rac1 or east (Table 2; see Figure S6 and Figure S7). Collectively, these genetic interactions are consistent with the notion that Dlg, aPKC, RhoGEF2, and Pbl act upstream of Rho1 and Rac in their cooperative effects with RasACT. These results also suggest that in their cooperation with RasACT, Rib acts upstream of Rho1 and East acts downstream or independently of Rho1 and Rac.
TABLE 2.
Summary of genetic interactions of Rho1, Rac1, aPKC, or bsk with ey>RasACT + cooperating genes
| ey>RasACT | ey>RasACT Rac1DN | ey>RasACT Rho1RNAj | ey>RasACT aPKCDN | ey>RasACT bskDN | |
| UAS-dlgRNAj | Enhanced | Slight suppression | Slight suppression | Slight suppression | Suppression |
| UAS-aPKCΔN | Enhanced | Suppression | Suppression | No suppression | Suppression |
| UAS-Rac1 | Enhanced | Suppression | No suppression | No suppression | Suppression |
| GS12503(Rho1) | Enhanced | No suppression | Suppression | No suppression | Slight suppression |
| UAS-Rho1ACT | Enhanced | No suppression | Suppression | No suppression | Suppression |
| UAS-RhoGEF2 | Enhanced | Slight suppression | Suppression | No suppression | Suppression |
| UAS-pblGEF#8 | Enhanced | Slight suppression | Slight suppression | No suppression | Suppression |
| GS9641(rib) | Enhanced | No suppression | Slight suppression | No suppression | No suppression |
| UAS-rib | Enhanced | No suppression | No suppression | No suppression | No suppression |
| UAS-east | Enhanced | No suppression | No suppression | No suppression | No suppression |
The requirement of aPKC activity for the cooperation with RasACT:
Since we have previously shown that the scrib mutant clonal phenotype depends on aPKC and that aPKC contributes to the cooperative tumorigenesis of scrib mutants with RasACT or NotchACT (Leong et al. 2009), we tested whether the RasACT-cooperating genes also required aPKC for their cooperative effects. We blocked aPKC activity by expression of a kinase-dead (dominant-negative) transgene (aPKCDN)(Sotillos et al. 2004), which in clones can suppress the defects of scrib or lgl mutants (Grzeschik et al. 2007; Leong et al. 2009). aPKCDN exhibited no effect upon the ey>RasACT phenotype (see Figure S5); however, it partially suppressed the cooperative effect of dlgRNAi with RasACT (Table 2 and see Figure S5), consistent with the antagonistic relationship between these proteins (as described previously). Surprisingly, aPKCDN did not suppress the ey>RasACT aPKCΔN phenotype (Table 2 and see Figure S5), perhaps due to high expression of aPKCΔN, although it can suppress weaker activated aPKC phenotypes due to expression of a membrane-tethered aPKC construct (aPKCCAAX-WT; Sotillos et al. 2004 and data not shown). aPKCDN was unable to suppress the cooperative effects of any of the other RasACT-cooperating genes (Table 2, see Figure S6 and Figure S7), suggesting that aPKC acts upstream or independently of these genes.
JNK is upregulated and is required for the cooperative effect of Rho-GTPases and Rho-family regulators with RasACT:
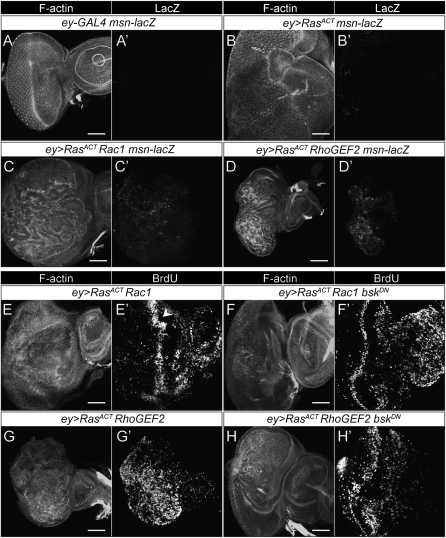
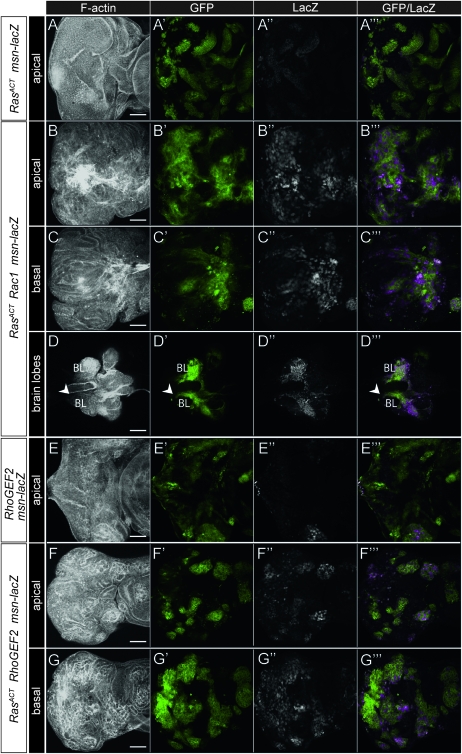
Activation of JNK is critical for cooperative tumorigenesis of scrib mutants with RasACT or NotchACT (Leong et al. 2009). To determine the involvement of JNK signaling in the cooperation of ey>RasACT with RhoGEF2 and Rac1, we first tested whether JNK activity was increased in these eye discs, using the msn-lacZ reporter to monitor JNK pathway activity (Mattila et al. 2005). Expression of RasACT via ey-GAL4 resulted in a weak induction of msn-lacZ in some cells in the eye disc (Figure 3, A and B), which was expected because of previous findings on the regulation of Jun and Fos activity via the Ras-MAPK signaling pathway (Kockel et al. 1997; Ciapponi et al. 2001). However, coexpression of Rac1 or RhoGEF2 with RasACT resulted in a more consistent and stronger upregulation of msn-lacZ throughout the eye disc (Figure 3, C and D). Thus, in the ey>RasACT system, JNK activity is induced by Rac1 or RhoGEF2 expression.
Figure 3.—
- The JNK pathway is upregulated in ey>RasACT + Rac1 or ey>RasACT + RhoGEF2, and blocking JNK reduces the overgrowth by decreasing the number of S phases: (A–D) LacZ staining of eye discs from female larvae of: (A) ey-GAL4 msn-lacZ, (B) ey>RasACT msn-lacZ, (C) ey>RasACT Rac1 msn-lacZ, (D) ey>RasACT RhoGEF2 msn-lacZ. Rac1 or RhoGEF2-expressing eye discs show increased LacZ staining (msn expression). (E–H) BrdU labeling of eye discs from: (E) ey>RasACT Rac1, (F) ey>RasACT Rac1 bskDN, (G) ey>RasACT RhoGEF2, (H) ey>RasACT RhoGEF2 bskDN. The ectopic S phases are suppressed by blocking JNK signaling with bskDN. In E, the arrowhead marks the second mitotic wave. Scale bars, 50 μm.
We then tested if blocking JNK signaling, by expression of kinase-dead (dominant negative) transgene (bskDN), could affect the cooperation of the RasACT-cooperating genes with RasACT on the adult eye phenotypes. As expected on the basis of our findings in the clonal setting (Leong et al. 2009), bskDN strongly suppressed the cooperation of RasACT with dlgRNAi or aPKCΔN, but did not affect the ey>RasACT phenotype (Table 2 and see Figure S5). Expression of bskDN also suppressed the cooperation of RhoGEF2, pbl, Rac1, and Rho1ACT and partially suppressed the stronger phenotype of Rho1GS12503 with RasACT (Table 2, see Figure S6, and Figure S7). Consistent with this, expression of bskDN resulted in a suppression of the ectopic S phases observed in posterior region of ey>RasACT + Rac1 or RhoGEF2 eye discs (Figure 3, E–H). Thus, in the ey>RasACT system, JNK activity is required for the increased proliferation observed in Rac1 or RhoGEF2 + RasACT eye discs. However, bskDN failed to suppress the cooperative effects of east or rib with RasACT (Table 2, see Figure S7). Since it is conceivable that bskDN could function by acting on other MAPK-family signaling pathways, such as p38, to confirm that these interactions were due specifically to blocking the JNK signaling pathway, we also tested whether reducing the dosage of bsk would suppress the ey>RasACT + RhoGEF2 or Rac1 phenotypes. Indeed, bsk2/+ suppressed the cooperative overgrowth phenotypes of Rac1 or RhoGEF2 with RasACT (see Figure S8). Collectively, these data suggest that RhoGEF2, pbl, Rac1, and Rho1 require JNK activity for their cooperation with RasACT, but that east and rib cooperate with RasACT independently of JNK.
Finally, to determine whether upregulation of JNK signaling was sufficient for cooperation with RasACT, we coexpressed RasACT (via the ey driver) with various transgenes encoding components of the JNK signaling pathway (Stronach 2005); Bsk (JNK), Hep (JNK kinase), HepACT (activated version of Hep), Msn (JNK kinase kinase kinase), and Eiger (tumor necrosis factor, TNF, homolog, which signals through the JNK pathway). We also knocked down a negative regulator of the pathway, the JNK phosphatase, Puc (using UAS-pucRNAi), in the ey>RasACT background. Expression of these transgenes or RNAi had no discernable effect when expressed alone (data not shown) and did not enhance the ey>RasACT phenotype (see Figure S8 and data not shown). Thus, JNK signaling is required, but is not sufficient, for the cooperation with RasACT in the whole eye tissue setting.
In a clonal setting, Rac1, Rho1ACT, RhoGEF2, and pbl cooperate with RasACT in tumorigenesis:
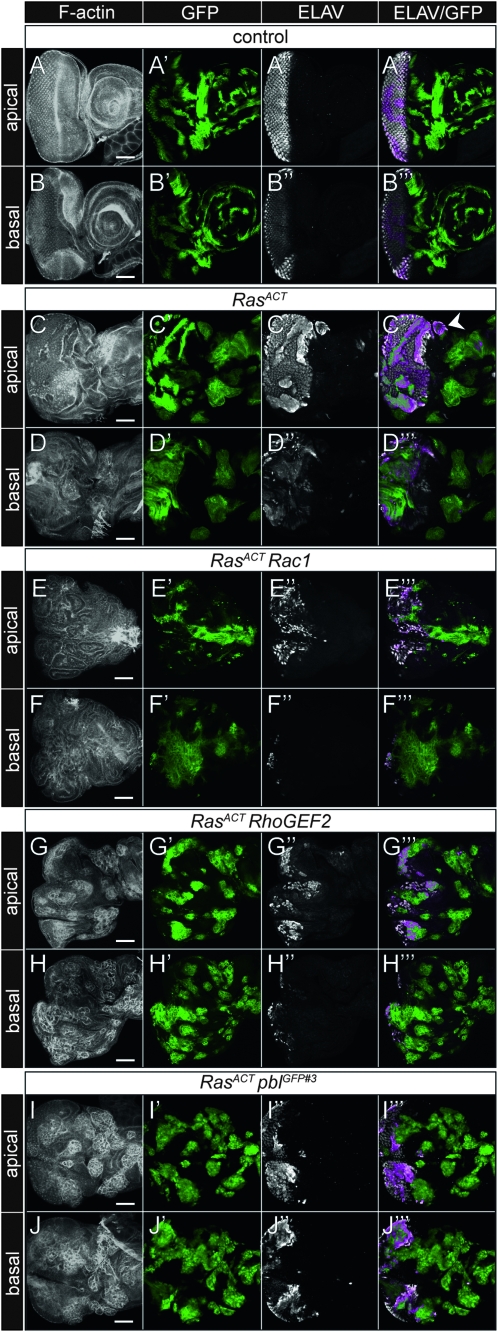
Mutations in genes, such as scrib, that affect cell morphology, result in tumors when the whole tissue is mutant, but are unable to do so when mutant cells are generated in clones surrounded by wild-type tissue (Brumby and Richardson 2003; Pagliarini and Xu 2003). This phenomenon is due to induction of cell-competitive mechanisms leading to JNK-mediated cell death (Adachi-Yamada and O'connor 2004; Igaki 2009; Johnston 2009). However, while RasACT itself in clones results in some hyperplasia and ectopic differentiation in the eye field relative to wild type (Figure 4, A–D), when it is expressed in scrib- clones in the eye disc, mutant clones outgrow the wild-type tissue forming massive neoplastic tumors that invade between the brain lobes (Brumby and Richardson 2003; Pagliarini and Xu 2003; Leong et al. 2009). On the basis of these findings, we wished to test whether the ey>RasACT cooperating genes could cooperate with RasACT in a clonal setting.
Figure 4.—
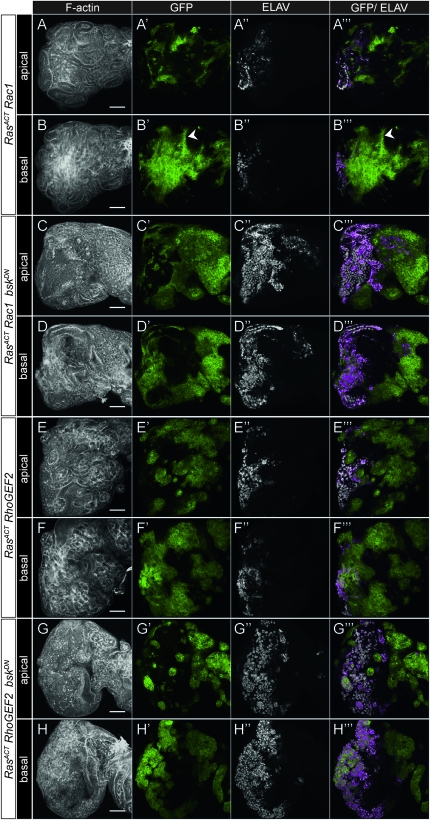
- Analysis of the cooperation of Rac1, RhoGEF2, and pbl with RasACT in eye disc clones: expression of RasACT with cooperating oncogenes in clones compared with controls from day 5 staged larvae stained for F-actin and ELAV. Clones are positively marked by GFP. (A) FRT82B control, apical section; (B) FRT82B control, basal section; (C) RasACT clones, apical section; (D) RasACT clones, basal section; (E) RasACT + Rac1, apical section; (F) RasACT + Rac1, basal section; (G) RasACT + RhoGEF2, apical section; (H) RasACT + RhoGEF2, basal section; (I) RasACT + pblGFP#3, apical section; (J) RasACT + pblGFP#3, basal section. In C, the arrowhead points to a patch of ectopic differentiation. Expression of Rac1, RhoGEF2, or pblGFP#3 with RasACT results in large overgrowths, more prominently in basal sections. Non-cell-autonomous overgrowth is also observed around the clones as evidenced by tissue folding seen by F-actin staining. Scale bars, 50 μm.
Rac1:
When expressed alone, Rac1 showed many small clones that were basally excluded with pyknotic features, suggesting that cells were dying or being outcompeted (see Figure S9). Rac1 cooperated with RasACT to form large neoplastic tumors, particularly in the basal sections, and differentiation was largely blocked (Figure 4, E and F). Larvae harboring these tumors showed an extended larval lifetime, over which the tumors continued to grow, reaching massive sizes, similar to scrib- + RasACT tumors (see Figure S10).
Rho1:
Rho1GS12503 expression resulted in very small clones, suggesting that they were dying or being outcompeted; however, coexpression of RasACT with Rho1GS12503 did not improve clonal survival (see Figure S11). Since activated Rho1 (Rho1ACT) was able to cooperate better than wild-type Rho1 when expressed in the whole eye tissue (Figure 2, D and E), we envisaged that Rho1ACT may be able to cooperate with RasACT in clones. Indeed, while Rho1ACT alone resulted in small clones and morphological defects, Rho1ACT + RasACT tumors showed overgrowth during the extended larval lifetime forming invasive tumors, as scored by invasion between the brain lobes (see Figure S12).
RhoGEF2:
Expression of RhoGEF2 alone resulted in small clones exhibiting features of dying cells (see Figure S9). RhoGEF2 cooperated with RasACT to form large neoplastic tumors, particularly in the basal sections, with reduced differentiation (Figure 4, G–H), and the tumors increased in size over the extended larval life span, although were not as large as scrib- + RasACT tumors (see Figure S10).
Pbl:
Expression of pbl alone produced wild-type sized clones, although some basally extruded differentiated cells were observed (see Figure S9). Similar to RhoGEF2 + RasACT, pbl cooperated with RasACT to form large neoplastic tumors, with reduced differentiation (Figure 4, I–J) and showed massive overgrowth over the extended larval stage (see Figure S10).
Rib:
rib expression via the transgene of GS line resulted in very small clones, suggesting that they were dying or being outcompeted (see Figure S11; data not shown). Coexpression of RasACT with rib mildly increased rib clonal size, but did not lead to tumor formation (see Figure S11). Interestingly, rib + RasACT eye discs showed non-cell-autonomous overgrowth effects, suggesting that RasACT may impart “un-dead” cell characteristics to the rib-expressing cells, allowing the release of morphogens that promote compensatory proliferation of the surrounding wild-type tissue, as has been previously described (reviewed by Fan and Bergmann 2008).
East:
east-expressing clones alone in the eye disc did not appear to show any morphological or differentiation abnormities and coexpression of east with RasACT resulted in a similar phenotype to RasACT alone (see Figure S11). Thus, unlike the situation in the whole eye disc, East did not cooperate with RasACT to promote hyperplasia or neoplasia in the clonal system.
Taken together, these data show that Rac1, an activated allele of Rho1 (Rho1ACT), RhoGEF2, and pbl, but not Rho1, rib, or east, were capable of cooperating with RasACT in a clonal setting. The differences observed between cooperative effects of these genes in the whole tissue vs. the clonal setting highlight the context dependent nature of RasACT-mediated cooperative tumorigenesis.
JNK is upregulated in eye disc clones of RasACT + Rac1 or RhoGEF2, and is required and sufficient for cooperative neoplastic overgrowth:
We then tested whether the JNK pathway was upregulated in eye disc clones upon the expression of Rac1 or RhoGEF2 with RasACT by monitoring the expression JNK pathway reporter, msn-lacZ. In RasACT + Rac1 or RhoGEF2 + RasACT-expressing clones, in either apical or basal sections, high levels of JNK signaling were observed (Figure 5, B, C, F, G) compared with RasACT-expressing clones alone (Figure 5A) or wild-type discs (see Figure 3A). Indeed, in RasACT + Rac1-expressing clones, high levels of msn-lacZ expression were also observed in the tissue invading between the brain lobes (Figure 5D), consistent with a role for JNK in promoting cell migration and invasion. The increased expression of msn-lacZ in the RhoGEF2 + RasACT-expressing clones (Figure 5, F and G), compared with RasACT clones alone, likely reflected increased levels of JNK activation due to RhoGEF2 activity, since expression of RhoGEF2 alone in clones also exhibited an upregulation of msn-lacZ expression (Figure 5E). This is likely to also be the case for Rac1, although we were unable to analyze the expression of msn-lacZ in clones expressing Rac1 alone, since in this genetic background the clones were poorly viable (data not shown).
Figure 5.—
- Rac1 or RhoGEF2 expression in clones results in upregulation of the JNK pathway: LacZ staining, to detect msn-lacZ expression, and F-actin staining of mosaic discs expressing Rac1 or RhoGEF2 +/− RasACT. Clones are positively marked by GFP. (A) RasACT clones in msn-lacZ/+ discs, (B) RasACT + Rac1 clones in msn-lacZ/+ discs, apical view, (C) RasACT + Rac1 clones in msn-lacZ/+ discs, basal view, (D) RasACT + Rac1 clones in msn-lacZ/+ discs with brain lobes, showing invasion of GFP+ tissue between the brain lobes, BL, (E) RhoGEF2 clones in msn-lacZ/+ discs, (F) RasACT + RhoGEF2 clones in msn-lacZ/+ discs, apical view, (G) RasACT +RhoGEF2 clones in msn-lacZ/+ discs, basal view. In D, the arrowhead points to GFP+ tissue invading between the brain lobes. RasACT results in mild ectopic msn-lacZ expression. RasACT +RhoGEF2 and RasACT + Rac1 clones show extensive upregulation of msn-lacZ. In some RhoGEF2-expressing clones, high levels of msn-lacZ expression is observed. Scale bars, A–C, E–G, 50 μm; D, 200 μm.
To determine the importance of JNK on the cooperative overgrowth in the clonal setting, we blocked the JNK pathway, using bskDN, in Rac1 + RasACT or RhoGEF2 + RasACT-expressing clones (Figure 6). Indeed, expression of bskDN increased differentiation and restored pupation of both Rac1 + RasACT (Figure 6, C and D compared with Figure 6, A and B) and RhoGEF2 + RasACT (Figure 6, G and H, compared with Figure 6, E and F)-expressing clones. Furthermore, bskDN reduced the invasive cell morphology of Rac1 + RasACT-expressing clones and decreased the invasive properties of the tumor (Figure 6, C and D, compared with Figure 6, A and B and data not shown). Furthermore, the expression of bskDN in Rho1ACT + RasACT-expressing clones also restored pupation, increased differentiation, and prevented invasion between the brain lobes (see Figure S13). Collectively, these data show that the activation of JNK is essential to preventing differentiation, for blocking pupation, and for the invasive behavior of RhoGEF2 + RasACT, Rac1 + RasACT, or Rho1ACT + RasACT tumors. However, at least in the case of Rac1 + RasACT + bskDN the tumors were still larger than RasACT clones alone. These data indicate that in Rac1 + RasACT tumors a JNK-independent signal appears to drive additional overgrowth. This is in contrast to the whole-tissue system in which the increased proliferation of Rac1 + RasACT eye discs was JNK dependent (Figure 3, E and F). RhoGEF2 + RasACT + bskDN or Rho1ACT + RasACT+ bskDN tumors were more similar to RasACT alone, so in these cases (and possibly also Rac1 + RasACT tumors) a JNK-dependent signal is required for additional overgrowth. The requirement for JNK in this additional overgrowth is likely to relate to JNKs ability to block differentiation and pupation in these RasACT-expressing clones, thereby enabling tumor overgrowth during an extended larval phase (see discussion).
Figure 6.—
- JNK signaling is required for cooperation of Rac1 or RhoGEF2 with RasACT in clones: clones expressing RasACT with Rac1 or RhoGEF2 +/− bskDN at day 5, stained for F-actin and ELAV. Clones are positively marked by GFP. (A) RasACT + Rac1 clones, apical view; (B) RasACT + Rac1 clones, basal view; (C) RasACT + Rac1 + bskDN clones, apical view; (D) RasACT + Rac1 + bskDN clones, basal view; (E) RasACT + RhoGEF2 clones, apical view; (F) RasACT + RhoGEF2 clones, basal view; (G) RasACT + RhoGEF2 + bskDN clones, apical view; (H) RasACT + RhoGEF2 + bskDN clones, basal view. In B, the arrowhead points to tissue with an invasive morphology. Expression of bskDN rescues the cooperation of Rac1 or RhoGEF2 with RasACT by increasing differentiation and reducing the invasive phenotype of Rac1 + RasACT clones. Scale bars, 50 μm.
Finally, to examine whether activation of JNK was sufficient to cooperate with RasACT in a clonal setting, we expressed a UAS-bsk transgene alone or in combination with UAS-RasACT in eye disc clones and analyzed clonal growth with time (see Figure S14). Expression of bsk alone in clones resulted in small clone size and many cells exhibited a pyknotic phenotype, suggesting that cells were undergoing apoptosis or being outcompeted (see Figure S14). By contrast, expression of RasACT with bsk rescued the cell-death phenotype of bsk-expressing clones and at day 5, eye discs were similar to RasACT expression alone (see Figure S14). However, some bsk + RasACT mosaic larvae exhibited an extended larval phase in which the tumor overgrew the surrounding wild-type tissue (see Figure S14). The tissue overgrowth was associated with altered cell morphology and aberrant differentiation. Moreover, in older larvae, tumor invasion was observed between the brain lobes (see Figure S14). Collectively, our data show that in a clonal setting, activation of JNK is sufficient to block pupation, promote RasACT–mediated proliferation, disrupt differentiation, and induce invasive properties.
Cooperation of Ha-RasV12 and JNK signaling in mammalian breast epithelial cells and in human cancer:
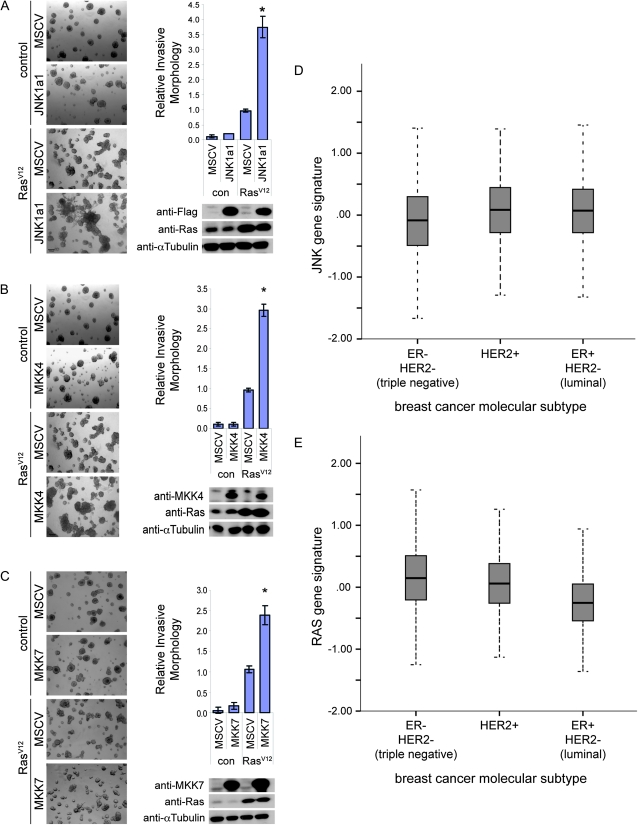
Given our findings of the importance of JNK signaling in Drosophila RasACT-mediated cooperative tumorigenesis with actin cytoskeletal regulators, we sought to investigate the requirement of JNK signaling for cooperation with oncogenic Ras in mammalian cell models and in human cancer. To explore the cooperation of JNK with activated Ras, we utilized MCF10A normal breast epithelial cells grown in 3D matrigel cultures. MCF10A cells form acini in matrigel; however, upon low-level expression of activated Harvey–Ras (Ha-RasV12) the lumens become filled with cells and with the concomitant knockdown of cell polarity regulators, such as hScrib, cells form invasive clusters (Dow et al. 2008)—thus this system is a useful model with which to examine cooperative tumorigenesis. We established MCF10A cell populations overexpressing JNK1a1 and the JNK kinase genes, MKK4 or MKK7, with or without Ha-RasV12 and examined their behavior in matrigel (Figure 7, A–C). MCF10A lines expressing JNK1a1, MKK4, or MKK7 alone were similar to controls, while low-level Ha-RasV12 expression alone showed some invasive acini. Coexpression of JNK1a1, MKK4, or MKK7 with Ha-RasV12 resulted in a ∼4-fold, ∼3-fold and ∼2.5-fold increase in invasive acini relative to Ha-RasV12 alone, respectively. Thus, similar to Drosophila, upregulation of JNK in mammalian epithelial cells cooperates with RasV12 to promote invasive properties upon normal human epithelial cells.
Figure 7.—
- Cooperation of JNK and Ras signaling in normal mammalian epithelial cells and human cancer: (A–C) JNK1a1, MKK4, and MKK7 cooperate with Ha-RasV12 in promoting invasion of MCF10A cells in 3D matrigel cultures. A representative of three independent experiments with three independently derived MCF10A cell line sets overexpressing JNK1a1 (A), MKK4 (B), or MKK7 (C) in the context of Ha-RasV12 expression is shown. Bright-field images of acini morphology (left) (scale bar, 100 μm). Invasive morphology quantitation expressed relative to Ha-RasV12 control [*P < 0.05; Student’s t-test, two tailed, unpaired; error bars represent standard deviation] (right). Western blot of whole-cell lysates probed with antibodies as indicated on the right side. α-Tubulin was used as loading control. (D, E) Box-plots representing expression of a gene signature of JNK activation and Ras activation in human breast cancers. The relative expression of JNK signature genes (D) and Ras signature genes (E) were compared using gene expression data and divided according to the three major molecular breast cancer subtypes: ER−/HER2− (triple negative), HER2+, and ER+/HER2− (luminal). The correlation with high JNK signature expression in HER2+ and ER+/HER2− is higher than in the other breast cancer subtypes, and of these the Ras signature is higher in the HER2+ subtype (P < 0.0001).
We also examined the effect of expressing JNK1a1, MKK4, or MKK7 in MCF10A cells on anchorage-independent growth (growth in soft agar; see Figure S15). Expression of these genes alone could not confer anchorage-independent growth to MCF10A cells or modify the ability of Ha-RasV12 to promote growth in soft agar. In 2D cultures, expression of JNK1a1, MKK4, or MKK7 also did not enhance the proliferation rate alone or in combination with Ha-RasV12, and indeed MKK4 resulted in a decreased proliferation rate of Ha-RasV12-expressing cells (see Figure S15). These data indicate that upregulation of JNK signaling cooperates with RasV12 in 3D cultures to promote invasion, but does not enhance cell proliferation rates in 2D cultures or promote anchorage independent growth.
To further examine the relevance of our findings to human cancer, we investigated a gene signature related to JNK signaling (Han et al. 2002) for its association with gene expression in breast cancer using publicly available data sets (see File S1). Breast cancers are now divided into three major molecular subtypes, according to estrogen receptor and HER2 expression, for clinical and research purposes (Sorlie et al. 2001; Sorlie et al. 2003; Sotiriou et al. 2003), which are recognized to have different biological mechanisms of tumor growth and progression. We found that in the breast cancer subtype that overexpresses the human epidermal growth factor receptor (HER2+), there was a moderate and positive correlation with the JNK signature relative to the other breast cancer subtypes (HER2+ Spearman’s ρ = 0.15, P < 0.001; ER+/HER2− ρ = 0.05, P = 0.05; ER−/HER2− ρ = 0.02, P = 0.6; Figure 7D). As HER2 upregulation is known to activate Ras/Erk signaling (P < 0.0001; Figure 7E), this observation is in agreement with our data, highlighting cooperation between Ras and JNK signaling. The association of a high JNK signature in ER+/HER2− (“luminal” subtype) breast cancers is also consistent with reports from previous clinical studies and xenograft models of tamoxifen resistance, which have reported a positive association with activated/phosphorylated JNK (Johnston et al. 1999; Schiff et al. 2000), although these tumors do not show high expression of the Ras signature (Figure 7E). While Ras is not an established oncogene in breast cancer, Ras pathway upregulation is recognized to be important for breast cancer growth and tumorigenesis (reviewed by Whyte et al. 2009), and our data support a link between Ras and JNK signaling in HER2+ breast cancers. Together these data support further investigation into the relationship between JNK and Ras signaling in human cancers.
DISCUSSION
In a genome-wide overexpression screen for RasACT-cooperating genes in the developing eye, we have identified Rac1, Rho1, RhoGEF2, pbl, rib, and east, which all have roles in regulation of cell morphology. We showed that in a clonal setting, which reveals the competitive ability of mutant tissue, that Rac1, an activated allele of Rho1 (Rho1ACT), RhoGEF2, and pbl exhibit cooperativity with RasACT. Our studies reveal that JNK signaling is required for the cooperation of these genes with RasACT; however, the role of JNK is gene and context dependent. In a whole-tissue setting, we show that (1) expression of Rac1 + RasACT or RhoGEF2 + RasACT leads to upregulation of the JNK-Jun/Fos (AP-1) target gene, msn, (2) that JNK signaling is required for the increased proliferative potential of Rac1 or RhoGEF2 with RasACT, and (3) that the eye phenotypes of Rac1, Rho1 RhoGEF2, and pbl require JNK, but (4) JNK is not sufficient for cooperation. By contrast in a clonal setting, upregulation of JNK is both necessary and sufficient for cooperative tumorigenesis of Rac1, Rho1ACT, or RhoGEF2 with RasACT: (1) JNK is upregulated in Rac1 + RasACT or RhoGEF2 +/− RasACT clones, (2) blocking JNK reduces the tumorigenic potential of Rac1, RhoGEF2, or Rho1ACT with RasACT, and (3) upregulation of JNK alone cooperates with RasACT, although was less aggressive than scrib-, Rac1, Rho1ACT, RhoGEF2, or pbl with RasACT. This role for JNK is conserved in mammalian cells, since JNK upregulation cooperates with activated Ha-Ras to promote invasive growth of MCF10A normal breast epithelial cells in 3D cultures, and upregulation of the JNK signature correlates with HER2+ human breast cancers, where Ras signaling is upregulated. However, upregulation of JNK signaling in mammalian cells did not increase the proliferation or anchorage-independent growth properties of Ha-RasV12, consistent with our analysis that JNK was not sufficient to promote hyperproliferation in the ey>RasACT system. Collectively, our data reveal the importance of the RhoGEF/Rho-family/JNK pathway for cooperative tumorigenesis with RasACT. Moreover, our data reveal that the cooperation of JNK with oncogenic Ras in tumorigenesis is conserved between Drosophila and humans and highlights the relevance of Drosophila screens and genetic analysis to human cancer biology.
Context dependent effects of JNK activation on cell behavior:
Our analysis revealed that the RasACT-cooperating genes resulted in different effects in different contexts; when expressed alone within the whole eye tissue the spectrum of phenotypes ranged from little effect (Rac1, pbl, east) to reduced eyes with morphological and differentiation defects (Rho1, Rho1ACT, RhoGEF2, rib), and with RasACT from increased hyperplasia (Rac1, pbl, east) or more severe morphological and differentiation defects (Rho1, Rho1ACT, RhoGEF2, rib), while in the clonal setting expression of the RasACT-cooperating genes alone ranged from little effect (pbl, east) to small clones with evidence of apoptosis (Rac1, Rho1, Rho1ACT, RhoGEF2, rib), and with RasACT either did not cooperate (Rho1, rib and east) or resulted in neoplastic invasive tumors (Rac1, Rho1ACT, RhoGEF2, pbl). We hypothesize that these spectrums of phenotypic outcomes are related to the severity of cell-morphology disruption and to different levels of Rho1 and JNK signaling, although we have not been able to measure this directly due to the absence of reliable Drosophila reagents for Western analysis.
The reduced eye phenotype of Rho1, Rho1ACT, RhoGEF2, and rib when expressed alone in the whole eye tissue, is consistent with strong activation of JNK, since ey-driven expression of hepACT also results in reduced eyes (data not shown). Furthermore, in cooperation with RasACT in the whole eye disc, Rac1, Rho1, Rho1ACT, RhoGEF2, and pbl required JNK. Indeed, Rac1 + RasACT and RhoGEF2 + RasACT eye discs upregulated Jun/Fos (AP-1) activity and JNK was required for the increased numbers of S phase cells in these discs. Thus, RhoGEF2 and Rac1 require the activation of JNK to cooperate with RasACT to result in increased hyperplasia. A role for JNK in promoting proliferation has recently been revealed in tissue regeneration after wounding (Bergantinos et al. 2010), and the SWH tissue growth control pathway has been implicated in this process (Grusche et al. 2011; Sun and Irvine 2011). Whether the SWH pathway is also required for cooperation of JNK with RasACT to increase hyperplasia remains to be determined.
In the clonal setting the cooperation of Rac1, Rho1ACT, RhoGEF2, and pbl, but not Rho1, rib or east, with RasACT could be related to their ability to upregulate JNK to an appropriate level. Indeed the degree of overgrowth and invasive properties may be related to the level of JNK upregulation; Rac1 + RasACT and scrib− + RasACT tumors show a more consistent upregulation of JNK (revealed by msn-lacZ expression) than in RhoGEF2 + RasACT tumors, which correlates with the more severe overgrowth and invasion of Rac1 + RasACT or scrib− + RasACT tumors. Moreover, the expression of bsk (jnk) alone was sufficient to cooperate with RasACT to produce large neoplastic tumors, consistent with the previous report that upregulation of JNKK (Hep) expression can also cooperate with RasACT (Uhlirova and Bohmann 2006). Uhlirova and Bohmann also showed that the level of JNK pathway activation appears to be important for this cooperation, since overexpression of an activated version of hep (hepACT), which in contrast to bsk or hep upregulation, promotes high levels of cell death when expressed in clones (Brumby and Richardson 2003; Leong et al. 2009), was unable to cooperate with RasACT (Uhlirova and Bohmann 2006). These observations may explain why Rho1 and rib (situations in which we hypothesize that JNK signaling may be higher) did not cooperate with RasACT in the clonal situation; the high levels of cell death triggered by strong JNK activation may not be able to be overcome by expression of RasACT. Upregulation of the Ras–MAPK signaling pathway blocks apoptosis via phosphorylation of the cell-death inducer, Hid, as well as downregulation of hid transcription (Bergmann et al. 1998; Kurada and White 1998). When high levels of JNK activity are induced, the activation of Hid or other cell death inducers may not be able to be blocked by RasACT. By contrast, since expression of east alone in clones did not exhibit signs of cell death, east may be unable to induce sufficient activation of JNK in the clonal setting to enable cooperation with RasACT.
In a clonal setting, we showed that JNK is required to block differentiation and pupation and to promote the invasive phenotypes of RhoGEF2, Rac1, and Rho1ACT in cooperation with RasACT, although not the cell-morphology defects. The effect of JNK on invasion has been shown to be due to upregulation of targets important in cell migration, such as Paxillin, and in breakdown of the extracellular matrix, such as MMP1 (Beaucher et al. 2006; Uhlirova and Bohmann 2006; Srivastava et al. 2007; Leong et al. 2009), but how JNK blocks differentiation and pupation is currently unknown. Expression of bskDN also reduced tumor overgrowth to a level commensurate with RasACT alone for all except Rac1 + RasACT. The reduced differentiation and delayed pupation mediated by JNK most likely contributes to the overgrowth phenotypes, since the overgrowth manifests during the extended larval phase. The JNK-mediated overgrowth in these tumors may depend upon the JAK–STAT pathway, since JNK signaling in scrib− cells has been shown to induce expression of the cytokine, Unpaired (Upd), which can lead to activation of the JAK–STAT tissue growth control signaling pathway in scrib− cells, but also in adjacent cells wild type (Wu et al. 2010). Rac1, Rho1ACT, RhoGEF2, and pbl + RasACT mosaic discs exhibited some non-cell-autonomous tissue growth, suggesting that such a mechanism involving JAK–STAT signaling may be occurring.
For Rac1 + RasACT + bskDN the tumors were still larger than RasACT alone, suggesting that a JNK-independent mechanism must be triggered to drive the overgrowth of these tumors and their competitive advantage over the surrounding wild-type tissue. This is similar to what occurs in scrib- + RasACT tumors when JNK signaling is blocked; although the overgrowth is reduced, tumors are still considerably larger than with RasACT alone (Leong et al. 2009). Pertinent to this is that while activation of JNK alone can cooperate with RasACT (this study; Uhlirova and Bohmann 2006), the cooperative effect is not as potent as with Rac1 + RasACT or scrib− + RasACT, raising the possibility that these genes are affecting other processes to mediate cooperative overgrowth. This may include the integrity of the E-cadherin–β-catenin complex, which has been revealed to also contribute to RasACT-mediated cooperative tumourigenesis (Igaki et al. 2006). Furthermore, we and others have recently shown that the SWH pathway, which inhibits both cell proliferation and survival, is deregulated by loss-of-function of the polarity regulator, Lgl, in the eye disc (Grzeschik et al. 2010), and in lgl− + RasV12 clones in the wing disc (Menendez et al. 2010). Therefore deregulated SWH signaling could contribute to the increased proliferative potential of Rac1 + RasACT or scrib− + RasACT tumors independently of JNK. Other factors, such as the relative level of the Myc cell growth protein, which has been shown to affect the survival of lgl− clones in the wing disc (Froldi et al. 2010), or the recently discovered membrane protein isoform, Flower-Lose, which is associated with dying cells in cell competition (Rhiner et al. 2010), may also be involved in the overgrowth of Rac1 + RasACT or scrib− + RasACT tumors.
Hierarchical relationships between the RasACT-cooperating genes:
Our genetic analysis of the RasACT-cooperating genes with Rho1, Rac1, aPKC, and bsk (jnk) in the whole eye (Table 2) has revealed an interaction relationship between these genes (Figure 8). We found that blocking aPKC, with the kinase-dead (dominant-negative) form, partially suppressed the dlgRNAi + RasACT cooperative phenotype, but not other cooperative interactions, suggesting that aPKC acts downstream of Dlg (consistent with the antagonistic relationship between basal-lateral cell polarity regulators and aPKC, reviewed by Humbert et al. 2008). Analysis of the genetic interactions of the RasACT-cooperating genes with JNK, revealed that JNK acts downstream of dlgRNAi, aPKCΔN, Rac1, Rho1 (and Rho1ACT), RhoGEF2, and pbl in cooperation with RasACT (Table 2 and Figure 8). The cooperation of east with RasACT was epistatic to rho1, rac1, bsk, and aPKC, and therefore east must act downstream or independently of these genes in its cooperation with RasACT to result in increased hyperplasia.
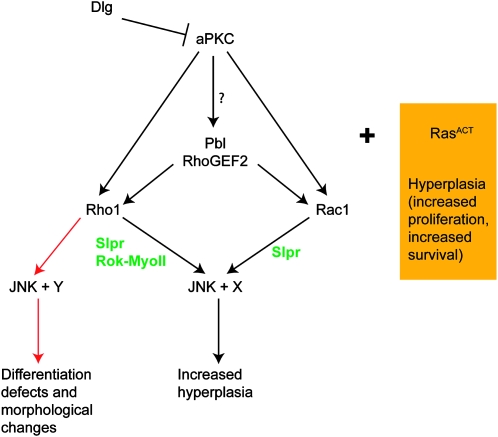
Figure 8.—
Model of interactions of RasACT-cooperating genes: genetic epistasis tests reveal a possible model of how the RasACT-cooperating genes may function in a pathway to lead to the upregulation of JNK in the ey-GAL4 system. Expression of RasACT alone via ey-GAL4 leads to hyperplasia. Pathways shown in black lead to increased hyperplasia, while those in red lead to differentiation defects and morphological changes, which we propose is due to higher levels of Rho1 and JNK signaling. Proteins shown in green are hypothesized to be required for the effects, on the basis of information in other systems (see text). Since JNK is required, but not sufficient, to cooperate with ey>RasACT, we hypothesize that other factors (X or Y) are altered by Rho1 or Rac1 signaling to enable cooperation with RasACT. Whether knockdown of Dlg or activation of aPKC requires Pbl or RhoGEF2 is not known (indicated by ?). See text for further details.
Analysis of the epistatic relationships of the RasACT-cooperating genes (Table 2) revealed that RhoGEF2 and pbl required both Rac1 and Rho1 activity for their cooperation with RasACT (Figure 8). The requirement for Rho1 is consistent with previous studies (Barrett et al. 1997; Hacker and Perrimon 1998; O'keefe et al. 2001); however, the requirement of Rac1 for RhoGEF2 or Pbl function is novel and may be manifest only in the presence of RasACT. We also found that dlgRNAi and aPKCΔN cooperation with RasACT required Rac1 and Rho1 function (Table 2 and Figure 8); however, whether their cooperation with RasACT requires RhoGEF2 or pbl remains to be determined. The mammalian homolog of Pbl (Ect2) can bind to the aPKC (PKCζ)/Par3/Par6 complex, but Ect2 was shown to regulate aPKC activity (Liu et al. 2004) rather than vice versa, as we would predict.
The cooperation of rib with RasACT was not suppressed by bskDN, but was suppressed by Rho1RNAi such that the female eyes now exhibited a hyperplastic phenotype and male lethality was rescued (Table 2). It is difficult to explain in relation to the model (Figure 8) why the rib + RasACT phenotype was suppressed by Rho1RNAi, but not bskDN. It is possible that JNK is upregulated so highly by rib expression that it cannot be blocked by the bskDN transgene, but the Rho1RNAi transgene is more effective in downregulating Rho1. The genetic interaction of rib + RasACT cooperation by Rho1RNAi suggests that Rho1 acts downstream of Rib to mediate cooperation with RasACT. Since Rib is a nuclear-localized protein, thought to be a transcription factor (Bradley and Andrew 2001; Shim et al. 2001), it is possible that Rib may upregulate the expression of Rho1 or Rho1 regulators to mediate its known effects on epithelial migration and morphogenesis (Jack and Myette 1997; Blake et al. 1998), as well as in cooperation with RasACT. Interestingly, Rib has been shown to upregulate the expression of the apical polarity regulator, Crb, and downregulate Moesin (Moe) to facilitate salivary gland and tracheal tube elongation (Kerman et al. 2008). Since Moe is a negative regulator of Rho1 (Speck et al. 2003), upregulation of Rib would be predicted to increase Rho1 activity to mediate its affects on cell morphology. Since Rib also upregulates crb transcription (Kerman et al. 2008), and we and others have shown that high Crb levels deregulates the SWH signaling pathway to promote tissue growth (Grzeschik et al. 2010; Robinson et al. 2010), it is possible that suppression of Rho1 activity in rib + RasACT eye discs unleashes this cryptic activity of Rib in tissue growth via its effect on Crb.
Taken together, our genetic interaction data are consistent with a model whereby RhoGEF2 and Pbl act through Rho1 and Rac1 to activate JNK and increase hyperplasia or promote morphological and differentiation defects in a whole-tissue context. However, our data have revealed that while JNK is required, it is not sufficient for this cooperation with RasACT, suggesting that Rho1 or Rac1 require another unidentified factor (X or Y) for cooperation with RasACT to promote hyperplasia or morphological and differentiation defects (Figure 8). In the case of dlgRNAi, aPKCΔN, pbl, and Rac1 this interaction results in a hyperplastic response through JNK activation and the effector, X. By contrast, Rho1GS12503 and Rho1ACT result in morphological and differentiation defects via JNK activation and the Rho1 effector Y (which may be equivalent to X), while RhoGEF2 has intermediary effects. We propose that stronger activation of Rho1 and therefore JNK in Rho1-expressing eye discs leads to induction of a different effector, Y, or to a higher induction of effector, X. High activation of JNK could also contribute to the cell-morphology changes and differentiation defects observed for Rho1, since JNK has a well-described role in cell-morphology changes during Drosophila development (Martin-Blanco 1997; Harden 2002) and has been shown to contribute to loss of apico-basal cell polarity in wing imaginal discs in lgl mutants (Zhu et al. 2010).
Whether this hierarchical pathway linking polarity regulators, through Rho1 and Rac1, to JNK activation (Figure 8) occurs in a clonal setting remains to be determined. JNK is activated in scrib− clones by upregulation of Eiger (Igaki et al. 2009), and the Drosophila macrophage-like blood cells (hemocytes) are able to recognize the mutant cells and provide the source of Eiger (Cordero et al. 2010). However, since Rho1 and Rac1 can activate JNK during morphogenesis in Drosophila development (Settleman 2001), deregulation of cell polarity regulators may also lead to JNK activation cell intrinsically, via activation of Rho-family regulators, to contribute to RasACT-mediated tumorigenesis. Indeed, Rho1 may directly activate JNK signaling, since it can form a complex with Slipper (Slpr, a JNKKK) in imaginal discs to active JNK-mediated cell death (Neisch et al. 2010). Moreover, Rho1, via Rho kinase (Rok) and Myosin II (Zipper), activates JNK to mediate compensatory proliferation in imaginal discs (Warner et al. 2010). Rac1 can also activate the JNK pathway directly in dorsal closure via Slpr (Garlena et al. 2010). Whether the RasACT-cooperating genes lead to JNK activation in either the whole-tissue or clonal context via these mechanisms remains to be determined.
Cooperation of oncogenic Ras and JNK in mammalian cancer:
Our analysis has revealed that the importance of JNK activation for oncogenic Ras-mediated tumorigenesis extends to mammalian cells, since upregulation of JNK1a or its activators MKK4 or MKK7 cooperates with Ha-RasV12 in the MCF10A normal breast epithelial cell line, to induce invasive growth in 3D matrigel cultures. However, upregulation of the JNK signaling pathway did not cooperate with Ha-RasV12 to increase anchorage-independent growth or cell proliferation in culture. Thus in this context, JNK upregulation is acting simply by promoting the invasive properties of Ha-RasV12-expressing MCF10A cells. Our previous studies have shown that in this system, the cooperation of scrib loss-of-function with Ha-RasV12 is due to further upregulation of Ras signaling (Dow et al. 2008). Whether this is also the case for JNK pathway upregulation in cooperation with Ha-RasV12 will require further analysis.
Our analysis has also revealed a correlation of the JNK signaling signature with the HER2+ breast cancer subtype, which shows upregulation of Ras signaling. This finding provides evidence that upregulation of Ras with JNK may be important for the development of certain types of human cancer. In mammalian cells and human cancer, the role of JNK signaling is complex and context dependent (Kennedy and Davis 2003; Weston and Davis 2007; Whitmarsh and Davis 2007; Wagner and Nebreda 2009). However, our experiments support previous evidence that JNK pathway activation can cooperate with oncogenic Ras in mammalian cell transformation (Nielsen et al. 2007; Zhang et al. 2007; Ke et al. 2010); therefore, our analysis, together with these findings, highlights the need for further research into the association of Ras and JNK status in cancer cell lines and the involvement of JNK signaling in Ras-dependent tumors.
Mammalian homologs exist for Pbl (Ect2), RhoGEF2 (LARG, PDZ-RhoGEF, and p115-RhoGEF), and Rac1/Rho1-family proteins (Table 1). Upregulation of these proteins have been shown to induce cell transformation and are associated with human cancer (Miki et al. 1993; Fukuhara et al. 1999; Fukuhara et al. 2000; Fukuhara et al. 2001; Sahai and Marshall 2002; Ridley 2004; Gomez Del Pulgar et al. 2005; Titus et al. 2005). Indeed, upregulation of Rho-family proteins has been shown to cooperate with oncogenic Ras in enabling cell transformation, by overcoming Ras-induced cellular senescence due to upregulation of the cell-cycle inhibitor p21 (Olson et al. 1998; Coleman et al. 2004). Recently, the Rac1 effector, Pak1, has been found to cooperate with ErbB2–MAPK and PI3K signaling in promoting growth factor-independent proliferation in 3D cultures and to be associated with estrogen-receptor positive human breast cancers (Arias-Romero et al. 2010). Whether JNK signaling is also involved in these cases has not been investigated. Given our findings from this study, further analysis of the association of mammalian Ect2, RhoGEF2-related proteins, and Rac1/Rho1-family members with JNK signaling, in Ras-dependent human cancers, is warranted. Since the Ras signaling pathway is upregulated in 30% of human cancers, but Ras itself is not sufficient for tumorigenesis due to induction of cellular senescence (Olson et al. 1998; Coleman et al. 2004; DeNicola and Tuveson 2009), our identification of the importance of JNK for cooperation of Ras with Rho-family regulators suggests new avenues of investigation in the understanding and treatment of Ras-dependent cancers.
Acknowledgments
We thank Gary Hime and Hannah Robertson for coordinating the shipments of GS lines from Japan and initial discussions on the RasACT genetic screen, Alyssa Harley and Peter Burke for stock maintenance and help with setting up crosses for the genetic screen, Simon Crawford for help with the SEM, and Linda Parsons, Nezaket Turkel, Nathalie Martinek, and Karen Doggett for help with sexing larvae. We are grateful to Ben Rashleigh for developing the FileMaker Pro database used for compiling data from the genetic screen and Benjamin Haibe-Kains and Izi Haviv for bioinformatic assistance. We thank Barry Dickson, Udo Hacker, and Deborah Andrews for sharing prior to publication the dlg-RNAi, UAS-RhoGEF2, and UAS-rib flies, respectively. We also thank Robert Saint, Gary Hime, Akinao Nose, L. S. Shashidhara, and the Kyoto and the Bloomington stock center for various fly stocks, and the Developmental Studies Hybridoma Bank for antibodies. We acknowledge Flybase for the wealth of information that facilitated this study. We thank Sarah Russell, Kieran Harvey, members of the Richardson lab, Harvey lab, and Tissue Architecture groups for discussions and helpful suggestions during the course of this research, and to Linda Parsons and Nicola Grzeschik for critique of the manuscript. This genetic screen was supported by a R21 grant from the National Institutes of Health, NIH (1R21CA098997-01), and the follow-up analysis was funded in part from National Health & Medical Research (NHMRC) grants 350369 and 400211 and funds from the Peter MacCallum Cancer Center to H.E.R. and NHMRC grants to P.O.H. H.E.R. was supported by a NHMRC Senior Research Fellowship, P.O.H. by a NHMRC Biomedical Career Development Award Fellowship, A.M.B. by funds from the NIH and NHMRC 350396 and 509051 grants, S.L. by a NHMRC Neil Hamilton Farley Fellowship, and P.K. and R.G. by Australian Postgraduate Awards.
LITERATURE CITED
- Adachi-Yamada T., O'Connor M. B., 2004. Mechanisms for removal of developmentally abnormal cells: cell competition and morphogenetic apoptosis. J. Biochem. 136: 13–17 [DOI] [PubMed] [Google Scholar]
- Aigaki T., Ohsako T., Toba G., Seong K., Matsuo T., 2001. The gene search system: its application to functional genomics in Drosophila melanogaster. J. Neurogenet. 15: 169–178 [DOI] [PubMed] [Google Scholar]
- Arias-Romero L. E., Villamar-Cruz O., Pacheco A., Kosoff R., Huang M., et al. , 2010. A Rac–Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 29: 5839–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baonza A., Freeman M., 2005. Control of cell proliferation in the Drosophila eye by Notch signaling. Dev. Cell 8: 529–539 [DOI] [PubMed] [Google Scholar]
- Barrett K., Leptin M., Settleman J., 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91: 905–915 [DOI] [PubMed] [Google Scholar]
- Beaucher M., Hersperger E., Page-McCaw A., Shearn A., 2006. Metastatic ability of Drosophila tumors depends on MMP activity. Dev. Biol. 303: 625–634 [DOI] [PubMed] [Google Scholar]
- Bergantinos C., Corominas M., Serras F., 2010. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development 137: 1169–1179 [DOI] [PubMed] [Google Scholar]
- Bergmann A., Agapite J., McCall K., Steller H., 1998. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 95: 331–341 [DOI] [PubMed] [Google Scholar]
- Betschinger J., Mechtler K., Knoblich J. A., 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422: 326–330 [DOI] [PubMed] [Google Scholar]
- Bilder D., Perrimon N., 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N., 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289: 113–116 [DOI] [PubMed] [Google Scholar]
- Billuart P., Winter C. G., Maresh A., Zhao X., Luo L., 2001. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 107: 195–207 [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Radisky D., 2001. Putting tumours in context. Nat. Rev. Cancer 1: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake K. J., Myette G., Jack J., 1998. The products of ribbon and raw are necessary for proper cell shape and cellular localization of nonmuscle myosin in Drosophila. Dev. Biol. 203: 177–188 [DOI] [PubMed] [Google Scholar]
- Bradley P. L., Andrew D. J., 2001. ribbon encodes a novel BTB/POZ protein required for directed cell migration in Drosophila melanogaster. Development 128: 3001–3015 [DOI] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E., 2003. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22: 5769–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E., 2005. Using Drosophila melanogaster to map human cancer pathways. Nat. Rev. Cancer 5: 626–639 [DOI] [PubMed] [Google Scholar]
- Ciapponi L., Jackson D. B., Mlodzik M., Bohmann D., 2001. Drosophila Fos mediates ERK and JNK signals via distinct phosphorylation sites. Genes Dev. 15: 1540–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. L., Marshall C. J., Olson M. F., 2004. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell Biol. 5: 355–366 [DOI] [PubMed] [Google Scholar]
- Cordero J. B., Macagno J. P., Stefanatos R. K., Strathdee K. E., Cagan R. L., et al. , 2010. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev. Cell 18: 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S. K., Brugge J. S., 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30: 256–268 [DOI] [PubMed] [Google Scholar]
- DeNicola G. M., Tuveson D. A., 2009. RAS in cellular transformation and senescence. Eur. J. Cancer 45(Suppl. 1): 211–216 [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156 [DOI] [PubMed] [Google Scholar]
- Dow L. E., Elsum I. A., King C. L., Kinross K. M., Richardson H. E., et al. , 2008. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene 27: 5988–6001 [DOI] [PubMed] [Google Scholar]
- Fan Y., Bergmann A., 2008. Apoptosis-induced compensatory proliferation: the Cell is dead. Long live the Cell! Trends Cell Biol. 18: 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferres-Marco D., Gutierrez-Garcia I., Vallejo D. M., Bolivar J., Gutierrez-Avino F. J., et al. , 2006. Epigenetic silencers and Notch collaborate to promote malignant tumours by Rb silencing. Nature 439: 430–436 [DOI] [PubMed] [Google Scholar]
- Froldi F., Ziosi M., Garoia F., Pession A., Grzeschik N. A., et al. , 2010. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Murga C., Zohar M., Igishi T., Gutkind J. S., 1999. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 274: 5868–5879 [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Chikumi H., Gutkind J. S., 2000. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 485: 183–188 [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Chikumi H., Gutkind J. S., 2001. RGS-containing RhoGEFs: The missing link between transforming G proteins and Rho? Oncogene 20: 1661–1668 [DOI] [PubMed] [Google Scholar]
- Garlena R. A., Gonda R. L., Green A. B., Pileggi R. M., Stronach B., 2010. Regulation of mixed-lineage kinase activation in JNK-dependent morphogenesis. J. Cell Sci. 123: 3177–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateff E., 1978. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200: 1448–1459 [DOI] [PubMed] [Google Scholar]
- Gateff E., Schneiderman H. A., 1969. Neoplasms in mutant and cultured wild-tupe tissues of Drosophila. Natl. Cancer Inst. Monogr. 31: 365–397 [PubMed] [Google Scholar]
- Gomez del Pulgar T., Benitah S. A., Valeron P. F., Espina C., Lacal J. C., 2005. Rho GTPase expression in tumourigenesis: evidence for a significant link. BioEssays 27: 602–613 [DOI] [PubMed] [Google Scholar]
- Greco V., Hannus M., Eaton S., 2001. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106: 633–645 [DOI] [PubMed] [Google Scholar]
- Grusche F. A., Degoutin J. L., Richardson H. E., Harvey K. F., 2011. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev. Biol. 350: 255–266 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Amin N., Secombe J., Brumby A. M., Richardson H. E., 2007. Abnormalities in cell proliferation and apico-basal cell polarity are separable in Drosophila lgl mutant clones in the developing eye. Dev. Biol. 311: 106–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., Richardson H. E., 2010. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20: 573–581 [DOI] [PubMed] [Google Scholar]
- Hacker U., Perrimon N., 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12: 274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., et al. , 2002. Rac function and regulation during Drosophila development. Nature 416: 438–442 [DOI] [PubMed] [Google Scholar]
- Han S. Y., Kim S. H., Heasley L. E., 2002. Differential gene regulation by specific gain-of-function JNK1 proteins expressed in Swiss 3T3 fibroblasts. J. Biol. Chem. 277: 47167–47174 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A., 2000. The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Harden N., 2002. Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila. Differentiation 70: 181–203 [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Bilder D., 2006. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu. Rev. Genet. 40: 335–361 [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wolff T., Rubin G. M., 1994. Expression of baculovirus P35 prevents cell death in Drosophila. Development 120: 2121–2129 [DOI] [PubMed] [Google Scholar]
- Humbert P. O., Grzeschik N. A., Brumby A. M., Galea R., Elsum I., et al. , 2008. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27: 6888–6907 [DOI] [PubMed] [Google Scholar]
- Igaki T., 2009. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis 14: 1021–1028 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pagliarini R. A., Xu T., 2006. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16: 1139–1146 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pastor-Pareja J. C., Aonuma H., Miura M., Xu T., 2009. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16: 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J., Myette G., 1997. The genes raw and ribbon are required for proper shape of tubular epithelial tissues in Drosophila. Genetics 147: 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. A., 2009. Competitive interactions between cells: death, growth, and geography. Science 324: 1679–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. R., Lu B., Scott G. K., Kushner P. J., Smith I. E., et al. , 1999. Increased activator protein-1 DNA binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin. Cancer Res. 5: 251–256 [PubMed] [Google Scholar]
- Kanuka H., Hiratou T., Igaki T., Kanda H., Kuranaga E., et al. , 2005. Gain-of-function screen identifies a role of the Sec61alpha translocon in Drosophila postmitotic neurotoxicity. Biochim. Biophys. Acta 1726: 225–237 [DOI] [PubMed] [Google Scholar]
- Karim F. D., Rubin G. M., 1998. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125: 1–9 [DOI] [PubMed] [Google Scholar]
- Ke H., Harris R., Coloff J. L., Jin J. Y., Leshin B., et al. , 2010. The c-Jun NH2-terminal kinase 2 plays a dominant role in human epidermal neoplasia. Cancer Res. 70: 3080–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N. J., Davis R. J., 2003. Role of JNK in tumor development. Cell Cycle 2: 199–201 [PubMed] [Google Scholar]
- Kerman B. E., Cheshire A. M., Myat M. M., Andrew D. J., 2008. Ribbon modulates apical membrane during tube elongation through Crumbs and Moesin. Dev. Biol. 320: 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J. A., Sauer K., Jones L., Richardson H., Saint R., et al. , 1994. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77: 107–120 [DOI] [PubMed] [Google Scholar]
- Kockel L., Zeitlinger J., Staszewski L. M., Mlodzik M., Bohmann D., 1997. Jun in Drosophila development: redundant and nonredundant functions and regulation by two MAPK signal transduction pathways. Genes Dev. 11: 1748–1758 [DOI] [PubMed] [Google Scholar]
- Kurada P., White K., 1998. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell 95: 319–329 [DOI] [PubMed] [Google Scholar]
- Laviolette M. J., Nunes P., Peyre J. B., Aigaki T., Stewart B. A., 2005. A genetic screen for suppressors of Drosophila NSF2 neuromuscular junction overgrowth. Genetics 170: 779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Treisman J. E., 2001. The role of Wingless signaling in establishing the anteroposterior and dorsoventral axes of the eye disc. Development 128: 1519–1529 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L., 2001. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24: 251–254 [DOI] [PubMed] [Google Scholar]
- Lee T., Winter C., Marticke S. S., Lee A., Luo L., 2000. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron 25: 307–316 [DOI] [PubMed] [Google Scholar]
- Leong G. R., Goulding K. R., Amin N., Richardson H. E., Brumby A. M., 2009. scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs. BMC Biol. 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. F., Ishida H., Raziuddin R., Miki T., 2004. Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Czeta (PKCzeta) and regulates PKCzeta activity. Mol. Cell Biol. 24: 6665–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N., 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8: 1787–1802 [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E., 1997. Regulation of cell differentiation by the Drosophila Jun kinase cascade. Curr. Opin. Genet. Dev. 7: 666–671 [DOI] [PubMed] [Google Scholar]
- Massarwa R., Schejter E. D., Shilo B. Z., 2009. Apical secretion in epithelial tubes of the Drosophila embryo is directed by the Formin-family protein Diaphanous. Dev. Cell 16: 877–888 [DOI] [PubMed] [Google Scholar]
- Mattila J., Omelyanchuk L., Kyttala S., Turunen H., Nokkala S., 2005. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int. J. Dev. Biol. 49: 391–399 [DOI] [PubMed] [Google Scholar]
- Menendez J., Perez-Garijo A., Calleja M., Morata G., 2010. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc. Natl. Acad. Sci. USA 107: 14651–14656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Smith C. L., Long J. E., Eva A., Fleming T. P., 1993. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature 362: 462–465 [DOI] [PubMed] [Google Scholar]
- Mulinari S., Barmchi M. P., Hacker U., 2008. DRhoGEF2 and diaphanous regulate contractile force during segmental groove morphogenesis in the Drosophila embryo. Mol. Biol. Cell 19: 1883–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch A. L., Speck O., Stronach B., Fehon R. G., 2010. Rho1 regulates apoptosis via activation of the JNK signaling pathway at the plasma membrane. J. Cell Biol. 189: 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Thastrup J., Bottzauw T., Jaattela M., Kallunki T., 2007. c-Jun NH2-terminal kinase 2 is required for Ras transformation independently of activator protein 1. Cancer Res. 67: 178–185 [DOI] [PubMed] [Google Scholar]
- O'Keefe D. D., Prober D. A., Moyle P. S., Rickoll W. L., Edgar B. A., 2007. Egfr/Ras signaling regulates DE-cadherin/Shotgun localization to control vein morphogenesis in the Drosophila wing. Dev. Biol. 311: 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe L., Somers W. G., Harley A., Saint R., 2001. The pebble GTP exchange factor and the control of cytokinesis. Cell. Struct. Funct. 26: 619–626 [DOI] [PubMed] [Google Scholar]
- Olson M. F., Paterson H. F., Marshall C. J., 1998. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394: 295–299 [DOI] [PubMed] [Google Scholar]
- Padash Barmchi M., Rogers S., Hacker U., 2005. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol. 168: 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini R. A., Xu T., 2003. A genetic screen in Drosophila for metastatic behavior. Science 302: 1227–1231 [DOI] [PubMed] [Google Scholar]
- Patch K., Stewart S. R., Welch A., Ward R. E., 2009. A second-site noncomplementation screen for modifiers of Rho1 signaling during imaginal disc morphogenesis in Drosophila. PLoS One 4: e7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober D. A., Edgar B. A., 2000. Ras1 promotes cellular growth in the Drosophila wing. Cell 100: 435–446 [DOI] [PubMed] [Google Scholar]
- Prober D. A., Edgar B. A., 2002. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 16: 2286–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko S. N., Brumby A., O'Keefe L., Prior L., He Y., et al. , 1999. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 13: 2301–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C., Lopez-Gay J. M., Soldini D., Casas-Tinto S., Martin F. A., et al. , 2010. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell 18: 985–998 [DOI] [PubMed] [Google Scholar]
- Richardson H., O'Keefe L. V., Marty T., Saint R., 1995. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development 121: 3371–3379 [DOI] [PubMed] [Google Scholar]
- Richardson H. E., O'Keefe L. V., Reed S. I., Saint R., 1993. A Drosophila G1-specific cyclin E homolog exhibits different modes of expression during embryogenesis. Development 119: 673–690 [DOI] [PubMed] [Google Scholar]
- Ridley A. J., 2004. Rho proteins and cancer. Breast Cancer Res. Treat. 84: 13–19 [DOI] [PubMed] [Google Scholar]
- Robinson B. S., Huang J., Hong Y., Moberg K. H., 2010. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20: 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E., Marshall C. J., 2002. Rho-GTPases and cancer. Nat. Rev. Cancer 2: 133–142 [DOI] [PubMed] [Google Scholar]
- Schiff R., Reddy P., Ahotupa M., Coronado-Heinsohn E., Grim M., et al. , 2000. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J. Natl. Cancer Inst. 92: 1926–1934 [DOI] [PubMed] [Google Scholar]
- Settleman J., 2001. Rac 'n Rho: the music that shapes a developing embryo. Dev. Cell 1: 321–331 [DOI] [PubMed] [Google Scholar]
- Shim K., Blake K. J., Jack J., Krasnow M. A., 2001. The Drosophila ribbon gene encodes a nuclear BTB domain protein that promotes epithelial migration and morphogenesis. Development 128: 4923–4933 [DOI] [PubMed] [Google Scholar]
- Somers W. G., Saint R., 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4: 29–39 [DOI] [PubMed] [Google Scholar]
- Sorlie T., Perou C. M., Tibshirani R., Aas T., Geisler S., et al. , 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98: 10869–10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T., Tibshirani R., Parker J., Hastie T., Marron J. S., et al. , 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 100: 8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S., Diaz-Meco M. T., Caminero E., Moscat J., Campuzano S., 2004. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166: 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C., Neo S. Y., McShane L. M., Korn E. L., Long P. M., et al. , 2003. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 100: 10393–10398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O., Hughes S. C., Noren N. K., Kulikauskas R. M., Fehon R. G., 2003. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature 421: 83–87 [DOI] [PubMed] [Google Scholar]
- Srivastava A., Pastor-Pareja J. C., Igaki T., Pagliarini R., Xu T., 2007. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. USA 104: 2721–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach B., 2005. Dissecting JNK signaling, one KKKinase at a time. Dev. Dyn. 232: 575–584 [DOI] [PubMed] [Google Scholar]
- Sun G., Irvine K. D., 2011. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev. Biol. 350: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus B., Schwartz M. A., Theodorescu D., 2005. Rho proteins in cell migration and metastasis. Crit. Rev. Eukaryot. Gene Expr. 15: 103–114 [DOI] [PubMed] [Google Scholar]
- Toba G., Ohsako T., Miyata N., Ohtsuka T., Seong K. H., et al. , 1999. The gene search system: a method for efficient detection and rapid molecular identification of genes in Drosophila melanogaster. Genetics 151: 725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M., Bohmann D., 2006. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25: 5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Warner S., Read R., Cagan R. L., 2007. Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res. 67: 10278–10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Nebreda A. R., 2009. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9: 537–549 [DOI] [PubMed] [Google Scholar]
- Warner S. J., Yashiro H., Longmore G. D., 2010. The Cdc42/Par6/aPKC polarity complex regulates apoptosis-induced compensatory proliferation in epithelia. Curr. Biol. 20: 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser M., Chia W., 2000. The EAST protein of Drosophila controls an expandable nuclear endoskeleton. Nat. Cell. Biol. 2: 268–275 [DOI] [PubMed] [Google Scholar]
- Weber U., Paricio N., Mlodzik M., 2000. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development 127: 3619–3629 [DOI] [PubMed] [Google Scholar]
- Weston C. R., Davis R. J., 2007. The JNK signal transduction pathway. Curr. Opin. Cell. Biol. 19: 142–149 [DOI] [PubMed] [Google Scholar]
- Whitmarsh A. J., Davis R. J., 2007. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene 26: 3172–3184 [DOI] [PubMed] [Google Scholar]
- Whyte J., Bergin O., Bianchi A., McNally S., Martin F., 2009. Key signalling nodes in mammary gland development and cancer: mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 11: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann T. J., Dahmann C., 2009. Dpp signaling promotes the cuboidal-to-columnar shape transition of Drosophila wing disc epithelia by regulating Rho1. J. Cell Sci. 122: 1362–1373 [DOI] [PubMed] [Google Scholar]
- Woodhouse E., Hersperger E., Shearn A., 1998. Growth, metastasis, and invasiveness of Drosophila tumors caused by mutations in specific tumor suppressor genes. Dev. Genes Evol. 207: 542–550 [DOI] [PubMed] [Google Scholar]
- Wu M., Pastor-Pareja J. C., Xu T., 2010. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature 463: 545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Lu Q., Fang X., Adler P. N., 2009. Rho1 has multiple functions in Drosophila wing planar polarity. Dev. Biol. 333: 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhou W., Yin C., Chen W., Ozawa R., et al. , 2006. Misexpression screen for genes altering the olfactory map in Drosophila. Genesis 44: 189–201 [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Adams A. E., Ridky T. W., Tao S., Khavari P. A., 2007. Tumor necrosis factor receptor 1/c-Jun-NH2-kinase signaling promotes human neoplasia. Cancer Res. 67: 3827–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Xin T., Weng S., Gao Y., Zhang Y., et al. , 2010. Activation of JNK signaling links lgl mutations to disruption of the cell polarity and epithelial organization in Drosophila imaginal discs. Cell. Res. 20: 242–245 [DOI] [PubMed] [Google Scholar]