Abstract
OBJECTIVE
The study objectives were 1) to assess the effectiveness and safety of a standardized protocol for the transition to subcutaneous insulin and oral feeding in diabetic or hyperglycemic patients with acute coronary syndrome (ACS) who were receiving intravenous insulin and glucose at the time of the transfer from the intensive cardiac care unit to a general ward and 2) to identify predictors of transition outcome.
RESEARCH DESIGN AND METHODS
This was a prospective observational study. The protocol specifies that patients receive a 100% of their daily subcutaneous insulin requirement from the first day of oral feeding, calculated from the intravenous insulin rate during the final 12 h divided into two: 50% basal and 50% prandial.
RESULTS
In 142 patients (93 male, 49 female, age range 47–88 years, 135 with known diabetes) the first day after transition, 44.8% of blood glucose (BG) measurements were within the strict range of 100–140 mg/dL before meals and 100–180 mg/dL after meals, and 70.8% were within the broader ranges of 80–160 mg/dL and 80–200 mg/dL, respectively. Pre- or postprandial hypoglycemia (BG <70 mg/dL) occurred in 11 patients (7.7%) on the first day and in 38 patients (26.8%) on the first 3 days after transition. Old age, high doses of intravenous insulin, and wide BG variations in the 24 h before insulin infusion was stopped were predictive of poor BG control after transition.
CONCLUSIONS
This study shows the effectiveness and safety of a standardized protocol for the transition from intravenous to subcutaneous insulin in patients with ACS when regular oral feeding was resumed.
In critically ill patients with diabetes or hyperglycemia who are admitted to intensive care units, intravenous infusion of insulin is the recommended treatment (1–6). During the postacute phase, many guidelines and recommendations suggest switching to subcutaneous insulin when patients begin eating regular meals and are moved to a lower-intensity care setting (1–6).
There are few observational and intervention studies on the procedure for the transition from intravenous to subcutaneous insulin, and almost all concerned patients who had undergone operation and took little if any food (7–11). The transition is delicate because of the patients’ clinical condition and the organizational context in which they are transferred from an intensive care unit to a general ward. The few studies that have examined the course of blood glucose (BG) after interruption of intravenous insulin have documented inadequate control in the absence of a standardized transition protocol (9,12). In addition, the literature reporting the predictors of optimal transition is scarce and refers mainly to patients postsurgery (7,9,10).
The objectives of this prospective observational study were to
assess the effectiveness and safety of a standardized protocol for conversion from intravenous to subcutaneous insulin therapy in patients with acute coronary syndrome (ACS) during the transfer from the intensive cardiac care unit (ICCU) to the general ward; and
identify predictive factors of transition outcome.
RESEARCH DESIGN AND METHODS
Desio Diabetes Diagram transition protocol
The Desio Diabetes Diagram (DDD) transition protocol is part of a more comprehensive original protocol (Organization and optimization of Care in Team to improve Outcomes in Patients with hyperglycemia admitted to coronary care Unit/cardiology division for acute coronary Syndromes [OCTOPUS]) for the full acute and subacute metabolic management of patients with diabetes or hyperglycemia admitted to the Ospedale di Desio ICCU for suspected ACS. The protocol is the outcome of collaboration between the cardiologist responsible for the ICCU (FA) and the diabetologist responsible for the Diabetes and Metabolic Diseases Unit (GM). The name “OCTOPUS” reflects the multiple therapeutic approaches (intravenous insulin infusion at first, followed by subcutaneous doses, combined with controlled nutrition such as intravenous glucose during the acute phase, followed by oral carbohydrates) and the several health teams involved (medical, nursing, and support personnel of the ICCU and Cardiology Department; physicians, nurses, and dietitians of the Diabetes and Metabolic Disease Unit) (13).
The OCTOPUS project adopted the nurse-implemented DDD insulin infusion protocol for intensive glycemic control during the acute phase, with target BG between 100 and 139 mg/dL (13).
At admission to the ICCU, each diabetic patient received glucose solution by infusion, parallel with, but separately from, the intravenous insulin to guarantee the necessary carbohydrate supply, as suggested by recent nutritional recommendations (4,14). The glucose dose was calculated on the basis of the estimated amount of carbohydrate needed in the diet for the postacute phase, to supply 20 Kcal per kilogram of ideal weight (of which ∼60% are carbohydrates), taking into account the 10% lower bioavailability of the oral route. To guarantee the 24-h glucose intravenous intake, glucose concentrations and infusion rates varied in relation to the patient’s clinical condition and the availability of venous access (13).
The conversion from intravenous infusion to subcutaneous insulin was scheduled when most of the BG measurements remained within the target range for at least 24 h. Table 1 reports the DDD protocol for conversion.
Table 1.
Conversion protocol
| DDD protocol for conversion from continuous intravenous insulin and glucose infusions to subcutaneous insulin and oral diet | |
|---|---|
| An estimate of the combined basal and nutritional subcutaneous insulin requirements can be extrapolated from the average amount of intravenous insulin infused during the hours preceding the conversion if: | |
| 1) the intravenous glucose infusion is kept constant and equivalent to the amount of carbohydrate in the oral diet and | |
| 2) the BG level is stable in the target range between 100 and 139 mg/dL (most of the BG measurements on target for at least 24 h) | |
| Protocol | Example |
| Step 1. Calculate the average insulin intravenous infusion rate in the last 12 h to obtain the mean hourly rate and multiply by 24 to get the total daily insulin requirement. | → 1.5 units/h × 24 = 36 units/24 h |
| Step 2. Halve this 24-h insulin dose to obtain the long-acting insulin analog dose and total daily rapid-acting insulin analog dose. | → 36 units/2 = 18 units |
| Step 3. Give the long-acting insulin analog subcutaneous monodose 2 h before the first meal and the discontinuation of intravenous insulin and intravenous glucose infusions. | → give glargine 18 units s.c. 2 h before the first meal and stop intravenous insulin and glucose infusions at meal |
| Step 4. Split the total daily rapid-acting subcutaneous insulin analog dose into 20% at breakfast, 40% at lunch, and 40% at dinner, according to a similar distribution of carbohydrates in the typical Mediterranean diet. | → give lispro 4 units s.c. before breakfast, give lispro 7 units s.c. before lunch, give lispro 7 units s.c. before dinner |
Every 1–2 days the subcutaneous glargine and lispro insulin scheduled doses were titrated, increasing or decreasing the previous dose by 10–20% depending on pre- and postprandial BG, respectively (BG preprandial target <120 mg/dL and postprandial target <160 mg/dL). If pre- or postprandial hyperglycemia occurred, a correction dose of subcutaneous insulin lispro was administered according to an algorithm that takes into account the total daily dose of insulin (15).
The conversion to subcutaneous insulin was started the day of the first evening meal, giving the first dose of insulin glargine 2 hours before dinner. Immediately before dinner, intravenous insulin and glucose were stopped, and the first dose of insulin lispro was given. On the next day, the insulin glargine injection was given at lunch.
Training
Before starting the DDD insulin infusion and transition from the intravenous to subcutaneous protocols, all nurses and physicians attended a 2–3-h training meeting on the use of protocols. Some months from the start of application of the protocols, then yearly, further meetings were held with the whole staff to assess the results, discuss problems, and decide on solutions.
Patients
All patients consecutively admitted to the Ospedale di Desio ICCU from May 2006 to May 2008 for suspected ACS and known diabetes, or with BG >200 mg/dL, were treated with the DDD insulin infusion protocol. Those whose BG remained within the target interval (100–139) in the last 24 h with <0.5 units/h of insulin, thus with estimated daily insulin needs <12 units, were excluded and did not shift to subcutaneous insulin.
Measurements
BG was measured in venous blood every 1–2 h during intravenous insulin infusion and in capillary blood before and 2 h after each meal during subcutaneous insulin using a bedside glucometer (Ascensia Elite XL, Bayer, Tarrytown, NY).
Outcomes
The primary outcome was the percentage of BG in the range of 100–140 mg/dL before meals and 100–180 mg/dL after meals (2–4) on the first day after transition. Secondary outcomes were the percentage of BG in the wider range of 80–160 mg/dL before meals and 80–200 mg/dL after meals on the first day after transition; the percentage of hypoglycemia (defined in this study as BG <70 mg/dL) the first day after transition; the percentage of BG in the tighter and wider ranges before and after meals on the second and third days after transition; the percentage of hypoglycemia on the second and third days after transition; and deaths in hospital and main nonlethal cardiovascular complications (myocardial reinfarction, heart failure, ventricular fibrillation, sustained ventricular tachycardia, stroke).
Statistical analysis
Quantitative variables were described using mean ± SD if normally distributed. When this condition was not satisfied the median was used instead of the mean, and the first and third quartiles were used to obtain a CI (25th –75th percentile). Qualitative variables are expressed as percentages.
We used a stepwise logistic multivariate analysis to identify the variables associated with an unsuccessful transition to subcutaneous insulin (patients with <50% of BG in the range of 100–140 mg/dL before meals and 100–180 after meals on the first day after transition from intravenous to subcutaneous insulin) (2–4). Selected variables of clinical interest included in the model were sex, age, BMI, smoking habit, history of hypertension, hypercholesterolemia and diabetes, duration of diabetes, previous insulin treatment, reason for hospitalization (ST elevation myocardial infarction vs. other), BG and HbA1c at admission, mean BG and mean intravenous insulin dose administered in the last 12 and 24 h, and the total dose of subcutaneous insulin given the first day after transition. Selected indexes of variability in BG and insulin doses during the last 12 and 24 h of intravenous insulin included in the model were SD, coefficient of variation (SD/mean) and trend (difference of mean values in first and second half of the interval) for BG and insulin dose, percentage of BG at target, and mean amplitude of the glycemic excursion (MAGE) (16). The MAGE (16) was calculated by measuring the arithmetic mean of the difference between consecutive peaks and nadirs, provided that the difference was greater than the SD around the BG mean. All the quantitative covariates analyzed were introduced in the model in categories (higher and lower than the median). The results of multivariate analysis are reported as odds ratio (OR) with 95% CI.
RESULTS
In the first 2 years of application of the OCTOPUS protocol, 172 patients (60 women, 112 men), with a mean age of 69.8 ± 10.7 years (range 42–90 years), were treated with intravenous insulin infusion. The transition from intravenous to subcutaneous insulin involved 145 patients (84.3%): in 27 patients, the transition was not done because the insulin infusion protocol was stopped prematurely (2 patients died, 11 patients had to be transferred to other hospitals or wards, other reasons in 2 patients) or the transition was not considered necessary because BG was at the target with low insulin doses (12 patients). Among the 145 patients who were shifted from intravenous to subcutaneous insulin, 3 (2.1%) were excluded from the analysis because subcutaneous insulin was interrupted on the first day: one patient refused to continue, one patient was transferred to another hospital, and one patient died suddenly.
Table 2 shows the main details of the 142 patients in the study. In all, 135 patients (95.1%) were known to have diabetes and 92 patients (64.8%) had BG >200 mg/dL at admission. At discharge, the diagnosis of ACS was confirmed in 118 patients (83.1%), ST-segment elevation acute myocardial infarction was confirmed in 38 patients (26.8%), non–ST-segment elevation acute myocardial infarction was confirmed in 69 patients (48.6%), and unstable angina was confirmed in 11 patients (7.7%). During a median hospital stay of 10 days (7–14, 25–75th percentiles of median), there were 3 deaths due to cardiogenic shock and 15 major nonlethal cardiovascular complications in 14 patients (3 myocardial reinfarctions, 2 ventricular fibrillations, 2 sustained ventricular tachycardias, 7 heart failures, and 1 ischemic stroke).
Table 2.
Main characteristics of the population (142 patients)
| Age (years) | |
| Mean ± SD | 69.8 ± 10.1 |
| Range | 47–88 |
| Male (n, %) | 93 (65.4%) |
| History of diabetes before hospital admission (n, %) | |
| None | 7 (4.9%) |
| Type 1 diabetes | 3 (2.1%) |
| Type 2 diabetes | 130 (91.6%) |
| Other types | 2 (1.4%) |
| Glucose-lowering drugs (n, %) | |
| None | 12 (8.5%) |
| Oral | 90 (63.4%) |
| Insulin | 35 (24.6%) |
| Insulin and oral drugs | 5 (3.5%) |
| BMI (kg/m2, mean ± SD) | 28.2 ± 4.4 |
| Glycated hemoglobin (%, mean ± SD) | 7.7 ± 1.6 |
| BG (mg/dL, mean ± SD) | |
| At hospital admission | 248.1 ± 99.6 |
| At the end of the insulin infusion | 110.1 ± 20.3 |
| Insulin infusion (median, 25th–75th percentile of median) | |
| Duration (h) | 51 (45–70) |
| Hourly insulin dose at the end of the infusion (units) | 1.5 (1.1–2.2) |
| Subcutaneous insulin (median, 25th–75th percentile of median) | |
| Total daily insulin dose the first day (units) | 36 (24–52) |
| Total daily insulin dose the second day (units) | 40 (26–56) |
| Total daily insulin dose the third day (units) | 40 (26–59) |
| Carbohydrate intake (g/day, mean ± SD) | |
| Glucose intravenous in the last 24 h of insulin infusion | 166.8 ± 9.9 |
| Oral intake of carbohydrates in the first day after transition | 185.6 ± 13.2 |
Effectiveness of the protocol
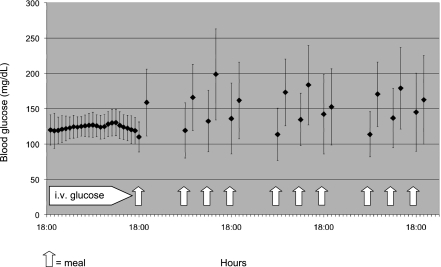
Figure 1 shows the mean ± SD of BG in the last 24-h infusion, 2 hours after the first dinner, and before and 2 hours after breakfast, lunch, and dinner on the first 3 days after the transition from intravenous to subcutaneous insulin.
Figure 1.
BG concentrations (mean ± SD) in the last 24 h of intravenous insulin infusion and before and 2 h after breakfast, lunch, and dinner on the first 3 days of subcutaneous insulin.
On the first day after shifting from intravenous insulin, 826 BG measurements were performed, 423 preprandial and 403 postprandial of the 852 measurements scheduled (96.9%), with an average of 5.8 BG measurements per patient. Of the 423 preprandial BG values, 164 (38.8%) were 100–140 mg/dL and 302 (71.4%) were 80–160 mg/dL; of the 403 postprandial BG values, 206 (51.1%) were 100–180 mg/dL and 283 (70.2%) were 80–200 mg/dL.
On the second day of subcutaneous insulin, 39.3 and 67.2% of BG measurements before meals (159 and 272/405) were in the ranges of 100–140 and 80–160 mg/dL, respectively, and 51.4 and 68.6% of BG measurements after meals (198 and 264 of 385) were in the ranges of 100–180 and 80–200 mg/dL, respectively.
On the third day, there were 38.8 and 70.1% of preprandial BG measurements within the range of 100–140 and 80–160 mg/dL (148 and 267 of 381) and 52.8 and 67.2% of postprandial BG within the ranges of 100–180 and 80–200 mg/dL (195 and 248 of 369), respectively.
Safety of the protocol
Pre- or postprandial hypoglycemia (BG <70 mg/dL) was observed in 11 patients (7.7%) on the first day after transition from intravenous to subcutaneous insulin, in 21 patients (14.8%) on the second day, and in 20 patients (14.1%) on the third day. Overall, 38 patients (26.8%) presented at least one episode of hypoglycemia in the first 3 days after transition.
BG was <70 mg/dL 13 times on the first day (2.8% of BG measured before meals and 0.2% of BG measured after meals), 25 times on the second day (3.7% of pre- and 2.6% of postprandial BG measurements), and 23 times on the third day (3.7% of pre- and 2.4% of postprandial BG measurements).
Of all the 61 hypoglycemic episodes in the first 3 days after transition, 2 (3.3%) were <40 mg/dL, 3 (4.9%) were 40–49 mg/dL, 11 (18.0%) were 50–59 mg/dL, and the majority (45, 73.8%) were 60–69 mg/dL.
All hypoglycemic episodes were asymptomatic, with no manifest clinical consequences. Among the 38 patients with hypoglycemic episodes during the first 3 days after transition to subcutaneous insulin, there was one death (2.6%) and six major nonlethal cardiovascular complications (15.8%). The corresponding figures for 104 patients without hypoglycemic events were 2 (1.9%) and 9 (7.7%).
Predictors of outcomes of the transition from intravenous to subcutaneous insulin
The transition to subcutaneous insulin was successful (half or more of all first-day BG values in the strict ranges of 100–140 mg/dL before meals and 100–180 after meals) in 75 patients (52.8%) and unsuccessful in 67 patients (47.2%). Variables associated with unsuccessful transition by multivariate analysis were old age (≥72 years, OR 2.292, 95% CI 1.082–4.856), high mean dose of intravenous insulin infused in the 24 h preceding the transition (≥1.6 units/h, OR 2.202, 95% CI 1.045–4.640), and wide BG coefficient of variation in the 24 h preceding the transition (≥11.9%, OR 1.751, 95% CI 0.841–3.646).
CONCLUSIONS
Effectiveness
The model of transition from intravenous to subcutaneous insulin therapy used in this study achieved from the first day approximately half of BG values within the strict ranges of 100–140 mg/dL before meals and 100–180 after meals. The data refer to a population almost entirely made up of patients with a history of diabetes admitted to an ICCU for an ACS. The protocol gives the whole daily insulin requirement, calculated from the intravenous insulin dose infused during the last 12 h, administered as a basal-bolus in combination with regular oral feeding. Other published protocols calculate the subcutaneous insulin dose to be given the first day after transition on the basis of shorter reference intravenous infusion periods (6–8 h) and at first usually give only basal subcutaneous insulin (generally 80% of the projected 24-h requirement calculated on the basis of the insulin intravenous infusion rate), adding rapid-acting insulin when the patients begin to eat (7,8,15). However, these protocols have usually been set for patients postsurgery who are eating little and have been used in different clinical settings (surgical intensive care units or surgical departments) (7,8,15).
In 2006, Schmeltz et al. (11) published the only randomized clinical trial to our knowledge to determine the optimal dose of insulin for the transition. The patients were mostly nondiabetic, had recently undergone operation, and were taking little food if any. A dose of insulin glargine corresponding to 80% of the total daily requirement, calculated on the basis of the last 6-h intravenous infusion, tended to achieve a better glycemic control in the first 24 h in comparison with conversion at 40 and 60%, with average BG (before every meal and at bedtime) of 153.2 ± 66.2 mg/dL and 48% of target BG between 80 and 140 mg/dL.
Our data on the control of BG in the first days of subcutaneous insulin therapy are comparable to those observed during the whole hospital stay in the two major trials on the basal-bolus insulin therapy in inpatients with diabetes. In the Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes (RABBIT 2) trial on patients admitted to general medical wards, mean BG values (fasting, premeal, and at bedtime) were 166 ± 32 mg/dL in subjects randomized to receive glargine and glulisine, and 66% of these patients had mean BG <140 mg/dL (17). In the RABBIT 2 Surgery trial on general surgery patients, the mean daily BG was 157 ± 32 mg/dL in subjects randomized to glargine and glulisine and the proportion of BG values <140 mg/dL was 53 ± 30% (18).
Safety
With our transition protocol, hypoglycemic episodes occurred in approximately one quarter of patients on the first 3 days of subcutaneous therapy, a rate similar to that observed in other studies. In the Schmeltz et al. trial (11), patients enrolled in the arm with the best transition from intravenous to subcutaneous insulin had 4% of BG ≤70 mg/dL among all measurements on the first day. In the observational study by Czosnowski et al. (9), 8% of patients had at least one hypoglycemic event (defined as BG <60 mg/dL) the first day after interruption of intravenous insulin, whereas in the study by Weant et al. (10) 8 hypoglycemic events (defined as BG <80 mg/dL) occurred in the first 48 h after transition in 19 patients with diabetes and 13 hypoglycemic events occurred in 60 patients without diabetes.
Safety data in our population compare well with those observed in the two main trials on the basal-bolus insulin therapy in hospitalized patients with diabetes: in the RABBIT 2 trial, 2 of 65 patients (3.1%) had BG <60 mg/dL before meals or at bedtime (17); in the RABBIT 2 Surgery trial, 23.1 and 3.8% of patients had BG <70 and <40 mg/dL, respectively (18). When our protocol was first used, hypoglycemia was not a major concern, but the safety issue is now considered important because severe hypoglycemia in critically ill patients, particularly in ACS, is associated with a worse prognosis (19–21). In addition, recent trials have found neutral or even negative results, with a significant excess of hypoglycemic events, with more aggressive treatment in critically ill patients (22–24) and diabetic outpatients (25). Even if all the hypoglycemic episodes in our population were asymptomatic and with no immediate manifest clinical consequences, the patients with hypoglycemic episodes had a higher incidence of major cardiovascular complications than those without. Until proven otherwise, hypoglycemia should be considered a possible cause for the increase in cardiac events and every effort to prevent hypoglycemia should be made in patients with ACS, including the adoption of higher glucose targets (2–4).
Predictors of a successful transition
In our population, the variables predictive of an unsuccessful transition were old age, high doses of intravenous insulin, and wide BG variations in the 24 h before insulin infusion was stopped. These variables are all indicative of diabetes that is difficult to control.
Few studies have been published on the predictors of optimal transition, and most refer to patients different from ours, often nondiabetic, recently surgically operated, and with little or no food intake (7,9,10). In these patients, in addition to the complexity of the surgical intervention and the presence of other clinical complications, the variables predictive of unsuccessful control of BG after the transition to subcutaneous insulin were poor glycemic control during intravenous insulin, high doses of intravenous insulin required to control BG, high subcutaneous insulin doses, and history of diabetes, especially type 1.
Feasibility
Despite the complexity of the transition process that demands various nursing and medical specialties (choosing the best time to stop intravenous insulin; formulating and planning basal, prandial, and correction insulin doses; shifting from intravenous feeding to an oral diet calibrated to meet the patient’s needs; performing frequent blood sampling for bedside BG monitoring before and 2 hours after meals) and the logistic difficulties arising from the frequent overlap of these interventions when the patient is moved from the ICCU to the general ward, this study shows that the protocol is feasible for medical and nursing staff.
During the transition from intravenous to subcutaneous insulin, errors in the application of the protocol were made in 16% of patients, generally as a consequence of wrong calculation of the subcutaneous dose. To limit this risk, a computer program is now available to make application of the protocol simpler and more precise, and consequently to improve its effectiveness and safety.
Study limits
The main limit of this study lies in the peculiarity of the population and setting, which may not allow extrapolation of the results to patients with conditions other than ACS and settings with organizational differences from an ICCU/cardiology ward. The majority of our population were known patients with diabetes, and the results will not necessarily apply to patients who do not have diabetes. Nevertheless, we believe the model of the ACS is representative enough of diabetic patients admitted to a medical ICU, and our findings could help fill the gap in the literature where data on the transition from intravenous to subcutaneous insulin mainly refer to surgical settings.
The current study shows the effectiveness and safety of a standardized protocol for the transition from intravenous to subcutaneous insulin in patients with ACS at the moment of their transfer from the ICCU to a general ward. This protocol can already achieve optimal glycemic control on the first day in approximately half the pre- and postprandial BG values, with a rate of hypoglycemic events similar to that obtained with other protocols. The variables predictive of unsuccessful transition were old age, high doses of intravenous insulin, and wide BG variations in the 24 h preceding the switch from intravenous to subcutaneous insulin.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
F.A. participated in the planning of the study, collection and analysis of data, and drafting of the manuscript. G.M. participated in the planning of the study, analysis of data, and drafting of the manuscript. W.D. participated in the planning of the study and drafting of the manuscript. G.B. participated in the collection of data and drafting of the manuscript. S.C. participated in the collection of data and drafting of the manuscript. L.B. participated in the collection of data and drafting of the manuscript. E.L.C. participated in the collection of data and drafting of the manuscript. R.F. participated in the analysis of data and drafting of the manuscript. E.R. participated in the planning of the study, analysis of data, and drafting of the manuscript. M.C.R. participated in the planning of the study, analysis of data, and drafting of the manuscript. M.D.M. participated in the planning of the study, collection and analysis of data, and drafting of the manuscript.
The authors acknowledge the editorial assistance of J.D. Baggott, Istituto di Ricerche Farmacologiche “Mario Negri,” Milan, Italy.
APPENDIX
The DDD Study Group: 1) Medical staff of the Division of Cardiology/ICCU: M. De Martini, A. Alberzoni, F. Avanzini, G. Balestri, P. Bertocchi, P. Camisasca, W. Donzelli, G. Iacuitti, G. Mantovani, M. Mistò, E. Planca, G. Pozzoli, R. Rogatska, D. Saltafossi, S. Tresoldi, P. Vandoni. 2) Nursing staff of the Division of Cardiology/ICCU: S. Tomasello, R. Amodeo, S. Baldo, M. Berizzi, J. Bertazzolo, S. Bottan, G. Busi, S. Carbone, M. Caspani, L. Ciotta, A. Colaianni, R. Cotza, A. De Ponti, E. Di Rocco, V. Donè, G. Feroleto, A.M. Gagliardi, M. Klajn, M. Ilardi, A. Ledda, R. Mamo, M. Mancuso, M. Mulieri, F. Orsenigo, E. Palazzo, A. Pintonello, C. Radaelli, I. Saltarel, L. Sorbara, G. Stelluti, S. Sutera, L. Tonelli, F Tullio, A. Zucchini. 3) Support staff of the Division of Cardiology/ICCU: A. Agrusti, L. Battaglia, M. Blandino, M. Calati, C. Lovisi, S. Montaperto, P. Sala. 4) Medical staff of the Diabetes and Metabolic Diseases Unit: G. Marelli, E. Fochesato. 5) Nursing staff of the Diabetes and Metabolic Diseases Unit: L. Bellato, M. Fedeli, A. Merlini, G. Pinelli. 6) Dieticians of the Diabetes and Metabolic Diseases Unit: E. Colombo, E. De Luca, C. Galimberti.
Footnotes
A complete list of the Desio Diabetes Diagram Study Group can be found in APPENDIX.
References
- 1.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 2006;355:1903–1911 [DOI] [PubMed] [Google Scholar]
- 2.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists; American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Associazione Medici Diabetologi - Società Italiana di Diabetologia Standard Italiani per la Cura del Diabete Mellito 2009-2010. Torino, Edizioni Infomedica, 2010 [Google Scholar]
- 5.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 6.Deedwania P, Kosiborod M, Barrett E, et al. American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2008;117:1610–1619 [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Braithwaite SS. Effects of outcome on in-hospital transition from intravenous insulin infusion to subcutaneous therapy. Am J Cardiol 2006;98:557–564 [DOI] [PubMed] [Google Scholar]
- 8.DeSantis AJ, Schmeltz LR, Schmidt K, et al. Inpatient management of hyperglycemia: the Northwestern experience. Endocr Pract 2006;12:491–505 [DOI] [PubMed] [Google Scholar]
- 9.Czosnowski QA, Swanson JM, Lobo BL, Broyles JE, Deaton PR, Finch CK. Evaluation of glycemic control following discontinuation of an intensive insulin protocol. J Hosp Med 2009;4:28–34 [DOI] [PubMed] [Google Scholar]
- 10.Weant KA, Ladha A. Conversion from continuous insulin infusions to subcutaneous insulin in critically ill patients. Ann Pharmacother 2009;43:629–634 [DOI] [PubMed] [Google Scholar]
- 11.Schmeltz LR, DeSantis AJ, Schmidt K, et al. Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia. Endocr Pract 2006;12:641–650 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004;27:461–467 [DOI] [PubMed] [Google Scholar]
- 13.Avanzini F, Marelli G, Donzelli W, et al. ; DDD study group Hyperglycemia during acute coronary syndrome: a nurse-managed insulin infusion protocol for stricter and safer control. Eur J Cardiovasc Nurs 2009;8:182–189 [DOI] [PubMed] [Google Scholar]
- 14.Bantle JP, Wylie-Rosett J, Albright AL, et al. ; American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 15.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract 2004;10(Suppl. 2):71–80 [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–655 [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Smiley D, Zisman A, et al. Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes (RABBIT 2 Trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 18.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes Undergoing General Surgery (RABBIT 2 Surgery). Diabetes Care 2011;34:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007;35:2262–2267 [DOI] [PubMed] [Google Scholar]
- 20.Svensson AM, McGuire DK, Abrahamsson P, Dellborg M. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J 2005;26:1255–1261 [DOI] [PubMed] [Google Scholar]
- 21.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 2008;117:1018–1027 [DOI] [PubMed] [Google Scholar]
- 22.Brunkhorst FM, Engel C, Bloos F, et al. ; German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–139 [DOI] [PubMed] [Google Scholar]
- 23.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35:1738–1748 [DOI] [PubMed] [Google Scholar]
- 24.Finfer S, Chittock DR, Su SY, et al. ; NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 25.Skyler JS, Bergenstal R, Bonow RO, et al. ; American Diabetes Association; American College of Cardiology Foundation; American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation 2009;119:351–357 [DOI] [PubMed] [Google Scholar]