Despite the well-known influence of maternal glucose on infant birth weight (BW), the prevalence of large for gestational age (LGA) infants (≥90th percentile for age) has been increasing steadily over decades, particularly in pregnancies complicated by pregestational or gestational diabetes mellitus (1). Although the overall prevalence of macrosomia (BW ≥4,000 g) is 17–29% in women with untreated gestational diabetes, the majority of macrosomic infants are born to women with obesity but no gestational diabetes (2,3). Moreover, epidemiologic data show that a higher BW is associated with higher BMI and glucose intolerance later in life (4,5), suggesting life-long metabolic implications for offspring.
Recent data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study suggested that concentrations of maternal glucose below the previously accepted diagnostic thresholds for gestational diabetes are predictive of LGA and fetal hyperinsulinemia (6). On the basis of this landmark study, the International Association of Diabetes in Pregnancy Study Group and the American Diabetes Association (ADA) recommended new lower diagnostic criteria for gestational diabetes (7,8). However, a significant number of women with gestational diabetes whose glucose values are within the current targeted therapeutic ranges deliver macrosomic infants (9). Although glucose plays a major role in fetal growth, this paradox underscores the likely role of other nutrients in fetal growth, but also the need to critically reexamine our definition of “normal” maternal patterns of glycemia and the effects on fetal growth. The new diagnostic criteria recommended by the International Association of Diabetes in Pregnancy Study Group and ADA are expected to increase the prevalence of gestational diabetes to 18%. Thus, treatment targets may need to be reevaluated.
Historically, the treatment goal in pregnancies complicated by diabetes has been to mimic patterns of glycemia in normal pregnancy (1). Although the HAPO study better defined abnormal glycemic thresholds for the diagnosis of gestational diabetes based on fetal outcomes, the current clinical guidelines for defining treatment targets (10–13) are less rigorous given that optimal therapeutic targets remain untested in randomized trials (14). Further, there has been a reluctance to compare descriptive data in “normal” pregnant women because of the difficulty of comparing major differences in study design, patient characteristics, and methodology. Nevertheless, ∼5 decades of research have helped define “normal” maternal glucose metabolism. The intent of this review is to offer the clinician 1) a clear graphic representation of available glucose data collected in “normal” pregnancy (i.e., a pooled analysis of weighted averages across 12 studies involving nonobese patients); 2) a full discussion of study methodologies and limitations; and 3) a proposal of more aggressive therapeutic targets that may be prospectively tested for the prevention of fetal macrosomia.
RESEARCH DESIGN AND METHODS
Literature search strategy and inclusion of evidence
PubMed was searched broadly using keywords such as pregnancy, glycemia, glucose, diurnal patterns, and gestational diabetes. Reference lists in review papers, original manuscripts, and expert reports were compared with findings in PubMed. Data were included if they provided information on glycemic patterns in normal pregnancy, excluding type 1 or 2 diabetes or gestational diabetes. Patterns needed to have been established using diurnal profiling techniques such as hospital admission with frequent blood sampling, serial measures of self-monitored blood glucose (SMBG), or a continuous glucose monitoring system (CGMS). The patterns of glycemia required characterization during controlled or ad libitum dietary intake to include the effect of incretin hormones and the enteroinsular axis (15). Thus, investigations using a glucose challenge or glucose infusion were not considered.
Graphic portrayal of data and weighted averages
Exact data were plotted as reported in text or tables by the original authors. When data were only shown in figures, the mean and variance were taken from the graphs. In some cases, complete patterns of glycemia were not reported between meals. For graphic purposes, if a premeal value was not reported, the 24-h mean was used as the between-meal glucose concentration. In the graphs, postprandial (PP) spikes are 1 h (60–70 min) and 2 h after a meal as reported.
Because test statistics were not uniformly reported and methodologies for measuring glucose concentrations were variable, a formal meta-analysis was not possible. Thus, this is a pooled analysis of 12 studies that met our inclusion criteria. Weighted means and SDs were calculated as the product of the mean reported value and sample size for each study. The sum of the products across studies was then calculated and divided by the total number of study participants. In addition, mean 1- and 2-h PP glucose concentrations were calculated across three meals (breakfast, lunch, and dinner). Data are presented as weighted mean ± SD.
RESULTS
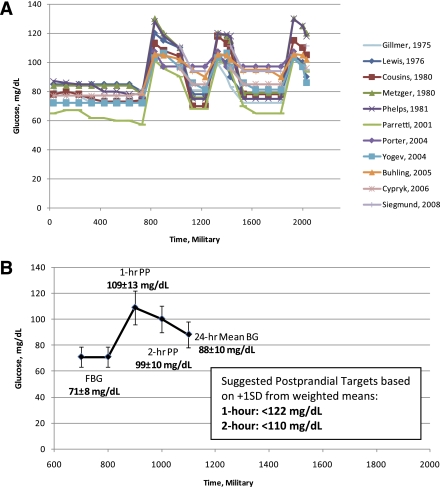
Twelve studies met the criteria for inclusion with a total of 255 pregnant women with normal weight and glucose tolerance (Table 1). Table 2 reports data from the included studies, and Fig. 1A graphically depicts the patterns of glycemia. The mean gestational week of study was 33.8 ± 2.3 weeks (range 24–40.8 ± 0.09–8.1 weeks). Most of the women had a BMI <25 kg/m2, although prepregnancy BMI versus BMI at the time of study was variably reported and specific BMI ranges were often unreported. On average, fasting blood glucose (FBG) was 70.9 ± 8 mg/dL (n = 195) (Table 2). The weighted mean pattern of glycemia (including FBG, 1- and 2-h PP, and 24-h mean glucose) is shown in Fig. 1B.
Table 1.
Summary of 12 studies that met criteria for inclusion*
| Author and date | Purpose | Research design/instruments | Subjects | Glucose measure/method | Diet |
|---|---|---|---|---|---|
| Hospital admission studies | |||||
| Gillmer et al. (16) | To characterize diurnal glucose and insulin profiles in normal vs. pregnancies affected by diabetes | Observational admission to hospital at 1000 h; hourly blood during day/every 2 h at night; 50-g 3-h OGTT 0900 h next morning | n = 24 normal; n = 13 “chemical diabetes” | Venous plasma glucose oxidase-peroxidase | Total CHO intake 180 g; 40 g for breakfast before admission |
| Lewis et al. (17) | To characterize differences in diurnal glucose, insulin, and C-peptide in control vs. pregnant women with diabetes over 24 h | Observational; 72-h hospital admission; hourly blood during day/five samples during night | n = 6 normal; n = 3 “mild” diabetes; n = 4 type 1 diabetes | Venous serum; rapid photoelectric | 125 g CHO/day for all women |
| Cousins et al. (18) | To characterize the effect of second-/third-trimester pregnancy on glucose, insulin, and C-peptide hourly for 24 h | Observational; hospital admission; hourly blood samples began after 10-h fast | n = 6 nonobese “normal” women; passed OGTT | Venous plasma; glucose oxidase | Standard hospital meals |
| Metzger et al. (19) | To characterize the effect of gestational diabetes on diurnal profiles of glucose, lipids, and AAs in late pregnancy compared with normal pregnancy | Observational; 24-h hospital admission; hourly blood during day/every 2 h at night | n = 8 normal; n = 7 gestational diabetes (FBG <105); n = 6 gestational diabetes (FBG ≥105) | Venous plasma; glucose oxidase | Liquid meal diet: 2,110 kcal; 275 g CHO; 76 g PRO equally over three meals (0800, 1300, and 1800 h) |
| Phelps et al. (20) | To characterize diurnal profiles of glucose, insulin, FFA, TG, cholesterol, and AAs in late normal pregnancy compared with matched nonpregnant control subjects | Observational; 24-h hospital admission | n = 8 nonpregnant women; n = 8 “normal” pregnant women | Venous plasma; glucose oxidase | Liquid meal diet: 2,110 kcal; 275 g CHO; 76 g PRO equally over three meals (0800, 1300, and 1800 h) |
| SMBG studies | |||||
| Parretti et al. (21) | To assess diurnal glucose profiles in normal-weight women without diabetes and to assess correlations between maternal glucose and fetal growth parameters | Observational; SMBG every 2 h during day/night; fixed meal times; 28, 30, 32, 34, 36, and 38 weeks’ gestation | n = 51 | Accutrend-α (Boehringer Mannheim, Mannheim, Germany); reflectance photometry; plasma-corrected | Free-living diet |
| CGMS studies | |||||
| Porter et al. (23) | To compare patterns of glycemia between women with history of macrosomia or polyhydramnios vs. women without | Observational/correlational; CGMS for 72 h | n = 28; all without diabetes; n = 17 history of polyhydramnios or macrosomia; n = 11 no macrosomia | Medtronic (Minneapolis, MN) Minimed CGMS | Free-living diet |
| Yogev et al. (22) | To characterize the glycemic profile in normal-weight and obese pregnant women | Observational; CGMS for 72 h | n = 57; no diabetes; obese was ≥27 kg/m2 | Medtronic Minimed CGMS | Free-living diet |
| Bühling et al. (25) | To assess the frequency of hyperglycemia using SMBG vs. CGMS in nonpregnant, normal pregnant, and women with gestational diabetes or IGT | Observational; CGMS for 72 h with SMBG 7× daily | n = 8 nonpregnant; n = 56 pregnant (n = 24 no diabetes, 17 diet-controlled gestational diabetes; 17 IGT) | Medtronic Minimed CGMS; Accu-Chek Sensor (Roche Diagnostics, Mannheim, Germany); SMBG: FBG, premeal, 2-h PP, hs | Free-living diet for no diabetes; with diabetes: 50% CHO, 35% fat, 15% PRO as counseled |
| Bühling et al. (24) | To characterize in pregnancies affected by diabetes vs. no diabetes: 1) time of PP glucose peak; 2) PP glucose profiles; and 3) optimal time for PP glucose according to clinical outcome | Observational; CGMS for 72 h | n = 53; n = 36 (no diabetes); n = 17 with diabetes (13 gestational diabetes; 4 type 1 diabetes) | Medtronic Minimed CGMS | Free-living diet for no diabetes; with diabetes: 50% CHO, 35% fat, 15% PRO as counseled |
| Cypryk et al. (26) | To characterize blood glucose concentrations in women with gestational diabetes using CGMS | Observational; CGMS for 72 h | n = 19; n = 7 diet-controlled gestational diabetes; n = 5 diet + insulin-controlled gestational diabetes; n = 7 normal controls | Medtronic Minimed CGMS | Free-living diet |
| Siegmund et al. (27) | To characterize the glucose profile in healthy pregnant women and determine cutoff values | Observational, longitudinal; CGMS for 72 h | n = 32; prepregnancy BMI = 22.4 ± 2.5 kg/m2 | Medtronic Minimed CGMS | Free-living diet; kept diet records |
AA, amino acid; CHO, carbohydrate; FFA, free fatty acid; hs, bedtime; IGT, impaired glucose tolerance; PRO, protein; TG, triglyceride.
*Because of the complexity in study designs and space constraints, it was not possible to fully characterize all studies beyond the scope of this article.
Table 2.
Selected participant characteristics with compiled glucose variables of interest across 12 studies (weighted mean ± SD)
| Study | N | Week of gestation | BMI kg/m2 | FBG | 1-h Breakfast | 2-h Breakfast | 1-h Lunch | 2-h Lunch | 1-h Dinner | 2-h Dinner | 24-h Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gillmer et al. (16) | 24 | 33.8 ± 2.2 | 28* | 72 ± 4 | 99 ± 18 | 83 ± 16 | 104 ± 18 | 95 ± 16 | 84.4 ± 6.7 | ||
| Lewis et al. (17) | 6 | 40.8 ± 0.9 | 24.8* | 80 ± 2.5 | 120 ± 5 | 105 ± 2.5 | 90 ± 2 | 105 ± 11 | 90 ± 2 | 93 ± 10 | |
| Cousins et al. (18) | 6 | 36 ± 1 | “non-obese” | 74 ± 6.61 | 113 ± 9.8 | 104 ± 14 | 118 ± 9.8 | 108 ± 9.8 | 117 ± 12.2 | 105 ± 9.8 | 87.3 ± 4.1 |
| Metzger et al. (19) | 8 | 36† | 89 ± 4.6% of ideal‡ | 78 ± 5.66 | 130 ± 14.14 | 110 ± 19 | 120 ± 11.31 | 115 ± 19 | 130 ± 14.14 | 120 ± 17 | 96 ± 8.49 |
| Phelps et al. (20) | 8 | 36.3 ± 2.3 | 89 ± 4.6% of ideal‡ | 78 ± 8.49 | 128 ± 8.49 | 110 ± 11 | 120 ± 8.49 | 118 ± 14 | 130 ± 16.97 | 118 ± 8 | 96 ± 8.49 |
| Parretti et al. (21) | 51 | 36 | 21 | 57.2 ± 3.9 | 101.2 ± 4.9 | 90.1 ± 4.9 | 101.9 ± 3.4 | 94.2 ± 4.1 | 102.2 ± 3.2 | 93.5 ± 5.1 | 77.4 ± 4.7 |
| Porter et al. (23) | 11 | 34.6 ± 2.6 | 22.8 ± 2.7‡ | 77 ± 6.4 | 107.3 ± 13.1 | 107.3 ± 13.1 | 107.3 ± 13.1 | 94.1 ± 10.5 | |||
| Yogev et al. (22) | 42 | 28.9 ± 8.1 | 23.7 ± 1.8 | 72.1 ± 13 | 103.2 ± 13 | 96.8 ± 12 | 103.2 ± 13 | 96.8 ± 12 | 103.2 ± 13 | 96.8 ± 12 | 83.6 ± 18 |
| Bühling et al. (25) | 24 | 34 ± 3.7 | 23.0 ± 5.7‡ | 97 ± 9 | |||||||
| Bühling et al. (24) | 36 | 32 ± 4.6 | 23.0 ± 5.5‡ | 124.2 ± 23.4 | 117 ± 21.6 | 118.8 ± 28.8 | 95.4 ± 14.4 | ||||
| Cypryk et al. (26) | 7 | 24–28 | 27.2 ± 6.3 | 79 ± 13§ | 96 ± 11 | 85 ± 6 | |||||
| Siegmund et al. (27) | 32 | 36 | 22.4 ± 2.5‡ | 81.1 ± 10.8 | 110.6 ± 12.6 | 110.6 ± 12.6 | 110.6 ± 12.6 | 94 ± 9 | |||
| Weighted mean, all | 70.9 ± 7.8 | 110.8 ± 12.5 | 99.4 ± 9.9 | 107.1 ± 12.2 | 98.5 ± 10.4 | 108.9 ± 14.1 | 99.9 ± 10.3 | 88.2 ± 10.0 | |||
| Weighted mean, inpatient only | 75.0 ± 5.1 | 123.6 ± 11.2 | 108.7 ± 12.6 | 108.3 ± 13.4 | 97 ± 13.8 | 113.6 ± 15.8 | 102.9 ± 12.6 | 89.3 ± 7.3 | |||
| Weighted mean, CGMS only | 76.3 ± 11.4 | 112.2 ± 17.2 | 102.2 ± 12.1 | 109.3 ± 16.5 | 102.2 ± 12.1 | 110.0 ± 19.4 | 102.2 ± 12.1 | 91.5 ± 12.7 |
*Third-trimester BMI calculated from reported height/weights. Prepregnancy height/weights and gestational weight gain are not reported.
†SD not available.
‡Prepregnancy.
§SMBG value was not different from CGMS value.
Figure 1.
A: Patterns of glycemia in normal pregnancy (gestational week 33.8 ± 2.3) across 11 studies published between 1975 and 2008. One study provided useful information but required exclusion from the figure because data could not be regraphed. Methodologies used diurnal pattern characterization during inpatient admission (five studies), SMBG via reflectance photometry (one study), and CGMS (six studies) (n = 168–255; BMI range 22–28 at time of study). B: Mean pattern of glycemia across 12 studies (n = 168–255) during 33.8 ± 2.3 weeks’ gestation (weighted average ± SD, values rounded to whole numbers for clinical use). Suggested 1- and 2-h PP targets are <122 and <110 mg/dL, respectively.
In Table 2, the data from five inpatient studies (16–20), one SMBG study (21), and six CGMS studies (22–27) are reported. The inpatient studies were conducted decades earlier than the CGMS studies, and women were admitted to a metabolic ward. The average FBG concentrations were similar across all of the studies, with the exception of the single SMBG study (21) (Fig. 1A). Across studies, the 24-h mean glucose ranged from 77.4 ± 4.7 to 97 ± 9 mg/dL. The single SMBG study reported the lowest glucose concentrations compared with all other studies. However, by excluding these data, the weighted means changed minimally and these data were included because of the structured study design and controlled variance. One-h PP glucose excursions were higher in the inpatient studies compared with the SMBG and CGMS studies (115.2 ± 13.4 compared with 101.8 ± 3.8 and 110.5 ± 17.7 mg/dL, respectively). However, 2-h PP glucose concentrations were similar for inpatient and CGMS studies (103 ± 13 vs. 102.2 ± 12.2 mg/dL, respectively) but were lower in the SMBG study (92.6 ± 4.7 mg/dL). With all studies included, the average 1- and 2-h PP glucose concentrations across meals were 108.9 ± 12.9 and 99.3 ± 10.2 mg/dL, respectively (n = 160–192; Fig. 1B). The ±1 SD range above the weighted mean for a 1-h PP was 96.0–121.8 mg/dL, and the ±2 SD range was 83.1–134.7 mg/dL. For the 2-h PP target, ±1 SD above the weighted mean ranged from 89.1 to 109.5 mg/dL, and ±2 SDs above the weighted mean ranged from 78.9 to 119.7 mg/dL. Time to peak glucose concentration after a meal, based on the CGMS articles (22,24,25), was an average of 69.4 ± 23.9 min (n = 102).
Review of evidence and discussion
The most compelling finding from our review of the available literature is that glucose concentrations during normal pregnancy in the absence of obesity are lower than the current suggested normal therapeutic targets. As depicted in Fig. 1B, the weighted mean pattern of glycemia reveals an FBG of 71 ± 8 mg/dL, followed by 1- and 2-h PP glucose concentrations of 109 ± 13 and 99 ± 10 mg/dL, respectively, and a 24-h glucose of 88 ± 10 mg/dL. These weighted mean values are appreciably lower than the currently recommended therapeutic targets of ≤95 mg/dL for FBG, <140 and <120 mg/dL at 1- and 2-h PP, respectively (13). Although HAPO provided rigorous outcome data to determine the thresholds appropriate for the diagnosis of gestational diabetes, there are a paucity of data on appropriate therapeutic targets for women with diabetes or gestational diabetes. Until prospective outcome data are generated by examining the most appropriate therapeutic targets to minimize both LGA and small for gestational age (SGA) infants (infants <10th percentile for gestational age), mimicking patterns of normal glycemia in pregnancy, in women without obesity, remains a reasonable goal. The data presented would argue that lower targets be tested in future prospective trials.
Differences in methodology
There has been a reluctance to compare data characterizing glycemia in pregnancy, largely because of differences in study design and methodologies. Studies in women admitted to a hospital have been criticized because of the highly controlled nature of the design (28), the lack of repeated measures throughout pregnancy, and the small numbers of participants (21). These studies removed women from the free-living environment, which changed their physical activity pattern. However, dietary carbohydrate intake was controlled and a liberal amount (190–275 g/day) was administered (Table 1). The controlled physical activity and liberal carbohydrate intake may explain, in part, the somewhat higher PP peaks, particularly by Phelps et al. (20) (Table 2, Fig. 1). Nevertheless, the patterns of glycemia across inpatient studies remain remarkably similar. The CGMS studies may be criticized for opposite reasons. With the exception of one study (27), investigations using CGMS tended to poorly control for the week of pregnancy, with variance up to ±8 weeks reported (22). This makes glucose concentrations difficult to compare because of the variably increasing insulin resistance with each passing week of the third trimester (29,30). Also, with the one exception (27), no effort was made to control dietary intake or physical activity. Further, the methods for interpreting the large volume of data points generated by CGMS are not well described in regard to how FBG and PP values were defined.
There has been further reluctance to compare data among studies because of variations in the methodologies used to measure glucose concentrations. Glucose is unstable in whole blood, including capillary blood. Compared with serum or plasma, glucose concentrations are 11–12% lower in whole blood because of erythrocyte metabolism of glucose via glycolysis. Moreover, compared with plasma, serum glucose concentrations can decrease ∼0.6 mmol/L/h as the result of glycolysis during sample preparation (31). Since 1987 (32), glucometers have been standardized (31) to report plasma-adjusted glucose values (within ±15%) (33). Current CGMS technology measures glucose concentrations in interstitial fluid, which are highly correlated with glucometer-derived plasma-corrected glucose measures (34,35). Thus, although capillary and interstitial glucose concentrations are not recommended for diagnostic purposes (7,33,34), they seem reliable for determination of glucose trends (33,34).
On the basis of our detailed review of the published studies, we think the pooled studies for our analyses were comparable according to the following criteria. First, the inpatient studies reported laboratory-derived glucose concentrations measured in plasma (glucose oxidase) (16,18–20) or serum (photoelectric) (17) (Table 1). None used whole blood measurements, which would have produced lower glucose concentrations. Second, the single SMBG study (21) used a reflectance photometry-based glucometer that reported plasma-corrected values comparable (<10% error) to the standard laboratory glucose oxidase method (36). Thus, the lower glucose concentrations in the SMBG study (21) cannot be explained by use of this glucometer. Third, the CGMS interstitial glucose concentrations are correlated with now standardized glucometer-derived, plasma-corrected glucose measures (34,35). Therefore, although there is undoubtedly a degree of variance among methodologies, it is reasonable to compare the data for therapeutic purposes.
Early descriptive studies of glycemia in pregnancy
The earliest study of glucose metabolism in pregnancy revealed that PP glucose concentrations were higher despite higher insulin levels (37), implying a degree of maternal insulin resistance. These early observations were confirmed in later studies (16–18,20,37) (Tables 1 and 2). Gillmer et al. (16) showed that during normal pregnancy, mean diurnal glucose concentrations increased only 4 mg/dL and rarely exceeded 100 mg/dL. Cousins et al. (18) demonstrated that a lower 24-h glucose area under the curve could be explained by relative nocturnal hypoglycemia. In addition, third-trimester, 2-h postmeal glucose excursions were higher compared with those at 22 to 26 weeks’ gestation.
Studies using SMBG
Parretti et al. (21) published the only study in which SMBG was used to characterize patterns of glycemia (51 pregnancies) controlling for gestational age. Women measured their blood glucose every 2 h throughout the day and night and ate their meals within defined time periods. This structured study tried to approach what had previously been possible only during hospital admission. However, glucose concentrations were far lower than those in any other study (Table 2, Fig. 1A). Although the glucometer used does not seem to explain the lower concentrations, it is possible the women in this study were more physically active or consumed less simple carbohydrates compared with other studies. Furthermore, the women were not blinded to the glucometer measurements, and it is possible that they changed their behavior on the basis of their SMBG concentrations.
Studies using CGMS
The advent of CGMS made it possible to measure continuous patterns of glycemia over a 24-h period, rather than hourly. By using CGMS, it was recognized that women with gestational diabetes had occult periods of hyperglycemia not captured using SMBG (38). Moreover, the glucose peak occurred 90 min PP (rather than 70 min) with the tendency to remain elevated for 3 h without returning to baseline (39). In another CGMS study, obese pregnant (BMI >27 kg/m2) women were shown to have higher preprandial and PP glucose, compared with normal-weight control subjects, and the PP peak was delayed 15 min (22). Unexpectedly, these obese women also had lower nocturnal glycemia compared with control subjects (22). In contrast, Porter et al. (23) did not observe differences in nocturnal glycemia between women with and without a history of macrosomia (Table 1). The former study is the most highly cited study (22) in support of current clinical practice for PP glucose monitoring (13). However, the week of pregnancy during which CGMS was worn varied between 21 and 37 weeks of gestation, diet was not controlled (or reported), and the CGMS analysis of the data was generally not well described (22). Thus, interpretation of the data is limited.
Other groups using CGMS have attempted to control sources of variance (24), but even women with normal pregnancies seem to have a wide range of glucose concentrations (Table 1) (26). In the most controlled CGMS study to date (27), PP blood glucose concentrations increased up to 36 weeks of gestation despite a self-reported constant caloric intake; macronutrient composition of the diet was not reported. Because the study was well controlled for the week of pregnancy, this report provides perhaps the best interpretable data from CGMS yet published.
In summary, the inpatient studies were less “real-world,” but the physical activity and dietary intake were highly controlled, so they likely best captured the normal physiology of pregnancy. On the other hand, the CGMS studies, although less controlled, better depict free-living conditions. Both types of studies require careful interpretation but provide important information. Taken together, the data reveal a range of glycemia in glucose-tolerant women, yet a remarkably similar pattern among studies (Fig. 1A).
Basis for the currently recommended therapeutic targets
The current clinical recommendations for treatment targets in pregnancies complicated by diabetes are not uniform internationally or primarily based on the studies listed in Table 1. Although the therapeutic targets were chosen to attenuate the risk for fetal macrosomia, they have never been prospectively tested compared with lower targets (12,13). Even when current glucose targets are achieved in the pregnancy affected by diabetes, macrosomia still occurs and in utero programming may have a lasting metabolic impact on the offspring (40,41).
The current therapeutic targets of ≤95 mg/dL for FBG, <140 mg/dL for 1-h PP glucose, and <120 mg/dL for 2-h PP glucose (13) were established on the basis of data from women with pregestational and gestational diabetes. In 1986, Willman et al. (42) reported data in 95 women with medication-treated gestational diabetes (A2 gestational diabetes) that indicated a mean 24-h blood glucose concentration >130 mg/dL was associated with a high risk of fetal macrosomia. In 1992, Combs et al. (43) reported that in pregnant women with class B through RF diabetes, a 1-h PP target equal to 130 mg/dL during gestational weeks 29–32 suggested a reduction in macrosomia incidence without increasing the incidence of SGA. Previously, it had been thought that monitoring preprandial versus PP glucose concentrations were equally effective in preventing macrosomia (44). However, data from women with pregestational diabetes (45,46) and later with gestational diabetes (44) established the relation between PP glucose and infant body weight/fetal macrosomia, as well as other infant outcomes (47). The randomized study of de Veciana et al. in 1995 (44) demonstrated that targeting a 1-h PP glucose (compared with preprandial) in women with gestational diabetes was superior in the reduction of macrosomia incidence. In this study, an FBG threshold of <105 mg/dL and a 1-h PP threshold of <140 mg/dL were used, but they were not compared with any other threshold. In 1998, this evidence was cited as the basis for 1- and 2-h PP thresholds of <140 and <120 mg/dL, respectively (14). As a result, these targets were used in most studies published after the year 2000 (21,48–50). As recognized by others (13,51), these cutoffs are not based on randomized studies of alternate treatment targets. In the most recent guidelines from the ADA 5th International Conference on Gestational Diabetes, two studies were cited to support maintaining the current therapeutic targets (21,22) without citation of the inpatient (16–20) or other CGMS studies (23–26).
Although the prevention of macrosomia is clearly an important target for therapy during the pregnancy complicated by diabetes, it is equally important to consider the risk for SGA when testing lower therapeutic targets. Langer and Mazze (52) and Langer et al. (53) observed that a range of 87–104 mg/dL for mean 24-h blood glucose can minimize both SGA and LGA incidence. It has further been observed that overtreatment of pregnant women with diabetes with insulin can cause SGA (54). However, the considerations for type 1, type 2, and gestational diabetes may be different. In long-standing type 1 diabetes, placental perfusion may be compromised to a variable degree because of abnormal placentation from underlying vascular disease or hypertension that may limit glucose availability to the fetus (55). Thus, if glucose concentrations are too low in pregnant women with long-standing diabetes accompanied by vascular disease or placental insufficiency, the glucose gradient to the fetus may be further compromised. This is unlikely to be the case with uncomplicated gestational diabetes. It is also critical to acknowledge that increasing evidence suggests other factors, such as free fatty acids and triglycerides, are extremely important in influencing fetal growth (56,57) as are prepregnancy BMI and, to a lesser extent, gestational weight gain (58). None of the trials that have examined therapeutic glycemic targets to prevent macrosomia have controlled for these significant confounders.
Challenge to reconsider current therapeutic targets
In this critical review and pooled analysis of 12 studies (n = 255 women), we have for the first time graphically compiled patterns of glycemia in normal pregnancy (Fig. 1A and B) and derived weighted means and SDs for clinical application. As seen in Table 2 and Fig. 1A, despite the differences in study design and methodology for measuring glucose, the data fall into a remarkably similar range. The weighted average for FBG was 70.9 ± 7.8 mg/dL (n = 195), well below the current therapeutic target of ≤95 mg/dL (13) and below the HAPO-based diagnostic threshold of <92 mg/dL (7,8). The weighted average for 1- and 2-h PP glucose concentrations across meals were 108.9 ± 12.9 and 99.3 ± 10.2 mg/dL, respectively, also far below the current targets of <140 and <120 mg/dL. Finally, the weighted mean 24-h glucose was 88 ± 10 mg/dL, on the lower end of the observed 87–104 mg/dL range that is thought to minimize SGA and LGA incidence (52,53).
The HAPO study documented a mean FBG of 80.9 mg/dL in >25,000 women with an average BMI of 28 kg/m2 and determined that LGA and a cord C-peptide ≥90th percentile were 1.75 times higher at an FBG ≥92 mg/dL (6). Thus, there are strong data to support that this fasting diagnostic threshold might be adopted as an FBG therapeutic target. However, there are no equivalent data for PP targets. On the basis of our pooled analysis, we suggest prospective controlled studies are needed to test more aggressive therapeutic targets for PP glycemia in pregnancies affected by diabetes, uncomplicated by underlying vascular disease, hypertension, or smoking, and controlled for BMI and gestational age. On the basis of the weighted means, the ±1 SD above the weighted mean for a 1-h PP glucose ranges from 96 to 122 mg/dL and the ±2 SD ranges from 83 to 135 mg/dL. For the 2-h PP target, ±1 SD above the weighted mean ranges from 89 to 110 mg/dL, and ±2 SDs above the weighted mean ranges from 79 to 119 mg/dL. Although the glucose targets 2 SDs above the weighted means (135 mg/dL for 1 h and 119 mg/dL for 2 h) are similar to the current therapeutic targets, it is important to keep in mind that these values are the high end of the SD range. Thus, if a woman with gestational diabetes consistently demonstrates a glucose concentration at or ∼135 mg/dL 1 h after a meal, her average value will significantly exceed the population mean of 110 mg/dL. Although observing that +2 SD values are helpful for understanding normality in a population, it is possible that targeting the mean or the +1 SD range of values will result in a lower risk of macrosomia and, more important, excess neonatal adiposity (59). Therefore, we suggest testing PP targets at +1 SD above the calculated weighted means, 122 and 110 mg/dL (120 and 110 mg/dL, rounded for clinical use) for 1 and 2 h, respectively (Fig. 1B), in women without significant risks for placental insufficiency. We also strongly recommend that recent data supporting a role for maternal lipids as a significant contributor to excess fetal growth be concurrently evaluated in such future studies (60).
CONCLUSIONS
The results of 45 years of data characterizing glycemia in normal pregnancy strongly support the need for future prospective studies that specifically test lower therapeutic PP glycemic targets for gestational diabetes, and possibly obese patients, to potentially limit a looming epidemic of fetal macrosomia.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
T.L.H. researched and analyzed data and wrote the manuscript. J.E.F. contributed to discussion and reviewed and edited the manuscript. R.E.V. contributed to discussion and data interpretation and reviewed and edited the manuscript. L.A.B. assisted in data analysis, contributed to discussion and data interpretation, and reviewed and edited the manuscript.
References
- 1.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 2.Langer O. Fetal macrosomia: etiologic factors. Clin Obstet Gynecol 2000;43:283–297 [DOI] [PubMed] [Google Scholar]
- 3.Brody SC, Harris R, Lohr K. Screening for gestational diabetes: a summary of the evidence for the U.S. Preventive Services Task Force. Obstet Gynecol 2003;101:380–392 [DOI] [PubMed] [Google Scholar]
- 4.Rogers I; EURO-BLCS Study Group The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord 2003;27:755–777 [DOI] [PubMed] [Google Scholar]
- 5.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc 2007;66:423–434 [DOI] [PubMed] [Google Scholar]
- 6.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE, Gabbe SG, Persson B, et al. ; International Association of Diabetes and Pregnancy Study Groups Consensus Panel International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss PA, Scholz HS, Haas J, Tamussino KF. Effect of fetal hyperinsulinism on oral glucose tolerance test results in patients with gestational diabetes mellitus. Am J Obstet Gynecol 2001;184:470–475 [DOI] [PubMed] [Google Scholar]
- 10.ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol 2001;98:525–538 [PubMed] [Google Scholar]
- 11.American Diabetes Association Gestational diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S88–S90 [DOI] [PubMed] [Google Scholar]
- 12.Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008;31:1060–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl. 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 14.Jovanovic L. American Diabetes Association’s Fourth International Workshop-Conference on Gestational Diabetes Mellitus: summary and discussion. Therapeutic interventions. Diabetes Care 1998;21(Suppl. 2):B131–B137 [PubMed] [Google Scholar]
- 15.Mari A, Ferrannini E. Beta-cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab 2008;10(Suppl. 4):77–87 [DOI] [PubMed] [Google Scholar]
- 16.Gillmer MD, Beard RW, Brooke FM, Oakley NW. Carbohydrate metabolism in pregnancy. Part I. Diurnal plasma glucose profile in normal and diabetic women. BMJ 1975;3:399–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis SB, Wallin JD, Kuzuya H, et al. Circadian variation of serum glucose, C-peptide immunoreactivity and free insulin normal and insulin-treated diabetic pregnant subjects. Diabetologia 1976;12:343–350 [DOI] [PubMed] [Google Scholar]
- 18.Cousins L, Rigg L, Hollingsworth D, Brink G, Aurand J, Yen SS. The 24-hour excursion and diurnal rhythm of glucose, insulin, and C-peptide in normal pregnancy. Am J Obstet Gynecol 1980;136:483–488 [DOI] [PubMed] [Google Scholar]
- 19.Metzger BE, Phelps RL, Freinkel N, Navickas IA. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care 1980;3:402–409 [DOI] [PubMed] [Google Scholar]
- 20.Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am J Obstet Gynecol 1981;140:730–736 [PubMed] [Google Scholar]
- 21.Parretti E, Mecacci F, Papini M, et al. Third-trimester maternal glucose levels from diurnal profiles in nondiabetic pregnancies: correlation with sonographic parameters of fetal growth. Diabetes Care 2001;24:1319–1323 [DOI] [PubMed] [Google Scholar]
- 22.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol 2004;191:949–953 [DOI] [PubMed] [Google Scholar]
- 23.Porter H, Lookinland S, Belfort MA. Evaluation of a new real-time blood continuous glucose monitoring system in pregnant women without gestational diabetes. A pilot study. J Perinat Neonatal Nurs 2004;18:93–102 [DOI] [PubMed] [Google Scholar]
- 24.Bühling KJ, Winkel T, Wolf C, et al. Optimal timing for postprandial glucose measurement in pregnant women with diabetes and a non-diabetic pregnant population evaluated by the Continuous Glucose Monitoring System (CGMS). J Perinat Med 2005;33:125–131 [DOI] [PubMed] [Google Scholar]
- 25.Bühling KJ, Kurzidim B, Wolf C, et al. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycemia by comparing the self-monitoring of blood glucose (SMBG) in non-pregnant women and in pregnant women with impaired glucose tolerance and gestational diabetes. Exp Clin Endocrinol Diabetes 2004;112:556–560 [DOI] [PubMed] [Google Scholar]
- 26.Cypryk K, Pertyńska-Marczewska M, Szymczak W, Wilcyński J, Lewiński A. Evaluation of metabolic control in women with gestational diabetes mellitus by the continuous glucose monitoring system: a pilot study. Endocr Pract 2006;12:245–250 [DOI] [PubMed] [Google Scholar]
- 27.Siegmund T, Rad NT, Ritterath C, Siebert G, Henrich W, Buhling KJ. Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur J Obstet Gynecol Reprod Biol 2008;139:46–52 [DOI] [PubMed] [Google Scholar]
- 28.Hod M, Yogev Y. Goals of metabolic management of gestational diabetes: is it all about the sugar? Diabetes Care 2007;30(Suppl. 2):S180–S187 [DOI] [PubMed] [Google Scholar]
- 29.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000;71(Suppl.):1256S–1261S [DOI] [PubMed] [Google Scholar]
- 30.Watson WJ. Serial changes in the 50-g oral glucose test in pregnancy: implications for screening. Obstet Gynecol 1989;74:40–43 [PubMed] [Google Scholar]
- 31.D’Orazio P, Burnett RW, Fogh-Andersen N, et al. ; International Federation of Clinical Chemistry Scientific Division Working Group on Selective Electrodes and Point of Care Testing Approved IFCC recommendation on reporting results for blood glucose (abbreviated). Clin Chem 2005;51:1573–1576 [DOI] [PubMed] [Google Scholar]
- 32.Consensus statement on self-monitoring of blood glucose. Diabetes Care 1987;10:95–99 [PubMed] [Google Scholar]
- 33.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436–472 [PubMed] [Google Scholar]
- 34.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 35.Yogev Y, Chen R, Ben-Haroush A, Phillip M, Jovanovic L, Hod M. Continuous glucose monitoring for the evaluation of gravid women with type 1 diabetes mellitus. Obstet Gynecol 2003;101:633–638 [DOI] [PubMed] [Google Scholar]
- 36.Solnica B, Naskalski JW. Quality control of SMBG in clinical practice. Scand J Clin Lab Invest Suppl 2005;240:80–85 [DOI] [PubMed] [Google Scholar]
- 37.Bleicher SJ, O’Sullivan JB, Freinkel N. Carbohydrate metabolism in pregnancy. V. The interrelations of glucose, insulin and free fatty acids in late pregnancy and post partum. N Engl J Med 1964;271:866–872 [DOI] [PubMed] [Google Scholar]
- 38.Chen R, Yogev Y, Ben-Haroush A, Jovanovic L, Hod M, Phillip M. Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2003;14:256–260 [DOI] [PubMed] [Google Scholar]
- 39.Ben-Haroush A, Yogev Y, Chen R, Rosenn B, Hod M, Langer O. The postprandial glucose profile in the diabetic pregnancy. Am J Obstet Gynecol 2004;191:576–581 [DOI] [PubMed] [Google Scholar]
- 40.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med 2000;9:83–88 [DOI] [PubMed] [Google Scholar]
- 41.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 1995;18:611–617 [DOI] [PubMed] [Google Scholar]
- 42.Willman SP, Leveno KJ, Guzick DS, Williams ML, Whalley PJ. Glucose threshold for macrosomia in pregnancy complicated by diabetes. Am J Obstet Gynecol 1986;154:470–475 [DOI] [PubMed] [Google Scholar]
- 43.Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care 1992;15:1251–1257 [DOI] [PubMed] [Google Scholar]
- 44.de Veciana M, Major CA, Morgan MA, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995;333:1237–1241 [DOI] [PubMed] [Google Scholar]
- 45.Jovanovic-Peterson L, Peterson CM, Reed GF, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development—Diabetes in Early Pregnancy Study. Am J Obstet Gynecol 1991;164:103–111 [DOI] [PubMed] [Google Scholar]
- 46.Parfitt VJ, Clark JD, Turner GM, Hartog M. Maternal postprandial blood glucose levels influence infant birth weight in diabetic pregnancy. Diabetes Res 1992;19:133–135 [PubMed] [Google Scholar]
- 47.Demarini S, Mimouni F, Tsang RC, Khoury J, Hertzberg V. Impact of metabolic control of diabetes during pregnancy on neonatal hypocalcemia: a randomized study. Obstet Gynecol 1994;83:918–922 [DOI] [PubMed] [Google Scholar]
- 48.Landon MB, Spong CY, Thom E, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sivan E, Weisz B, Homko CJ, Reece EA, Schiff E. One or two hours postprandial glucose measurements: are they the same? Am J Obstet Gynecol 2001;185:604–607 [DOI] [PubMed] [Google Scholar]
- 50.Weisz B, Shrim A, Homko CJ, Schiff E, Epstein GS, Sivan E. One hour versus two hours postprandial glucose measurement in gestational diabetes: a prospective study. J Perinatol 2005;25:241–244 [DOI] [PubMed] [Google Scholar]
- 51.Metzger BE, Coustan DR; The Organizing Committee Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21(Suppl. 2):B161–B167 [PubMed] [Google Scholar]
- 52.Langer O, Mazze R. The relationship between large-for-gestational-age infants and glycemic control in women with gestational diabetes. Am J Obstet Gynecol 1988;159:1478–1483 [DOI] [PubMed] [Google Scholar]
- 53.Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus—how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol 1989;161:646–653 [DOI] [PubMed] [Google Scholar]
- 54.Kjos SL, Schaefer-Graf U, Sardesi S, et al. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care 2001;24:1904–1910 [DOI] [PubMed] [Google Scholar]
- 55.Howarth C, Gazis A, James D. Associations of type 1 diabetes mellitus, maternal vascular disease and complications of pregnancy. Diabet Med 2007;24:1229–1234 [DOI] [PubMed] [Google Scholar]
- 56.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbour LA, Hernandez TL, Reece MS, et al. Change in fasting triglycerides from early to late gestation are highly predictive of neonatal adiposity independent of maternal BMI (Abstract). Diabetes 2009;58(Suppl. 1):A84 [Google Scholar]
- 58.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol 2007;109:419–433 [DOI] [PubMed] [Google Scholar]
- 59.HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catalano PM, Hauguel-De MS. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2 February 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed]