Abstract
OBJECTIVE
It is important to identify modifiable factors that may lower gestational diabetes mellitus (GDM) risk. Dietary iron is of particular interest given that iron is a strong prooxidant, and high body iron levels can damage pancreatic β-cell function and impair glucose metabolism. The current study is to determine if prepregnancy dietary and supplemental iron intakes are associated with the risk of GDM.
RESEARCH DESIGN AND METHODS
A prospective study was conducted among 13,475 women who reported a singleton pregnancy between 1991 and 2001 in the Nurses’ Health Study II. A total of 867 incident GDM cases were reported. Pooled logistic regression was used to estimate the relative risk (RR) of GDM by quintiles of iron intake controlling for dietary and nondietary risk factors.
RESULTS
Dietary heme iron intake was positively and significantly associated with GDM risk. After adjusting for age, BMI, and other risk factors, RRs (95% CIs) across increasing quintiles of heme iron were 1.0 (reference), 1.11 (0.87–1.43), 1.31 (1.03–1.68), 1.51 (1.17–1.93), and 1.58 (1.21–2.08), respectively (P for linear trend 0.0001). The multivariate adjusted RR for GDM associated with every 0.5-mg per day of increase in intake was 1.22 (1.10–1.36). No significant associations were observed between total dietary, nonheme, or supplemental iron intake and GDM risk.
CONCLUSIONS
These findings suggest that higher prepregnancy intake of dietary heme iron is associated with an increased GDM risk.
Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications affecting approximately 7% of all pregnancies and up to 14% of pregnancies in high-risk populations (1). Overweight and obesity are the major modifiable risk factors of GDM. However, the overall population attributable fraction among the general U.S. population because of overweight and obesity is estimated to be less than 50% (2), implying the need to identify additional risk factors, particularly modifiable risk factors, that may help lower GDM risk.
Although the underlying mechanism remains unclear, available evidence suggests that the main defect in the pathogenesis of GDM is relatively diminished insulin secretion coupled with pregnancy-induced insulin resistance (3). Iron, a redox-active transitional metal, is a strong prooxidant. Accumulating evidence from experimental studies has demonstrated that iron overload can lead to β-cell toxicity, β-cell dysfunction, and impaired glucose metabolism (4). Moreover, several epidemiological studies have documented a positive association of circulating levels of ferritin (a marker of body iron stores) with circulating levels of glucose and insulin, and risk of type 2 diabetes melletus (T2DM) (5) and GDM (6–9). The major source of body iron is from the diet. Dietary iron exists as heme (mainly from meat and meat products) or nonheme iron. A positive association has been observed between dietary heme iron intake and T2DM (10). However, to our knowledge, there are no published studies evaluating dietary iron intake and GDM risk. Studies of supplemental iron and GDM risk are also scarce, and findings are inconsistent (11,12). The aim of this study was to evaluate the association between iron intake, including varying sources of iron (heme, nonheme, and supplemental) and GDM risk in a large prospective cohort.
RESEARCH DESIGN AND METHODS
The Nurses’ Health Study II (NHSII) is a prospective cohort study of 116,671 female U.S. nurses recruited between 22 and 44 years of age beginning in 1989. The cohort is followed by biennial mailed questionnaires to update data on health-related behaviors and to identify incident disease. Food frequency questionnaires (FFQs) were mailed every 4 years. The follow-up rate has been approximately 90% for every 2-year period. Women reporting a pregnancy lasting at least 6 months between 1991 and 2001 were included in the study. Women were excluded from the present analyses if they reported a multiple gestation, an implausible total energy intake (<500 or >3,500 kcal/day), a diagnosis of diabetes, GDM, cancer, cardiovascular disease, were peri-menopausal at baseline, or were missing information on age, iron intake, or vital status. The final sample included 13,475 eligible women.
Ascertainment of GDM
GDM cases were identified based on self-reported information in the biennial questionnaire. The validity of self-reported diagnosis of GDM has been demonstrated against a medical record review. Briefly, of 114 women who reported their first diagnosis of GDM in a singleton pregnancy between 1989 and 1991 on a supplemental questionnaire, 94% were confirmed to have a physician diagnosis. Supplementary questionnaires were also sent to 100 women reporting a pregnancy uncomplicated by GDM during the same interval. Of 93 responders who confirmed a singleton pregnancy during this period, 83% reported a glucose loading test, and 100% reported frequent urine screening in pregnancy (13).
Assessment of nutrient intake
Dietary information was collected by a 133-item semiquantitative food frequency questionnaire (SFFQ) every 4 years. Information on the average frequency of consumption of selected foods and beverages during the previous year was reported. The food composition database used to calculate nutrient values is based primarily on U.S. Department of Agriculture data and is supplemented with data from manufacturers. Participants reported the use and dose of multivitamin and iron supplements. Total iron intake was calculated as the sum of all dietary and supplemental intakes. All food-derived nutrient intakes (except supplemental iron and alcohol intake) were energy adjusted by the residual method. The validity and reliability of the SFFQ to assess nutrient intake were measured in a similar cohort (NHSI). The Pearson correlation coefficient for total iron intake between the SFFQ and four 1-week diet records administered 3 months apart in a subset of 150 women from NHSI was 0.55 (14). In addition to total, dietary, and supplemental iron, we estimated intakes of heme (mainly derived from animal meat sources) and nonheme iron (calculated as the difference between total iron and heme iron).
Assessment of nondietary covariates
Information on sociodemographic, clinical, and lifestyle factors was collected at baseline and was updated every 2 years. BMI was calculated from self-reported weight and height (weight in kilograms divided by the square of height in meters). In a similar cohort (NHSI), self-reported body weight was highly correlated (r = 0.96) with technician-measured weight (15,16). Physical activity was assessed in 1989, 1991, and 1997 in which participants were asked to report weekly activities. From this information, weekly energy expenditure in MET hours was calculated. We calculated the cumulative average of total recreational physical activity in the analyses. Family history of diabetes and other diseases were reported on the initial questionnaire (1989).
Statistical analysis
All statistical analyses were performed with SAS software (SAS Institute, Cary, NC). Means with SD for continuous baseline characteristics and proportions for categorical characteristics were calculated by quintile of total iron and heme iron intake. Analyses of dietary intake of total, total dietary, heme, nonheme, and supplemental iron were conducted in the full cohort of women (n = 13,475) using a cumulative average measurement of iron intake prior to GDM diagnosis. For example, the 1991 intake was used for the follow-up between 1991 and 1995, and the average of the 1991 and the 1995 intake was used for the follow-up between 1995 and 1999 to reduce within-person variation as well as to represent habitual intake of dietary factors (17). Iron intake was assessed as a categorical (quintiles of cumulative average intake) and continuous variable. The significance of linear trends across categories of dietary intake was evaluated using the median value for each category of dietary intake analyzed as a continuous variable in multivariate models.
Pooled logistic regression was used to estimate the relative risk (RR) of incident GDM for each iron category. All models were adjusted for age, parity, BMI, physical activity, glycemic load, cereal fiber, polyunsaturated fat, current smoking, alcohol, total calories, and family history of diabetes. In addition, we used restricted cubic spline regressions to model the association between continuous dietary heme iron intake and GDM risk.
By conducting stratified analyses and evaluating interaction terms, we evaluated whether associations between dietary iron intakes and GDM risk were modified by other risk factors of GDM associated with iron storage, oxidative stress, and/or insulin resistance. These factors included BMI (<25, 25–29.9, or ≥30 kg/m2), physical activity (highest 2 quintiles vs. lowest 3), family history of diabetes (yes or no), current cigarette smoking (yes or no), and dietary vitamin C (high vs. low). All statistical analyses were performed by using SAS statistical software (version 8.2; SAS Institute, Cary, NC). (Fig. 2 was created using Stata 9.0 [StataCorp. 2005, Stata Statistical Software: Release 9; StataCorp LP, College Station, TX].)
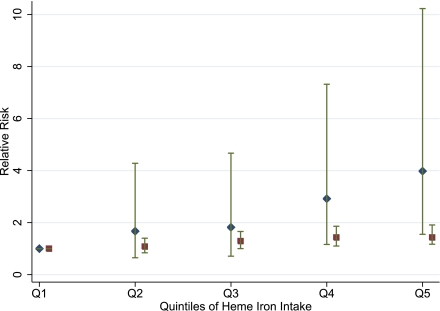
Figure 2.
RRs of GDM in association with prepregnancy dietary heme iron intake stratified by smoking status. RRs are adjusted for age, parity, BMI, physical activity, glycemic load, polyunsaturated fat intake, cereal fiber, alcohol, total calories, and family history of diabetes. Smokers are represented by diamonds, and nonsmokers are represented by squares. (A high-quality color representation of this figure is available in the online issue.)
RESULTS
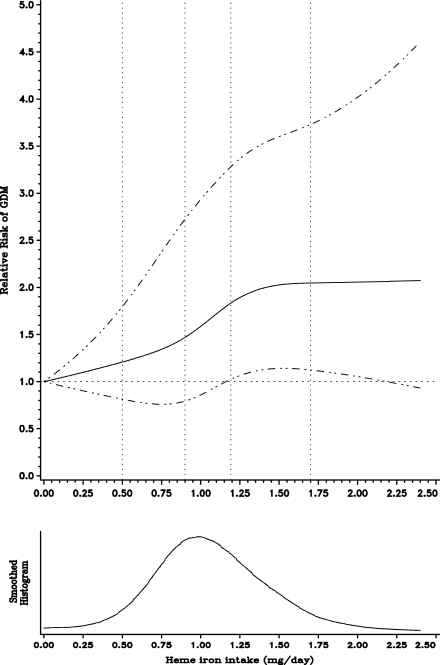
During 10 years of follow-up (1991–2001), 867 women reported a first diagnosis of GDM. Women in the highest quintile of heme iron had a higher glycemic load and had higher intakes of meat and fish (Table 1). In addition, women in the highest quintile of intake were more likely to smoke and consume more caffeine and alcohol. In age-adjusted models, total iron intake was inversely associated with GDM; however this association attenuated and was not significant after adjustment for both dietary and nondietary confounding factors (Table 2). Heme iron intake was significantly and positively associated with incident GDM in both age- and fully adjusted models (Table 2). The age-adjusted RR between extreme quintiles of cumulative heme iron intake was 2.13 (95% CI 1.70–2.67; Ptrend < 0.0001), which was attenuated to 1.58 (1.21–2.08; Ptrend = 0.0001) in the fully adjusted model. When heme iron was modeled as a continuous variable, the multivariate RR for every 0.5-mg per day of increase in intake was 1.22 (1.10–1.36). The regression splines demonstrated linear associations between heme iron (P = 0.22 for curvature) and the risk for GDM (Fig. 1).
Table 1.
Baseline characteristics by quintiles of prepregnancy dietary total iron and heme iron intakes among 13,475 women
| Characteristic* |
Total iron |
Heme iron |
||||
|---|---|---|---|---|---|---|
| Q1 |
Q3 | Q5 | Q1 | Q3 | Q5 | |
| Mean intake (mg/day) | 7.85 (1.50) | 15.42 (1.38) | 67.85 (31.81) | 0.53 (0.18) | 1.05 (0.05) | 1.80 (0.36) |
| Age (years) | 31.36 (3.37) | 31.57 (3.26) | 31.50 (3.29)** | 31.57 (3.40) | 31.46 (3.18) | 31.42 (3.21) |
| Race (%) | ||||||
| White | 2,095 (93.86) | 2,224 (94.36) | 3,758 (95.04)** | 3,188 (94.52) | 2,287 (94.43) | 2,454 (93.17)** |
| African American | 19 (0.85) | 17 (0.72) | 32 (0.81) | 19 (0.56) | 21 (0.87) | 37 (1.40) |
| Hispanic | 30 (1.34) | 33 (1.40) | 52 (1.32) | 52 (1.54) | 33 (1.36) | 37 (1.40) |
| Asian | 45 (2.02) | 47 (1.99) | 51 (1.29) | 61 (1.81) | 37 (1.53) | 54 (2.05) |
| Other | 43 (1.93) | 36 (1.53) | 61 (1.54) | 53 (1.57) | 44 (1.82) | 52 (1.97) |
| Mean physical activity (MET/week) | 21.35 (29.03) | 23.65 (27.84) | 24.13 (30.56)** | 24.56 (30.29) | 22.39 (28.22) | 23.29 (29.65) |
| Family history of diabetes (%) | 291 (13.04) | 291 (12.35) | 493 (12.47)** | 385 (11.41) | 291 (12.01) | 361 (13.71) |
| Mean BMI (kg/m2) | 23.09 (4.22) | 23.29 (4.18) | 23.79 (4.27) | 22.61 (3.61) | 23.43 (4.25) | 24.28 (4.67) |
| Current smokers (%) | 292 (13.08) | 246 (10.44) | 204 (5.16) | 249 (7.38) | 217 (8.96) | 266 (10.10) |
| Mean alcohol intake (g/day) | 2.93 (5.53) | 3.27 (4.93) | 2.41 (4.65) | 2.76 (4.96) | 3.08 (5.22) | 3.19 (5.50)** |
| Mean total calories (kcal/day) | 1,271.80 (300.62) | 1,974 (439.53) | 1,999.23 (542.31)** | 1,448.24 (430.40) | 1,833.33 (448.28) | 2,279.55 (503.41) |
| Calories from carbohydrates (%) | 48.32 (7.86) | 50.80 (6.84) | 52.08 (6.79) | 54.62 (7.41) | 50.32 (6.35) | 46.62 (6.41) |
| Calories from total fat (%) | 32.50 (5.77) | 30.87 (5.36) | 29.99 (5.13) | 29.12 (5.73) | 30.85 (5.08) | 32.88 (5.09) |
| Calories from protein (%) | 19.24 (3.67) | 19.13 (3.17) | 19.23 (3.14) | 17.29 (2.97) | 19.55 (2.97) | 21.04 (3.21) |
| Calories from trans fat (%) | 1.78 (0.71) | 1.58 (0.57) | 1.49 (0.53) | 1.54 (0.65) | 1.57 (0.58) | 1.64 (0.56) |
| Calories from saturated fat (%) | 11.84 (2.51) | 11.06 (2.33) | 10.93 (2.30) | 10.65 (2.66) | 11.09 (2.22) | 11.82 (2.23) |
| Calories from polyunsaturated fat (%) | 5.62 (1.42) | 5.47 (1.27) | 5.26 (1.24) | 5.28 (1.42) | 5.51 (1.25) | 5.49 (1.20) |
| Polyunsaturated-to-saturated ratio | 0.49 (0.15) | 0.51 (0.14) | 0.50 (0.15) | 0.52 (0.19) | 0.51 (0.14) | 0.48 (0.14) |
| Cereal fiber (g/day) | 3.40 (1.37) | 6.58 (2.71) | 7.21 (4.72) | 5.58 (3.39) | 6.13 (3.36) | 6.79 (3.33) |
| Magnesium (mg/day) | 201.29 (50.26) | 325.17 (70.98) | 384.18 (116.26) | 275.32 (105.24) | 323.61 (100.01) | 375.09 (107.83) |
| Vitamin C (mg/day) | 146.24 (203.65) | 215.18 (214.32) | 331.06 (283.80) | 240.27 (276.57) | 247.93 (257.03) | 254.08 (235.04) |
| Vitamin E (mg/day) | 20.77 (82.02) | 23.35 (77.54) | 49.09 (107.86) | 35.90 (101.16) | 31.55 (97.43) | 32.47 (83.39) |
| Caffeine intake (mg/day) | 192.22 (188.83) | 209.55 (197.73) | 151.92 (164.06) | 170.12 (175.04) | 189.95 (187.58) | 201.66 (189.76) |
| Mean glycemic load | 82.74 (27.70) | 136.09 (37.26) | 141.43 (43.43) | 107.76 (41.04) | 126.87 (44.03) | 146.81 (46.61) |
| Intake of total meat (servings/day) | 0.54 (0.36) | 0.84 (0.57) | 0.77 (0.55) | 0.35 (0.26) | 0.72 (0.38) | 1.31 (0.65) |
| Intake of red meat (servings/day) | 0.39 (0.26) | 0.59 (0.42) | 0.54 (0.38) | 0.24 (0.16) | 0.49 (0.24) | 0.96 (0.46) |
| Intake of chicken/turkey (servings/day) | 0.33 (0.22) | 0.50 (0.30) | 0.48 (0.30) | 0.23 (0.15) | 0.49 (0.22) | 0.68 (0.39) |
| Intake of fish meat (servings/day) | 0.19 (0.16) | 0.29 (0.23) | 0.29 (0.25)** | 0.17 (0.15) | 0.28 (0.21) | 0.38 (0.32) |
| Intake of fruits and vegetables (servings/day) | 3.19 (1.69) | 5.50 (2.65) | 5.73 (2.96) | 4.30 (2.61) | 5.17 (2.67) | 5.99 (3.08) |
| Whole grain intake (g/day) | 0.57 (0.59) | 1.22 (1.03) | 1.33 (1.15) | 1.05 (1.06) | 1.11 (0.99) | 1.23 (1.05) |
| Multivitamin supplement users (%) | 500 (22.4) | 778 (33.01) | 3637 (91.98) | 1,804 (53.48) | 1,336 (55.16) | 1,368 (51.94)** |
| Iron supplement use (g/day) | 0.03 (0.27) | 0.52 (1.82) | 50.27 (32.63) | 15.14 (27.28) | 17.32 (30.22) | 16.16 (28.49) |
| Parous (%) | 1,135 (51.95) | 1,383 (60.10) | 2,452 (64.92) | 1,653 (50.37) | 1,458 (62.04) | 1,683 (65.95) |
MET, metabolic equivalent hours per week; Q, quintile.
*Means (SD) for continuous variables and n (%) for categorical variables.
**Differences between categories of iron intake were not statistically different (P > 0.05).
Table 2.
Prepregnancy dietary iron intakes and RRs of GDM among 13,475 women
| Quintiles of iron intake (median, mg/day) | Total (n) | Cases (n) | Age-adjusted | Multivariate-adjusted | ||
|---|---|---|---|---|---|---|
| 13,475 | 867 | RR (95% CI) | P value | RR (95% CI)* | P value | |
| Heme iron | ||||||
| Q1 (0.66) | 2,581 | 122 | 1.00 (ref) | n/a | 1.00 (ref) | n/a |
| Q2 (0.90) | 2,987 | 168 | 1.20 (0.95–1.52) | 0.13 | 1.11 (0.87–1.43) | 0.40 |
| Q3 (1.10) | 2,933 | 171 | 1.42 (1.13–1.78) | 0.003 | 1.31 (1.03–1.68) | 0.03 |
| Q4 (1.30) | 2,779 | 201 | 1.74 (1.39–2.19) | <0.0001 | 1.51 (1.17–1.93) | 0.001 |
| Q5 (1.60) | 2,195 | 205 | 2.13 (1.70–2.67) | <0.0001 | 1.58 (1.21–2.08) | 0.001 |
| Trend | <0.0001 | 0.0001 | ||||
| Total iron | ||||||
| Q1 (10.70) | 2,380 | 180 | 1.00 (ref) | n/a | 1.00 (ref) | n/a |
| Q2 (13.00) | 2,128 | 161 | 0.85 (0.68–1.07) | 0.17 | 0.86 (0.68–1.10) | 0.24 |
| Q3 (16.13) | 2,376 | 144 | 0.80 (0.64–1.00) | 0.05 | 0.85 (0.67–1.09) | 0.20 |
| Q4 (24.15) | 2,673 | 154 | 0.72 (0.58–0.90) | 0.004 | 0.84 (0.66–1.07) | 0.15 |
| Q5 (49.80) | 3,918 | 228 | 0.78 (0.64–0.96) | 0.02 | 0.90 (0.72–1.12) | 0.33 |
| Trend | 0.12 | 0.95 | ||||
| Dietary total iron | ||||||
| Q1 (10.30) | 2,708 | 183 | 1.00 (ref) | n/a | 1.00 (ref) | n/a |
| Q2 (11.90) | 2,695 | 211 | 1.00 (0.82–1.23) | 0.99 | 0.99 (0.80–1.24) | 0.96 |
| Q3 (13.30) | 2,506 | 148 | 0.77 (0.62–0.95) | 0.02 | 0.83 (0.66–1.06) | 0.14 |
| Q4 (15.00) | 2,684 | 163 | 0.84 (0.68–1.04) | 0.11 | 0.97 (0.77–1.24) | 0.83 |
| Q5 (18.90) | 2,882 | 162 | 0.83 (0.68–1.02) | 0.08 | 1.12 (0.87–1.45) | 0.38 |
| Trend | 0.05 | 0.26 | ||||
| Supplemental iron | ||||||
| Q1 (0) | 7,949 | 544 | 1.00 (ref) | n/a | 1.00 (ref) | n/a |
| Q2 (5.10) | 603 | 40 | 0.96 (0.76–1.22) | 0.76 | 0.98 (0.76–1.25) | 0.84 |
| Q3 (15.00) | 1,654 | 95 | 0.89 (0.72–1.11) | 0.30 | 0.95 (0.76–1.19) | 0.66 |
| Q4 (30.00) | 1,134 | 59 | 0.80 (0.64–1.01) | 0.07 | 0.86 (0.68–1.10) | 0.23 |
| Q5 (60.00) | 2,135 | 129 | 0.99 (0.81–1.20) | 0.89 | 1.04 (0.84–1.28) | 0.72 |
| Trend | 0.56 | 0.97 | ||||
| Nonheme iron | ||||||
| Q1 (7.58) | 2,324 | 184 | 1.00 (ref) | n/a | 1.00 (ref) | n/a |
| Q2 (10.55) | 2,189 | 153 | 0.86 (0.69–1.08) | 0.20 | 0.95 (0.74–1.22) | 0.68 |
| Q3 (13.39) | 2,381 | 150 | 0.80 (0.64–1.00) | 0.05 | 0.92 (0.71–1.19) | 0.52 |
| Q4 (21.03) | 2,648 | 147 | 0.69 (0.55–0.86) | 0.001 | 0.85 (0.67–1.10) | 0.21 |
| Q5 (45.33) | 3,933 | 233 | 0.79 (0.65–0.96) | 0.02 | 0.97 (0.78–1.20) | 0.75 |
| Trend | 0.15 | 0.86 |
*Covariates include age, parity, BMI, physical activity, glycemic load, polyunsaturated fat intake, cereal fiber, smoking, alcohol, total calories, and family history of diabetes. n/a, not applicable.
Figure 1.
Smoothed histogram and spline plot displaying the RRs of GDM according to dietary heme iron intake (mg/day, continuous). RRs adjusted for age, parity, BMI, physical activity, glycemic load, polyunsaturated fat intake, cereal fiber, smoking, alcohol, total calories, and family history of diabetes. The solid lines represent point estimates; the dashed lines illustrate 95% CIs.
Because red meat is one of the primary sources of heme iron and its intake was positively associated with GDM in this cohort, other components of red meat may confound the heme iron and GDM association. When we further adjusted for other components of red meat that could be related to glucose metabolism—including saturated fat and dietary cholesterol—by adding these variables to the fully adjusted models, the RR was slightly attenuated but remained significant (RR 1.55 [95% CI 1.13–2.13] for the highest quintile vs. the lowest quintile; Ptrend = 0.002) (data not shown). When we additionally adjusted for red meat, the RR was further attenuated (1.29 [0.95–1.75]; Ptrend = 0.02) (data not shown).
In stratified analyses, the association of heme iron with GDM risk appeared to be stronger among current cigarette smokers than nonsmokers although the test for interaction was not significant (P value for interaction = 0.26). For example, the RR comparing the extreme quintiles was 3.98 (95% CI 1.55–10.23; Ptrend = 0.0008) among current smokers and 1.43 (1.17–1.91; Ptrend = 0.003) among current nonsmokers (Fig. 2). The association did not vary by BMI, physical activity, family history of diabetes, or vitamin C intake (data not shown).
CONCLUSIONS
In this large prospective cohort study, we identified a significant and positive association between prepregnancy dietary heme iron intake and GDM. The association remained significant even after adjustment for other dietary and nondietary risk factors of GDM. We observed no significant association of total, nonheme, and supplemental iron intakes with GDM risk.
Accumulating evidence suggests that the main defect in the pathogenesis of GDM is relatively diminished insulin secretion coupled with pregnancy-induced insulin resistance, although the underlying molecular mechanism remains unclear (3). Pregnancy-related metabolic challenges unmask a predisposition to glucose metabolic disorders in some women. Therefore, factors that contribute to insulin resistance or impaired insulin secretion before pregnancy and in early pregnancy can have a deleterious effect during pregnancy and may be risk factors for GDM. Iron, a redox-active transitional metal, is a powerful prooxidant that promotes the formation of hydroxyl radicals and increases oxidative stress. The pancreatic β-cell is particularly susceptible to oxidative stress because of a weak antioxidant defense (3). Since little iron is excreted from the body, iron surplus is a potential consequence of dietary and supplemental intake (3). Therefore, it is plausible that the observed association of dietary iron intakes with GDM risk is mediated through the impact of iron on β-cell toxicity (18).
A role of iron in diabetic pathogenesis was first suggested by the increased rates of diabetes among individuals with iron-related disorders. Frequent blood donations, which reduce iron stores, were associated with a decreased prevalence of diabetes (19), and a lower incidence of GDM was observed in women with versus women without anemia (20). Emerging data from some, though not all, studies (6–9) also support the significant associations between measures of body iron stores and T2DM and GDM. Serum ferritin, the most frequently studied measure of iron stores, was significantly and positively associated with GDM risk in several studies (6–9). For example, Chen et al. (21) identified and multivariate adjusted RR = 1.84 (95% CI 0.95–3.58), comparing the 5th quintile of serum ferritin to all other quintiles in a prospective cohort of 1,456 Caucasian women. However, it should be noted that the specificity of serum ferritin as a measure for body iron store is questioned as other factors, such as chronic inflammation, can affect its levels as well.
Dietary intake of iron is a critical determinant of body iron stores. Despite accumulating evidence from both experimental and epidemiological studies supporting potential links between iron and GDM risk, studies on the association of dietary iron and specific forms of iron with GDM risk are sparse. The positive association between heme, but not nonheme, iron intake and GDM risk observed in the current study is consistent with findings from studies on T2DM in men (19) and women (10). For example, Rajpathak et al. (10), in a prospective study of women accumulating 1,578,982 person-years and 4,599 T2DM cases, found a 28% increased risk for T2DM for the highest compared with the lowest quintile of heme iron intake, but no increased risk for total iron.
Dietary iron exists as heme (mainly from meat and meat products) or nonheme iron. Iron supplements are primarily nonheme iron. Previous studies focusing on supplementary iron intakes during pregnancy and GDM risk have produced inconsistent findings (11,12,22). Similar to the current study, no significant association was observed between iron supplementation and GDM risk in a recent clinical trial (12). Although precise molecular mechanisms are unclear, the different bioavailability of the two forms of iron could explain the discrepancy in their association with diabetes risk. Absorption of heme iron is more efficient and not significantly affected by other components of the meal, whereas the absorption of nonheme iron is well regulated and affected substantially by accompanying dietary factors (23). It has been observed that the absorption of heme iron is 10–15 times higher than nonheme iron. Therefore, it is plausible that chronically high intake of heme iron is more likely to lead to higher body iron stores and thus is more likely to be associated with increased risk of GDM. It is also plausible that women who used iron supplements may have had a much lower baseline iron status, such that their subsequent increase in iron stores resulting from supplemental iron intakes may not have raised their iron levels enough to be pathologic. Future studies integrating measures of body iron store status are warranted.
In the current study, the positive association of heme iron and GDM risk was suggested to be stronger among current cigarette smokers than nonsmokers. Among smokers, the risk of GDM comparing extreme quintiles of heme iron intake was fourfold, while the risk was only 1.5-fold for nonsmokers. Cigarette smoke contains multiple oxidants and may, therefore, exacerbate a prooxidant effect of iron. In rats and human tissue, cigarette smoke has been shown to cause iron accumulation, altering systematic iron homeostasis and inducing oxidative stress (24). If confirmed in other studies, these results may suggest that women who smoke may be particularly susceptible to the adverse effect on GDM associated with greater heme iron intakes.
There are several unique strengths to the study including the large sample size, prospective study design, repeated dietary assessments, and a high rate of follow-up and comprehensive information on covariates, which together minimize sources of measurement error and bias. However, several potential limitations merit discussion. Because of the observational nature of the study, we cannot rule out the possibility of unmeasured and unknown confounders leading to residual confounding. Heme iron intake may be affected by other lifestyle variables that also increase the risk of GDM. However, the association persisted after we adjusted for major dietary and nondietary risk factors of GDM. As in other observational studies, dietary data measured by FFQs are subject to measurement error; however, due the prospective design, misclassification is likely to be nondifferential, which is likely to bias results toward the null. In addition, this FFQ has been validated, and the three repeated assessments over 8 years of follow-up help reduce the extent of this error. Dietary intakes specifically during pregnancy were not measured in the current study. Rather, dietary iron measurements in the present analyses represent long-term or habitual intakes. It should be noted that iron deficiency in early pregnancy was associated with adverse pregnancy outcomes such as a greater risk of preterm delivery (25), and therefore further studies are needed to examine associations between dietary iron intakes before and during pregnancy and other pregnancy outcomes, including fetal growth and development.
In summary, findings from the large prospective study suggest that greater dietary heme iron intake is associated with an elevated risk for GDM. The results present another potential modifiable risk factor for GDM and suggest that the reduction in prepregnancy intake of heme iron may potentially reduce GDM risk. Future metabolic and clinical studies with data on dietary iron intakes and measures of body iron stores are warranted to confirm these findings and to decipher underlying molecular mechanisms.
Acknowledgments
This study was funded by research grants CA50385 and DK58845 from the National Institutes of Health (NIH). K.B., C.Z., and E.Y. were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
No potential conflicts of interest relevant to this article were reported.
K.B. analyzed data and wrote the manuscript. E.Y. contributed to the analyses and reviewed and edited the manuscript. M.A.W., L.Q., D.K.T., and F.B.H. reviewed and edited the manuscript. C.Z. conceived the hypothesis and reviewed and edited the manuscript.
Footnotes
References
- 1.American Diabetes Association Gestational diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S88–S90 [DOI] [PubMed] [Google Scholar]
- 2.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health 2010;100:1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care 2007;30(Suppl. 2):S105–S111 [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Sun L, Tan Y, Wang G, Lin X, Cai L. Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Curr Med Chem 2009;16:113–129 [DOI] [PubMed] [Google Scholar]
- 5.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004;291:711–717 [DOI] [PubMed] [Google Scholar]
- 6.Afkhami-Ardekani M, Rashidi M. Iron status in women with and without gestational diabetes mellitus. J Diabetes Complications 2009;23:194–198 [DOI] [PubMed] [Google Scholar]
- 7.Lao TT, Tam KF. Maternal serum ferritin and gestational impaired glucose tolerance. Diabetes Care 1997;20:1368–1369 [DOI] [PubMed] [Google Scholar]
- 8.Tarim E, Kilicdag E, Bagis T, Ergin T. High maternal hemoglobin and ferritin values as risk factors for gestational diabetes. Int J Gynaecol Obstet 2004;84:259–261 [DOI] [PubMed] [Google Scholar]
- 9.Lao TT, Chan PL, Tam KF. Gestational diabetes mellitus in the last trimester: a feature of maternal iron excess? Diabet Med 2001;18:218–223 [DOI] [PubMed] [Google Scholar]
- 10.Rajpathak S, Ma J, Manson J, Willett WC, Hu FB. Iron intake and the risk of type 2 diabetes in women: a prospective cohort study. Diabetes Care 2006;29:1370–1376 [DOI] [PubMed] [Google Scholar]
- 11.Bo S, Menato G, Villois P, et al. Iron supplementation and gestational diabetes in midpregnancy. Am J Obstet Gynecol 2009;201:158–, e1–e6. [DOI] [PubMed] [Google Scholar]
- 12.Chan KKL, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes—a randomised placebo-controlled trial. BJOG 2009;116:789–797; discussion 797–798 [DOI] [PubMed] [Google Scholar]
- 13.Solomon CG, Willett WC, Rich-Edwards J, et al. Variability in diagnostic evaluation and criteria for gestational diabetes. Diabetes Care 1996;19:12–16 [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–199 [DOI] [PubMed] [Google Scholar]
- 15.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–1083 [PubMed] [Google Scholar]
- 16.Willett W, Hennekens CH, Castelli W, et al. Effects of cigarette smoking on fasting triglyceride, total cholesterol, and HDL-cholesterol in women. Am Heart J 1983;105:417–421 [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–540 [DOI] [PubMed] [Google Scholar]
- 18.Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr 2003;78:1160–1167 [DOI] [PubMed] [Google Scholar]
- 19.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr 2004;79:70–75 [DOI] [PubMed] [Google Scholar]
- 20.Lao TT, Ho LF. Impact of iron deficiency anemia on prevalence of gestational diabetes mellitus. Diabetes Care 2004;27:650–656 [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care 2006;29:1077–1082 [DOI] [PubMed] [Google Scholar]
- 22.Palma SP-I, Perez-Iglesias R, Prieto D, Pardo R, Llorca J, Delgado-Rodriguez M. Iron but not folic acid supplementation reduces the risk of low birthweight in pregnant women without anaemia: a case-control study. J Epidemiol Community Health 2008;62:120–124 [DOI] [PubMed] [Google Scholar]
- 23.Cook JD. Adaptation in iron metabolism. Am J Clin Nutr 1990;51:301–308 [DOI] [PubMed] [Google Scholar]
- 24.Ghio AJ, Hilborn ED, Stonehuerner JG, et al. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am J Respir Crit Care Med 2008;178:1130–1138 [DOI] [PubMed] [Google Scholar]
- 25.Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs. iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr 1992;55:985–988 [DOI] [PubMed] [Google Scholar]