Abstract
OBJECTIVE
To evaluate the long-term intervention effects of oral insulin on the development of type 1 diabetes and to assess the rate of progression to type 1 diabetes before and after oral insulin treatment was stopped in the Diabetes Prevention Trial–Type 1 (DPT-1).
RESEARCH DESIGN AND METHODS
The follow-up included subjects who participated in the early intervention of oral insulin (1994–2003) to prevent or delay type 1 diabetes. A telephone survey was conducted in 2009 to determine whether diabetes had been diagnosed and, if not, an oral glucose tolerance test (OGTT), hemoglobin A1c (HbA1c), and autoantibody levels were obtained on all subjects who agreed to participate.
RESULTS
Of 372 subjects randomized, 97 developed type 1 diabetes before follow-up; 75% of the remaining 275 subjects were contacted. In the interim, 77 subjects had been diagnosed with type 1 diabetes and 54 of the remainder have had an OGTT; 10 of these were diagnosed with type 1 diabetes, subsequently. Among individuals meeting the original criteria for insulin autoantibodies (IAAs) (≥80 nU/mL), the overall benefit of oral insulin remained significant (P = 0.05). However, the hazard rate in this group increased (from 6.4% [95% CI 4.5–9.1] to 10.0% [7.1–14.1]) after cessation of therapy, which approximated the rate of individuals treated with placebo (10.2% [7.1–14.6]).
CONCLUSIONS
Overall, the oral insulin treatment effect in individuals with confirmed IAA ≥80 nU/mL appeared to be maintained with additional follow-up; however, once therapy stopped, the rate of developing diabetes in the oral insulin group increased to a rate similar to that in the placebo group.
In the Diabetes Prevention Trial–Type 1 (DPT-1), conducted from 1994 to 2003, oral insulin or placebo was administered to nonaffected relatives of type 1 probands ascertained to have a 26–50% risk of developing diabetes over a 5-year period (1,2). In this trial, 103,391 relatives of type 1 diabetic patients were screened and 97,273 samples for antibodies (Abs) were analyzed. There were 372 subjects enrolled and randomized. After approximately one-third of the subjects were recruited, the insulin autoantibody (IAA) entry criteria were lowered from 80 to 39 nU/mL. At study end, there was no beneficial effect observed overall (1). However, it was noted that oral insulin resulted in a significant delay in type 1 diabetes (P = 0.04) in individuals recruited before the change in eligibility criteria (i.e., having an IAA level ≥80 nU/mL) and all those accrued who met the original eligibility criteria (IAA level ≥80 nU/mL) (P = 0.015); the annualized type 1 diabetes rate was 6.2% during oral insulin treatment and 10.4% with placebo, with a delay in diabetes progression by 4.5 years (1).
In this follow-up study, we evaluated the long-term effects of oral insulin on the development of type 1 diabetes and assessed the rate of progression to type 1 diabetes before and after oral insulin treatment was stopped.
RESEARCH DESIGN AND METHODS
Screening, staging, and randomization of DPT-1 subjects and other study methods have been described (1). The original double-masked oral insulin trial enrolled 372 subjects with a projected 5-year risk of diabetes of 26–50% (60% male, 88% Caucasian, median age 10.3 years) between 1994 and 2002 (median follow-up of 4.3 years). Participants were randomly assigned to 7.5 mg oral insulin or placebo intervention once a day.
Follow-up study
In 2009, the Type 1 Diabetes TrialNet Network funded a follow-up study of the DPT-1 oral insulin trial subjects to determine whether the beneficial effect was prolonged. Each of the eight DPT-1 centers contacted those subjects eligible for recontact on the basis of the following criteria: 1) they were diabetes-free at time of last contact and 2) they agreed to future contact on the DPT-1 consent form. The study was completed in two phases. Phase 1 consisted of a questionnaire follow-up by phone to eligible subjects to determine current diabetes status, date of diagnosis, diabetes treatment, symptoms at time of diagnosis, most recent diabetes-associated laboratory work before diagnosis, and willingness to return for a clinical visit if diabetes-free. Subjects who were diabetes-free were eligible for phase 2, which consisted of an in-person clinic visit for a one-time oral glucose tolerance test (OGTT) to assess glycemic status, hemoglobin A1c (HbA1c), C-peptide, and Ab testing.
The protocol was approved by the institutional review boards at participating locations, and all participants provided written informed consent.
Laboratory measures
All test procedures and assays performed have been described (3). The TrialNet biochemical antibody laboratory had sensitivities and specificities of 76/99% for GAD, 64/100% for islet cell antibody (ICA)512, and 58/99% for micro-IAA (mIAA) on the basis of the 2005 Diabetes Antibody Standardization Program (4). The OGTT includes samples for glucose and C-peptide levels at 0, 30, 60, 90, and 120 min. HbA1c was measured in a single reference laboratory.
Statistical analysis
Data were analyzed using the Statistical Analysis System Software (version 9.2; SAS Institute, Cary, NC). All variables not normally distributed were log-transformed for analysis. The area under the curve (AUC) C-peptide was calculated using the trapezoid rule. The intention-to-treat principle was used for this analysis. Kaplan-Meier life tables were used to determine the time to type 1 diabetes onset by treatment group and were compared using the log-rank χ2 statistic. Categorical variables were analyzed using Pearson χ2 tests or Fisher exact test. Mean differences were tested using ANOVA. Data were summarized using mean (SD) or median (interquartile range). All tests for significance were two-tailed. Cox proportional hazards models were used to estimate the risk for type 1 diabetes by treatment adjusted for time on study and time since treatment ended.
RESULTS
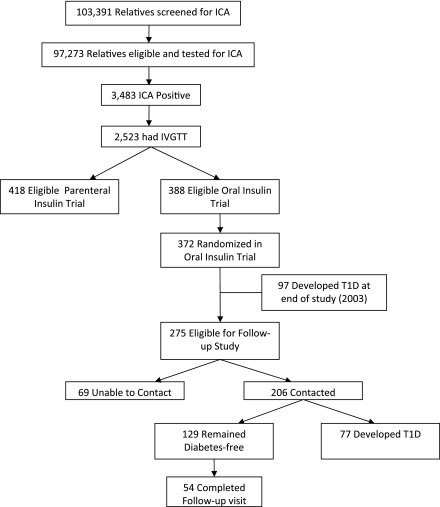
Of the 372 subjects who participated in the original DPT-1 study, 275 were eligible for the follow-up study; 97 developed type 1 diabetes at the end of the study in 2003 (0.02 [Q1–Q3: 0–0.5] median years after treatment to type 1 diabetes diagnosis) (Fig. 1). Through the 2009 follow-up study, 206 (75%) of the eligible subjects were contacted with the median follow-up time of 9.1 years (107 were on oral insulin and 99 were on placebo). A total of 37% (n = 77) had developed type 1 diabetes (median 3.7 [2.0–5.3] years after treatment to type 1 diabetes diagnosis); 71% (n = 92, 49 were on oral insulin and 44 on placebo during trial) of the 129 subjects diabetes-free on contact agreed to a clinic visit to complete an OGTT, HbA1c, and Ab testing and 59% (n = 54) completed a follow-up clinic visit. Of these (28 were on oral insulin and 26 on placebo during the trial), OGTT testing identified 26% (n = 14) with impaired glucose tolerance, 11% (n = 6) with asymptomatic type 1 diabetes, and 7% (n = 4) with symptomatic type 1 diabetes. There were no significant changes between baseline and follow-up measures of HbA1c (P = 0.99), GAD65 positivity (P = 0.11), mIAA positivity (P = 0.99), or ICA512 positivity (P = 0.43) in subjects who completed a follow-up visit. Significant changes were noted for mean C-peptide AUC during OGTT (baseline AUC: 491 [SD 185]; follow-up AUC: 647 [SD 233], P < 0.0001) and ICA positivity (P < 0.0001), where all 54 subjects were ICA positive at baseline and 19 (35%) reverted to being ICA negative at the follow-up visit.
Figure 1.
Flow diagram of all subjects recruited into the original DPT-1 study and results of the follow-up study. IVGTT, intravenous glucose tolerance test; T1D, type 1 diabetes.
Over the entire study and follow-up, individuals who did not develop type 1 diabetes (n = 198) had a significantly lower median ICA titer (80 vs. 160 Juvenile Diabetes Foundation Units, P < 0.0001), lower mean IAA titer (309.3 vs. 426.5 nU/mL, P = 0.02), lower proportion with ICA512 (45 vs. 60%, P = 0.004), higher mean first-phase insulin response (173.1 vs. 145.6 µU/mL, P = 0.002), higher mean C-peptide peak (5.7 vs. 5.1 ng/mL, P = 0.003), higher mean C-peptide AUC (530.1 vs. 470.7, P = 0.005) measured by OGTT, were more likely to be black or Hispanic (10.5 vs. 4.5%, P = 0.03), and were older at time of randomization in study (median age 11 vs. 9 years, P < 0.0001) compared with individuals who developed type 1 diabetes (n = 174).
Table 1 provides the baseline characteristics at the time of randomization into the DPT-1 study of the subjects eligible for the follow-up study by whether or not contact was achieved. Individuals contacted were more likely to be white (P < 0.0001) compared with those unable to be contacted.
Table 1.
Baseline characteristics (at time of randomization) of subjects eligible for follow-up study
| Subjects contacted in DPT-1 follow-up | Subjects unable to contact in DPT-1 follow-up | P | |
|---|---|---|---|
| n | 206 | 69 | |
| Median age‡ | 10 (7–14) | 11 (8–16) | 0.0962 |
| Average first-phase insulin response (μU/mL)* | 158.7 (93.6) | 182.4 (75.2) | 0.0574 |
| Race† | <0.0001 | ||
| White | 193 (93.6) | 52 (75.3) | |
| African American | 1 (0.4) | 4 (5.8) | |
| Hispanic | 5 (2.4) | 12 (17.3) | |
| Other | 7 (3.2) | 1 (1.4) | |
| Sex† | 0.9082 | ||
| Male | 127 (61.6) | 42 (60.8) | |
| Female | 79 (38.3) | 27 (39.1) | |
| Relationship to index patient with diabetes† | 0.1139 | ||
| Sibling | 108 (52.4) | 47 (68.1) | |
| Offspring | 63 (30.5) | 13 (18.8) | |
| Parent | 13 (6.3) | 2 (2.9) | |
| Second-degree relative | 22 (10.6) | 7 (10.1) | |
| Antibody levels | |||
| Median ICA (Juvenile Diabetes Foundation Units)‡ | 80 (40–160) | 40 (20–160) | 0.1175 |
| Mean IAA (nU/mL)* | 329.59 (411.6) | 349.48 (671.0) | 0.7703 |
| GAD antibodies† | 0.8384 | ||
| Positive | 146 (74.9) | 51 (76.1) | |
| Negative | 49 (25.1) | 16 (23.9) | |
| ICA512 antibodies† | 0.5207 | ||
| Positive | 93 (47.7) | 35 (52.2) | |
| Negative | 102 (52.3) | 32 (47.8) | |
| mIAA antibodies† | 0.7028 | ||
| Positive | 13 (20.3) | 6 (24.0) | |
| Negative | 51 (79.7) | 19 (76.0) | |
| HbA1c (%)* | 5.31 (0.38) | 5.36 (0.36) | 0.3095 |
| C-peptide AUC (during OGTT)* | 505.77 (208.0) | 532.75 (190.4) | 0.3447 |
Data are *mean (SD), †n (%), or ‡median (interquartile range).
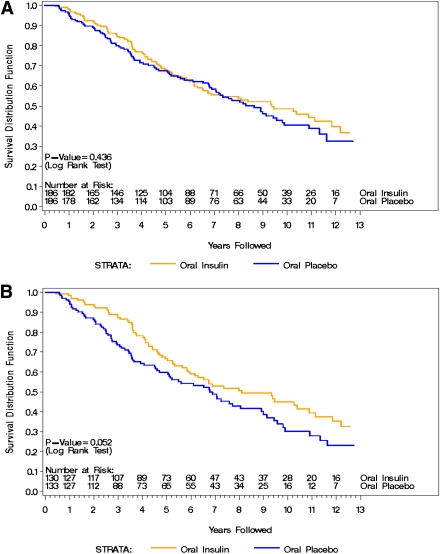
Figure 2 shows the Kaplan-Meier curve from the start of the DPT-1 to the end of the follow-up for both the entire oral insulin population (Fig. 2A, n = 372) and subjects with a baseline confirmed IAA ≥80 nU/mL (Fig. 2B, n = 263). The overall median follow-up was 9.1 (Q1–Q3: 8.7–10.1) years. The annualized rate of type 1 diabetes development for the entire population was 7.4% per year in the oral insulin group and 8.2% in the placebo group (hazard ratio [HR] 1.125, 95% CI 0.837–1.511; P = 0.436). In subjects with a baseline confirmed IAA level ≥80 nU/mL, the annualized rate of type 1 diabetes development was 7.7% per year in the oral insulin group and 10.1% in the placebo group (HR 1.384, 95% CI 0.995–1.925; P = 0.052). Even after discontinuing oral insulin treatment, individuals with a confirmed IAA level ≥80 nU/mL who received oral insulin appeared to benefit overall by significantly delaying type 1 diabetes onset by 2.2 years.
Figure 2.
A: Time to type 1 diabetes for the entire DPT-1 population from 1994 to 2009. B: Time to type 1 diabetes for subjects with a baseline IAA level confirmed ≥80 nU/mL from 1994 to 2009. (A high-quality color representation of this figure is available in the online issue.)
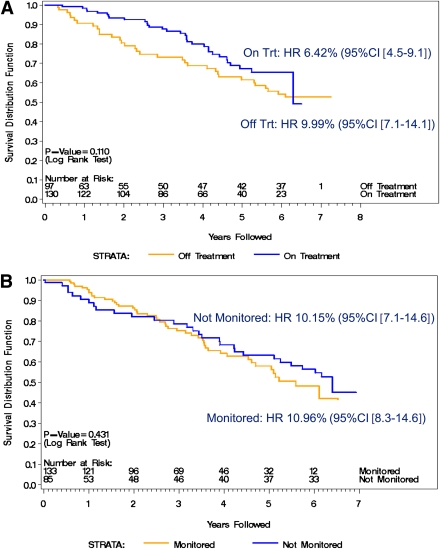
Figure 3 breaks down the patient subgroups with confirmed IAAs ≥80 nU/mL into the following: time on (all subjects treated with oral insulin) and off (only subjects contacted through the follow-up) treatment for the oral insulin group (Fig. 3A) and time on (monitored on study receiving placebo) and off (only subjects contacted through follow-up and no longer monitored on study) treatment for the placebo group (Fig. 3B). There were 130 subjects who received oral insulin during the study, and 97 (75%) of those subjects were contacted in the follow-up; 133 subjects received placebo and 85 (64%) were contacted in the follow-up. The annualized rate of diabetes while on oral insulin was 6.2%; after treatment ended, the rate increased to 9.5%, a rate similar to the placebo group (HR 1.492, 95% CI 0.911–2.444; P = 0.110). Figure 3A clearly shows that the reduced rate of diabetes development depicted in Fig. 2 is only apparent during the time the subjects received oral insulin. During the time on oral insulin compared with after oral insulin treatment, type 1 diabetes onset was delayed by 3.9 years. After oral insulin ended, the median estimated survival for subjects treated was similar to placebo during the original trial (6.32 years for placebo vs. 6.94 years for oral insulin after oral insulin treatment ended). Figure 3B shows the proportion of the placebo group without diabetes while on study and off study. The annual type 1 diabetes rate on study was 10.4% and off study was 9.7% (HR 0.826, 95% CI 0.513–1.330; P = 0.431). The hazard rate remained relatively constant for subjects in the placebo group over the study and follow-up periods.
Figure 3.
Proportion of subjects with IAA levels confirmed ≥80 nU/mL who are diabetes-free by time of study and after study. A: Oral insulin treatment group (where “on treatment [Trt]” is defined as time receiving oral insulin). B: Placebo group (where “monitored” is defined as time receiving placebo and “not monitored” is defined as time no longer receiving placebo and monitored by study). (A high-quality color representation of this figure is available in the online issue.)
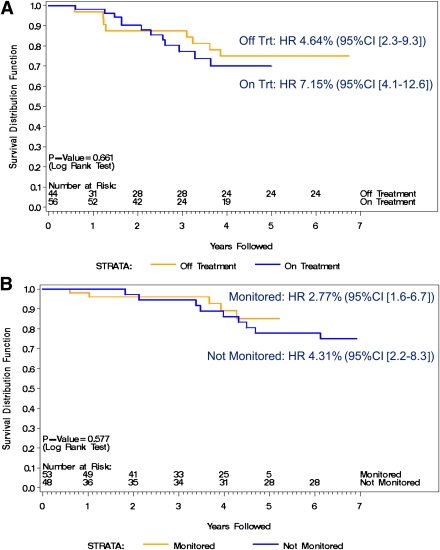
Figure 4A and B shows the proportion of subjects diabetes-free in the subgroup who did not have confirmed IAA ≥80 nU/mL for those who received oral insulin (Fig. 4A) or placebo (Fig. 4B). In Fig. 4A, the annualized type 1 diabetes rate for the time on oral insulin was 6.9 and 4.5% during the follow-up time after therapy was withdrawn (HR 0.817, 95% CI 0.330–2.022; P = 0.661). Figure 4B describes the proportion of subjects in the placebo group diabetes-free both during study and after study. The annualized rate was 2.7% during the study and 4.2% after study (HR 1.379, 95% CI 0.444–4.286; P = 0.577).
Figure 4.
Proportion of subjects with IAA levels not confirmed ≥80 nU/mL who are diabetes-free by time of study and after study. A: Oral insulin treatment group (where “on treatment [Trt]” is defined as time receiving oral insulin). B: Placebo group (where “monitored” is defined as time receiving placebo and “not monitored” is defined as time no longer receiving placebo and monitored by study). (A high-quality color representation of this figure is available in the online issue.)
CONCLUSIONS
The follow-up of the DPT-1 study showed that level of IAA titer is associated with responsiveness to oral insulin. The overall oral insulin effect in individuals with confirmed IAA levels ≥80 nU/mL was maintained with additional follow-up; however, once oral insulin was stopped, the rate of developing diabetes in the oral insulin group became similar to the rate in the placebo group. This regression provides evidence that subjects with IAA levels ≥80 nU/mL who received oral insulin before type 1 diabetes onset have delayed progression while on oral insulin, but once treatment ended, the risk of type 1 diabetes increased to the level observed in the placebo group. Because the loss of benefit coincided with the discontinued oral insulin, it is hypothesized that the immunological effect is tied temporally to the insulin intervention. In contrast, individuals with IAA levels <80 nU/mL displayed the opposite effect with a nonstatistically significant increasing risk of type 1 diabetes with oral insulin, while similarly the effect is attenuated, once therapy stopped.
The mechanism through which oral insulin mediates the apparent treatment response is unclear, but tolerance induction through this route may operate via effects on the adaptive immune system (5). Several nonclinical studies of the response to oral antigen have identified the induction of T cells that are associated with, and can adoptively transfer, protection from autoimmune disease (6). These cells are typically antigen specific, are CD4+, secrete the immune suppressive cytokines interleukin (IL)-10 and transforming growth factor (TGF)-β (7,8), and have been termed TH3 cells or regulatory T cells (Tregs) (9). In other settings in which antigen-induced tolerance was achieved via a mucosal route (intranasal), there is evidence of activation-induced cell death of responder T cells (10), a possibility that should be explored in future studies of oral antigen administration. Preliminary evidence has recently shown that these processes could be counterproductive, at least theoretically, if Tregs are also deleted or sequestered (M. von Herrath, personal communication) and that the balance of induction/deletion is strongly influenced by the dose (frequency), with more frequent dosing being more harmful (11–13). A further intriguing, and hitherto unexplained, finding of the oral insulin study has been the reliance of the therapeutic effect on the presence of high levels of IAAs. It is possible that the mechanistic pathway requires the induction of adaptive Tregs from cohorts of preexisting insulin-specific T cells, or perhaps that antigen presentation by insulin-specific B cells is important (14). In summary, the T-cell field has matured sufficiently to allow rational hypotheses as to the mechanistic effects of oral insulin to be proposed and examined experimentally.
In previous mouse model studies, such as those that influenced the initiation of the DPT-1 study (15–17), oral insulin therapy was successful in preventing type 1 diabetes onset; however, therapy was dose dependent, with a reduction in insulitis seen at lower doses (1 mg), disappearing at higher doses (5 mg) (18). Although the majority of human studies reported no beneficial effect (1,19,20), one study (21) that focused on evaluating time of intervention, antigen dosing, and administration interval reported that oral insulin given in low doses (1 mg) delays β-cell failure in new-onset patients >20 years of age. This double-blind placebo-controlled study randomized 191 subjects who were 5–60 years of age to receive 1 mg insulin dose, 10 mg insulin dose, or placebo. Ergun-Longmire et al. (21) demonstrated that age and duration of diabetes in combination with different immunological and metabolic markers have varying responsiveness to varying doses of oral insulin. The addition of the DPT-1 follow-up study finding that IAA titer is associated with drug therapy responsiveness will have a considerable impact on future human studies, such as those with antigen-specific immunotherapy, where understanding that variation in biomarker levels may have a large impact on immunotherapy success (22).
The results of the randomized clinical trials designed to delay or prevent type 1 diabetes progression to date have indicated that timing of intervention, dosing and frequency, age at diagnosis, baseline C-peptide level, and number and type of Ab are important factors that need further investigation when designing effective treatment interventions. This study of a subgroup within the DPT-1 study provided new evidence that IAA titer affects responsiveness to oral insulin therapy. These results and advances in the immunological field (i.e., antigen-specific immunotherapy) using the TrialNet Network will provide ample opportunity to retest this hypothesis as well as others that evaluate varying dose and frequency of oral insulin therapy, evaluate other immunologic and metabolic biomarkers, and assess responsiveness to drug therapy as a prevention strategy in type 1 diabetes.
Acknowledgments
This work was supported by cooperative agreements with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute of Allergy and Infectious Diseases; the National Institute of Child Health and Human Development; the National Center for Research Resources; the American Diabetes Association; the Juvenile Diabetes Research Foundation; and the U.K. Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London.
No potential conflicts of interest relevant to this article were reported.
K.V. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. D.C. and H.R. researched data. D.A.S. reviewed and edited the manuscript. M.P. contributed to discussion and reviewed and edited the manuscript. J.P.K. reviewed and edited the manuscript.
References
- 1.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Trial–Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Miao D, Babu S, et al. Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 2007;92:88–92 [DOI] [PubMed] [Google Scholar]
- 4.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 5.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol 2006;13:143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007;148:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells: they’re back and critical for regulation of autoimmunity! Immunol Rev 2001;182:149–163 [DOI] [PubMed] [Google Scholar]
- 8.Waldmann H, Cobbold S. Regulating the immune response to transplants: a role for CD4+ regulatory cells? Immunity 2001;14:399–406 [DOI] [PubMed] [Google Scholar]
- 9.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 2006;25:195–201 [DOI] [PubMed] [Google Scholar]
- 10.Laliotou B, Duncan L, Dick AD. Intranasal administration of retinal antigens induces transient T cell activation and apoptosis within drainage lymph nodes but not spleen. J Autoimmun 1999;12:145–155 [DOI] [PubMed] [Google Scholar]
- 11.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004;113:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homann D, Dyrberg T, Petersen J, Oldstone MB, von Herrath MG. Insulin in oral immune “tolerance”: a one-amino acid change in the B chain makes the difference. J Immunol 1999;163:1833–1838 [PubMed] [Google Scholar]
- 13.Petersen JS, Bregenholt S, Apostolopolous V, et al. Coupling of oral human or porcine insulin to the B subunit of cholera toxin (CTB) overcomes critical antigenic differences for prevention of type I diabetes. Clin Exp Immunol 2003;134:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol 2010;22:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir A, Schatz D, Maclaren N. Antigen-specific immunotherapy: oral tolerance and subcutaneous immunization in the treatment of insulin-dependent diabetes. Diabetes Metab Rev 1993;9:279–287 [DOI] [PubMed] [Google Scholar]
- 16.Ploix C, Bergerot I, Fabien N, Perche S, Moulin V, Thivolet C. Protection against autoimmune diabetes with oral insulin is associated with the presence of IL-4 type 2 T-cells in the pancreas and pancreatic lymph nodes. Diabetes 1998;47:39–44 [DOI] [PubMed] [Google Scholar]
- 17.Bergerot I, Fabien N, Maguer V, Thivolet C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J Autoimmun 1994;7:655–663 [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A 1991;88:10252–10256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaillous L, Lefèvre H, Thivolet C, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabète Insuline Orale group. Lancet 2000;356:545–549 [DOI] [PubMed] [Google Scholar]
- 20.Pozzilli P, Pitocco D, Visalli N, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). Diabetologia 2000;43:1000–1004 [DOI] [PubMed] [Google Scholar]
- 21.Ergun-Longmire B, Marker J, Zeidler A, et al. Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann N Y Acad Sci 2004;1029:260–277 [DOI] [PubMed] [Google Scholar]
- 22.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity 2010;32:437–445 [DOI] [PubMed] [Google Scholar]