Abstract
Visual habit formation in monkeys, assessed by concurrent visual discrimination learning with 24-h intertrial intervals (ITI), was found earlier to be impaired by removal of the inferior temporal visual area (TE) but not by removal of either the medial temporal lobe or inferior prefrontal convexity, two of TE's major projection targets. To assess the role in this form of learning of another pair of structures to which TE projects, namely the rostral portion of the tail of the caudate nucleus and the overlying ventrocaudal putamen, we injected a neurotoxin into this neostriatal region of several monkeys and tested them on the 24-h ITI task as well as on a test of visual recognition memory. Compared with unoperated monkeys, the experimental animals were unaffected on the recognition test but showed an impairment on the 24-h ITI task that was highly correlated with the extent of their neostriatal damage. The findings suggest that TE and its projection areas in the ventrocaudal neostriatum form part of a circuit that selectively mediates visual habit formation.
The inferior temporal cortical area (TE), which in monkeys comprises a late station in the occipitotemporal visual-processing stream, sends direct and dense projections to several different cerebral targets, two of which are known now to play selective roles in visual learning and memory. One of these targets, the inferior prefrontal convexity (1–5), is essential for short-term visual memory (6–8), whereas the second, the perirhinal/entorhinal cortices in the medial temporal lobe (9–13), is critical for the long-term recognition of visual stimuli and of their associations with other stimuli (14–22). Neither of these areas, however, seems to contribute substantially to still another type of visual learning known as visual habit formation (23–28). Unlike the various forms of short- and long-term memory that allow cognitive retention of stimuli and of stimulus–stimulus associations, often after only a single presentation and on the basis of visual observation alone, habits are considered to be stimulus–response connections formed gradually through trial and error on the basis of differential reinforcement of the instrumental response (29, 30). The test of visual habit formation that was used in the studies cited above requires the animal to learn a large set of object discriminations presented concurrently but with each of the object pairs appearing just once daily, such that the repetition of each pair occurs only on successive days, i.e., at 24-h intertrial intervals (ITI). Performance on this test, as on all of the memory tests cited, critically depends on TE (31) in large part, presumably, because this area is necessary for visual stimulus processing, per se (32). Therefore, like the other types of visual learning and memory, this one too is likely to require the participation of additional structures that receive the highly processed visual information from TE. The recipient structures in this case, however, would be predicted to use that information in the service of visual habit formation. Because neither the prefrontal nor medial temporal projection zones of TE proved to be important for this type of learning, as assessed by the 24-h ITI task, we examined the possibility that it is mediated instead by two other projection targets of TE, namely, the rostral part of the tail of the caudate nucleus (TC) and the ventrocaudal part of the putamen (VP) (33–36).
These two ventrocaudal neostriatal areas, TC and VP, were considered good candidates not only because both receive input from TE, but also because earlier lesion studies in monkeys (37, 38) had found that damage to each impaired performance on a visual pattern-discrimination task. Although these studies were conducted before the neurobehavioral distinction between memory and habit had been proposed, their results could be interpreted as supporting the possibility examined here that the ventrocaudal neostriatum participates specifically in the formation of visual-discrimination habits. However, although these earlier results are highly suggestive, they are not definitive for several reasons. First, the lesions had been made with radiofrequency in one study and by the electrolytic method in the other, and it was uncertain therefore whether the deficit in each case arose from damage to the small neostriatal target itself or to fibers of passage coursing through the surrounding white matter. To clarify this issue in the present experiment, lesions were made by injecting a neurotoxin, ibotenic acid, which spares white matter. Second, the behavioral measure used earlier, namely learning (or relearning) of a single visual-discrimination problem, may not have assessed habit formation alone, because the method it involves—repeatedly presenting a single stimulus pair at relatively short intervals within a session—could have allowed contributions as well from short-term memory, recognition memory, and/or associative memory. Because all these forms of stimulus memory seem precluded in the 24-h ITI task, as evidenced by this task's insensitivity to the lesions that impair stimulus memory, use of this task provides an especially clear test of the possibility that rostral TC and VP serve visual habit formation specifically. Finally, as noted, the earlier studies were not designed to determine whether the role of the targeted neostriatal areas in long-term memory is selective for habit formation or whether it extends to cognitive forms of memory as well. To address this issue, some of the animals in the present study were assessed also on delayed nonmatching-to-sample (DNMS) with trial-unique objects, a measure of one-trial visual recognition. A preliminary report of some of these data has been published.‖
Methods
Animals.
Ten young rhesus monkeys (Macaca mulatta) weighing 3.5–5 kg at the beginning of the experiment were used. Seven of the monkeys were assigned to the experimental group given neostriatal lesions, and the three others served as unoperated controls. The study was conducted under a protocol approved by the Animal Care and Use Committee of the National Institute of Mental Health and in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the National Institutes of Health.
Behavioral Testing.
The tasks were presented in a Wisconsin General Testing apparatus inside a darkened sound-shielded room. Additional sound masking was provided by a white-noise generator. A sliding opaque screen separated the unlit monkey compartment from the lit compartment containing the test tray, and a sliding one-way vision screen concealed the experimenter from the monkey when the opaque screen was raised. The test tray contained three food wells spaced 17 cm apart and aligned 12 cm in front of the cage.
In the 24-h ITI task, each of 20 pairs of easily discriminable junk objects was presented in succession at 30-s intervals, one object in each pair covering the left well and the other covering the right well (the central well was unused in this task). One object in each pair was designated arbitrarily as the positive one, i.e., baited with a food reward in the underlying well with its position varying pseudorandomly across the 20 pairs within a session and across the daily sessions. The positive object in each pair, as well as the order of presentation of the pairs, remained the same across sessions, which continued until the monkeys reached a criterion of 90 correct responses in 100 trials (five sessions). Before surgery, the animals were trained on one such set of 20 concurrent object discriminations (set A), and 2 weeks after surgery or after an equivalent rest period for the controls, they were trained in succession on three new sets (B, C, and D), each consisting of 20 pairs of new objects. Subsequently, three of the experimental animals together with the three controls received a fifth set of 20 new object pairs (set E), whereas the four other experimental animals were tested on DNMS with trial-unique objects. The DNMS scores of the latter animals were compared with those of four unoperated monkeys that had been subjects in a pharmacological study (39) after having been trained and tested on DNMS by the same methods as those described below.
In the DNMS task, the animals were trained first in the nonmatching rule as follows. The central well of the three-well test tray was covered with a baited sample object that the animal displaced to obtain the reward. The sample object and a new one were presented 10 sec later for choice over the lateral wells with a food reward now located only under the novel object. Another trial was presented 20 sec later in the same way (i.e., baited sample followed by a choice trial in which only the novel object was baited), and so on for 20 trials a day, each trial with a new pair of objects. The left-right position of the novel object varied pseudorandomly across trials. When the animals had learned the nonmatching rule to a criterion of 90 correct choices in 100 trials, they received a memory performance test: (i) the delay between sample and choice was increased gradually (in blocks of five 20-trial sessions) from the initial 10 sec to 30, 60, and finally 120 sec; then (ii) the list of objects to be remembered was increased gradually (in blocks of five 30-trial sessions) from the initial single object to 3, 5, 10, and finally (in a block of five 20-trial sessions) 20 objects.
Surgical Procedures.
After the animals attained criterion on set A of the 24-h ITI task, the seven monkeys assigned to the experimental group were scanned by using MRI to determine the stereotaxic coordinates of rostral TC and of VP. Details of the MRI procedure have been presented elsewhere (40), and the target areas are detailed below (see Histological Analysis). Before surgery, which was performed under aseptic conditions, the animal was anesthetized with ketamine hydrochloride (10 mg/kg, i.m.) followed by isofluorane and then treated with atropine sulfate to reduce secretions. Vital signs including heart rate, respiration, temperature, expired CO2, blood oxygen level, and blood pressure were monitored continuously. The monkey's temperature was maintained between 36 and 37.5°C with a heating pad, and the isofluorane level was adjusted to maintain an adequate level of anesthesia. The monkey's head was placed in the same stereotaxic headframe that was used for MRI scanning and positioned to match landmark coordinates recorded at that time. The surgical area was cleaned and sterilized, the scalp was incised, and a rectangular calvarium flap overlying the target area was turned on each side. Small incisions were made in the dura to allow the needle of a 10-μl syringe, held in a Kopf micromanipulator (Kopf Instruments, Tujunga, CA) attached to the stereotaxic frame, to be lowered to the target area. Ibotenic acid dissolved in sterile PBS (pH 7.2) to a final concentration of 10 mg/ml then was injected into six to nine sites per hemisphere aimed at rostral TC and VP; each injection consisted of l.0 μl of ibotenate injected at the rate of 0.2 μl per minute. On completion of the surgery, the bone flaps were replaced, the wounds were closed in anatomical layers with silk sutures, and the animals were placed in an incubator for maintenance of body temperature in a postoperative treatment room. The monkeys received dexamethasone sodium phosphate (0.4 mg/kg) to reduce swelling, Di-Trim (0.1 ml/kg, 24% solution) the day before and after surgery to prevent infection, and acetaminophen (40 mg) for 3 days after surgery to reduce any pain.
Histological Analysis.
On completion of postoperative testing, the experimental animals were anesthetized with ketamine hydrochloride (10 mg/kg, i.m.) and given a lethal dose of sodium pentobarbital (50 mg/kg, i.p.). Once the animals were deeply anesthetized, they were perfused intracardially with saline followed by formalin. The brains were removed and prepared for coronal sectioning at 50 μm on a freezing microtome. Every fifth section was mounted and stained with thionine. The sections were examined microscopically for gliosis, cell loss, and signs of the needle tracks. Assessment of the locus and extent of damage was based on analysis of stained sections taken every 1 mm through the rostrocaudal extent of the lesion.
The target areas had been determined on the basis of anatomical connectional studies (33, 35, 36) that showed the locus and extent of the projections from TE to rostral TC and VP. These projection zones were outlined on drawings of coronal sections 0.5 mm apart of a normal brain, and the area of each zone on each drawn section, measured with a planimeter, was averaged across the sections and multiplied by the distance between them. The total projection volume was estimated to consist of 55% TC and 45% VP. The necrosis in each of these structures then was transferred to the standard coronal drawings, and the percentage of damage in each structure relative to the target area in each was measured again with a planimeter. For each hemisphere, the percentage of damage to rostral TC and VP then was added in the proportion of 55 and 45%, respectively, and the two-hemisphere average was calculated by using the equation M% = (L% + R%)/2, in which M% is the mean percentage of bilateral damage to the target area, and L% and R% are the mean percentages of damage to that area in the left and right hemispheres, respectively. An alternative method of assessing percentage of damage, recommended by Hodos and Bobko (41) as a correction for asymmetrical damage in the two hemispheres, is a weighted index (WI) obtained using the formula WI = (L% × R%)/100.
Results
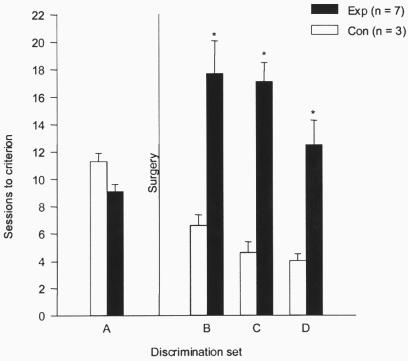
Sessions to criterion on sets A–D of the 24-h ITI task (Fig. 1) were subjected to a two-way ANOVA (with Huynh-Feldt correction for repeated measures), which yielded a significant interaction between discrimination sets and lesion groups [F(3,24) = 9.54, P < 0.001]. Paired comparisons indicated that before surgery, the two groups did not differ significantly (set A: means of 9.1 and 11.3 sessions for experimental and control groups, respectively). After surgery, however, the experimental group was retarded significantly in learning relative to the control group on all three sets combined (sets B–D: means of 15.8 and 5.1 sessions, respectively, P = .003) as well as on each set separately (Ps < 0.05). The same results were obtained when errors to criterion, instead of sessions to criterion, were used as the learning measure.
Figure 1.
Effects of ventrocaudal neostriatal lesions on performance of the 24-h ITI task. Scores on sets A–D are means and standard errors. Con, control animals; Exp, experimental animals; *, P < 0.05.
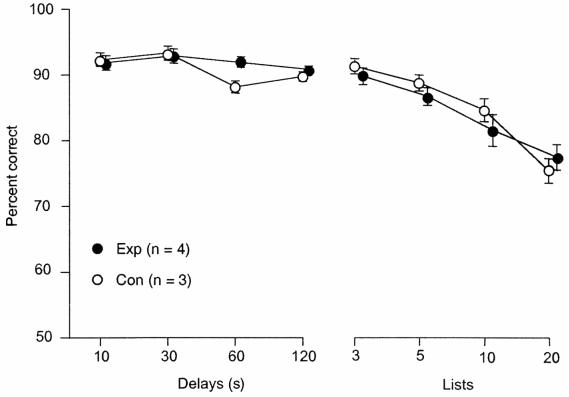
On the final postoperative set (set E), which was given to three of the experimental animals (E1–3) and the control group, the impairment still was evident (means of 10.7 vs. 5.3 sessions, respectively, P < 0.05). The four remaining experimental animals (E4–7) were trained on the DNMS task, and their scores on the memory performance test were compared with a separate group of unoperated animals (see Behavioral Testing). As shown in Fig. 2, these two groups did not differ under the condition of either increasing delays or increasing list lengths.
Figure 2.
Effects of ventrocaudal neostriatal lesions on performance of the DNMS task. Scores are means and standard errors. Con, control animals; Exp, experimental animals.
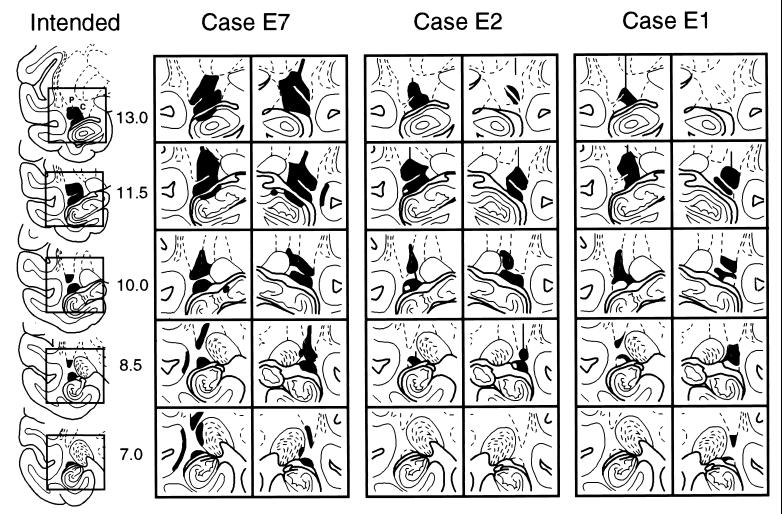
Histological analysis of the lesions revealed that each of the experimental animals had sustained damage to both rostral TC and VP (Table 1). In most cases, these two neostriatal structures were damaged about equally, but in one case (E4) there was nearly complete necrosis of VP with more limited loss in rostral TC, whereas in another (E7; Fig. 3), this pattern of necrosis was reversed. Minor unintended damage occurred in four of the experimental animals: in E4, the ventral claustrum and deep layers of the fundus of the superior temporal sulcus were invaded on the left; in E5 and E6, these areas were encroached bilaterally; and in E7, there was damage to the deep layers of the fundus of the left superior temporal sulcus, the lateral edge of the left lateral geniculate nucleus, and the dorsal edges of the pes hippocampi (Fig. 3, E7).
Table 1.
Percentage of damage to each structure in each hemisphere
| Exp. Case | Left
|
Right
|
M | WI | Sessions | ||
|---|---|---|---|---|---|---|---|
| TC | VP | TC | VP | ||||
| 1 | 47.9 | 31.1 | 26.8 | 58.4 | 40.7 | 16.5 | 11.3 |
| 2 | 60.1 | 54.2 | 54.5 | 40.7 | 52.9 | 27.7 | 16.6 |
| 3 | 30.1 | 16.9 | 19.5 | 14.7 | 20.8 | 4.2 | 10.6 |
| 4 | 25.8 | 93.0 | 34.7 | 97.0 | 59.4 | 35.2 | 23.3 |
| 5 | 53.3 | 29.1 | 22.0 | 67.2 | 42.4 | 18.0 | 14.0 |
| 6 | 79.6 | 56.2 | 33.7 | 41.3 | 53.1 | 25.6 | 16.3 |
| 7 | 90.0 | 90.0 | 90.0 | 35.0 | 63.0 | 39.6 | 18.3 |
| Ave. | 55.3 | 52.9 | 40.2 | 50.6 | 47.5 | 23.8 | 15.8 |
M, mean percentage of bilateral damage [(L% + R%)/2]; WI, weighted index [(L% × R%)/100]; Sessions, mean number of sessions to criterion on sets B–D; TC, tail of caudate nucleus; VP, ventrocaudal putamen.
Figure 3.
Intended and actual lesions (in black) in three experimental animals (E7, E2, and E1, with mean percentage of bilateral damage at 63.0, 52.9, and 40.7, respectively; refer to Table 1) transferred to drawings of coronal sections of a normal brain. The areas within the square outlines (left-hand column) that encompass the intended lesion in the left hemisphere are shown slightly enlarged for both left and right hemispheres of the three experimental cases. Numerals (left-hand column) indicate millimeters anterior to the interaural coronal plane. C, rostral part of tail of caudate nucleus; P, ventrocaudal putamen.
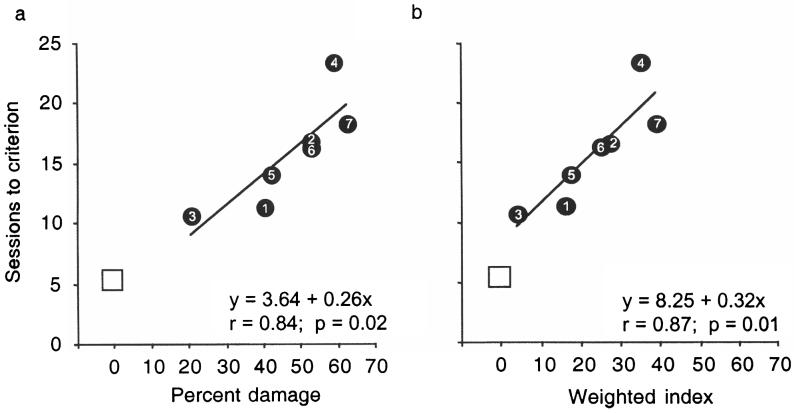
Estimates of the proportion of damage to the two targeted areas combined, averaged for the two hemispheres, ranged 20.8–63.0% across the seven experimental animals (Table 1). Correcting for hemispheric asymmetry of each lesion by means of the weighted index (see Histological Analysis) yielded indices for the targeted structures that ranged 4.2–39.6. The correlation between mean sessions to criterion on sets B–D and each of these measures of lesion extent (Fig. 4) was significant (two-hemisphere average: r = 0.84, P = 0.02; weighted index: r = 0.87, P = 0.01). When the control group's mean number of sessions to criterion was added as a single data point (with a value of 0 for percentage of damage), both correlations rose to 0.92 (P < 0.01). Correlations between mean sessions to criterion on the 24-h ITI task and percentage of damage to either rostral TC or VP alone or between mean sessions to criterion and extent of damage to the other structures that were invaded by a few of the lesions (i.e., ventral claustrum and fundus of the superior temporal sulcus) were not significant.
Figure 4.
Correlations between extent of lesions and mean sessions to criterion on three postoperative discrimination sets (B–D; Fig. 1). (a) Percentage of damage is the two-hemisphere average. (b) Weighted index provides a correction for hemispheric asymmetry of the lesions (see Histological Analysis). Numerals inside the filled circles refer to the experimental case numbers (see Table 1). Unfilled squares depict average score of the three control animals.
Discussion
The results demonstrate that lesions of the ventrocaudal neostriatum produce impairment on the 24-h ITI task, a measure of visual habit formation, but not on DNMS, a measure of visual recognition memory. This dissociation of effects is striking particularly in that the four experimental animals (E4–7) that were assessed and found to be unaffected in recognition memory ability were among the most retarded in visual habit formation (see Fig. 4). Although DNMS was their last test, their lack of impairment on it cannot be ascribed to recovery from the effects of the lesions, because during this same postoperative period, the three other less-retarded experimental animals (E1–3) continued to show significant impairment on the 24-h ITI task (set E). The findings thus clarify and extend those of the earlier neostriatal studies in monkeys (37, 38) and suggest that, like the 24-h ITI task, pattern-discrimination tasks are measures of habit learning. Together, the earlier studies and the current one provide strong support for the view that the ventrocaudal neostriatum participates in visual habit formation as opposed to visual recognition memory.
As already noted, damage to the perirhinal/entorhinal cortices produces a behavioral pattern that is just the opposite of the one observed here, namely an impairment in visual recognition memory but not in visual habit formation (14–24, 26–28). Further, each of these patterns differs from the one produced by damage to TE, which yields impairments in both types of visual learning and memory (31, 32). A result mirroring the nonselective effects of TE damage was reported recently in animals whose medial temporal removals encroached on the ventrocaudal neostriatum (42); that is, unlike separate lesions of these two regions, the combined damage produced impairments both in recognition memory and on a measure of habit formation similar to the one used here (with the exception that many of the ITIs occurred within rather than only across sessions; see ref. 43 for evidence that this version of the concurrent visual-discrimination task, like the 24-h ITI version, is insensitive to the effects of medial temporal damage). Taken together with the anatomical connectional evidence (9–13, 33–36), these several different patterns of lesion-induced deficit support the proposal (29, 30) that long-term visual learning and memory are mediated by at least two parallel pathways diverging from a common source, with a TE-limbic projection forming part of a neural circuit critical for visual recognition and a TE-neostriatal projection comprising part of a circuit mediating visual habit formation. (As indicated at the outset, a third projection from TE to the inferior prefrontal convexity seems to be part of yet another circuit, this one serving short-term visual memory.)
The notion supported by the present study in monkeys, that long-term learning and memory can be separated into a limbic-dependent cognitive form and a neostriatal-dependent behavioral form (29, 30), has received confirmation already both from lesion and pharmacological studies in rodents as well as from neuropsychological investigations in patients with brain disorders. Thus, many studies in rats (e.g., 44–47) now have shown that cue-response maze learning, a type of visual habit formation, depends on the neostriatum and not on medial temporal limbic structures, which is just the opposite of the pattern of effects these same studies found for place learning, the type of maze learning that is presumed to rely on cognitive spatial mapping, a form of stimulus memory. Similarly, studies in patients have shown that the acquisition of a wide variety of habits and motor skills including mirror-reading, pursuit-rotor tracking, adaptation level during weight judgments, and implicit category learning are impaired in patients with disorders involving the neostriatum (48–51) but not in amnesic patients with medial temporal pathology (50–53); this pattern of effects again is the opposite of the one these two groups of patients exhibit on cognitive memory tests requiring recognition or recall (48, 51).
Besides providing additional support for a neostriatal habit system, the present results suggest some new proposals regarding the operation of the system. First, the system's plasticity is such that the effects of training can continue to accumulate gradually on the basis of trials presented at the rate of only once per day. Apparently, unlike the one-trial learning but relatively rapid forgetting that commonly occurs in the limbic-dependent cognitive memory system (the reason, presumably, that this system is not useful for mastering the 24-h ITI task), a single daily stimulus-response reinforcement or extinction trial can have a durable, even if relatively small, effect that permits slow accretion of the habit over a period of several days. These differences in the learning and retention of cognitive information versus habits imply that there are major differences in the rate and durability of synaptic modification in the two systems.
Second, the neuroanatomical basis of this study lead to some proposals about the neural circuitry underlying visual habit formation. An important aspect of the present result is that the degree of impairment was highly correlated with lesion extent. The damage sustained by the two animals with the largest lesions (E4 and E7) affected approximately 60% of the target areas, and these two cases required about four times the mean number of sessions taken by the control group to learn the postoperative sets of concurrent object discriminations. Extrapolation of the regression lines in Fig. 4 beyond 60% damage suggests that greater impairment might have resulted from greater destruction of the targeted areas than was achieved here. However, even complete bilateral ablation of TE, the major origin of the projections to the neostriatal targets in this study, does not prevent learning of the 24-h ITI task completely (31). It could be significant in this connection that extrastriate visual areas other than TE also project to the neostriatum, and that they do so in a roughly topographic manner with the more caudal visual areas projecting most densely to the more caudal and dorsal portions of TC and VP (33). Given this evidence of a widespread extrastriate-neostriatal projection system, a testable proposal is that the residual ability in visual habit formation observed after lesions limited to either TE or its neostriatal targets is mediated by the remaining more-caudal portions of this system. Finally, the ventrocaudal neostriatum, in receipt of visual information from extrastriate cortex, is in a position to transmit it further to the premotor cortex via a basal ganglia-thalamic circuit (54) and perhaps thereby mediate the reinforcement of stimulus–response bonds. Where within this circuit such linkage might take place is unknown presently, but it may not occur within the neostriatum itself (55), suggesting that its neuronal signal should be sought also in one or more later stations of the proposed circuit.
Acknowledgments
We thank S. McBride for assistance with analysis of the histological material and R. C. Saunders for help with preparation of the illustrations.
Abbreviations
- TE
inferior temporal cortical area
- ITI
intertrial interval
- TC
tail of the caudate nucleus
- VP
ventrocaudal part of the putamen
- DNMS
delayed nonmatching-to-sample
Footnotes
Wang, J., Aigner, T. & Mishkin, M. (1990) Soc. Neurosci. Abstr. 16, 617 (abstr.).
References
- 1.Kuypers H G J M, Szwarcbart M K, Mishkin M, Rosvold H E. Exp Neurol. 1965;11:245–262. doi: 10.1016/0014-4886(65)90016-6. [DOI] [PubMed] [Google Scholar]
- 2.Barbas H. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 3.Ungerleider L G, Gaffan D, Pelak V S. Exp Brain Res. 1989;76:373–384. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- 4.Webster M J, Bachevalier J, Ungerleider L G. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael S T, Price J L. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 6.Mishkin M, Manning F J. Brain Res. 1975;143:313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- 7.Passingham R. Brain Res. 1975;92:89–102. doi: 10.1016/0006-8993(75)90529-6. [DOI] [PubMed] [Google Scholar]
- 8.Fuster J M, Bauer R H, Jervey J P. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- 9.Shiwa T. Arch Ital Biol. 1987;125:139–154. [PubMed] [Google Scholar]
- 10.Webster M J, Ungerleider L G, Bachevalier J. J Neurosci. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Elkins C L, Horel J A. J Comp Neurol. 1992;32:177–192. doi: 10.1002/cne.903210202. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki W A, Amaral D G. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 13.Saleem K S, Tanaka K. J Neurosci. 1996;16:4757–4775. doi: 10.1523/JNEUROSCI.16-15-04757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray E A, Mishkin M. J Neurosci. 1986;6:1991–2003. doi: 10.1523/JNEUROSCI.06-07-01991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George P J, Horel J A, Cirillo R A. Behav Brain Res. 1989;34:163–178. doi: 10.1016/s0166-4328(89)80100-7. [DOI] [PubMed] [Google Scholar]
- 16.Zola-Morgan S, Squire L R, Amaral D G, Suzuki W A. J Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meunier M, Bachevalier J, Mishkin M, Murray E A. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki W A, Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray E A, Gaffan D, Mishkin M. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray E A, Gaffan E A, Flint R W., Jr Behav Neurosci. 1996;110:30–42. [PubMed] [Google Scholar]
- 21.Meunier M, Hadfield W, Bachevalier J, Murray E A. J Neurophysiol. 1996;75:1190–1205. doi: 10.1152/jn.1996.75.3.1190. [DOI] [PubMed] [Google Scholar]
- 22.Buffalo E A, Ramus S J, Clark R E, Teng E, Squire L R, Zola S M. Learn Mem. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malamut B L, Saunders R C, Mishkin M. Behav Neurosci. 1984;98:759–769. doi: 10.1037//0735-7044.98.5.759. [DOI] [PubMed] [Google Scholar]
- 24.Overman W H, Ormsby G, Mishkin M. Exp Brain Res. 1990;79:18–24. doi: 10.1007/BF00228870. [DOI] [PubMed] [Google Scholar]
- 25.Kowalska D M, Bachevalier J, Mishkin M. Neuropsychologia. 1991;29:583–600. doi: 10.1016/0028-3932(91)90012-w. [DOI] [PubMed] [Google Scholar]
- 26.Gaffan D, Murray E A. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Malkova L, Mishkin M, Bachevalier J. Behav Neurosci. 1995;109:212–226. doi: 10.1037//0735-7044.109.2.212. [DOI] [PubMed] [Google Scholar]
- 28.Thornton J A, Rothblat L A, Murray E A. J Neurosci. 1997;17:8536–8549. doi: 10.1523/JNEUROSCI.17-21-08536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishkin M, Malamut B, Bachevalier J. In: Neurobiology of Learning and Memory. Lynch G, McGaugh J L, Weinberger N M, editors. New York: Guilford; 1984. pp. 65–77. [Google Scholar]
- 30.Mishkin M, Petri H L. In: Neuropsychology of Memory. Squire L R, Butters N, editors. New York: Guilford; 1984. pp. 287–296. [Google Scholar]
- 31.Phillips R R, Malamut B L, Bachevalier J, Mishkin M. Behav Brain Res. 1988;27:99–107. doi: 10.1016/0166-4328(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 32.Mishkin M. Philos Trans R Soc London Ser B. 1982;298:85–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- 33.Saint-Cyr J A, Ungerleider L G, Desimone R. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- 34.Steele G E, Weller R E. Visual Neurosci. 1993;10:563–583. doi: 10.1017/s0952523800004776. [DOI] [PubMed] [Google Scholar]
- 35.Webster M J, Bachevalier J, Ungerleider L G. J Comp Neurol. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- 36.Yeterian E H, Pandya D N. J Comp Neurol. 1995;352:436–457. doi: 10.1002/cne.903520309. [DOI] [PubMed] [Google Scholar]
- 37.Divac I, Rosvold H E, Szwarcbart M K. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- 38.Buerger A A, Gross C G, Rocha-Miranda C E. J Comp Physiol Psychol. 1974;86:440–446. doi: 10.1037/h0036142. [DOI] [PubMed] [Google Scholar]
- 39.Aigner T G, Mishkin M. Behav Neural Biol. 1986;45:81–87. doi: 10.1016/s0163-1047(86)80008-5. [DOI] [PubMed] [Google Scholar]
- 40.Saunders R C, Aigner T G, Frank J A. Exp Brain Res. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- 41.Hodos W, Bobko P A. J Neurosci Methods. 1984;12:43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- 42.Teng E, Stefanacci L, Squire L R, Zola S M. J Neurosci. 2000;20:3853–3863. doi: 10.1523/JNEUROSCI.20-10-03853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buffalo E A, Stefanacci L, Squire L R, Zola S M. Behav Neurosci. 1998;112:3–14. doi: 10.1037//0735-7044.112.1.3. [DOI] [PubMed] [Google Scholar]
- 44.Packard M G, Hirsh R, White N M. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald R J, White N M. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 46.Kesner R P, Boland B L, Dakis M. Exp Brain Res. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- 47.Packard M G, McGaugh J L. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 48.Martone M, Butters N, Payne M, Becker J, Sax D S. Arch Neurol (Chicago) 1984;41:965–970. doi: 10.1001/archneur.1984.04050200071020. [DOI] [PubMed] [Google Scholar]
- 49.Heindel W C, Salmon D P, Shults C W, Walicke P A, Butters N. J Neurosci. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heindel W C, Salmon D P, Butters N. J Clin Exp Neuropsychol. 1991;13:189–203. doi: 10.1080/01688639108401037. [DOI] [PubMed] [Google Scholar]
- 51.Knowlton B J, Mangels J A, Squire L R. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 52.Cohen N, Squire L R. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 53.Eslinger P J, Damasio A R. J Neurosci. 1986;6:3006–3009. doi: 10.1523/JNEUROSCI.06-10-03006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander G E, DeLong M R, Strick P L. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 55.Brown V J, Desimone R, Mishkin M. J Neurophysiol. 1995;74:1083–1094. doi: 10.1152/jn.1995.74.3.1083. [DOI] [PubMed] [Google Scholar]