Abstract
OBJECTIVE
Increased glomerular filtration rate (GFR), also called hyperfiltration, is a proposed mechanism for renal injury in diabetes. The causes of hyperfiltration in individuals without diabetes are largely unknown, including the possible role of borderline hyperglycemia. We assessed whether impaired fasting glucose (IFG; 5.6–6.9 mmol/L), elevated HbA1c, or hyperinsulinemia are associated with hyperfiltration in the general middle-aged population.
RESEARCH DESIGN AND METHODS
A total of 1,560 individuals, aged 50–62 years without diabetes, were included in the Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6). GFR was measured as single-sample plasma iohexol clearance. Hyperfiltration was defined as GFR >90th percentile, adjusted for sex, age, weight, height, and use of renin-angiotensin system inhibitors.
RESULTS
Participants with IFG had a multivariable-adjusted odds ratio of 1.56 (95% CI 1.07–2.25) for hyperfiltration compared with individuals with normal fasting glucose. Odds ratios (95% CI) of hyperfiltration calculated for a 1-unit increase in fasting plasma glucose (FPG) and HbA1c, after multivariable-adjustment, were 1.97 (1.36–2.85) and 2.23 (1.30–3.86). There was no association between fasting insulin levels and hyperfiltration. A nonlinear association between FPG and GFR was observed (df = 3, P < 0.0001). GFR increased with higher glucose levels, with a steeper slope beginning at FPG ≥5.4 mmol/L.
CONCLUSIONS
Borderline hyperglycemia was associated with hyperfiltration, whereas hyperinsulinemia was not. Longitudinal studies are needed to investigate whether the hyperfiltration associated with IFG is a risk factor for renal injury in the general population.
Chronic kidney disease (CKD) is recognized as a global health problem. The prevalence of CKD is estimated to exceed 10% in Western societies and in many Asian countries (1). Concurrently, the incidence of obesity and prediabetes, defined as impaired fasting glucose (IFG) or impaired glucose tolerance, has reached epidemic proportions worldwide (2). Growing evidence links prediabetes and insulin resistance to microalbuminuria and CKD, but the pathophysiologic mechanisms for renal injury have not been elucidated (3,4). However, studies in animals and humans indicate that an abnormally elevated glomerular filtration rate (GFR), or hyperfiltration, may increase the susceptibility to renal injury in obesity and in diabetes (5,6).
At the single-nephron level, hyperfiltration is hypothesized to be an early link in the chain of events that lead from intraglomerular hypertension to albuminuria and, subsequently, to reduced GFR (7). This paradigm has received attention in experimental research, but is difficult to study at the population level because obtaining accurate measurements of GFR is complicated and time-consuming. GFR estimated from creatinine or cystatin C levels is imprecise in the normal or upper range of GFR and is biased in individuals with atypical body composition or creatinine production (8). Accordingly, although hyperglycemia is known to mediate hyperfiltration in diabetes, the causes of hyperfiltration in the general population are largely unknown; particularly, whether prediabetes or insulin resistance is associated with hyperfiltration is unknown.
The current study investigated whether IFG, elevated HbA1c, hyperinsulinemia, or insulin resistance are associated with hyperfiltration in a general middle-aged population. To avoid the problems of estimating GFR from creatinine or cystatin C values, we measured GFR as iohexol clearance, which is recognized as an accurate method (9).
RESEARCH DESIGN AND METHODS
The Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6) is a part of the population-based sixth Tromsø study (Tromsø 6) in the municipality of Tromsø, Northern Norway. Tromsø 6 was conducted in 2007 through 2008 and included an age-stratified representative sample of 12,984 inhabitants of Tromsø. Among the 5,464 invited persons in the group aged 50 to 62 years, 3,564 (65%) met and completed the main part of Tromsø 6, which included a self-administered questionnaire on health status, a physical examination, and collection of three separate morning spot urine samples. From this group, the 2,825 subjects without previous myocardial infarction, angina pectoris, stroke, diabetes, or renal disease were invited to participate in RENIS-T6 (Supplementary Fig. 1).
The age-group of 50 to 62 years was chosen to study a relatively healthy population, but with a sufficient risk of CKD and cardiovascular disease for a later end point study. A detailed description of RENIS-T6 has been published elsewhere (10). Briefly, 2,107 (75%) responded positively and 72 were excluded. A total of 1,632 subjects were included according to a predetermined target size. The characteristics of the RENIS-T6 cohort were comparable with the 2,825 eligible recruits, as previously reported (10). For the present analyses, subjects with fasting plasma glucose (FPG) ≥7.0 mmol/L or HbA1c ≥6.5% were considered to have diabetes and were excluded. We also excluded subjects with an iohexol clearance <60 mL/min/1.73 m2 according to the definition of CKD.
Study participants met in the morning after an overnight fast, including abstinence from tobacco. Blood pressure (BP) was measured three times with an automatic device (model UA-799, A&D Medical, San Jose, CA), and the last two readings were averaged. A Teflon catheter was placed in an antecubital vein and fasting plasma samples were drawn for biochemical analyses. Iohexol (5 mL) was injected, and the syringe was weighed before and after injection. The venous catheter was flushed with 30 mL of isotonic saline. The iohexol blood sample was drawn from the same catheter and the iohexol concentration was measured by high-performance liquid chromatography.
GFR was calculated as described by Jacobsson (11). Details about the iohexol clearance measurements were published previously (10). Plasma creatinine levels were analyzed by the enzymatic method that was standardized against isotope dilution mass spectroscopy. Cystatin C was measured by particle-enhanced turbidimetric immunoassay (Gentian, Moss, Norway). We estimated GFR (eGFR) from creatinine or cystatin C by using the recalibrated four-variable Modification of Diet in Renal Disease (MDRD) equation, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, and Rule’s equation of 2006 (12,13). Values for FPG, triglycerides, and cholesterol were measured on the Modular model P800 (Roche Diagnostics Corp., Indianapolis, IN). IFG was defined according to the American Diabetes Association criteria of 5.6–6.9 mmol/L for FPG.
The insulin samples were measured with an ELISA kit (DRG Instruments, Marburg, Germany). The intraassay and interassay coefficients of variation were 4.7 and 6.3%. Insulin resistance (IR) was expressed by homeostasis model assessment (HOMA)-IR: [FPG (mmol/L) × fasting insulin (μU/L)]/22.5.
HbA1c, urinary albumin excretion (UAE), and urinary creatinine were measured in the main part of Tromsø 6. HbA1c was measured with a liquid chromatographic method. UAE and urinary creatinine were measured with commercial kits, as described in a previous study (14). The albumin/creatinine ratio (ACR) was calculated for each urine specimen, and the mean ACR value was used in the analyses (14).
We estimated age- and sex-adjusted means or median values across two groups: those with normal FPG and those with IFG. Differences across groups were tested by linear regression for mean values, quantile regression for median values, and multiple logistic regression for dichotomous variables. The associations between GFR expressed in mL/min/1.73 m2 and FPG, HbA1c, fasting insulin, and HOMA-IR were assessed by multiple linear regression analysis, adjusting for the following known or possible determinants of GFR: age, sex, height, weight, current smoking, diastolic BP, and current use of ACE inhibitors or angiotensin receptor blockers (ARB). The same analyses were repeated for the absolute GFR expressed in mL/min.
To investigate a possible nonlinear association between FPG and GFR, we used local regression smoothing in a generalized additive model, adjusting for the same variables as in the linear regression analyses. Renal hyperfiltration was defined as an absolute GFR >90th percentile after adjusting for sex, age, weight, height, and the use of ACE inhibitors or ARB. This was done by selecting all subjects >90th percentile in the distribution of residuals from a multiple linear regression analysis where we used the logarithm of absolute GFR as a dependent variable and sex, use of ACE inhibitors or ARB, and the logarithm of age, weight, and height as independent variables.
Multiple logistic regression analyses were performed to determine the odds ratios of hyperfiltration associated with the same independent variables, and adjusted for age, sex, height, weight, current smoking, diastolic BP, and use of ACE inhibitors or ARB. The same linear and logistic regression analyses were repeated with adjustment for BMI instead of for height and weight. We tested for interactions between the independent variables and sex, age, and BMI in all analyses. Stata 11 software (Stata Corp., College Station, TX) was used for the statistical analysis. Generalized additive models were analyzed using PROC GAM in SAS 9.2 software (SAS Institute, Cary, NC). Statistical significance was set at P < 0.05. The study was approved by the regional ethics committee of Northern Norway. All subjects provided informed written consent.
RESULTS
The study excluded 33 individuals with diabetes according to their FPG or HbA1c results, 34 with measured GFR <60 mL/min/1.73 m2, and 5 with a failure in the iohexol measurement (Supplementary Fig. A1).
IFG was present in 311 men (40%) and 141 women (18%). Table 1 reports the characteristics of the study population divided by glucose status, adjusted for age and sex. Individuals with IFG had higher BMI, insulin levels, and BP, but not higher ACR compared with those with normal FPG. Measured GFR, but not creatinine- or cystatin C–based eGFR, was higher in individuals with IFG (P = 0.002).
Table 1.
General characteristics of the study population grouped by glycemic category*
| Fasting glucose |
|||
|---|---|---|---|
| Normal† | Impaired‡ | ||
| Variable | n = 1,108 | n = 452 | P |
| Male sex | 42 | 69 | <0.001 |
| Age (years) | 57.8 ± 3.7 | 58.5 ± 3.9 | 0.001 |
| BMI (kg/m2) | 26.7 ± 3.8 | 28.4 ± 3.9 | <0.001 |
| Overweight | 48 | 48 | 0.86 |
| Obese | 18 | 32 | <0.001 |
| Current daily smoking | 23 | 18 | 0.02 |
| Systolic BP (mmHg) | 128.6 ± 16.6 | 131.4 ± 16.9 | 0.003 |
| Diastolic BP (mmHg) | 83.0 ± 9.4 | 84.5 ± 9.6 | 0.005 |
| Triglyceride level (mmol/L) | 0.9 (0.7–1.4) | 1.1 (0.8–1.6) | <0.001 |
| HDL cholesterol level (mmol/L) | 1.55 ± 0.40 | 1.50 ± 0.40 | 0.03 |
| Fasting glucose (mmol/L) | 5.1 ± 0.3 | 5.9 ± 0.3 | |
| HbA1c level (% unit) | 5.5 ± 0.3 | 5.7 ± 0.3 | <0.001 |
| Fasting insulin level (μU/mL) | 7.7 (5.4–10.6) | 10.8 (7.8–14.7) | <0.001 |
| HOMA-IR (index) | 1.7 (1.2–2.4) | 2.9 (2.1–3.9) | <0.001 |
| ACR (mg/mmol) | 0.33 (0.19–0.59) | 0.31 (0.18–0.58) | 0.18 |
| Measured GFR§ | 100.7 ± 15.0 | 106.1 ± 15.2 | <0.001 |
| Adjusted for BSA | 91.8 ± 12.4 | 94.0 ± 12.6 | 0.002 |
| eGFR | |||
| MDRD|| | 94.4 ± 16.2 | 94.2 ± 16.4 | 0.8 |
| CKD-EPI¶ | 95.2 ± 8.8 | 95.0 ± 8.9 | 0.69 |
| Cystatin C# | 92.6 ± 16.4 | 92.7 ± 16.7 | 0.91 |
Values are expressed as means ± SD, percentages, or medians (interquartile range).
BSA, body surface area.
*Values are adjusted by age and sex.
†Normal fasting glucose: <5.6 mmol/L (<100 mg/dL).
‡IFG: 5.6–6.9 mmol/L (100–125 mg/dL).
§GFR (mL/min/1.73 m2) measured by single-sample iohexol clearance.
||GFR (mL/min/1.73 m2) estimated by the MDRD equation (12).
#GFR (mL/min/1.73 m2) estimated by Rule’s cystatin C–based equation of 2006 (13).
¶GFR (mL/min/1.73 m2) estimated by the CKD-EPI equation (12).
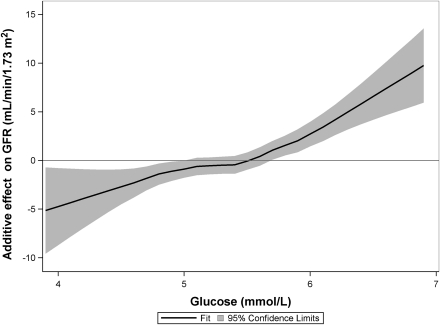
Multiple linear regression analyses with measured GFR as a dependent variable are reported in Table 2. FPG, HbA1c, fasting insulin, and HOMA-IR were positively associated with GFR in separate models when adjusted for age, sex, weight, height, diastolic BP, current smoking, and use of ACE inhibitors or ARB. Because of collinearity, HOMA-IR and insulin were analyzed in separate models. Regression diagnostics did not indicate problems with collinearity between FPG and insulin or HOMA-IR. The effect estimates of insulin and HOMA-IR were no longer significant in models including FPG. There were no significant interactions among age, sex, or BMI and the predictor variables listed in Table 2. The pattern of statistically significant estimates was similar when we used the logarithm of absolute GFR as the dependent variable and the same independent variables but with log-transformed age, weight, and height (not shown). A nonlinear association between FPG and GFR was observed by using local regression smoothing in a generalized additive model, after multivariable adjustment (df = 3, P < 0.0001; Fig. 1). GFR increased with higher glucose levels, with a steeper slope beginning at FPG ≥5.4 mmol/L.
Table 2.
Multiple linear regression analyses with measured GFR as the dependent variable
| Independent variable | β Coefficient | 95% CI | P |
|---|---|---|---|
| Model 1 | |||
| BMI, per unit | 0.04 | −0.12 to 0.21 | 0.64 |
| Model 2 | |||
| Fasting glucose, per mmol/L (18 mg/dL) | 3.67 | 2.29–5.06 | <0.001 |
| Model 3 | |||
| HbA1c, per % unit | 2.38 | 0.46–4.31 | 0.015 |
| Model 4 | |||
| Fasting insulin, per μU/mL | 0.16 | 0.03–0.29 | 0.015 |
| Model 5 | |||
| HOMA-IR, per unit | 0.80 | 0.31–1.29 | 0.001 |
| Model 6 | |||
| Fasting glucose, per mmol/L (18 mg/dL) | 3.46 | 2.02–4.89 | <0.001 |
| Fasting insulin, per μU/mL | 0.08 | −0.05 to 0.21 | 0.230 |
| Model 7 | |||
| Fasting glucose, per mmol/L (18 mg/dL) | 3.28 | 1.78–4.78 | <0.001 |
| HOMA-IR, per unit | 0.36 | −0.16 to 0.89 | 0.177 |
All models except model 1 were adjusted for age, sex, weight, height, diastolic BP, current smoking, and the use of ACE inhibitors or ARB. Model 1 was adjusted for the same variables except for weight and height. GFR was measured by iohexol clearance and expressed as mL/min/1.73 m2.
Figure 1.
A nonlinear effect of fasting glucose on measured GFR, calculated by local regression smoothing in a generalized additive model (df = 3, P < 0.0001), and adjusted for age, sex, height, weight, current smoking, diastolic BP, and the use of ACE inhibitors or ARB.
The 79 women and 77 men with hyperfiltration had mean GFRs of 110.1 (range 98.7–138.6) and 118.2 (107.5–137.3) mL/min/1.73 m2, compared with 86.5 and 93.6 mL/min/1.73 m2 for women and men with normal filtration. Multivariable-adjusted odds ratios for hyperfiltration in relation to metabolic factors are reported in Table 3. Higher levels of FPG, HbA1c, and HOMA-IR, and having IFG, were significantly associated with an increased odds ratio of hyperfiltration. Fasting insulin level was not associated with hyperfiltration. The effect of FPG and IFG remained strong and significant after additional adjustment for HDL cholesterol, triglycerides, insulin, ACR, and BMI (not shown). HOMA-IR, however, was not associated with hyperfiltration after adjusting for FPG (model 8). All the logistic regression models were repeated with a stricter definition of hyperfiltration by defining only those with adjusted absolute GFR >95th percentile as having hyperfiltration. These analyses yielded similar findings. Some degree of hemolysis was found in 180 serum samples (11%), and this significantly influenced the mean insulin levels but not the mean glucose levels. However, both the linear and logistic regression estimates remained essentially the same after excluding individuals with hemolysis in serum samples.
Table 3.
Multiple logistic regression analyses of odds ratio for hyperfiltration
| Independent variable | Odds ratio* (95% CI) | P |
|---|---|---|
| Model 1 | ||
| BMI, per unit | 1.02 (0.98–1.06) | 0.38 |
| Model 2 | ||
| Fasting glucose, per mmol/L (18 mg/dL) | 1.97 (1.36–2.85) | <0.001 |
| Model 3 | ||
| HbA1c, per % unit | 2.23 (1.30–3.86) | 0.004 |
| Model 4 | ||
| IFG†, yes/no | 1.56 (1.07–2.25) | 0.019 |
| Model 5 | ||
| Insulin, per μU/mL | 1.03 (1.00–1.06) | 0.08 |
| Model 6 | ||
| IFG†, yes/no | 1.48 (1.01–2.25) | 0.04 |
| Insulin, per μU/mL | 1.02 (0.99–1.05) | 0.20 |
| Model 7 | ||
| HOMA-IR, per unit | 1.14 (1.01–1.28) | 0.033 |
| Model 8 | ||
| Fasting glucose, per mmol/L (18 mg/dL) | 1.86 (1.25–2.76) | 0.002 |
| HOMA-IR, per unit | 1.06 (0.93–1.20) | 0.41 |
*All models except model 1 were adjusted for age, sex, weight, height, diastolic BP, current smoking, and the use of ACE inhibitors or ARB. Model 1 was adjusted for the same variables except weight and height. Models 6 and 8 were also adjusted for the other variable in the same model.
†IFG: 5.6–6.9 mmol/L (100–125 mg/dL).
CONCLUSIONS
In this middle-aged population without diabetes, we found that IFG was associated with hyperfiltration independent of age, sex, BMI, BP, smoking status, and insulin levels. A similar association was found between HbA1c and hyperfiltration, which indicates not only an acute effect but also an effect of chronically elevated glucose levels on GFR. Furthermore, we observed a nonlinear association between FPG and GFR, with steepening of the regression curve at FPG ≥5.4 mmol/L.
Experimental studies in healthy subjects have shown increased GFR was induced by acute glucose infusion, but plasma glucose in these experiments was increased to >7.0 mmol/L (15). In dogs, a continuous glucose infusion for 6 days, producing a modest rise in serum glucose from 6.5 to 7.1 mmol/L, increased GFR significantly (16). To our knowledge, no previous human studies have found that glucose levels in the nondiabetic range significantly and independently influence GFR. A few studies found that FPG in the nondiabetic range was associated with increased GFR, but these studies did not adjust GFR for sex, body size, or body weight (17). FPG was not associated with hyperfiltration in two previous hyperfiltration studies in nondiabetic individuals (18,19). However, these studies used creatinine clearance or creatinine-based eGFR, which are limited by low precision and bias, especially in the upper GFR range (8).
The estimating formulas are also influenced by non-GFR factors such as body composition and glycemic status (20). In the current study, we found a significantly higher measured GFR but not higher creatinine- or cystatin C–based eGFR in persons with IFG. This demonstrates the difficulty of studying hyperfiltration with eGFR. Cystatin C was recently proposed as a new and better marker of renal function in the normal GFR range. However, cystatin C is also influenced by non-GFR factors. We recently showed that cystatin C–based eGFR did not perform better than creatinine-based eGFR when validated against iohexol clearance in the general population (10). We are aware of only one previous study that measured GFR to assess the association between IFG and hyperfiltration. In a study that included 363 participants of African descent with a positive family history of hypertension, individuals with IFG had an increased risk of hyperfiltration, although not statistically significant (21). Hyperfiltration in this study was defined as GFR >140 mL/min/1.73 m2, without adjusting for age and sex.
In the current study, fasting insulin levels and HOMA-IR were associated with increased GFR in the linear regression analysis, but not after adjusting for FPG. Furthermore, insulin levels were not associated with hyperfiltration. Previous population studies of insulin levels and GFR are scarce, none included an exact method to measure GFR, and the results are divergent (22). Nevertheless, hyperinsulinemia and insulin resistance are both proposed as mediators of hyperfiltration and subsequent renal injury in obesity (22). Our results are inconsistent with the hypothesis that hyperinsulinemia causes hyperfiltration in the general population where overweight and obesity is prevalent. Thus as previously suggested, mechanisms other than hyperfiltration, such as inflammatory cytokines or lipotoxicity, may link insulin resistance to kidney damage (5).
Unlike most previous hyperfiltration studies (18,19,21), we adjusted for age, sex, height, and body weight when defining hyperfiltration. There is no consensus on how to define hyperfiltration. The clinical relevance of hyperfiltration is based on a proposed pathologic effect of increased single-nephron GFR, which cannot be measured in humans. Instead, whole-kidney hyperfiltration, with a threshold arbitrarily set in different studies from 125 to 140 mL/min/1.73 m2, was assumed to reflect hyperfiltration at the glomerular level (6). However, because the number of nephrons varies significantly between individuals, whole-kidney GFR will reflect variation in nephron number as well as in single-nephron GFR. Moreover, GFR and nephron number both decrease with age and are lower in women than in men (23). A noncorrected threshold for hyperfiltration would mask hyperfiltration at older ages and in women. Because body weight could confound the association between IR or prediabetes and hyperfiltration, we chose to adjust GFR not only for age, sex, and height but also for body weight, in the definition of hyperfiltration.
The mechanism behind hyperfiltration in hyperglycemia is not fully understood, but studies in diabetic animals indicate a key role of increased sodium reabsorption through sodium-glucose cotransport in the proximal renal tubules (24). Raised proximal sodium reabsorption is also found in individuals with IFG compared with subjects with normal FPG (21). Furthermore, other factors associated with hyperglycemia, such as nitric oxide, vascular inflammation, oxidative stress, or activation of the renin-angiotensin system, could alter renal vascular tone, and consequently, increase GFR (24).
There is solid evidence that increased glomerular pressure causes kidney damage, but there is less evidence that glomerular hyperfiltration per se is harmful (5). For example, the long-term risk of proteinuria and end-stage renal disease after kidney donation, a state that implies hyperfiltration in the remaining kidney, is similar to that in the general population. However, transplant donors are carefully selected individuals without other CKD risk factors; therefore, their risk of renal failure should be low. In contrast, a high risk of developing proteinuria was found after unilateral nephrectomy for reasons other than kidney donation, particularly in overweight individuals (25). We are aware of only one prospective study of hyperfiltration in nondiabetic individuals. In a study of subjects with stage 1 hypertension, the risk of developing microalbuminuria was increased in those with hyperfiltration at baseline (19). These findings are consistent with the “multi-hit hypothesis” of CKD, where hyperfiltration in concert with other CKD risk factors causes kidney injury. In diabetes, some but not all studies showed an association between hyperfiltration and the subsequent development of nephropathy (6).
IFG is present in approximately 30% of the adult U.S. population, and CKD was recently found in 17% of individuals with IFG compared with 12% of those with normal FPG (2,4). Moreover, an increase in FPG within the normal range, or increased HbA1c, were both associated with progression of UAE in the general nondiabetic population (3,14). In the current study, which included a relatively healthy population, IFG was not associated with ACR. Longitudinal studies with an exact method of measuring GFR are needed to explore the temporal relationship between IFG, hyperfiltration, UAE, and CKD.
Some limitations in our study should be noted. The cross-sectional design limits inferences on causality. The study population consisted of middle-aged Caucasians only, thus the results cannot automatically be generalized to other age-groups or populations. In addition, IR was not measured with the gold standard euglycemic clamp method. However, the HOMA-IR correlates well with values obtained with the euglycemic clamp technique and remains the preferred method in epidemiologic studies.
The strength of this study includes the use of an exact method to measure GFR in a large sample of the general population. We conclude that IFG is associated with an increased risk of hyperfiltration in the middle-aged nondiabetic population. Hyperfiltration caused by IFG may be one of several mechanisms for renal injury in the general population. Longitudinal studies are needed to explore whether hyperfiltration increases the risk of CKD in nondiabetic individuals.
Supplementary Material
Acknowledgments
The study was funded by the Northern Norway Regional Health Authority.
No potential conflicts of interest relevant to this article were reported.
T.M. researched data and wrote the manuscript. U.D.M. researched data, contributed to discussion, and reviewed and edited the manuscript. O.C.I., T.G.J., I.N., M.D.S., and I.T. contributed to discussion and reviewed and edited the manuscript. B.O.E. researched data, contributed to discussion, and reviewed and edited the manuscript.
The authors thank Britt-Ann Winther Eilertsen, Bjørg Skog Høgset, Saskia van Heusden, and the rest of the staff at the Clinical Resarch Unit (University Hospital of North Norway) for performing the study; Harald Strand and the staff at the Department of Medical Biochemistry (University Hospital of North Norway) for high-performance liquid chromatography analyses of iohexol; Åse Lund and Gro Bolstad (Metabolic Research Laboratory, Department of Clinical Medicine, University of Tromsø) for insulin analyses; Inger Sperstad and Ingrid Dorthea Sandstad (Clinical Research Centre, University Hospital of North Norway) for database support; and Tom Wilsgaard, Sriharan Sivasingarajah, and Kurt Jøran Nyland (Institute of Community Medicine, University of Tromsø) for identifying eligible subjects from the Tromsø 6 cohort.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0235/-/DC1.
References
- 1.Tsukamoto Y, Wang H, Becker G, et al. Report of the Asian Forum of Chronic Kidney Disease Initiative (AFCKDI) 2007. “Current status and perspective of CKD in Asia”: diversity and specificity among Asian countries. Clin Exp Nephrol 2009;13:249–256 [DOI] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brantsma AH, Atthobari J, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT. What predicts progression and regression of urinary albumin excretion in the nondiabetic population? J Am Soc Nephrol 2007;18:637–645 [DOI] [PubMed] [Google Scholar]
- 4.Plantinga LC, Crews DC, Coresh J, et al. ; CDC CKD Surveillance Team Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010;5:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol 2008;294:F685–F696 [DOI] [PubMed] [Google Scholar]
- 6.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009;52:691–697 [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774–1777 [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009;20:2305–2313 [DOI] [PubMed] [Google Scholar]
- 9.Bird NJ, Peters C, Michell AR, Peters AM. Comparison of GFR measurements assessed from single versus multiple samples. Am J Kidney Dis 2009;54:278–288 [DOI] [PubMed] [Google Scholar]
- 10.Eriksen BO, Mathisen UD, Melsom T, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 2010;78:1305–1311 [DOI] [PubMed] [Google Scholar]
- 11.Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 1983;3:297–305 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–937 [DOI] [PubMed] [Google Scholar]
- 14.Solbu MD, Kronborg J, Eriksen BO, Jenssen TG, Toft I. Cardiovascular risk-factors predict progression of urinary albumin-excretion in a general, non-diabetic population: a gender-specific follow-up study. Atherosclerosis 2008;201:398–406 [DOI] [PubMed] [Google Scholar]
- 15.Greene SA, Dalton RN, Turner C, Haycock GB, Chantler C. Hyperglycemia with and without glycosuria: effect on inulin and para-amino hippurate clearance. Kidney Int 1987;32:896–899 [DOI] [PubMed] [Google Scholar]
- 16.Brands MW, Bell TD, Rodriquez NA, Polavarapu P, Panteleyev D. Chronic glucose infusion causes sustained increases in tubular sodium reabsorption and renal blood flow in dogs. Am J Physiol Regul Integr Comp Physiol 2009;296:R265–R271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerchman F, Tong J, Utzschneider KM, et al. Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J Clin Endocrinol Metab 2009;94:3781–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomaszewski M, Charchar FJ, Maric C, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 2007;71:816–821 [DOI] [PubMed] [Google Scholar]
- 19.Palatini P, Mormino P, Dorigatti F, et al. ; HARVEST Study Group Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int 2006;70:578–584 [DOI] [PubMed] [Google Scholar]
- 20.Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol 2004;15:1316–1322 [PubMed] [Google Scholar]
- 21.Pruijm M, Wuerzner G, Maillard M, et al. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol Dial Transplant 2010;25:2225–2231 [DOI] [PubMed] [Google Scholar]
- 22.Oterdoom LH, de Vries AP, Gansevoort RT, de Jong PE, Gans RO, Bakker SJ. Fasting insulin modifies the relation between age and renal function. Nephrol Dial Transplant 2007;22:1587–1592 [DOI] [PubMed] [Google Scholar]
- 23.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 2003;(83):S31–S37 [DOI] [PubMed] [Google Scholar]
- 24.Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 2010;200:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González E, Gutiérrez E, Morales E, et al. Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int 2005;68:263–270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.