Abstract
OBJECTIVE
The goals of this study were to examine trajectories of blood pressure (BP) in adults with diabetes and investigate the association of trajectory patterns with mortality.
RESEARCH DESIGN AND METHODS
A nonconcurrent longitudinal design was used to monitor 3,766 Medicare patients with diabetes from 2005 through 2008. Data were extracted from a registry of Medicare beneficiaries, which was developed by a large academic practice that participated in the Physician Group Practice Medicare Demonstration. The relationship between BP trajectories and all-cause mortality was modeled using multilevel mixed-effects linear regression.
RESULTS
During the 4-year study period, 10.7% of the patients died, half of whom were aged ≥75 years. The crude and adjusted models both showed a greater decline in systolic and diastolic BP in patients who died than in those who did not die. In a model adjusted for age, sex, race, medications, and comorbidities, the mean systolic BP decreased by 3.2 mmHg/year (P < 0.001) in the years before death and by 0.7 mmHg/year (P < 0.001) in those who did not die (P < 0.001 for the difference in slopes). Similarly, diastolic BP declined by 1.3 mmHg/year for those who died (P < 0.001) and by 0.6 mmHg/year for those who did not die (P < 0.001); the difference in slopes was significant (P = 0.021).
CONCLUSIONS
Systolic and diastolic BP both declined more rapidly in the 4 years before death than in patients who remained alive.
Blood pressure (BP) is usually recorded at every visit to the physician, yet patterns of BP over time in populations are not often published. BP trajectories have been reported in longitudinal studies of children (1,2), but trajectories in adults with diabetes are usually ancillary to treatment trials for hypertension, and the results are explained in terms of the assigned medication (3,4). In healthy adults evaluated longitudinally, systolic BP is relatively stable until approximately age 50, after which there is a steady increase with age (5). Diastolic BP increases longitudinally with age in healthy adult men, but in women, it increases in middle age and declines slightly after age 70 (5). In the Framingham population, systolic BP increased from age 30 through 84 but diastolic BP increased in middle age (40–60 years) and then declined afterward (6). This follows a similar pattern to that reported with cross-sectional data from the National Health and Nutrition Examination Study (7,8). In the Göteberg population, systolic BP increased until age 75 and decreased between 75 and 79 in those without antihypertensive therapy, whereas diastolic BP increased during middle age and then decreased after age 75 (9).
Missing from the medical literature are longitudinal BP trajectories in populations with diabetes. In particular, BP trajectories before death have yet to be reported. Therefore, we investigated patterns of BP over time in a longitudinal study of Medicare patients within a diabetes registry.
RESEARCH DESIGN AND METHODS
Type of study
We conducted a nonconcurrent longitudinal study using secondary data analysis of electronic medical record information linked to Medicare claims.
Study population
We studied 3,766 Medicare beneficiaries with diabetes, attributed by the Centers for Medicare & Medicaid Services (CMS) to the University of Michigan Faculty Group Practice based on their participation in the Physician Group Practice Demonstration (10), who were entered into the University of Michigan diabetes registry from 2005 through 2008. Patients in the diabetes registry are adults (aged ≥18 years) who have met the following three criteria:
have a diagnosis of diabetes in the Problem Summary List or a diagnosis of diabetes in the past 3 years with at least two face-to-face encounters in an ambulatory care setting or one face-to-face encounter in an acute care setting such as the emergency department;
have received antihyperglycemic medication, diabetic supplies (strips/lancets/glucometer), have a HbA1c >6.4% or two or more glucose results ≥200 mg/dL; and
were seen by a primary care physician, geriatric, or an endocrinology specialist at least twice in the past 2 years, with one of those visits in the past 395 days.
Data
CMS provided complete claims data each year for the beneficiaries assigned to the Physician Group Practice Demonstration project. National Claims History files contained information on beneficiary diagnoses, utilization of services, and Medicare payments made to providers for these services. Medicare enrollment data included age, sex, and geographic information.
The diabetes registry is a central repository for clinical data from various systems related to disease management and preventive care services. During the study period, registry information was updated between every day and monthly (varying by data element), and patient-centered summary reports were produced every 6 months. Eight reporting cycles occurred from 2005 through 2008, two for each of the 4 years. Accordingly, patients in the study may have had as many as eight BP reports. To study trends longitudinally, patients must have had at least two reports to be included in this study cohort.
Variables and their measurement
Data used for this analysis included age, sex, race, Medicaid insurance, height and weight, medications (statins, insulin, ACE inhibitors, angiotensin receptor blockers [ARB]), and the most recent HbA1c and LDL cholesterol levels. BMI was calculated from height and weight. Death was determined from the Medicare enrollment file and the medical records. Comorbidities were obtained from Medicare claims, and included specific diseases as well as the annual Medicare assignment of Hierarchical Condition Categories (HCCs), which were used to derive the overall burden of disease (i.e., severity scores) (11). Individual comorbid conditions included congestive heart failure (CHF), renal failure or dialysis, severe malignancies (digestive tract, lung, and other severe cancers; acute leukemia; metastatic cancer), liver disease (end-stage liver disease, cirrhosis of the liver, or chronic hepatitis), and stroke (cerebral hemorrhage, ischemic or unspecified stroke, or hemiplegia/hemiparesis).
The BP measurements included in this study were the last BP recorded within each of the 6-month reporting periods. BP measurements during hospitalization or emergency department visits were excluded. For each patient, BP was determined by usual clinical practice during the outpatient office visit. Therefore, the procedure for measuring BP was similar to that used for clinical decision making at the point of care and was based on individual clinic processes and the judgment of the providers.
Analytic strategy
The participants represented a fluid cohort because various patients entered and/or left the study at various calendar times during the 4-year period. BP was recorded in 1,195 of the 3,766 subjects (32%) at each 6-month period during the course of the entire 4-year study (i.e., eight recorded BPs); BP was recorded at two periods for 410 (11%), with the remaining subjects observed for three to seven periods. To ensure that we were comparing a similar period before death for the patients who died, the BPs recorded before death were aligned (i.e., right-justified); thus, BP trajectories reflected the series of last BPs recorded before death. The main results are presented as right-justified for all patients, and sensitivity analyses were conducted using left-justification (i.e., BPs recorded as the patients entered the cohort).
Multilevel mixed-effects linear regression was the principal method of analysis. The likelihood ratio test for nested models was used to assess the significance of random intercepts and random coefficients (i.e., slopes). Trajectories were calculated using the fixed-portion linear predictor plus the contribution determined by the predicted random effects. An interaction term was used to evaluate significant differences in slopes between different subgroups. Age of the patient at the time of the report, ACE inhibitors or ARB drugs, statins, insulin, values of HbA1c and LDL cholesterol, BMI, and disease severity score (HCC) were treated as time-varying covariates in the final regression models. The α was set at 0.05 (two-tailed).
Some data were missing on various 6-month reports for HbA1c (5%), LDL cholesterol (16%), HCC score (22%), BMI (23%), and insulin (35%) during the course of the 4-year study. Because this was a large group, we chose not to impute values. Rather, we conducted a sensitivity analysis that included these additional variables in a fully adjusted regression model.
This study was approved by the University of Michigan IRB and conducted under a Data Use Agreement with CMS.
RESULTS
In this cohort, 32% of patients were aged <65 and 30% were ≥75 years at the time of the initial visit (Table 1). Women accounted for 54% of subjects, and 80% of the patients were white. At the time of entry into the study, 68% of patients were receiving ACE/ARB therapy, and 66% were receiving statin medications. There were 404 patients (10.7%) who died, half of whom were aged ≥75 years at the initial visit. Increasing age and male sex were risk factors for death, whereas statin therapy was protective. CHF, dialysis or renal failure, stroke, liver disease, and malignancy were significant predictors of death.
Table 1.
Characteristics of patients with diabetes at baseline and mortality during the study period
| Variables | All subjects | Mortality | P |
|---|---|---|---|
| Age (years) | |||
| <65 | 1,215 (32.3) | 96 (7.9) | |
| 65–69 | 745 (19.8) | 48 (6.4) | |
| 70–74 | 679 (18.0) | 57 (8.4) | |
| >75 | 1,127 (29.9) | 203 (18.0) | <0.001 |
| Sex | |||
| Male | 1,747 (46.4) | 212 (12.1) | |
| Female | 2,019 (53.6) | 192 (9.5) | 0.009 |
| Race | |||
| White | 3,026 (80.4) | 335 (11.1) | |
| African American | 482 (12.8) | 47 (9.8) | |
| Other | 258 (6.8) | 22 (8.5) | 0.340 |
| Payer | |||
| Medicaid* | 714 (19.0) | 79 (11.1) | |
| Other | 3,052 (81.0) | 325 (10.6) | 0.747 |
| ACE/ARB therapy | |||
| Yes | 2,572 (68.3) | 264 (10.3) | |
| No | 1,194 (31.7) | 140 (11.7) | 0.178 |
| Statin therapy | |||
| Yes | 2,473 (65.7) | 241 (9.7) | |
| No | 1,293 (34.3) | 163 (12.6) | 0.007 |
| CHF | |||
| Yes | 753 (20.0) | 179 (23.8) | |
| No | 3,013 (80.0) | 225 (7.5) | <0.001 |
| Dialysis or renal failure | |||
| Yes | 639 (17.0) | 169 (26.4) | |
| No | 3,127 (83.0) | 235 (7.5) | <0.001 |
| Stroke or hemiplegia | |||
| Yes | 246 (6.5) | 53 (21.5) | |
| No | 3,520 (93.5) | 351 (10.0) | <0.001 |
| Liver disease | |||
| Yes | 73 (1.9) | 23 (31.5) | |
| No | 3,693 (98.1) | 381 (10.3) | <0.001 |
| Metastatic cancer | |||
| Yes | 117 (3.1) | 39 (33.3) | |
| No | 3,649 (96.9) | 365 (10.0) | <0.001 |
Data are presented as n (%).
*Medicaid dually eligible and Medicaid only.
Systolic and diastolic BP both significantly declined before death in patients with diabetes. In an unadjusted model, mean systolic BP declined 3.1 mmHg (95% CI 1.9–4.3) and mean diastolic BP declined 1.6 mmHg (0.9–2.3) annually before death. In the adjusted model (Table 2), systolic pressure decreased by 3.2 mmHg/year (P < 0.001) in those who died and by 0.7 mmHg/year (P < 0.001) in those who did not die (P < 0.001 for the difference in slopes between trajectories). Diastolic pressure declined by 1.3 mmHg/year for those who died (P < 0.001) and by 0.6 mmHg/year for those who did not die (P < 0.001); the difference in slopes remained significant (P = 0.021). The addition of a quadratic term to detect a nonlinear change in slope was not significant for systolic (P = 0.965) or diastolic BP (P = 0.761) in the adjusted models. BP trajectories generated from the adjusted models are shown in Fig. 1 and Fig. 2, with stratification by mortality.
Table 2.
Annual mean change in BP in patients with diabetes, by mortality
| Group | Patients n | Annual change in BP (mmHg) | 95% CI | P interaction |
|---|---|---|---|---|
| Systolic BP | ||||
| Adjusted model:* | ||||
| Alive | 3,362 | −0.7 | −0.4 to −0.9 | |
| Died | 404 | −3.2 | −2.2 to −4.2 | <0.001 |
| Fully adjusted model† | ||||
| Alive | 2,251 | −0.5 | −0.02 to −1.1 | |
| Died | 169 | −6.2 | −3.0 to −9.4 | 0.001 |
| Diastolic BP | ||||
| Adjusted model* | ||||
| Alive | 3,362 | −0.6 | −0.5 to −0.7 | |
| Died | 404 | −1.3 | −0.7 to −1.9 | 0.021 |
| Fully adjusted model† | ||||
| Alive | 2,251 | −0.4 | −0.05 to −0.7 | |
| Died | 169 | −3.4 | −1.5 to −5.3 | 0.002 |
*Adjusted for age, sex, race, Medicaid, ACE inhibitors or ARBs, statins, congestive heart failure, dialysis or renal failure, malignancies, liver disease, and stroke.
†Adjusted for age, sex, race, Medicaid, ACE inhibitors or ARBs, statins, congestive heart failure, dialysis or renal failure, malignancies, liver disease, stroke, BMI, HbA1c, LDL cholesterol, insulin, and disease severity score (HCC).
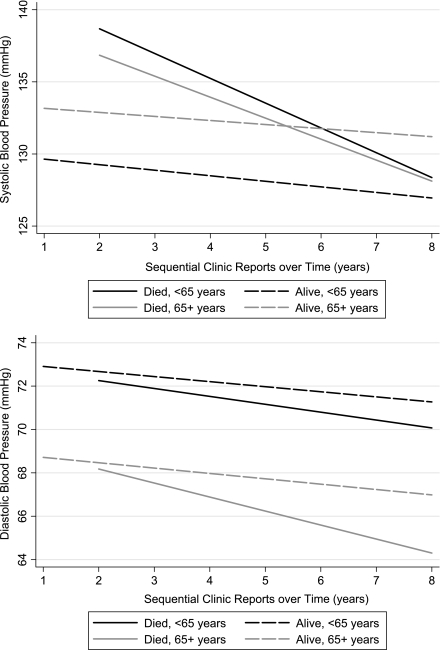
Figure 1.
Mean systolic and diastolic BP trajectories for patients with diabetes mellitus, by age and mortality.
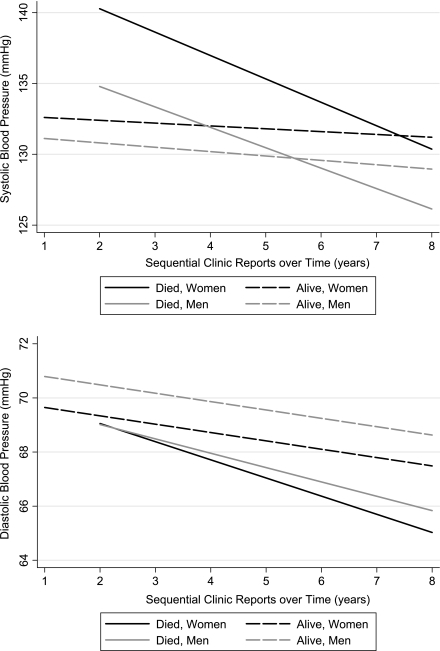
Figure 2.
Mean systolic and diastolic BP trajectories for patients with diabetes mellitus, by sex and mortality.
In the fully adjusted model, mean systolic BP significantly increased with age (0.23 mmHg for each year; P < 0.001), female sex (1.57 mmHg; P = 0.004), African American race (3.14 mmHg; P < 0.001), BMI (0.11 mmHg for each kg/m2 increase; P = 0.001), and for those with renal failure or receiving dialysis (5.08 mmHg; P < 0.001). Mean systolic BP significantly decreased with Medicaid insurance (−2.05 mmHg; P = 0.006) and in those with CHF (−4.03 mmHg; P < 0.001). Mean diastolic BP significantly increased with African American race (2.58 mmHg; P < 0.001) and BMI (0.07 mmHg; P = 0.001). Mean diastolic BP significantly decreased with age (−0.19 mmHg; P < 0.001), female sex (−1.09 mmHg; P = 0.001), and in those with CHF (−1.27 mmHg; P = 0.003). Some of these findings are illustrated in Fig. 1 and Fig. 2; the increased systolic BP with older age can be seen by the shift in the BP trajectories on the y-axis. Systolic BP is greater in older patients, whereas diastolic BP is lower in older patients. Women have higher systolic but lower diastolic BP than men.
In secondary analyses, the addition of HbA1c, LDL cholesterol, BMI, insulin, and severity risk score did not substantially change the major findings (Table 2, fully adjusted model). The decline in systolic BP was particularly evident (−6.2 mmHg/year) in those who died. We also evaluated potential differences in the slope of BP trajectories for those with and without CHF (regardless of mortality); the interaction term was not significant for systolic BP (P = 0.951) or diastolic BP (P = 0.056). Similarly, there was no effect modification by younger age (<65 years) versus older age (≥65 years); the interaction terms for differences in slope were not significant for systolic (P = 0.184) or diastolic BP (P = 0.979).
We repeated the statistical analyses using left-justification of the BP measurements; the results did not appreciably change. The rate of decline of BP was greater in those patients who died than in patients who remained alive (P < 0.001 for systolic BP; P = 0.013 for diastolic BP). Systolic BP decreased by 2.7 mmHg/year (95% CI 1.7–3.7) and diastolic BP decreased by 1.5 mmHg/year (95% CI 0.9–2.1) in those who died. Systolic BP declined by 0.7 mmHg/year (95% CI 0.5–0.9) and diastolic BP declined by 0.7 (95% CI 0.6–0.9) in the patients who remained alive.
Of the 404 patients who died, 62 (15%) died between the last clinic visit and the end of the reporting period, 86 (21%) died within 8 weeks of the last reporting period, 158 (39%) died within 8 to 24 weeks of the last reporting period, and 98 (24%) died >24 weeks from the last reporting period.
CONCLUSIONS
Mean systolic and diastolic BP both declined in the years before death in patients with diabetes. This annual rate of decline was significantly more marked (−3.2 mmHg systolic and −1.3 mmHg diastolic) than in the patients who remained alive. Moreover, the decline was evident after adjustment for major organ failure—heart, kidney, and liver—as well as for common causes of death such as malignancies and stroke. These findings may signal an underlying population of vulnerable patients, although it is unclear whether the BP decline is merely a symptom of this vulnerability or is one of several physiologic contributors to the dying process.
The rate of change in BP has been of lesser significance than threshold values that serve as guides to initiation or adherence to antihypertensive therapy (12). Studies of outcomes in those who experience significant changes (either increases or decreases) in BP would be appropriate. Although hypertension in middle age is a risk factor for death, it is not clear whether there is a point at which therapy should be re-evaluated in the very old or in those with a marked rate of BP decline. A similar phenomenon has been found in cohorts evaluated for the incidence of dementia: elevated BP in earlier life increases the risk, but a decline in BP is evident in the 5 years just before diagnosis (13).
There are few studies that plot the trajectories of BP in patients with diabetes. Maddox et al. (14) plotted BP trajectories over time in patients with diabetes and/or chronic kidney disease who were newly diagnosed with coronary artery disease. Their results indicated that good control of BP decreased the risk of myocardial infarction and the risk of revascularization, although overall mortality did not improve.
There is some corroborating evidence for our findings. Analysis of a cohort monitored for a median of 9.8 years showed the risk of death in patients with type 2 diabetes increased by 15% for every 10-mmHg decrease in systolic BP and increased by 22% for every 10-mmHg decrease in diastolic BP (15). This inverse relationship was particularly evident in the oldest age group. Similarly, Rönnback et al. (16) reported that low systolic and diastolic BP predicted elevated all-cause and cardiovascular deaths in patients with type 2 diabetes during a median follow-up of 9.5 years.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that intensive hypertensive therapy did not reduce mortality in patients with type 2 diabetes; the hazard ratio for intensive versus standard BP therapy was 1.07 (95% CI 0.85–1.35) for all-cause mortality (3). Likewise, Cooper-DeHoff et al. (17) found that tight control of systolic BP in patients with diabetes and coronary artery disease did not improve mortality. The hazard ratio for all-cause mortality was 1.20 (95% CI 0.99–1.45); again, suggesting a nonsignificant excess of deaths in patients with tight BP control compared with those receiving usual care. They also showed that systolic BP of <110 mmHg was associated with an increase in all-cause mortality compared with systolic BP of 125 to 130 mmHg.
The UK Prospective Diabetes Study Group found that tight BP control reduced diabetes-related deaths by 32% without a significant reduction in all-cause mortality after a median follow-up of 8.4 years (18). However, the Appropriate Blood Pressure Control in Diabetes (ABCD) trial demonstrated a decrease in all-cause mortality (P = 0.037) for intensive versus moderate BP therapy in patients with type 2 diabetes during a period of 5.3 years, despite showing no statistically significant differences in myocardial infarction, cerebrovascular, or congestive heart failure events (19).
Previous authors have noted a concern regarding aggressive treatment in older patients (20,21). A meta-analysis of randomized controlled trials showed treatment of hypertension did not yield a significant reduction in all-cause mortality in patients aged ≥80 years (20). Moreover, a nonsignificant relative excess of all-cause death was noted in those who received antihypertensive treatment. However, only 14% of the participants had a history of diabetes. Bulpitt et al. (21) reported similar findings in an open pilot trial of patients aged ≥80 years, although the proportion with diabetes was not stated. In contrast, antihypertensive treatment in mostly nondiabetic individuals aged ≥80 years aimed to a BP target of 150/80 mmHg significantly reduced the rate of death from any cause by 21% (22).
Our investigation did not determine the reason for the declining BP before death, and therefore, we advise caution in the interpretation. We have little evidence to indicate whether treatment intensification played a role or whether declining BP is a harbinger of the dying process in certain patients. Our results suggest that the decrease in BP is not merely due to the presence of CHF or increased age. It is important to recognize that the decline was observed in the mean pressures in a population and was not observed in all individuals. Because this was a registry-based study, the findings should be investigated in a large, population-based cohort of patients with diabetes. It is unclear whether declining BP could be a reliable predictor of mortality or whether the patients in this registry differ from the overall population in ways that this investigation did not capture.
In conclusion, it may be useful to plot BPs over time in populations, similar to what many physicians do for individual patients with diabetes. The trajectory of BP leading up to death can be revealing. Our results suggest that a significant decline occurs in both systolic and diastolic BP several years before death in populations of patients with diabetes.
Acknowledgments
This project was funded by the Agency for Health Care Research and Quality (AHRQ R21 HS017625-01), the Ann Arbor VA Geriatric Research, Education and Clinical Center, the Ann Arbor VA QUERI-Diabetes (DIB 98-001), and the Michigan Institute for Clinical & Health Research (UL1RR024986).
No potential conflicts of interest relevant to this article were reported.
M.A.M.R. contributed to the conception and design, writing the manuscript, and interpretation of the results and performed the statistical analyses. K.W. contributed to the conception, data extraction, database management, writing the manuscript, and interpretation of the results. T.R.G., H.M.C., and P.G.L. contributed to data collection, writing the manuscript, and interpretation of the results. S.J.B. and C.S.B. contributed to the conception and design, data collection, writing the manuscript, and interpretation of the results.
Parts of this work were presented in abstract form at the 3rd North American Congress of Epidemiology, Montreal, QC, Canada, 21–24 June 2011.
References
- 1.Hlaing WM, Prineas RJ, Zhu Y. Trajectory of systolic blood pressure in children and adolescents. Ann Epidemiol 2006;16:11–18 [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation 2006;114:2780–2787 [DOI] [PubMed] [Google Scholar]
- 3.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Neil HAW, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–1576 [DOI] [PubMed] [Google Scholar]
- 5.Pearson JD, Morrell CH, Brant LJ, Landis PK, Fleg JL. Age-associated changes in blood pressure in a longitudinal study of healthy men and women. J Gerontol A Biol Sci Med Sci 1997;52:M177–M183 [DOI] [PubMed] [Google Scholar]
- 6.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–315 [DOI] [PubMed] [Google Scholar]
- 7.Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021–1024 [DOI] [PubMed] [Google Scholar]
- 8.Joffres MR, Hamet P, MacLean DR, L’italien GJ, Fodor G. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens 2001;14:1099–1105 [DOI] [PubMed] [Google Scholar]
- 9.Landahl S, Bengtsson C, Sigurdsson JA, Svanborg A, Svärdsudd K. Age-related changes in blood pressure. Hypertension 1986;8:1044–1049 [DOI] [PubMed] [Google Scholar]
- 10.Kautter J, Pope GC, Trisolini M, Grund S. Medicare physician group practice demonstration design: quality and efficiency pay-for-performance. Health Care Financ Rev 2007;29:15–29 [PMC free article] [PubMed] [Google Scholar]
- 11.Pope G, Ellis RP, Ash AS, et al. Diagnostic cost group hierarchical condition category models for medicare risk adjustment [Internet]. Baltimore, MD, Centers for Medicare & Medicaid Services. Available from http://www.cms.gov/reports/reports/itemdetail.asp?itemid=CMS023176 Accessed 19 February 2011
- 12.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145 [DOI] [PubMed] [Google Scholar]
- 14.Maddox TM, Ross C, Tavel HM, et al. Blood pressure trajectories and associations with treatment intensification, medication adherence, and outcomes among newly diagnosed coronary artery disease patients. Circ Cardiovasc Qual Outcomes 2010;3:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Hateren KJ, Landman GW, Kleefstra N, et al. Lower blood pressure associated with higher mortality in elderly diabetic patients (ZODIAC-12). Age Ageing 2010;39:603–609 [DOI] [PubMed] [Google Scholar]
- 16.Rönnback M, Isomaa B, Fagerudd J, et al. ; Botnia Study Group Complex relationship between blood pressure and mortality in type 2 diabetic patients: a follow-up of the Botnia Study. Hypertension 2006;47:168–173 [DOI] [PubMed] [Google Scholar]
- 17.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 19.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(Suppl. 2):B54–B64 [PubMed] [Google Scholar]
- 20.Gueyffier F, Bulpitt C, Boissel JP, et al. ; INDANA Group Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. Lancet 1999;353:793–796 [DOI] [PubMed] [Google Scholar]
- 21.Bulpitt CJ, Beckett NS, Cooke J, et al. ; Hypertension in the Very Elderly Trial Working Group Results of the pilot study for the hypertension in the very elderly trial. J Hypertens 2003;21:2409–2417 [DOI] [PubMed] [Google Scholar]
- 22.Beckett NS, Peters R, Fletcher AE, et al. ; HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898 [DOI] [PubMed] [Google Scholar]