Abstract
OBJECTIVE
Higher heme iron intake is associated with increased type 2 diabetes risk. However, no previous study has evaluated gestational diabetes mellitus (GDM) risk in relation to heme iron intake during pregnancy. We investigated associations of maternal preconceptional and early pregnancy heme and nonheme iron intake with subsequent GDM risk.
RESEARCH DESIGN AND METHODS
We conducted a prospective cohort study of 3,158 pregnant women. A food frequency questionnaire was used to assess maternal diet. Multivariable generalized linear regression models were used to derive estimates of relative risks (RRs) and 95% CIs.
RESULTS
Approximately 5.0% of the cohort developed GDM (n = 158). Heme iron intake was positively and significantly associated with GDM risk (Ptrend = 0.04). After adjusting for confounders, women reporting the highest heme iron intake levels (≥1.52 vs. <0.48 mg per day) experienced a 3.31-fold–increased GDM risk (95% CI 1.02–10.72). In fully adjusted models, we noted that a 1-mg per day increase in heme iron was associated with a 51% increased GDM risk (RR 1.51 [95% CI 0.99–2.36]). Nonheme iron was inversely, though not statistically significantly, associated with GDM risk, and the corresponding RRs were 1.00, 0.83, 0.62, and 0.61 across quartiles of nonheme iron intake (Ptrend = 0.08).
CONCLUSIONS
High levels of dietary heme iron intake during the preconceptional and early pregnancy period may be associated with increased GDM risk. Associations of GDM risk with dietary nonheme iron intake are less clear. Confirmation of these findings by future studies is warranted.
Iron deficiency is the most common nutritional deficiency in the U.S. and worldwide (1). In recent years, concerns about iron overload in developed countries have spurred research designed to assess cardiometabolic risks secondary to excess body iron stores and high dietary iron intake (2,3). As a result, iron now is viewed as a double-edged sword for living systems. Increasingly, clinical and epidemiological evidence suggest that both iron deficiency and iron overload influence the production of reactive oxygen species, leading to oxidative stress, systemic inflammation, and alternations in mitochondrial function (4). Taken together, cellular and metabolic alterations secondary to iron overload are thought to contribute to increased risks of hypertension (2), cardiovascular disease (5), and type 2 diabetes (3,6–8).
The two kinds of dietary iron, heme and nonheme iron, with distinct metabolic pathways and intestinal absorption potential, are thought to play distinct roles in the pathophysiology of cardiometabolic disorders (4). Heme iron is exclusively present in hemoglobin and myoglobin from animal sources, including red meat and poultry. Nonheme iron, which is abundant in cereals, vegetables, fruits, beans, and dairy products, accounts for >85% of dietary iron intake. Although heme iron accounts for a smaller proportion of dietary iron, it is absorbed two to three times more readily than nonheme iron and is less affected by other dietary constituents. The bioavailability of heme and nonheme iron is influenced by dietary factors, including ascorbic acid, coffee, and whole grains (9). Body iron stores also are important determinants of intestinal absorption of heme and nonheme iron (10).
The expanding literature suggests that iron influences glucose metabolism (3). Statistically significant positive associations of dietary iron intake, particularly heme iron, with incident type 2 diabetes has been reported (6–8). These epidemiological associations are supported by findings documenting increased risks of incident type 2 diabetes among individuals with elevated serum ferritin concentrations (6,11). The relationship between nonheme iron intake and type 2 diabetes, however, has been far less consistent. Some (7), but not all (8), investigators have reported inverse associations of incident type 2 diabetes and dietary nonheme iron intake.
Although there have been several studies investigating the possible role of dietary iron and body iron stores on glucose metabolism, only a few have enrolled pregnant women, and the results have been inconsistent (12–14). The effect of iron supplement use on gestational diabetes mellitus (GDM) risk also is controversial (14,15). To the best of our knowledge, no previous study has examined the associations of dietary heme and nonheme iron with the risk of GDM. Given mounting available experimental and epidemiological evidence from studies of men and nonpregnant women supporting associations of heme iron and risk of type 2 diabetes, we hypothesized that higher preconceptional and early pregnancy dietary heme iron intake may be associated with increased GDM risk. We also hypothesized that diets high in nonheme iron may be associated with reduced GDM risk. We investigated these hypotheses among a well-characterized prospective cohort of pregnant women.
RESEARCH DESIGN AND METHODS
The Omega Study is a prospective cohort study designed to examine the dietary risk factors of adverse pregnancy outcomes. Participants were women attending prenatal care clinics affiliated with the Swedish Medical Center and Tacoma General Hospital in Seattle and Tacoma, WA (16). Eligible women were those who began prenatal care before 20 weeks’ gestation, spoke and read English, were aged ≥18 years, and planned to deliver at either of the two hospitals. During early pregnancy, participants were asked to complete an interviewer-administered questionnaire. Participants also completed a 121-item semiquantitative food frequency questionnaire (FFQ) (17). Pregnancy outcome information was abstracted from medical records. All procedures and study protocols were approved by the institutional review boards of the study hospitals. All participants provided written informed consent.
Analytical population
Women with pre-gestational diabetes (determined by self-report of physician-diagnosed diabetes) (n = 57), those with multifetal pregnancies (n = 118), those with pregnancies lasting <20 weeks (n = 58), and those who moved out of the study area (n = 170) were excluded. Also excluded were women with incomplete dietary intake information (n = 372) and those who reported extreme levels of daily energy intake (<500 [n = 24] or >3,500 [n = 43] calories per day). A cohort of 3,158 women remained for analysis.
Data collection
We obtained information of covariates, including maternal age, educational attainment, height, prepregnancy weight, and reproductive and medical histories. We also collected information on maternal smoking status and leisure time physical activity during pregnancy. Prepregnancy BMI was calculated as self-reported prepregnancy weight (in kilograms) divided by the square of height (in meters). We used the FFQ from the Women’s Health Initiative Clinical Trial (17) to assess maternal dietary intake during the 3-month period (before conception and during the first trimester). Participants were provided with instructions, including photos of portion sizes. The FFQ has documented reliability of accurately recording intake over an extended period of observation (17). Participants completed FFQs at an average of 15.3 weeks’ gestation. Dietary intake values of nutrients, vitamins, and minerals, including heme iron, nonheme iron (18), and total iron intake, were estimated using food composition tables from the University of Minnesota Nutrition Coding Center Nutrient Database (Nutrition Coordinating Center, Minneapolis, MN).
Medical records were reviewed to collect detailed clinical information. In our study settings, according to American Diabetes Association guidelines (19), pregnant women were screened at 24–28 weeks’ gestation using a 50-g 1-h oral glucose challenge test. Those who failed this screening test (≥7.8 mmol/L) were then followed-up within 1–2 weeks with a 100-g 3-h oral glucose tolerance test (OGTT). We also abstracted laboratory results from participants’ 50-g 1-h glucose challenge test and from the diagnostic 100-g 3-h OGTT. Women were diagnosed with GDM if two or more of the 100-g OGTT glucose concentrations exceeded American Diabetes Association criteria (19) (fasting ≥5.3 mmol/L, 1-h postchallenge ≥10.0 mmol/L, 2-h postchallenge ≥8.6 mmol/L, and 3-h postchallenge ≥7.8 mmol/L).
Statistical analysis
We classified each subject according to quartiles of dietary heme and nonheme iron intake. We examined frequency distributions of maternal characteristics and energy-adjusted nutrient intake according to these categories. We fitted generalized linear models, using a log-link function, to derive risk ratios (RRs) and 95% CIs. To assess confounding, we entered covariates into each model one at a time and compared adjusted and unadjusted RRs. Final models included covariates that altered unadjusted RRs by at least 10% and those that were identified a priori as potential confounders. Given that red and processed meats, saturated fat, and cholesterol intake have been implicated as potential risk factors for GDM, we report results from models adjusted for these covariates. In multivariable analyses, we evaluated linear trends in risk by treating heme and nonheme iron intake as continuous variables after assigning a score to each quartile. We also explored the possibility of a nonlinear relationship of heme iron intake with GDM risk by fitting a multivariable logistic regression model that implemented the generalized additive modeling method (20).
Given that nonheme iron absorption is known to be influenced by body iron stores, particularly iron deficiency anemia status (10,21), we repeated the analyses after excluding 55 women with iron deficiency anemia. We also repeated the analyses after excluding women (n = 75) who did not take prenatal-care vitamins during early pregnancy. S-Plus 6.1 (Insightful, Seattle, WA) was used for generalized additive modeling. All other analyses were performed using Stata 9.0 (Stata, College Station, TX).
RESULTS
Heme iron accounted for ~7% of total dietary iron intake (93% nonheme iron) in this study cohort. Red and processed meat consumption, a major food source of dietary heme iron, was highly correlated with heme iron intake (ρ = 0.85, P < 0.001) and explained 72% of the variability in heme iron intake in the cohort. Fruits, vegetables, and total fiber consumption explained 50% of the variability of nonheme iron. Women who reported higher heme iron intake tended to be heavier and multiparous (Table 1). Heme iron intake was positively associated with saturated fat, trans fat, red and processed meats, poultry meat, fruits, vegetables, and vitamin C intake. Heme iron intake was positively related to the percentage of dietary calories from fat and protein and was inversely related to the percentage of calories from carbohydrates (all Ptrends < 0.05). As expected, higher nonheme iron intake was associated with higher intake of fruits, vegetables, fiber, and vitamin C.
Table 1.
Participants’ characteristics according to quartiles of dietary heme and nonheme iron intake, Seattle and Tacoma, WA, Omega Cohort Study
| Characteristic |
Dietary heme iron (mg per day) |
Dietary nonheme iron (mg per day) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort |
Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | P for trend | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | P for trend | |
| n | 3,158 | 806 | 765 | 793 | 794 | 787 | 790 | 791 | 790 | ||
| Interval | — | <0.48 | 0.48–0.75 | 0.76–1.11 | ≥1.12 | <9.10 | 9.10–12.16 | 12.17–15.97 | ≥12.98 | ||
| Median | — | 0.30 | 0.61 | 0.93 | 1.43 | <0.001 | 7.16 | 10.65 | 13.81 | 19.60 | <0.001 |
| Maternal age (years) | 32.7 | 32.6 | 32.7 | 32.6 | 33.0 | 0.10 | 32.4 | 32.9 | 32.9 | 32.7 | 0.11 |
| Prepregnancy BMI (kg/m2) | 23.5 | 22.6 | 23.2 | 23.5 | 24.6 | <0.001 | 23.6 | 23.6 | 23.4 | 23.2 | 0.04 |
| Non-Hispanic white (%) | 87.7 | 87.6 | 89.5 | 88.8 | 84.8 | 0.08 | 80.9 | 87.6 | 89.9 | 92.2 | <0.001 |
| <12 years of education (%) | 3.1 | 3.7 | 2.2 | 3.4 | 3.0 | 0.72 | 3.6 | 3.0 | 3.2 | 2.7 | 0.35 |
| Not married (%) | 10.0 | 11.0 | 8.1 | 9.3 | 11.5 | 0.62 | 11.4 | 9.9 | 8.4 | 10.3 | 0.30 |
| Nullipara (%) | 62.2 | 67.0 | 63.5 | 62.2 | 56.1 | <0.001 | 60.2 | 61.8 | 63.6 | 63.2 | 0.17 |
| Smoked during pregnancy (%) | 5.4 | 5.1 | 4.4 | 5.4 | 6.7 | 0.11 | 7.1 | 4.7 | 4.8 | 5.1 | 0.10 |
| Prenatal vitamin use (%) | 97.6 | 97.8 | 97.3 | 98.1 | 97.4 | 0.87 | 97.5 | 98.4 | 97.1 | 97.6 | 0.72 |
| Iron deficiency anemia (%) | 2.3 | 3.1 | 2.4 | 1.8 | 1.8 | 0.051 | 2.3 | 2.0 | 1.6 | 3.0 | 0.43 |
| Physically inactive in pregnancy (%) | 12.1 | 11.8 | 11.1 | 13.1 | 12.5 | 0.44 | 11.9 | 13.9 | 11.5 | 11.1 | 0.35 |
| History of hypertension (%) | 4.2 | 3.5 | 3.7 | 3.5 | 6.2 | 0.02 | 4.3 | 5.1 | 4.3 | 3.2 | 0.19 |
| Family history of hypertension (%) | 49.2 | 45.9 | 49.2 | 48.9 | 52.8 | 0.01 | 52.5 | 48.2 | 48.3 | 47.7 | 0.08 |
| Family history of diabetes (%) | 13.5 | 12.3 | 13.7 | 13.6 | 14.4 | 0.26 | 14.6 | 13.8 | 12.8 | 12.8 | 0.23 |
| Total energy intake (kcal) | 1,717 | 1,420 | 1,563 | 1,786 | 2,096 | <0.001 | 1,185 | 1,606 | 1,857 | 2,216 | <0.001 |
| Calories from carbohydrate (%) | 53.0 | 57.1 | 54.4 | 52.0 | 48.4 | <0.001 | 51.3 | 52.2 | 53.5 | 55.0 | <0.001 |
| Calories from protein (%) | 17.4 | 15.9 | 17.1 | 17.8 | 18.8 | <0.001 | 17.5 | 17.3 | 17.5 | 17.2 | 0.28 |
| Calories from total fat (%) | 31.5 | 29.3 | 30.5 | 32.0 | 34.1 | <0.001 | 32.7 | 32.3 | 31.0 | 29.9 | <0.001 |
| Calories from saturated fat (%) | 11.2 | 10.6 | 10.9 | 11.4 | 12.0 | <0.001 | 11.7 | 11.5 | 11.1 | 10.6 | <0.001 |
| Calories from trans fat (%) | 1.11 | 0.97 | 1.05 | 1.15 | 1.25 | <0.001 | 1.16 | 1.14 | 1.08 | 1.05 | <0.001 |
| Calories from polyunsaturated fat (%) | 6.5 | 6.2 | 6.3 | 6.5 | 6.9 | <0.001 | 6.7 | 6.6 | 6.4 | 6.3 | <0.001 |
| Calories from n-3 fatty acids (%) | 0.76 | 0.71 | 0.75 | 0.77 | 0.82 | <0.001 | 0.82 | 0.78 | 0.73 | 0.71 | <0.001 |
| Dietary cholesterol (mg)* | 271 | 222 | 247 | 269 | 315 | <0.001 | 298 | 287 | 274 | 249 | <0.001 |
| Dietary vitamin C (mg)* | 136 | 148 | 142 | 138 | 123 | <0.001 | 118 | 129 | 129 | 151 | <0.001 |
| Dietary vitamin E (mg)* | 15.7 | 16.4 | 16.2 | 15.6 | 15.0 | 0.001 | 9.3 | 11.4 | 13.7 | 22.2 | <0.001 |
| Total dietary fiber (g)* | 20.9 | 22.7 | 21.8 | 20.9 | 19.2 | <0.001 | 16.7 | 19.2 | 20.9 | 23.4 | <0.001 |
| Dietary calcium (g)* | 1.41 | 1.54 | 1.49 | 1.42 | 1.27 | <0.001 | 1.32 | 1.32 | 1.38 | 1.51 | <0.001 |
| Dietary total iron (mg)* | 16.2 | 16.7 | 16.6 | 16.1 | 16.0 | 0.002 | 9.3 | 12.4 | 15.2 | 22.0 | <0.001 |
| Fruits and vegetables (servings per day) | 4.4 | 4.1 | 4.2 | 4.6 | 4.9 | <0.001 | 3.2 | 4.3 | 4.7 | 5.5 | <0.001 |
| Whole grains (servings per day) | 0.63 | 0.58 | 0.59 | 0.67 | 0.68 | <0.001 | 0.40 | 0.53 | 0.71 | 0.88 | <0.001 |
| Red and processed meats (servings per day) | 0.66 | 0.19 | 0.47 | 0.73 | 1.24 | <0.001 | 0.50 | 0.65 | 0.72 | 0.76 | <0.001 |
| Poultry meats (servings per day) | 0.23 | 0.09 | 0.19 | 0.26 | 0.37 | <0.001 | 0.18 | 0.22 | 0.24 | 0.27 | <0.001 |
| Fish meats (servings per day) | 0.20 | 0.11 | 0.18 | 0.22 | 0.28 | <0.001 | 0.15 | 0.18 | 0.20 | 0.25 | <0.001 |
Data are means or percentages.
*Energy adjusted (2,000 kcal/day).
Approximately 5.0% of the cohort developed GDM. GDM risk increased with increasing levels of heme iron (Ptrend = 0.04) (Table 2). Multivariate-adjusted RRs for GDM were 1.00, 1.17, 1.20, and 1.57 across successive quartiles of heme iron intake. Women reporting very high heme iron intake (≥1.52 mg per day, upper decile) had a 2.26-fold–increased (95% CI 1.09–4.69) GDM risk compared with women reporting lower levels of heme iron intake (<0.48 mg per day, lowest quartile). After additional adjustment for red and processed meats, saturated fat, and cholesterol intake, women reporting very high heme iron intake (≥1.52 mg per day) had a 3.31-fold–increased (1.02–10.72) GDM risk compared with women reporting lower levels of intake (<0.48 mg per day), suggesting that the additional covariates may have a diluting effect on the heme iron–GDM association. Adjustments for fish and poultry intake did not materially alter these associations.
Table 2.
RRs and 95% CIs of GDM according to quartiles of dietary heme and nonheme iron intake, Seattle and Tacoma, WA, Omega Cohort Study
| Dietary iron variables | Median | GDM (n) | Energy adjusted | Adjusted* | Adjusted† |
|---|---|---|---|---|---|
| Incidence (%) | RR (95% CI) | RR (95% CI) | RR (95% CI) | ||
| Dietary heme iron (mg per day) | |||||
| Quartile 1 (<0.48) | 0.30 | 33 (4.1) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Quartile 2 (0.48–0.75) | 0.61 | 36 (4.7) | 1.22 (0.76–1.96) | 1.17 (0.73–1.89) | 1.27 (0.77–2.09) |
| Quartile 3 (0.76–1.11) | 0.93 | 36 (4.5) | 1.28 (0.79–2.08) | 1.20 (0.73–1.95) | 1.41 (0.81–2.44) |
| Quartile 4 (≥1.12) | 1.43 | 53 (6.7) | 2.12 (1.31–3.43) | 1.57 (0.95–2.61) | 2.15 (1.09–4.27) |
| P for trend | 0.003 | 0.09 | 0.04 | ||
| Quartile 1 (<0.48) | 0.30 | 33 (4.1) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Upper decile (≥1.52) | 1.85 | 25 (7.8) | 3.32 (1.70–6.47) | 2.26 (1.09–4.69) | 3.31 (1.02–10.72) |
| Dietary nonheme iron (mg per day) | |||||
| Quartile 1 (<9.10) | 7.16 | 50 (6.4) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Quartile 2 (9.10–12.16) | 10.65 | 44 (5.6) | 0.83 (0.54–1.28) | 0.85 (0.54–1.33) | 0.83 (0.53–1.31) |
| Quartile 3 (12.17–15.97) | 13.81 | 33 (4.2) | 0.60 (0.36–1.00) | 0.63 (0.37–1.08) | 0.62 (0.36–1.06) |
| Quartile 4 (≥12.98) | 19.60 | 31 (3.9) | 0.54 (0.29–0.99) | 0.61 (0.32–1.17) | 0.61 (0.31–1.18) |
| P for trend | 0.03 | 0.08 | 0.08 | ||
| Quartile 1 (<9.10) | 7.16 | 50 (6.4) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Upper decile (≥21.13) | 25.19 | 11 (3.5) | 0.29 (0.10–0.84) | 0.34 (0.10–1.10) | 0.30 (0.09–0.99) |
*Adjusted for daily energy intake, maternal age, race/ethnicity, parity, physical activity, prepregnancy BMI, dietary fiber, and vitamin C intake.
†Adjusted for daily energy intake; maternal age; race/ethnicity; parity; physical activity; prepregnancy BMI; and dietary fiber, vitamin C, saturated fat, cholesterol, and red and processed meat intake.
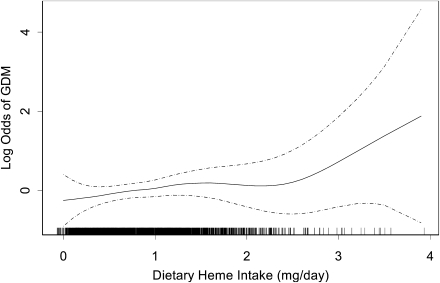
We also explored the possibility of a nonlinear relation of heme iron intake with GDM risk using regression procedures based on a generalized additive model. The results (Fig. 1) indicate an increasing risk of GDM with increasing dietary heme iron intake. In fully adjusted models, we noted that a 1-mg per day increase in heme iron intake was associated with a 51% increased GDM risk (RR 1.51 [95% CI 0.99–2.36]). Because the log odds of GDM risk appeared to raise steeply above daily heme iron intake levels >2.0 mg per day (Fig. 1), we repeated this analysis after restricting the study cohort to those women with daily reported heme iron take of 2.0 mg (n = 111). In this subgroup analysis, after adjusting for confounders including red and processed meat consumption, a 1-mg per day increase in daily heme iron intake was associated with a 186% increased GDM risk (2.86 [0.69–11.82]). Inferences from this subgroup analysis, however, are hindered by the relatively small sample size available for study.
Figure 1.
Relationship between maternal dietary heme iron intake in early pregnancy and risk of GDM (solid line) with 95% CIs (dotted lines) after adjusting for daily energy intake; maternal age; race/ethnicity; parity; physical activity; prepregnancy BMI; and dietary fiber, vitamin C, saturated fat, cholesterol, and red and processed meat intake. The vertical bars along the dietary heme iron intake axis indicate the distribution of study subjects.
GDM risks were reduced with increasing nonheme iron intake, although the association did not reach statistical significance (Ptrend = 0.08). Multivariable-adjusted RRs for GDM were 1.00, 0.83, 0.62, and 0.61, from the lowest to the highest quartiles of nonheme iron intake, respectively. Additional adjustments for red and processed meat consumption had little effect on the magnitudes of the observed associations (Table 2).
The above results were similar when we excluded women with early pregnancy iron deficiency anemia. For instance, the fully adjusted RRs for GDM were 1.00, 1.22, 1.39, and 2.09 for successive quartiles of heme iron intake after we excluded 55 women with early pregnancy iron deficiency anemia. Nonanemic women reporting very high heme iron intake (≥1.52 mg per day) had a 3.35-fold–increased (95% CI 1.04–10.79) GDM risk compared with nonanemic women reporting lower levels of heme iron intake (<0.48 mg per day). This association was slightly attenuated from that observed when the entire population was analyzed. The association of nonheme iron intake with GDM risk did not change substantially after further restriction of the cohort to nonanemic women. In a second series of sensitivity analyses, we excluded 75 women who reported taking no prenatal vitamins and repeated the above described analyses. Observed associations of dietary heme and nonheme iron intake with GDM were similar to those reported for the entire cohort (data not shown).
Given that the bioavailability of heme iron and body iron stores are influenced by dietary and nondietary factors such as ascorbic acid, dietary fiber, obesity, and cigarette smoking, we conducted multivariate analyses within the strata of each of these covariates. Although we found no evidence of statistically significant effect modification by these factors, we did note that GDM–heme iron associations were somewhat stronger among women who smoked during pregnancy. Among the cohort of nonsmokers (n = 2,987) the RR for GDM among those with high heme iron intake (≥1.12 mg per day) versus those with lower levels of intake, was 1.48 (95% CI 0.89–2.46). The corresponding RR for participants who smoked during pregnancy (n = 171) was 2.09 (0.42–10.41) (Pinteraction = 0.196).
CONCLUSIONS
We observed significant and positive associations of maternal dietary heme iron intake with the risk of GDM. Women with the highest levels of heme iron intake experienced at least a twofold higher risk of GDM compared with those who reported lower intake levels. This association was independent of established GDM risk factors, such as maternal age; race/ethnicity; prepregnancy BMI; parity; and other dietary factors, including saturated fat, cholesterol, and red and processed meat consumption; and was robust across study design and subanalyses restricted to women without iron deficiency anemia and those who did not consume prenatal vitamins. Nonheme iron appeared to be inversely associated with the risk of GDM; however, relative risk estimates generally were statistically nonsignificant.
To the best of our knowledge, this is the first study to explore the risk of GDM in relation to maternal dietary heme and nonheme iron intake during pregnancy. Our findings, however, are largely consistent with existing literature reporting associations of dietary heme iron intake with risk of incident type 2 diabetes in men and nonpregnant women (6–8). In addition, our findings are supported by studies documenting increased risks of incident GDM (13) or type 2 diabetes (6,11) among individuals with increased levels of serum ferritin, a biological marker of body iron stores. Chen et al. (13), in a prospective study of 1,456 healthy pregnant women, reported that maternal elevated serum ferritin concentrations were associated with a twofold increased GDM risk. However, the association was greatly attenuated after further adjustment for prepregnancy BMI.
The possible modest inverse relation between dietary nonheme iron and GDM risk suggested in our study is in general agreement with a report by Lee et al. (7), who noted that type 2 diabetes risks decreased across successive quintiles of nonheme iron intake among postmenopausal women in the Iowa Women’s Health Study. Among nondrinkers, adjusted RRs were 1.0, 0.83, 0.87, 0.72, and 0.67 across quintiles (Ptrend < 0.01) (7). Larger studies are needed to more formally and precisely assess GDM risk in relation to dietary nonheme iron intake.
Observed associations of increased GDM risk with increased heme iron consumption is biologically plausible. Iron, a strong pro-oxidant that catalyzes several reactions leading to the formation of reactive oxygen species such as hydroxyl radicals (22), is thought to contribute to an increased risk of diabetes through several potential mechanisms. First, increased accumulation of iron affects insulin synthesis and secretion in the pancreas and interferes with the insulin-extracting capacity of the liver so that hepatic neoglucogenesis suppression might be implicated (23). Second, excess iron deposition in muscle might decrease glucose uptake (23). Third, iron also may impair insulin action and interfere with glucose uptake in adipocytes (24). Finally, insulin stimulates cellular iron uptake through increased transferrin receptor externalization (25). Thus, insulin and iron might act synergistically, contributing, in a vicious cycle, to insulin resistance and diabetes (25). Future studies that empirically evaluate these mechanistic hypotheses are needed.
Our study has several strengths. The prospective design of the Omega Study and exclusion of women with diagnosed pregestational diabetes reduced the potential for bias from recall differences or dietary changes secondary to the disorder. Collection of dietary intake information in early pregnancy, before GDM was diagnosed, enhanced causal inference given our increased ability to infer the temporal relationship of heme and nonheme iron intake with subsequent GDM risk. In addition, the high follow-up rate of enrolled Omega Study participants (>95%) minimized possible selection bias. Nonetheless, several limitations of our study should be considered when interpreting study findings. Measures of body iron status, hemoglobin levels, and iron supplement use were not available. Although observed associations were robust across sensitivity analyses that excluded women with early pregnancy iron deficiency anemia and those who reported not taking prenatal vitamins during pregnancy, large-scale prospective studies in pregnant women, with detailed measures of dietary iron intake, supplemental intake, and body iron status, and studies of diet-genetic interactions are warranted. Second, because dietary iron intake was self-reported, we cannot exclude the possibility of reporting errors. However, because dietary intake information was collected before the testing and diagnosis of GDM, reporting errors are likely to have resulted in an attenuation of observed associations. Third, universal glucose tolerance testing in early pregnancy is not part of standard obstetric care. Hence, we cannot exclude the possibility that some subjects in our study had undiagnosed pregestational diabetes. Over 95% of study subjects reported having regular medical exams within a 24-month period before the index pregnancy, and the cumulative incidence of GDM in our study cohort is consistent with observations in other settings (19). These observations serve to attenuate concerns. Fourth, as with all observational studies, although we adjusted for known and suspected confounders, we cannot exclude the possibility of residual confounding from unmeasured covariates. Fifth, our relatively small number of incident GDM cases hindered inferences from some analyses. Finally, the generalizability of our findings may be limited to a largely white, well-educated obstetric population who registered for prenatal care early in pregnancy and who participate in regular annual medical exams. Their dietary behaviors, including dietary iron intake, are likely to differ from those of other socioeconomic, racial, and ethnic backgrounds.
In summary, we found significant associations between dietary heme iron intake and GDM risk. Confirmation of these findings in other populations and further exploration of possible underlying biological mechanisms of observed associations are warranted. Furthermore, given emerging evidence of harmful effects for unnecessary iron supplementation (14), studies designed to examine ways of increasing the intake of nonheme food or supplementation of vitamins and iron should be carried out to improve the iron status of pregnant women.
Acknowledgments
This research was supported by an award from the National Institutes of Health (R01-HD-32562). C.Z. was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
C.Q. analyzed data and drafted the manuscript. C.Z., B.G., D.A.E., and I.O.F. reviewed and edited the manuscript. M.A.W. drafted the manuscript, conceived and designed the study, and obtained funding for the study. All authors interpreted data, critically revised the draft for important intellectual content, and gave final approval of the manuscript to be published. C.Q. and M.A.W. had full access to all data in the study and take responsibility for the integrity of data, the accuracy of data analysis, and the decision to submit for publication.
The authors are indebted to the staff of the Center for Perinatal Studies for their expert technical assistance.
Footnotes
See accompanying original article, p. 1557 and editorial, p. 1676.
References
- 1.Yip R, Dallman PR. Iron. In Present Knowledge in Nutrition .7th ed. Ziegler EE, Filer LJ, Eds. Washington, DC, ILSI Press, 1996, p. 277–292 [Google Scholar]
- 2.Galan P, Vergnaud AC, Tzoulaki I, et al. Low total and nonheme iron intakes are associated with a greater risk of hypertension. J Nutr 2010;140:75–80 [DOI] [PubMed] [Google Scholar]
- 3.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 2009;1790:671–681 [DOI] [PubMed] [Google Scholar]
- 4.Cade JE, Moreton JA, O’Hara B, et al. Diet and genetic factors associated with iron status in middle-aged women. Am J Clin Nutr 2005;82:813–820 [DOI] [PubMed] [Google Scholar]
- 5.Klipstein-Grobusch K, Koster JF, Grobbee DE, et al. Serum ferritin and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr 1999;69:1231–1236 [DOI] [PubMed] [Google Scholar]
- 6.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004;291:711–717 [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Folsom AR, Jacobs DR., Jr Dietary iron intake and type 2 diabetes incidence in postmenopausal women: the Iowa Women’s Health Study. Diabetologia 2004;47:185–194 [DOI] [PubMed] [Google Scholar]
- 8.Rajpathak S, Ma J, Manson J, Willett WC, Hu FB. Iron intake and the risk of type 2 diabetes in women: a prospective cohort study. Diabetes Care 2006;29:1370–1376 [DOI] [PubMed] [Google Scholar]
- 9.Hallberg L, Hultén L, Gramatkovski E. Iron absorption from the whole diet in men: how effective is the regulation of iron absorption? Am J Clin Nutr 1997;66:347–356 [DOI] [PubMed] [Google Scholar]
- 10.Skikne B, Baynes RD. Iron absorption. In Iron Metabolism in Health and Disease. Brock JH, Halliday JW, Pippard MJ, Powell LW, Eds. London, WB Saunders, 1994, p. 151–187 [Google Scholar]
- 11.Jehn ML, Guallar E, Clark JM, et al. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2007;165:1047–1054 [DOI] [PubMed] [Google Scholar]
- 12.Lao TT, Chan LY, Tam KF, Ho LF. Maternal hemoglobin and risk of gestational diabetes mellitus in Chinese women. Obstet Gynecol 2002;99:807–812 [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden Study. Diabetes Care 2006;29:1077–1082 [DOI] [PubMed] [Google Scholar]
- 14.Bo S, Menato G, Villois P, et al. Iron supplementation and gestational diabetes in midpregnancy. Am J Obstet Gynecol 2009;201:158.e1–158.e6 [DOI] [PubMed] [Google Scholar]
- 15.Chan KK, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes: a randomised placebo-controlled trial. BJOG 2009;116:789–797 [DOI] [PubMed] [Google Scholar]
- 16.Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2005;70:134–142 [DOI] [PubMed] [Google Scholar]
- 17.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–187 [DOI] [PubMed] [Google Scholar]
- 18.Office of Dietary Supplements, National Institutes of Health. Dietary supplement fact sheet: iron [article online], 2007. Available from http://ods.od.nih.gov/factsheets/Iron_pf.asp Accessed 27 February 2011
- 19.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 20.Hastie TJ, Tibshirani RJ. Generalized Additive Models. London, Chapman-Hall, 1990 [Google Scholar]
- 21.Kang JO. Chronic iron overload and toxicity: clinical chemistry perspective. Clin Lab Sci 2001;14:209–219 [PubMed] [Google Scholar]
- 22.Reif DW. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med 1992;12:417–427 [DOI] [PubMed] [Google Scholar]
- 23.Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF. Potential role of increased iron stores in diabetes. Am J Med Sci 2003;325:332–339 [DOI] [PubMed] [Google Scholar]
- 24.Green A, Basile R, Rumberger JM. Transferrin and iron induce insulin resistance of glucose transport in adipocytes. Metabolism 2006;55:1042–1045 [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes 2002;51:2348–2354 [DOI] [PubMed] [Google Scholar]