Men with obesity, the metabolic syndrome, and type 2 diabetes have low total and free testosterone and low sex hormone–binding globulin (SHBG). Conversely, the presence of low testosterone and/or SHBG predicts the development of metabolic syndrome and type 2 diabetes. Visceral adiposity present in men with low testosterone, the metabolic syndrome, and/or type 2 diabetes acts through proinflammatory factors. These inflammatory markers contribute to vascular endothelial dysfunction with adverse sequelae such as increased cardiovascular disease (CVD) risk and erectile dysfunction. This review focuses on the multidirectional impact of low testosterone associated with obesity and the metabolic syndrome and its effects on erectile dysfunction and CVD risk in men with type 2 diabetes. Whenever possible in this review, we will cite recent reports (after 2005) and meta-analyses.
Epidemiological studies of low testosterone, obesity, metabolic status, and erectile dysfunction
Epidemiological studies support a bidirectional relationship between serum testosterone and obesity as well as between testosterone and the metabolic syndrome. Low serum total testosterone predicts the development of central obesity and accumulation of intra-abdominal fat (1–3). Also, low total and free testosterone and SHBG levels are associated with an increased risk of developing the metabolic syndrome, independent of age and obesity (1–3). Lowering serum T levels in older men with prostate cancer treated with androgen deprivation therapy increases body fat mass (4). Conversely, high BMI, central adiposity, and the metabolic syndrome are associated with and predict low serum total and to a lesser extent free testosterone and SHBG levels (1–3,5). Because obesity suppresses SHBG and as a result total testosterone concentrations, alterations in SHBG confound the relationship between testosterone and obesity.
Low total testosterone or SHBG levels are associated with type 2 diabetes, independent of age, race, obesity, and criteria for diagnosis of diabetes (6,7). In longitudinal studies, low serum total and free testosterone and SHBG levels were independent predictors of type 2 diabetes (6,8). In these studies, SHBG levels were stronger predictors of diabetes than total or free testosterone. Because type 2 diabetes is often associated with obesity, which suppresses SHBG and in turn total testosterone levels, both obesity and SHBG levels represent important confounding factors in the relationship between testosterone and type 2 diabetes. The prevalence of low free testosterone levels is higher in diabetic men compared with nondiabetic men (6). However, a recent longitudinal study found that free testosterone did not predict the development of type 2 diabetes. In this study, the association of total testosterone and of SHBG with diabetes was not significant after adjusting for waist circumference or central obesity (9). Also, low SHBG was found to be a strong independent predictor of type 2 diabetes (10,11). Finally, in prospective studies, androgen deprivation therapy either using bilateral orchidectomy or gonadotropin-releasing hormone agonist in older men with prostate cancer is associated with an increased risk of diabetes and CVD (12).
A number of epidemiological studies support associations of obesity (13,14), the metabolic syndrome (15,16), type 2 diabetes (17), and low serum testosterone (18) with sexual dysfunction including erectile dysfunction (ED) (19). These studies highlight the complex often multidirectional relationships among obesity, metabolic status, low testosterone, and ED in men.
Pathobiology of low testosterone in type 2 diabetes and its impact on sexual dysfunction and CVD risk
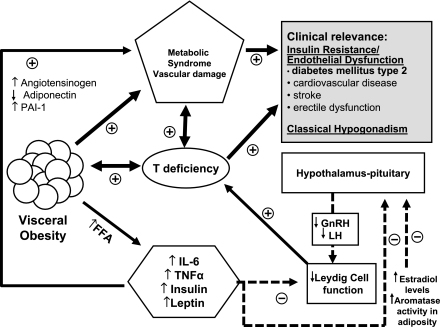
Obesity is a proinflammatory state resulting in increased release and secretion of proinflammatory cytokines and adipokines, free fatty acids, and estrogens from adipose tissue. These increases are important risk factors that may contribute to the development of metabolic syndrome and type 2 diabetes as well as androgen deficiency (20). Visceral fat is an active secretory tissue producing inflammatory cytokines, adipokines, biochemical modulators, and other proinflammatory factors including interleukin (IL)-6, IL-1β, plasminogen activator inhibitor-1, tumor necrosis factor (TNF)-α, angiotensinogen, vascular endothelial growth factor, and serum amyloid A (Fig. 1). These factors contribute to systemic and peripheral vascular inflammation and dysfunction (21). As shown in Fig. 1, one potential mechanism of how visceral adiposity and inflammatory response modulate insulin sensitivity involves the release of free fatty acids. Free fatty acids activate nuclear factor-κB pathways resulting in increased synthesis of TNF-α. TNF-α further activates lipolysis as well as increased synthesis of IL-6 and macrophage chemoattractant protein-1, which increases recruitment of more macrophages and modulates insulin sensitivity. Increased production of TNF-α also enhances expression of adhesion molecules in both endothelium and vascular smooth muscle cells. IL-6 stimulates hepatic synthesis of C-reactive protein, a nonspecific marker of vascular inflammation. In addition, TNF-α contributes to the dysregulation of insulin modulation of endothelin-1–mediated vasoconstriction and nitric oxide–mediated vasodilation, hence promoting vasoconstriction. Release of adipokines facilitates monocyte adhesion and migration into the vessel wall as well as the conversion of monoctyes to macrophages.
Figure 1.
Complex multidirectional interactions between testosterone and obesity, metabolic syndrome, and type 2 diabetes mediated by cytokines and adipokines leading to comorbidities such as ED and increased CVD risk. FFA, free fatty acids; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; PAI-1, plasminogen activator inhibitor-1.
Aromatase, the enzyme that converts testosterone to estradiol, is mainly located in adipose tissue. Obesity is associated with elevated estrogen in men activating hypothalamic estrogen receptors triggering inhibition of the hypothalamic-pituitary-gonadal axis. Treatment with aromatase inhibitors reverses the hypogonadotropic hypogonadism associated with obesity (22). Men with obesity and insulin resistance showed attenuated Leydig cell responsiveness to exogenous gonadotropin stimulation (23).
There are data supportive of a direct role of testosterone in insulin sensitivity. Acute withdrawal of testosterone in hypogonadal men for 2 weeks reduced insulin sensitivity without apparent changes in body composition, suggesting that sex steroids directly modulate insulin sensitivity (24). Others have reported that normalizing testosterone levels in older men had favorable effects on body composition, which could improve insulin sensitivity but not effects on postprandial triglyceride metabolism (25). Recently, it was demonstrated, using glucose clamp studies, that the interplay between insulin sensitivity, triglycerides, and sex steroids are almost immediate and not facilitated by changes in body composition. Concomitantly, increasing testosterone and decreasing estradiol levels for 1 week in young men improved postprandial triglyceride handling, postprandial glucose-dependent insulinotropic polypeptide (GIP) release, and insulin sensitivity (26). These studies indicate that interactions between low testosterone and visceral adiposity acting through proinflammatory agents (Fig. 1) result in insulin resistance and vascular endothelial dysfunction, which are potential causal factors for increased CVD and ED (20).
Sexual dysfunction and low testosterone in type 2 diabetes
A national survey of sexual activity in the U.S. found that over 60% of people with partners were sexually active, including individuals with diabetes (27). Similarly, 68.7% of 383 men with diabetes in the Look Ahead Study were sexually active (28). The clinical observation that ED occurs at an earlier age and with greater frequency in men with diabetes compared with nondiabetic men is supported by multiple population-based epidemiological studies (27) and by surveys of clinical practices (29). In the Look Ahead Study (28), 49.8% of men with diabetes reported mild or moderate ED. ED was associated with age (odds ratio 1.05, 95% CI 1.01–1.10), baseline hemoglobin A1c (1.31, 1.05–1.63), hypertension (2.41, 1.34–4.36), and the metabolic syndrome (3.05, 1.31–7.11). There are few studies evaluating the prevalence of reduced libido in men with diabetes. Decreased sexual desire is primarily affected by the presence of ED and by depression. An observational study of 253 men with type 2 diabetes in Sri Lanka found that after excluding men with ED (33%), the prevalence of reduced libido was 25% (30). In a population-based survey, premature ejaculation occurred in 36.3% (95% CI 26–48) of diabetic men and 22.9% (18–28.6) of nondiabetic men (27). Inability to climax was reported in 26% of diabetic men versus 15.9% of nondiabetic men. Premature ejaculation was reported in 40% of the patients from Sri Lanka who did not have severe or complete ED (30).
In the European Male Aging Study database of 3,369 men between the ages of 40 and 79 years, three sexual symptoms (poor morning erections, low sexual desire, and ED) had a syndromic relationship with decreased testosterone levels (18). Moreover, in the European Male Aging Study, low serum testosterone was more frequent in men with comorbidities such as obesity, metabolic syndrome, and type 2 diabetes. In studies from diabetes clinics, total, bioavailable, and free testosterone levels were low in men with type 2 diabetes (31). When comparing testosterone levels in men with and without ED and type 2 diabetes, these investigators found significantly lower serum bioavailable testosterone (P < 0.006) and free testosterone (P < 0.027) in men with ED, but there was no significant difference in total testosterone levels. The lower the serum testosterone, the greater the severity of ED (32). Corona et al. (33) evaluated 1,200 men with ED and reported that 16% had type 2 diabetes. Serum total testosterone levels were below the reference range (<300 ng/dL or <10.4 nmol/L) in 24.5% of men with diabetes versus 12.6% of nondiabetic subjects (P < 0.0001) after adjustment for age and BMI. In addition, hypogonadism in men with type 2 diabetes was associated with decreased sexual desire, more symptoms of depression, and lower luteinizing hormone levels.
ED in the past was ascribed to autonomic neuropathy or obliterative vascular disease; more recent studies identify endothelial dysfunction as an early abnormality that is potentially more amenable to therapy (20). Animal studies have demonstrated testosterone effects on nerve structure and function, nitric oxide synthase activity, and smooth muscle growth and differentiation, which mediate penile erections (34). Obesity and androgen deficiency are associated with increased proinflammatory cytokines, which also results in vascular endothelial dysfunction (20).
Men with type 2 diabetes can have other causes of ED. In a study of 8,373 men with type 2 diabetes (35), ED was associated with poor metabolic control, smoking, alcohol, antidepressants, antihypertensives, CVD medications, and histamine 2 receptor antagonists. There are multiple causes for low libido in the general population and in men with type 2 diabetes in addition to testosterone deficiency, including medications (e.g., serotonin reuptake inhibitors, antiandrogens), alcoholism, recreational drugs, fatigue, systemic illness, depression, relationship problems, other sexual dysfunction (fear of humiliation), hypoactive sexual disorder, and sexual aversion disorder.
The Look Ahead study reported that weight loss and increased physical activity were mildly beneficial in maintaining erections or improving ED in men with type 2 diabetes (36). Although improvement in glucose control is associated with some improvement in erectile function in some studies, most clinicians have not found this to be a reliable and effective treatment for ED. The Testosterone Replacement in Older Men with either Metabolic Syndrome or Type 2 Diabetes (TIMES 2) trial recruited hypogonadal men with total testosterone <318 ng/dL (11 nmol/L) or free testosterone <6.5 ng/dL (225 pmol/L) and either metabolic syndrome or type 2 diabetes. Testosterone treatment improved libido (37). Two meta-analyses of many clinical trials analyzed the effects of testosterone on different domains of sexual function (38,39). Testosterone treatment moderately improved the number of nocturnal erections, sexual thoughts and motivation, number of successful intercourse sessions, scores of erectile function, and overall sexual satisfaction in men with baseline serum testosterone <346 ng/dL (<12 nmol/L). The effects of testosterone on libido were more consistent than on erectile function. Testosterone replacement can restore nocturnal erections in hypogonadal men, but the effects are greater when testosterone and a phosphodiesterase (PDE)-5 inhibitor are administered together.
ED in many men with diabetes is improved by one of the PDE-5 inhibitors when used on demand. A recently published randomized double-blind placebo-controlled multicenter study evaluated the effectiveness of daily oral dosing of tadalafil in 298 men with diabetes and ED. Daily dosing of tadalafil showed significant improvement in vaginal penetration, completion of intercourse, and overall treatment satisfaction (40). Testosterone replacement therapy has been reported to improve erections in men who did not respond satisfactorily to a PDE-5 inhibitor alone (41). Larger trials using testosterone in addition to a PDE-5 inhibitor in hypogonadal men with ED who have testosterone levels <300 ng/dL (10.4 nmol/L) are needed.
Low testosterone, CVD risks, and type 2 diabetes
There is increasing evidence from multiple studies after adjustment of confounding variables that low serum testosterone is associated with an increase in all-cause mortality that is independent of the metabolic syndrome and diabetes (42–45) (Table 1). Low testosterone predicted the increased risk of CVD independent of age, obesity, hyperlipidemia, and lifestyle in men with or without type 2 diabetes (43–45). In patients with CVD, excess mortality was noted in the testosterone-deficient men compared with men with normal serum testosterone concentrations (46) (Table 1). Testosterone deficiency and CVD are both associated with visceral fat accumulation, metabolic syndrome, type 2 diabetes, increased inflammatory cytokines, hyperlipidemia, and abnormalities of coagulation (47).
Table 1.
Low testosterone is associated with increased mortality in older men
| Study design | n | Follow-up (years) | Mortality | Hazard ratio (95% CI) | Recent studies |
|---|---|---|---|---|---|
| Retrospective | 858 | 8 | All-cause | 1.88 (1.34–2.63)* | Shores et al. (42) |
| Prospective | 794 | 20 | All-cause and CVD | 1.40 (1.14–1.71)* 1.38 (1.02–1.85)* | Laughlin et al. (45) |
| Prospective | 2,314 | 10 | All-cause and CVD | 2.29 (1.60–3.26)* | Khaw et al. (43) |
| Prospective | 1,954 | 7.2 | All-cause and CVD | 2.32 (1.38–3.89)* | Haring et al. (44) |
| Prospective | 930 | 6.9 | All-cause and CVD in men with CVD | 2.27 (1.45–3.60)* | Malkin et al. (46) |
*On the basis of recent publications in which the number of subject is >500 and age of the subjects is >60 years.
In intervention studies on a small number of subjects, administration of testosterone caused coronary artery dilation, decreased myocardial ischemia, and improved angina during stress tests (48,49). Others suggest that testosterone may improve chronic heart failure (50,51). In a recent study, Testosterone in Older Men with Mobility Limitations (TOM), the older men had multiple comorbidities including obesity, hypertension, diabetes, and hyperlipidemia, the application of relatively high doses of transdermal testosterone gel was associated with significantly higher CVD event rates than in patients treated with placebo gel (52). An increase in CVD event rate was not observed in another study of frail elderly men treated with lower doses of transdermal testosterone gel compared with the placebo-treated men (53). The adverse CVD events in the TOM trial suggest that monitoring for cardiovascular adverse events is essential in a testosterone intervention study of older men with or without type 2 diabetes and we need a better understanding of testosterone effects on coagulation.
Clinical studies of testosterone replacement in men with obesity, metabolic syndrome, type 2 diabetes, and low testosterone concentrations
The major goal of testosterone replacement therapy is to increase serum testosterone concentrations to physiological concentrations with the purpose of resolving symptoms and biological sequelae of hypogonadism. The advent of new modes of testosterone delivery such as transdermal testosterone gels and depot intramuscular testosterone undecanoate injections have made physiological replacement possible. Testosterone replacement in the hypogonadal man with type 2 diabetes and/or metabolic syndrome should aim to have beneficial effects on multiple outcomes including sexual health; general well-being; body composition; and reducing CVD risk factors, including central adiposity, glycemic control, and atherogenic lipid profile.
Evidence from several studies (Table 2) demonstrated that testosterone promotes insulin sensitivity in hypogonadal men with and without type 2 diabetes. Mårin et al. (54) were the first to report that testosterone improved insulin sensitivity assessed by euglycemic clamp studies in obese men while reducing central adiposity. More recently, a randomized double-blind crossover trial demonstrated a significant reduction in insulin resistance in hypogonadal men with type 2 diabetes (55). This finding was confirmed in three further studies in men with metabolic syndrome and/or type 2 diabetes (37,56,57). Hypogonadism with either of these conditions was the major inclusion criterion. Subjects were not selected for poor diabetic control (Table 2). Even so, three studies reported decreases in HbA1c levels in the men with diabetes (37,55,56).
Table 2.
Randomized trials of testosterone replacement in hypogonadal men with metabolic syndrome or type 2 diabetes
| Study | Kapoor et al. (55) | Heufelder et al. (56) | Kalinchenko et al. (57) | Jones et al.* (37) |
|---|---|---|---|---|
| Subjects | Type 2 diabetes | New type 2 diabetes/metabolic syndrome | Type 2 diabetes/metabolic syndrome | Type 2 diabetes/metabolic syndrome |
| Study design | RCT-c | NRCT | RCT-p | RCT-p |
| n | 24 | 32 | 184 | 220 |
| Duration (months) | 3 | 12 | 6 | 6/12* |
| Medications for diabetes | Diet, oral, insulin | Naive | Diet, oral | Diet, oral |
| Baseline serum testosterone (nmol/L) | ≤8.6 | ≤10.5 | ≤6.7 | ≤10.2 |
| Testosterone formulation | TES injections (200 mg/2 weeks) | Testosterone gel (50 mg/day) | TU depot injections | Testosterone gel (40–80 mg/day) |
| Treatment effects (changes) | ||||
| HOMA-IR | −1.7 | −0.9 | −1.49 | −0.54 |
| Fasting glucose (nmol/L) | −1.6 | −0.3 (AS) | ↔ | −0.42 (AS) |
| Fasting insulin (mIU/mL) | ↔ | ↓ | ↔ | ↓(AS) |
| HbA1c | −0.37 | −0.80 | ND | ↔ [−0.45]† |
| Total cholesterol (nmol/L) | −0.4 | ND | ↔ | ↔ [−0.13] |
| LDL cholesterol (nmol/L) | ↔ | ND | ↔ | ↔‡ |
| HDL cholesterol (nmol/L) | ↔ | ↑§ | ↔ | −0.049‡ |
| Triglycerides | ↔ | ↓ | ↔ | ↔ |
| Lipoprotein a | ND | ND | ND | ↓ |
| BMI | ↔ | ↔ | ↔ | ↔ |
| Waist circumference | ↓ | ↓ | ↓ | ↔ |
| % Body fat | ND | ND | ND | ↔ |
| Blood pressure | ↔ | ↓‖ | ND | ↔ |
↔, No significant change; ↑, significant increase; ↓, significant decrease; AS, approaching significance (P = 0.05–0.07); ND, not done; NRCT, randomized open label, not placebo-controlled parallel trial; RCT-c, randomized placebo-controlled crossover; RCT-p, randomized placebo-controlled parallel; TES, mixed testosterone esters; TU, testosterone undecanoate depot injections after the first injection followed by another injection at 6 weeks and then injections every 12 weeks. Testosterone gel was dose-adjusted to give total testosterone level >17 nmol/L.
*The study by Jones et al. (TIMES2) had no medication changes in the first 6 months unless overriding clinical needs, but medication changes were allowed in the second 6 months for ethical reasons (intention-to-treat group, modified per protocol group where no changes in medications occurred; data not shown).
†Significant difference compared with placebo observed after 9 months, but result may be confounded by allowed medication changes.
‡Metabolic syndrome subgroup showed significant changes in total cholesterol (−0.34 mmol/L), LDL cholesterol (−0.21 mmol/L), and HDL cholesterol (−0.058 mmol/L).
§No figure quoted.
‖Diastolic blood pressure only.
Insulin resistance commonly occurs in chronic heart failure, and it has been shown to improve with testosterone replacement therapy (58). As discussed above, the mechanisms by which testosterone improves insulin sensitivity is multifactorial and likely to be due to a combination of testosterone effects on liver, muscle, and adipose tissues and by reducing the production of inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6), which cause insulin resistance (Fig. 1) (59).
It is well known that testosterone replacement reduces body fat mass and waist circumference in hypogonadal men with and without obesity (54,59). In men with the metabolic syndrome and/or type 2 diabetes, a decrease in central adiposity was observed in all but one study with testosterone treatment (55–57). BMI improved in only one trial (56) and body fat decreased in another in those men who did not have changes in medications that affect body weight (37). Leptin levels correlate with body fat content and have been shown to decrease with testosterone replacement in type 2 diabetes and the metabolic syndrome (57,60). The effect of testosterone on lipid profile was investigated in several studies including those on coronary heart disease, metabolic syndrome, and diabetes (59). The majority of studies have found that testosterone therapy results in a small but significant fall in total cholesterol and in some LDL cholesterol (37,55,59) (Table 2). HDL cholesterol may fall, rise, or remain unchanged (59). There is some evidence that after an initial decrease, HDL cholesterol levels then return to baseline (37). Most reports found no change in triglycerides. Lipoprotein a, which has the strongest positive correlation with premature coronary heart disease than any other component of the lipid profile, was found to fall significantly after testosterone treatment of men with the metabolic syndrome and/or type 2 diabetes (37).
Current evidence, albeit from mainly small-scale studies, does demonstrate some beneficial effects of testosterone on important CVD risk factors, which include insulin resistance, glycemic control, lipid profile, central adiposity, body composition, and inflammatory state in hypogonadal men with type 2 diabetes, as well as sexual health. None of these clinical trials reported any adverse effects on blood pressure, cardiovascular events, or mortality.
Conclusions
The multidirectional interrelationships between serum testosterone and SHBG with obesity, metabolic syndrome, and type 2 diabetes are complex. Obesity is accompanied by increased adipokines, cytokines, and other proinflammatory factor production from adipocytes and macrophages mainly in visceral fat. These factors may alter insulin responsiveness in fat, liver, muscle, and endothelial function resulting in metabolic syndrome, type 2 diabetes, ED, and CVD. Many men with type 2 diabetes, especially those who are obese, have low serum total testosterone and SHBG levels. Small-scale studies of testosterone treatment in men with metabolic syndrome or type 2 diabetes and borderline low or normal testosterone levels showed small improvement in glycemic control. Many of these studies in men with type 2 diabetes are associated with confounders, including changes in medications for diabetes. More randomized placebo-controlled interventional trials of testosterone treatment in testosterone-deficient men with the metabolic syndrome and poorly controlled type 2 diabetes are needed to evaluate the putative role of testosterone in the interruption of the vicious cycle contributed by metabolic imbalances. At present, it is important for the clinician to recognize that low testosterone and sexual dysfunction are commonly found in patients with obesity, metabolic syndrome, and type 2 diabetes. Testosterone replacement, in addition to diet, exercise, glycemic control, and PDE-5 inhibitors, should be considered in symptomatic hypogonadal men with type 2 diabetes and serum testosterone below the reference range. During testosterone treatment, monitoring should include assessment of improvement of symptoms, glycemic control, lipid levels, hematocrit, and potential adverse effects including CVD and prostate diseases in older men.
Acknowledgments
C.W. received research support from GlaxoSmithKline and Repros Therapeutics, received research materials from Besins Health Care, and is a consultant to GlaxoSmithKline and Lilly. T.H.J. is a consultant for Prostrakan and Bayer-Schering Pharma and has received research support and lecture honorarium from Bayer-Schering Pharma. R.S.S. is a consultant for Abbott (Solvay), Clarus Therapeutics, Endo Pharmaceuticals, GlaxoSmithKline, Lilly, and Repros Therapeutics and has received research support from Lilly, Repros, and Clarus Therapeutics. G.C. is a consultant for Abbott (Solvay), Endo (Indevus), and GlaxoSmithKline; has received research support from Abbott (Solvay) and Repros Therapeutics; and is on the speakers’ list for Abbott (Solvay). No other potential conflicts of interest relevant to this article were reported.
C.W. wrote sections of the manuscript, reviewed and revised the drafts, and edited the final manuscript. G.J., T.H.J., A.M.M., A.N., and M.A.P. wrote sections of the manuscript and reviewed and revised the drafts. R.S.S. wrote sections of the manuscript, reviewed and revised the drafts, and edited the final manuscript. A.T. and M.Z. wrote sections of the manuscript and reviewed and revised the drafts. G.C. wrote sections of the manuscript, reviewed and revised the drafts, and edited the final manuscript.
The authors thank the International Society of Men’s Health for organizing a meeting with discussions on the relevance of testosterone deficiency in obesity, metabolic syndrome, and type 2 diabetes. The authors based this review on the discussions.
References
- 1.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes 2010;17:224–232 [DOI] [PubMed] [Google Scholar]
- 2.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update 2010;16:293–311 [DOI] [PubMed] [Google Scholar]
- 3.Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011;40:189–207 [DOI] [PubMed] [Google Scholar]
- 4.Faris JE, Smith MR. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr Opin Endocrinol Diabetes Obes 2010;17:240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laaksonen DE, Niskanen L, Punnonen K, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005;90:712–719 [DOI] [PubMed] [Google Scholar]
- 6.Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 24 October 2010 [Epub ahead of print] [DOI] [PubMed]
- 7.Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: multi-ethnic study of atherosclerosis. Diabetes Care 2009;32:1049–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–1041 [DOI] [PubMed] [Google Scholar]
- 9.Vikan T, Schirmer H, Njølstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol 2010;162:747–754 [DOI] [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci 2010;65:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010;102:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajos N, Wellings K, Laborde C, Moreau C; CSF Group Sexuality and obesity, a gender perspective: results from French national random probability survey of sexual behaviours. BMJ 2010;340:c2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen SH, Wagner G, Heitmann BL. Sexual function and obesity. Int J Obes (Lond) 2007;31:1189–1198 [DOI] [PubMed] [Google Scholar]
- 15.Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol 2006;176:222–226 [DOI] [PubMed] [Google Scholar]
- 16.Bal K, Oder M, Sahin AS, et al. Prevalence of metabolic syndrome and its association with erectile dysfunction among urologic patients: metabolic backgrounds of erectile dysfunction. Urology 2007;69:356–360 [DOI] [PubMed] [Google Scholar]
- 17.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med 2009;6:1232–1247 [DOI] [PubMed] [Google Scholar]
- 18.Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–135 [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Arjonilla M, Schwarcz M, Swerdloff RS, Wang C. Obesity, low testosterone levels and erectile dysfunction. Int J Impot Res 2009;21:89–98 [DOI] [PubMed] [Google Scholar]
- 20.Traish AM, Feeley RJ, Guay A. Mechanisms of obesity and related pathologies: androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J 2009;276:5755–5767 [DOI] [PubMed] [Google Scholar]
- 21.Guzik TJ, Mangalat D, Korbut R. Adipocytokines: novel link between inflammation and vascular function? J Physiol Pharmacol 2006;57:505–528 [PubMed] [Google Scholar]
- 22.Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol 2008;158:741–747 [DOI] [PubMed] [Google Scholar]
- 23.Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 2005;90:2636–2641 [DOI] [PubMed] [Google Scholar]
- 24.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2007;92:4254–4259 [DOI] [PubMed] [Google Scholar]
- 25.Agledahl I, Hansen JB, Svartberg J. Impact of testosterone treatment on postprandial triglyceride metabolism in elderly men with subnormal testosterone levels. Scand J Clin Lab Invest 2008;68:641–648 [DOI] [PubMed] [Google Scholar]
- 26.Lapauw B, Ouwens M, ’t Hart LM, et al. Sex steroids affect triglyceride handling, glucose-dependent insulinotropic polypeptide, and insulin sensitivity: a 1-week randomized clinical trial in healthy young men. Diabetes Care 2010;33:1831–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindau ST, Tang H, Gomero A, et al. Sexuality among middle-aged and older adults with diagnosed and undiagnosed diabetes: a national, population-based study. Diabetes Care 2010;33:2202–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen RC, Wing RR, Schneider S, et al. Erectile dysfunction in type 2 diabetic men: relationship to exercise fitness and cardiovascular risk factors in the Look AHEAD trial. J Sex Med 2009;6:1414–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Berardis G, Franciosi M, Belfiglio M, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care 2002;25:284–291 [DOI] [PubMed] [Google Scholar]
- 30.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, Levy JC. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med 2008;5:2125–2134 [DOI] [PubMed] [Google Scholar]
- 31.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–917 [DOI] [PubMed] [Google Scholar]
- 32.Kapoor D, Clarke S, Channer KS, Jones TH. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl 2007;30:500–507 [DOI] [PubMed] [Google Scholar]
- 33.Corona G, Mannucci E, Petrone L, et al. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res 2006;18:190–197 [DOI] [PubMed] [Google Scholar]
- 34.Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007;52:54–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedele D, Bortolotti A, Coscelli C, et al. Erectile dysfunction in type 1 and type 2 diabetics in Italy. On behalf of Gruppo Italiano Studio Deficit Erettile nei Diabetici. Int J Epidemiol 2000;29:524–531 [PubMed] [Google Scholar]
- 36.Wing RR, Rosen RC, Fava JL, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med 2010;7:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (The TIMES2 Study). Diabetes Care 2011;34:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 2007;82:20–28 [DOI] [PubMed] [Google Scholar]
- 39.Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394 [DOI] [PubMed] [Google Scholar]
- 40.Hatzichristou D, Gambla M, Rubio-Aurioles E, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med 2008;25:138–146 [DOI] [PubMed] [Google Scholar]
- 41.Rochira V, Balestrieri A, Madeo B, Granata AR, Carani C. Sildenafil improves sleep-related erections in hypogonadal men: evidence from a randomized, placebo-controlled, crossover study of a synergic role for both testosterone and sildenafil on penile erections. J Androl 2006;27:165–175 [DOI] [PubMed] [Google Scholar]
- 42.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006;166:1660–1665 [DOI] [PubMed] [Google Scholar]
- 43.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 2007;116:2694–2701 [DOI] [PubMed] [Google Scholar]
- 44.Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J 2010;31:1494–1501 [DOI] [PubMed] [Google Scholar]
- 45.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008;93:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 2010;96:1821–1825 [DOI] [PubMed] [Google Scholar]
- 47.Maggio M, Basaria S. Welcoming low testosterone as a cardiovascular risk factor. Int J Impot Res 2009;21:261–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb CM, Adamson DL, de Zeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol 1999;83:437–439 [DOI] [PubMed]
- 49.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation 2000;102:1906–1911 [DOI] [PubMed] [Google Scholar]
- 50.Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J 2006;27:57–64 [DOI] [PubMed] [Google Scholar]
- 51.Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure: a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol 2009;54:919–927 [DOI] [PubMed] [Google Scholar]
- 52.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010;363:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010;95:639–650 [DOI] [PubMed] [Google Scholar]
- 54.Mårin P, Holmäng S, Gustafsson C, et al. Androgen treatment of abdominally obese men. Obes Res 1993;1:245–251 [DOI] [PubMed] [Google Scholar]
- 55.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154:899–906 [DOI] [PubMed] [Google Scholar]
- 56.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726–733 [DOI] [PubMed] [Google Scholar]
- 57.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612 [DOI] [PubMed] [Google Scholar]
- 58.Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur J Heart Fail 2007;9:44–50 [DOI] [PubMed] [Google Scholar]
- 59.Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis 2009;207:318–327 [DOI] [PubMed] [Google Scholar]
- 60.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2007;156:595–602 [DOI] [PubMed] [Google Scholar]