Abstract
Objective
Private cord blood banks are for-profit companies that facilitate storage of umbilical cord blood for personal or family use. Pediatric hematopoietic cell transplantation (HCT) physicians are currently best situated to use cord blood therapeutically. We sought to describe the experiences and views of these physicians regarding private cord blood banking.
Participants and Methods
Emailed cross-sectional survey of pediatric HCT physicians in the United States and Canada. 93/152 potentially eligible physicians (93/130 confirmed survey recipients) from 57 centers responded. Questions addressed the number of transplants performed using privately banked cord blood, willingness to use banked autologous cord blood in specific clinical settings, and recommendations to parents regarding private cord blood banking.
Results
Respondents reported having performed 9 autologous and 41 allogeneic transplants using privately banked cord blood. In 36/40 allogeneic cases for which data were available, the cord blood had been collected because of a known indication in the recipient. Few respondents would choose autologous cord blood over alternative stem cell sources for treatment of acute lymphoblastic leukemia in second remission. In contrast, 55% would choose autologous cord blood to treat high-risk neuroblastoma, or to treat severe aplastic anemia in the absence of an available sibling donor. No respondent would recommend private cord blood banking for a newborn with one healthy sibling when both parents were of Northern European descent; 11% would recommend banking when parents were of different minority ethnicities.
Conclusions
Few transplants have been performed using cord blood stored in the absence of a known indication in the recipient. Willingness to use banked autologous cord blood varies depending on disease and availability of alternative stem cell sources. Few pediatric HCT physicians endorse private cord blood banking in the absence of an identified recipient, even for mixed-ethnicity children for whom finding a suitably matched unrelated donor may be difficult.
Keywords: Hematopoietic Stem Cell Transplantation, Cord Blood Stem Cell Transplantation, Bioethics
Introduction
Private cord blood banks are for-profit companies that facilitate the collection and storage of umbilical cord blood for possible future use by the child from whom it was obtained, or by a member of the child’s immediate family. Initial collection and shipping fees are typically between $1500 and $2000, while annual storage fees typically range from $90 to $200.(1, 2) These fees are not generally reimbursed by health insurers.
Private cord blood banks advertise widely to the public and to physicians, and have established a presence in many obstetrical waiting rooms.(3) In their promotional materials, they indicate that stored cord blood may serve as a stem cell source for autologous or allogeneic hematopoietic cell transplantation (HCT). There are few published reports of transplants using privately banked autologous cord blood.(4–6) Banks also suggest that these cells might one day be modified through gene transfer or targeted differentiation for use in treating a host of degenerative disorders, such as Alzheimer’s disease, Parkinson’s disease and ischemic heart disease.(7, 8) While the use of banked cord blood for HCT involves existing technologies, the use of these cells for treating degenerative disorders is currently speculative.(9)
Families may choose to store cord blood in a private bank for prophylactic or preemptive reasons. When banked prophylactically (i.e., in the absence of a foreseeable indication for HCT in the family), the cord blood is perceived as a form of “biological insurance.” When banked preemptively, a family member is known to have, or to be at increased risk for, a potentially transplantable disease. In 1997, the American College of Obstetrics and Gynecology stated, “Parents should not be sold this service without a realistic assessment of their likely return on their investment.”(10) Subsequently, in 1999, the American Academy of Pediatrics (AAP) recommended that, “given the difficulty of making an accurate estimate of the need for autologous transplantation and the ready availability of allogeneic transplantation, private storage of cord blood as ‘biological insurance’ is unwise. However, banking should be considered if there is a family member with a current or potential need to undergo a stem cell transplantation."(11) The AAP reaffirmed this view in 2007, and the Council on Ethical and Judicial Affairs of the American Medical Association (AMA) and the American Society for Blood and Marrow Transplantation recently adopted similar positions.(12–15) Despite these recommendations, private cord blood banks have grown apace.(16–19)
Parents and clinicians need guidance about whether or not to consider or recommend private cord blood banking. Because HCT physicians choose among stem cell sources for transplantation, and because they are the only clinicians who might currently use these privately banked cord blood units therapeutically, their views about private cord blood banking are of particular interest. Furthermore, because of the young age of most potential recipients, decisions about use of privately banked autologous or allogeneic cord blood versus other available stem cell sources are most germane to pediatric transplant physicians. We therefore surveyed pediatric HCT physicians in the United States and Canada to determine: 1) their clinical experience with HCT using privately banked cord blood; 2) the circumstances in which they might consider using autologous cord blood as a stem cell source; and 3) how they would advise prospective parents about prophylactic cord blood banking.
Participants and Methods
Study Population
Because no comprehensive list of pediatric HCT physicians exists, we used the membership and subcommittee lists of the Center for International Blood and Marrow Transplant Research (CIBMTR) to identify prospective subjects. The CIBMTR was established in 2004 as a formal affiliation of the research division of the National Marrow Donor Program (NMDP, established in 1986) and the International Bone Marrow Transplant Registry (established in 1972). The CIBMTR is a working group of over 500 transplant centers worldwide that voluntarily contribute detailed patient-, disease- and, transplant-characteristics and outcome data on allogeneic HCT recipients to a Statistical Center at the Medical College of Wisconsin. In addition to the collection of demographic, disease and transplant characteristics, and outcome data on consecutive transplants facilitated by participating institutions, the CIBMTR maintains a list of transplant center directors and other physicians who are active participants in research activities of the CIBMTR.
Physicians were eligible if they were currently performing pediatric HCT in the United States (U.S.) or Canada. The list of U.S. and Canadian transplant physicians was generated from the membership directory for the following subcommittees of the CIBMTR: pediatric cancer, non-malignant marrow disorder and immune and metabolic diseases. These committees were chosen because most of their active participants are pediatric HCT physicians. Review of the membership directory for the above committees suggests that approximately one-third of transplant centers are represented by more than one physician per center.
The survey was mailed to 162 individuals. Of these, 10 were ineligible because they were not currently practicing pediatric HCT physicians, and 22 could not be reached due to change of address or inaccurate contact information. Thus 130 eligible physicians were confirmed to have received the survey. Of these, 93 responded, representing 57 centers (response rate 61% of 152 potentially eligible physicians, or 72% of 130 confirmed survey recipients).
The study was approved by the Institutional Review Board at the Dana-Farber Cancer Institute, which waived the requirement for documentation of informed consent.
Data Collection
After pilot-testing among 10 physicians at the investigators’ centers, surveys were distributed via email in June 2004. Non-respondents received one further email, one fax, and one phone call from a study team member at 2-week intervals. The survey consisted of 8 groups of questions addressing 5 broad domains: A) demographics; B) frequency of requests for advice about private cord blood banking; C) experience using privately banked cord blood for HCT; D) willingness to use privately banked autologous cord blood to treat three diseases that are among the most common indications for pediatric HCT; and E) advice to parents regarding prophylactic cord blood banking.
Statistical Methods
Data were analyzed using Stata 8 for Windows (Stata Corp., College Station, TX). Analyses were descriptive. We report results as proportions or as medians, ranges and interquartile ranges (IQRs), as appropriate to the data.
Results
Respondent Characteristics
Forty-eight respondents (52%) were program heads or clinical directors of transplant services. Respondents’ centers performed a median of 3.5 (IQR 1.5–6.5, range 0–78) unrelated-donor cord blood transplants per year. Respondents reported being asked for advice about private cord blood banking a median of 9.5 times (IQR 4.5–15, range 0–60) by prospective parents, and 5 times (IQR 2–10, range 0–200) by clinicians, during the previous 2 years.
Clinical Experience With Privately Banked Cord Blood
Respondents from 6 centers reported having performed a total of 9 autologous cord blood transplants. Indications included severe aplastic anemia (SAA; n=4), neuroblastoma (n=1), retinoblastoma (n=1), Shwachman-Diamond syndrome (after failure of an allogeneic transplant, n=1), brain tumor (n=1) and not stated (n=1) (Table 1). None of these grafts was supplemented with postnatally obtained peripheral blood stem cells (PBSC) or bone marrow. Primary failure of engraftment occurred after one of these transplants.
Table 1.
Hematopoietic cell transplants performed at respondents’ centers using privately banked cord blood
| Family-banked cord blood transplant type | Disease indication for transplant | Number of transplants |
|---|---|---|
| Autologous* | N = 9 | |
| Severe aplastic anemia | 4 | |
| Neuroblastoma | 1 | |
| Retinoblastoma | 1 | |
| Shwachman-Diamond syndrome† | 1 | |
| Brain tumor | 1 | |
| Diagnosis not stated | 1 | |
| Allogeneic‡ | N = 41 | |
| Acute leukemia | 20 | |
| Hemoglobinopathy | 7 | |
| Fanconi anemia | 7 | |
| Other§ | 7 |
9 autologous transplants were reported from 6 centers. Because of possible double-counting due to multiple respondents per center, between 7 and 9 autologous transplants were actually performed.
Patient with Shwachman-Diamond syndrome received autologous cord blood after primary failure of an allogeneic graft
41 allogeneic transplants were reported from 16 centers. Because of possible double-counting due to multiple respondents per center, between 36 and 41 allogeneic transplants were actually performed.
Other diagnoses: juvenile myelomonocytic leukemia (1), severe aplastic anemia (1), Wiskott-Aldrich syndrome (1), chronic granulomatous disease (1), hemophagocytic lymphohistiocytosis (1), Gaucher disease (1), and diagnosis not stated (1)
Respondents from 15 centers reported having performed 41 allogeneic privately banked cord blood transplants (one respondent did not identify the center). Indications included acute leukemias (n=20), hemoglobinopathies (n=7), Fanconi anemia (n=7) and other/not specified (n=7) (Table 1). The cord blood was derived from siblings in all but one case (a cousin). In 36/40 cases for which data were available, the cord blood had been collected in light of a known disease in the index patient (i.e., preemptively). Allogeneic bone marrow harvested postnatally was co-administered in 6/41 transplants. Primary failure of engraftment occurred in 7/41 cases.
Perceived Utility Of Privately Banked Autologous Cord Blood For HCT
To explore subjects’ willingness to use privately banked cord blood for pediatric HCT, we asked them to consider a hypothetical five-year-old child whose cord blood (with adequate cellularity) was banked prophylactically at birth and who subsequently developed a potentially transplantable illness.
We first asked about a child with acute lymphoblastic leukemia (ALL) in second remission after experiencing a bone marrow relapse while on initial therapy. No respondent would choose the autologous cord blood over a human leukocyte antigen-(HLA-) matched sibling donor (MSD) graft, and only 6% would choose the autologous cord blood over a suitably matched and cellular unrelated-donor (URD) marrow or cord blood graft. If no MSD or URD graft were available, 62% would use the autologous cord blood rather than pursue other therapy (Table 2).
Table 2.
Pediatric hematopoietic stem cell transplant physicians’ willingness to use banked autologous cord blood
| Disease* | Alternative stem cell source available | Respondent would perform transplant using banked autologous cord blood
|
||
|---|---|---|---|---|
| Yes Number (%) | No Number (%) | Combined† Number (%) | ||
| Acute lymphoblastic leukemia in 2nd remission after on-therapy relapse | ||||
| Matched sibling | 0 (0%) | 91 (100%) | N/A‡ | |
| Unrelated bone marrow or cord blood | 5 (6%) | 85 (94%) | N/A | |
| No suitable allogeneic stem cell source available | 55 (62%) | 34 (38%) | N/A | |
|
| ||||
| Severe aplastic anemia | ||||
| Newly diagnosed | Matched sibling | 25 (28%) | 65 (72%) | N/A |
| Unsuccessful immunosuppressive therapy | Unrelated bone marrow or cord blood | 47 (55%) | 38 (45%) | N/A |
|
| ||||
| High-risk neuroblastoma | ||||
| Adequate autologous cord blood cell dose | Autologous PBSC§ or bone marrow | 50 (55%) | 38 (42%) | 3 (3%) |
| Inadequate autologous cord blood cell dose ** | Autologous PBSC or bone marrow | 7 (8%) | 61 (67%) | 23 (25%) |
Respondents were asked to indicate their preferred stem cell source for a hypothetical 5 year old child whose cord blood had been stored prophylactically at birth (with adequate cell dose of 4 × 107 nucleated cells/kg recipient weight). The child now presents with the specified potentially transplantable disease.
Would choose autologous cord blood combined with available alternative stem cell source
N/A, option not offered
PBSC, peripheral blood stem cells
<2 × 107 nucleated cells/kg recipient weight
To treat a child with newly diagnosed severe aplastic anemia (SAA) and an available MSD, 28% would choose the autologous cord blood over the MSD graft. To treat a child with SAA refractory to immunosuppressive therapy in the absence of an available MSD, 55% would choose the autologous cord blood instead of an URD marrow or cord blood graft (Table 2).
To treat high-risk neuroblastoma, 55% would choose the autologous cord blood alone, 42% would choose autologous PBSC or marrow alone, and 3% would combine the autologous cord blood with autologous PBSC or marrow. If the cellularity of the unit was suboptimal, willingness to use the autologous cord blood diminished (Table 2).
Advice Regarding Prophylactic Private Cord Blood Banking
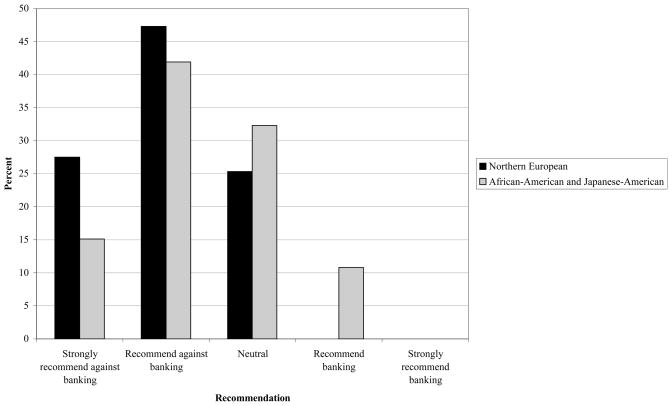
We asked physicians whether they would recommend private cord blood banking for a newborn with one healthy 3-year-old sibling. When both parents were of Northern European descent, no respondent would recommend banking. When parents were of mixed ethnicity (African-American father and Japanese-American mother), 11% would recommend banking (Figure).
Figure. Pediatric hematopoietic stem cell transplant physicians’ recommendations regarding prophylactic private cord blood banking.
“Parents are expecting their second child. Their first child is a healthy three-year-old. They ask your advice about private cord blood banking. What would you recommend?” In the first scenario, both parents are of Northern European origin. In the second scenario, one parent is African-American and the other is Japanese-American.
Discussion
Pediatric HCT physicians are currently the main group of clinicians who might employ privately banked cord blood in clinical care. They are often asked by families and by other clinicians for advice regarding prophylactic cord blood banking. We surveyed these physicians about their prior use of and views on privately banked cord blood. We found limited experience with and enthusiasm for prophylactic private cord blood banking among respondents.
Several observations from this survey stand out. First, despite the fact that cord blood banked prophylactically surely accounts for the preponderance of units stored in private cord blood banks, transplants using cord blood banked preemptively have substantially outnumbered transplants using cord blood banked prophylactically. Second, allogeneic transplants using privately banked cord blood have substantially outnumbered autologous transplants. In the allogeneic setting, because the sibling typically can serve postnatally as a hematopoietic stem cell donor, the transplant can generally proceed even if cord blood was not banked. Third, very few pediatric transplant physicians would recommend prophylactic cord blood banking to prospective parents. Importantly, willingness to recommend increases only marginally when the parents are of different minority ethnic groups, a circumstance that reduces the likelihood of finding a suitably matched unrelated-donor stem cell source should the need for HCT arise.(20)
Respondents were reluctant to use autologous cord blood to transplant a child with ALL, the most common malignancy of childhood and the most common indication for allogeneic HCT in children in North America (unpublished data, CIBMTR). This reluctance may be attributed to the established importance of an allogeneic “graft-versus-leukemia” effect for survival after HCT for ALL,(21) or to the observation that leukemic clone-specific molecular genetic markers may be present at birth in children who subsequently develop ALL.(22) The same considerations would likely affect clinical decision-making for pediatric acute myeloid leukemia.(23) The one circumstance in which a majority of respondents indicated willingness to use stored autologous cord blood to treat relapsed ALL is the child who lacks a suitable MSD or URD stem cell source. However, the likelihood that a healthy newborn will ultimately face such a situation is remote. First, approximately 25% of children who require an allogeneic HCT have an HLA-matched sibling.(12) For those who lack a matched sibling, availability of a suitable unrelated-donor graft varies according to ethnicity, with a match identified for approximately 85% of Caucasians and 60% of African-Americans.(20) Given the annual incidence of ALL in children aged 0–19 years (30.6/million for Caucasians and 15.9/million for African-Americans),(24) and relapse and re-induction rates of approximately 20% and 90%, respectively,(25, 26) the probabilities of a healthy newborn 1) developing ALL during childhood, 2) experiencing a relapse, 3) achieving a second remission, and 4) being unable to find a suitable allogeneic donor are approximately 12.4/million for Caucasians and 17.2/million for African-Americans.
Severe aplastic anemia, with an annual incidence for all ages combined of approximately 3/million,(27) is the most common non-malignant indication for allogeneic HCT in childhood (unpublished data, CIBMTR). Currently, MSD HCT is indicated for children with newly diagnosed SAA, whereas URD HCT is reserved for those with an inadequate response to immunosuppressive therapy.(28) Historically, survival has been approximately 90% after MSD HCT and 50–60% after URD HCT.(29, 30) The poorer reported outcomes after URD HCT likely underlie the willingness of 55% of respondents to use the autologous cord blood rather than an URD graft for transplantation. More recent reports, however, demonstrate improved survival after URD HCT for SAA.(31) Other factors, such as the absence of benefit related to graft-versus-tumor effect in SAA, may also contribute to respondents’ willingness to consider banked autologous cord blood as a stem cell source for treatment.
High-risk neuroblastoma, with an annual incidence of approximately 3–5/million children under 15 years of age in the United States,(32, 33) is the most common indication for autologous HCT in children (unpublished data, CIBMTR). Molecularly detectable contamination of the autologous stem cell graft with neuroblastoma cells is frequent,(34) and has been shown to contribute to relapse post-transplant.(35) The chance of graft contamination is likely lower with cord blood collected at birth, prior to the clinical diagnosis though not necessarily the development of neuroblastoma. Hence a majority (55%) of respondents said they would choose the autologous cord blood as a graft in this scenario. However, this majority decision was dependent on adequate cellularity of the stored cord blood unit, a variable affected by the skill of the personnel collecting and storing the cord blood as well as by other factors.(36, 37)
This study has several limitations. First, there is no comprehensive list of pediatric HCT physicians in the United States and Canada. The sampling frame for this survey included 152 pediatric HCT physicians who were listed with the CIBMTR. We believe this population to be representative of pediatric HCT physicians in these countries. Indeed, the preponderance of program leaders among respondents strengthens our confidence in our findings, both because these physicians are best able to report their prior institutional experience and because they are likely to make decisions regarding stem cell source in unusual clinical circumstances. Second, our findings might have been influenced by response bias if views on private banking of cord blood or on therapeutic use of autologous cord blood differed between respondents and non-respondents. Third, it is possible that additional transplants using privately banked cord blood have been performed, either by non-respondents to our survey, by physicians who were not included in our sample, or since the survey was fielded. However, these estimates are commensurate with those provided by cord blood banks themselves in their promotional materials.(17, 38) In addition, because several physicians responded from some centers, it is possible that we double-counted several transplants. Had we discounted transplants with the same type of graft and the same disease indication as one already reported by another respondent from the same center, there would have been a total of 7 autologous and 36 allogeneic transplants reported. Fourth, our data do not directly address the value of prophylactically stored cord blood for stem cell transplantation among adults. Finally, while pediatric HCT physicians are uniquely qualified to address the clinical experience with and utility of privately banked cord blood, their opinions concerning how prospective parents should spend their private funds should not be considered determinative. Nevertheless, the fact that pediatric HCT physicians are frequently asked for their advice regarding storage of cord blood suggests that their views on this issue are valued by parents and other physicians alike.
Although we did not ask HCT physicians about their views regarding public cord blood banking, there is growing recognition that unrelated-donor cord blood obtained from public banks represents an invaluable stem cell source for both pediatric and adult patients in need of transplantation.(39–41) Cord blood units are particularly useful for patients from racial and ethnic minority groups, due to the less stringent requirements for HLA matching with the use of cord blood as a stem cell source as compared with marrow from unrelated adult donors.(20) In recognition of the value of unrelated-donor cord blood, the U.S. Congress has funded a National Cord Blood Inventory Program, with plans to build an inventory of 150,000 cord blood units.(42) This source of funding, which supplements income derived from releasing cord blood units to recipients, is nevertheless inadequate to cover the full costs of maintaining a cord blood bank.(39) The role of alternative models of supporting public cord blood banks, including joint public-private banks,(43) remains to be clarified.
Conclusion
The debate about the utility of private cord blood banks is ongoing.(9, 12, 13, 19, 43–51) Numerous issues raised by private cord blood banking, including the vulnerability of expectant parents to the private banks’ marketing efforts, the accuracy of the information available to parents, the quality of parents’ understanding when deciding about banking,(52, 53) and the potential for competition with public cord blood banks that facilitate unrelated-donor transplantation,(54) warrant attention and concern. The data presented here suggest that clinicians who perform pediatric HCT endorse the recommendations of the AAP, the AMA and others against the private storage of cord blood in the absence of a foreseeable indication for transplant in the family of the newborn child.(10–15, 44, 48) Pediatricians, family physicians, obstetricians, nurse midwives and other professionals who work with families, together with their professional organizations, should educate prospective parents about this consensus view.
Acknowledgments
We thank our colleagues for taking the time to complete the survey, and gratefully acknowledge Robilyn Lake’s assistance with survey distribution from the CIBMTR, and Tina Gelsomino’s assistance with database design.
No external funding was obtained for the study reported here.
Abbreviations
- HCT
hematopoietic cell transplantation
- AAP
American Academy of Pediatrics
- AMA
American Medical Association
- CIBMTR
Center for International Blood and Marrow Transplant Research
- NMDP
National Marrow Donor Program
- IQR
interquartile range
- SAA
severe aplastic anemia
- PBSC
peripheral blood stem cells
- MSD
matched sibling donor
- URD
unrelated donor
- ALL
acute lymphoblastic leukemia
- HLA
human leukocyte antigen
Footnotes
Financial disclosures and conflict of interest:
The authors have no financial conflicts of interest. Dr. Eapen is the Associate Scientific Director, CIBMTR, and Scientific Director of the Pediatric Cancer, Immune and Metabolic Disease, Non-malignant Marrow Disease, and Graft Sources and Manipulation Committees of the CIBMTR. Dr. Lee is the Scientific Director of the Immunobiology Committee, CIBMTR, and Co-Chair of the Health Policy Working Committee, CIBMTR. Dr. Davies is a member of the Board and of the Executive Committee of the NMDP, Chair-elect of the CIBMTR Advisory Committee, and a member of the CIBMTR Executive Committee.
References
- 1.Cord Blood Registry. [Accessed June 5, 2008];Cord Blood Registry: our pricing. http://www.cordblood.com/cord_blood_banking_with_cbr/pricing_domestic.asp.
- 2.Viacord. [Accessed June 5, 2008];Viacord pricing and storage plans. http://www.viacord.com/pricing-storage-plans.htm.
- 3.Moise KJ. What to tell patients about banking cord blood stem cells. Contemporary OB/GYN. 2006 April 15;:42. [Google Scholar]
- 4.Ferreira E, Pasternak J, Bacal N, de Campos Guerra JC, Mitie Watanabe F. Autologous cord blood transplantation. Bone Marrow Transplant. 1999;24:1041. doi: 10.1038/sj.bmt.1702017. [DOI] [PubMed] [Google Scholar]
- 5.Hayani A, Lampeter E, Viswanatha D, Morgan D, Salvi SN. First report of autologous cord blood transplantation in the treatment of a child with leukemia. Pediatrics. 2007;119:e296–300. doi: 10.1542/peds.2006-1009. [DOI] [PubMed] [Google Scholar]
- 6.Fruchtman SM, Hurlet A, Dracker R, et al. The successful treatment of severe aplastic anemia with autologous cord blood transplantation. Biol Blood Marrow Transplant. 2004;10:741–2. doi: 10.1016/j.bbmt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Cord Blood Registry. [Accessed June 5, 2008];Why you should save cord blood for your family. http://www.cordblood.com/cord_blood_banking_with_cbr/banking/why_bank.asp.
- 8.Viacord. [Accessed June 5, 2008];Using stem cells to potentially treat heart disease. http://www.viacord.com/stem-cells-heart-disease.htm.
- 9.Healy M. The potential of stem cells' curative powers has spawned the creation of private tissue banks. But marketing is outpacing the medicine. Los Angeles Times. 2007 March 5;Sect 1 [Google Scholar]
- 10.Committee on Obstetric Practice ACoOaG. Routine storage of umbilical cord blood for potential future transplantation. Int J Gynaecol Obstet. 1997;58:257–9. [PubMed] [Google Scholar]
- 11.Cord blood banking for potential future transplantation: subject review. American Academy of Pediatrics. Work Group on Cord Blood Banking. Pediatrics. 1999;104:116–8. doi: 10.1542/peds.104.1.116. [DOI] [PubMed] [Google Scholar]
- 12.Lubin BH, Shearer WT. Cord blood banking for potential future transplantation. Pediatrics. 2007;119:165–70. doi: 10.1542/peds.2006-2901. [DOI] [PubMed] [Google Scholar]
- 13.Council on Ethical and Judicial Affairs of the American Medical Association. CEJA Report 9-I-07: Umbilical Cord Blood Banking (Recommendation E-2.165) Chicago: IL: [Accessed June 5, 2008]. 2007. Available at http://www.ama-assn.org/ama1/pub/upload/mm/369/ceja_9i07.pdf. [Google Scholar]
- 14.Ballen KK, Barker JN, Stewart SK, Greene MF, Lane TA. Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant. 2008;14:356–63. doi: 10.1016/j.bbmt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant. 2008;14:364. doi: 10.1016/j.bbmt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Grady D. The hype, and hope, of cord blood. New York Times. 1998 December 1;Sect 6 [Google Scholar]
- 17.Cord Blood Registry. Why choose Cord Blood Registry? [Accessed June 5, 2008];Most experienced. http://www.cordblood.com/cord_blood_banking_with_cbr/why_cbr/most_experienced.asp.
- 18.Viacord. [Accessed June 5, 2008];FAQ: How many families have banked samples with Viacord? http://www.viacord.com/general-faq.htm.
- 19.Neergaard L. Parents bank kids' umbilical cord blood. Associated Press Online. 2007 February 19; [Google Scholar]
- 20.Chell JW. Hematopoietic cell donor registries. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. 3. Malden, MA: Blackwell Publishing; 2004. pp. 624–631. [Google Scholar]
- 21.Champlin R, Giralt S, Gajewski J. T cells, graft-versus-host disease and graft-versus-leukemia: innovative approaches for blood and marrow transplantation. Acta Haematol. 1996;95:157–63. doi: 10.1159/000203871. [DOI] [PubMed] [Google Scholar]
- 22.Gale KB, Ford AM, Repp R, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci U S A. 1997;94:13950–4. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiemels JL, Xiao Z, Buffler PA, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99:3801–5. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 24.Gurney JG, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 1–14. [Google Scholar]
- 25.Silverman LB, Declerck L, Gelber RD, et al. Results of Dana-Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981–1995) Leukemia. 2000;14:2247–56. doi: 10.1038/sj.leu.2401980. [DOI] [PubMed] [Google Scholar]
- 26.Henze G, Fengler R, Hartmann R, et al. Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85). A relapse study of the BFM group. Blood. 1991;78:1166–72. [PubMed] [Google Scholar]
- 27.Szklo M, Sensenbrenner L, Markowitz J, Weida S, Warm S, Linet M. Incidence of aplastic anemia in metropolitan Baltimore: a population-based study. Blood. 1985;66:115–9. [PubMed] [Google Scholar]
- 28.Georges GE, Storb R. Allogeneic cell transplantation for aplastic anemia. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. 3. Malden, MA: Blackwell Publishing; 2004. pp. 981–1001. [Google Scholar]
- 29.Kojima S, Horibe K, Inaba J, et al. Long-term outcome of acquired aplastic anaemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol. 2000;111:321–8. doi: 10.1046/j.1365-2141.2000.02289.x. [DOI] [PubMed] [Google Scholar]
- 30.Margolis D, Camitta B, Pietryga D, et al. Unrelated donor bone marrow transplantation to treat severe aplastic anaemia in children and young adults. Br J Haematol. 1996;94:65–72. doi: 10.1046/j.1365-2141.1996.d01-1772.x. [DOI] [PubMed] [Google Scholar]
- 31.Kojima S, Inaba J, Yoshimi A, et al. Unrelated donor marrow transplantation in children with severe aplastic anaemia using cyclophosphamide, anti-thymocyte globulin and total body irradiation. Br J Haematol. 2001;114:706–11. doi: 10.1046/j.1365-2141.2001.02992.x. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein ML, Leclerc JM, Bunin G, et al. A population-based study of neuroblastoma incidence, survival, and mortality in North America. J Clin Oncol. 1992;10:323–9. doi: 10.1200/JCO.1992.10.2.323. [DOI] [PubMed] [Google Scholar]
- 33.Klaassen RJ, Trebo MM, Koplewitz BZ, Weitzman SS, Calderwood S. High-risk neuroblastoma in Ontario: a report of experience from 1989 to 1995. J Pediatr Hematol Oncol. 2003;25:8–13. doi: 10.1097/00043426-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Lode HN, Handgretinger R, Schuermann U, et al. Detection of neuroblastoma cells in CD34+ selected peripheral stem cells using a combination of tyrosine hydroxylase nested RT-PCR and anti-ganglioside GD2 immunocytochemistry. Eur J Cancer. 1997;33:2024–30. doi: 10.1016/s0959-8049(97)00243-8. [DOI] [PubMed] [Google Scholar]
- 35.Rill DR, Santana VM, Roberts WM, et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994;84:380–3. [PubMed] [Google Scholar]
- 36.Bayer-Zwirello LA, Hoffman DE, Adams LA, Wilder PT, Reece MT. The effect of processing and cryopreservation on nucleated umbilical cord blood cells. J Perinat Med. 2004;32:430–3. doi: 10.1515/JPM.2004.142. [DOI] [PubMed] [Google Scholar]
- 37.Hubel A, Carlquist D, Clay M, McCullough J. Liquid storage, shipment, and cryopreservation of cord blood. Transfusion. 2004;44:518–25. doi: 10.1111/j.1537-2995.2004.03238.x. [DOI] [PubMed] [Google Scholar]
- 38.Viacord. [Accessed June 5, 2008];FAQ: How many samples have been used for transplant from ViaCord? http://www.viacord.com/general-faq.htm#how%20many%20samples%20have%20been.
- 39.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 41.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–85. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 42.Health Resources and Services Administration. [Accessed June 5, 2008];Fiscal Year 2008 Justification of Estimates for Appropriations Committees; Healthcare Systems; National Cord Blood Inventory. http://www.hrsa.gov/about/budgetjustification08/NationalCordBloodInventory.htm.
- 43.Laurance J. Branson moves into biotechnology with launch of Virgin stem cell storage bank. The Independent. 2007 February 2;Sect 26 [Google Scholar]
- 44.Johnson FL. Placental blood transplantation and autologous banking--caveat emptor. J Pediatr Hematol Oncol. 1997;19:183–6. doi: 10.1097/00043426-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Sugarman J, Kaalund V, Kodish E, et al. Ethical issues in umbilical cord blood banking. Working Group on Ethical Issues in Umbilical Cord Blood Banking. JAMA. 1997;278:938–43. [PubMed] [Google Scholar]
- 46.Annas GJ. Waste and longing--the legal status of placental-blood banking. N Engl J Med. 1999;340:1521–4. doi: 10.1056/NEJM199905133401923. [DOI] [PubMed] [Google Scholar]
- 47.Burgio GR, Gluckman E, Locatelli F. Ethical reappraisal of 15 years of cord-blood transplantation. Lancet. 2003;361:250–2. doi: 10.1016/S0140-6736(03)12276-3. [DOI] [PubMed] [Google Scholar]
- 48.Fisk NM, Roberts IA, Markwald R, Mironov V. Can routine commercial cord blood banking be scientifically and ethically justified? PLoS Med. 2005;2:e44. doi: 10.1371/journal.pmed.0020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinbrook R. The cord-blood-bank controversies. N Engl J Med. 2004;351:2255–7. doi: 10.1056/NEJMp048283. [DOI] [PubMed] [Google Scholar]
- 50.Edozien LC. NHS maternity units should not encourage commercial banking of umbilical cord blood. BMJ. 2006;333:801–4. doi: 10.1136/bmj.38950.628519.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox NS, Chervenak FA, McCullough LB. Ethical considerations in umbilical cord blood banking. Obstet Gynecol. 2008;111:178–82. doi: 10.1097/01.AOG.0000295935.29407.4b. [DOI] [PubMed] [Google Scholar]
- 52.Fox NS, Stevens C, Ciubotariu R, Rubinstein P, McCullough LB, Chervenak FA. Umbilical cord blood collection: do patients really understand? J Perinat Med. 2007;35:314–21. doi: 10.1515/JPM.2007.084. [DOI] [PubMed] [Google Scholar]
- 53.Sugarman J, Kurtzberg J, Box TL, Horner RD. Optimization of informed consent for umbilical cord blood banking. Am J Obstet Gynecol. 2002;187:1642–6. doi: 10.1067/mob.2002.127307. [DOI] [PubMed] [Google Scholar]
- 54.Meyer EA, Hanna K, Gebbie K, editors. Cord Blood: Establishing a National Hematopoietic Stem Cell Bank Program. Washington, DC: National Academies Press; 2005. [Google Scholar]