SUMMARY
In the Sahel, the Anopheles gambiae complex consists of Anopheles arabiensis and the M and S molecular forms of A. gambiae sensu stricto. However, the composition of these malaria vectors varies spatially and temporally throughout the region and is thought to be linked to environmental factors such as rainfall, larval site characteristics and duration of the dry season. To examine possible physiological divergence between these taxa, we measured metabolic rates of mosquitoes during the wet season in a Sahelian village in Mali. To our knowledge, this study provides the first measurements of metabolic rates of A. gambiae and A. arabiensis in the field. The mean metabolic rate of A. arabiensis was higher than that of M-form A. gambiae when accounting for the effects of female gonotrophic status, temperature and flight activity. However, after accounting for their difference in body size, no significant difference in metabolic rate was found between these two species (whilst all other factors were found to be significant). Thus, body size may be a key character that has diverged in response to ecological differences between these two species. Alternatively, these species may display additional differences in metabolic rate only during the dry season. Overall, our results indicate that changes in behavior and feeding activity provide an effective mechanism for mosquitoes to reduce their metabolic rate, and provide insight into the possible strategies employed by aestivating individuals during the dry season. We hypothesize that female mosquitoes switch to sugar feeding while in dormancy because of elevated metabolism associated with blood digestion.
KEY WORDS: aestivation, gonotrophic cycle, malaria vector, respiration, seasonality, temperature
INTRODUCTION
Anopheles gambiae sensu lato (s.l.) consists of several morphologically cryptic species throughout sub-Saharan Africa. In the Sahel region of Mali, this complex includes Anopheles arabiensis (Patton 1905) and A. gambiae sensu stricto (s.s.) (Giles 1902), which is further divided into the M and S molecular forms; all are important malaria vectors (Coluzzi et al., 1979; Collins et al., 2001; della Torre et al., 2001; Coluzzi et al., 2002; della Torre et al., 2002). Although they are morphologically indistinguishable and can occur in sympatry (Lindsay et al., 1998; Coluzzi et al., 2002; della Torre et al., 2002; della Torre et al., 2005; Simard et al., 2009), these cryptic species often differ in abundance according to season, local rainfall, latitude and larval site characteristics (Gimnig et al., 2001; Edillo et al., 2002; Koenraadt et al., 2004; Diabaté et al., 2005; Edillo et al., 2006; Diabaté et al., 2008; Costantini et al., 2009), and thus local differentiation may occur between these taxa. Such ecological niche partitioning and adaptation to different environmental conditions may be reflected by differences in morphological, physiological and/or behavioral characters, such as diet, body size, metabolism and desiccation resistance.
In the Sahel region of Mali, West Africa, A. arabiensis and the M and S molecular forms of A. gambiae often are found together, but their relative proportions often vary seasonally and across years (Coluzzi et al., 1979; Coluzzi et al., 1985; Touré et al., 1994; Touré et al., 1998; Coluzzi et al., 2002; Edillo et al., 2002; della Torre et al., 2005; Costantini et al., 2009; Lehmann et al., 2010). Specifically, the M form of A. gambiae is the predominant species during the long dry season whereas A. arabiensis and the S form of A. gambiae are more common during the wet season but virtually disappear during the drier months (Lehmann et al., 2010; Adamou et al., 2011). Further, when the M and S forms are compared in sympatry, individuals of the M form are significantly more drought tolerant than individuals of the S form (Lee et al., 2009). In contrast to the Sahel region, studies in East Africa, where A. gambiae is only represented by the S form, have indicated that A. arabiensis is more arid tolerant than A. gambiae (Omer and Cloudsley-Thompson, 1968; Lindsay et al., 1998; Petrarca et al., 2000; Gray and Bradley, 2005). These comparisons suggest that the A. gambiae M form is more arid adapted than A. arabiensis, which is more arid adapted than the A. gambiae S form. It has been hypothesized that some populations need to be reestablished or enriched via migration each rainy season (Simard et al., 2000; Baber et al., 2010) whereas other populations may persist locally in a dormant state, known as aestivation, to survive the dry season (Holstein, 1954; Omer and Cloudsley-Thompson, 1968; Omer and Cloudsley-Thompson, 1970; Lehmann et al., 2010). However, few studies have provided concrete evidence for aestivation (Omer and Cloudsley-Thompson, 1970; Lehmann et al., 2010). Specifically, our earlier study suggests that in the Sahel, M-form A. gambiae undergo aestivation but that S-form A. gambiae and A. arabiensis may not (Lehmann et al., 2010; Adamou et al., 2011).
In addition to or as a component of aestivation, physiological and morphological differences that allow for reduced nutritional requirements, lengthening of the lifespan and/or higher desiccation resistance may explain the variation in the seasonal abundance and geographic distributions of these mosquito species. For example, aestivating mosquitoes could reduce their metabolic rate (MR), thus reducing energy consumption, caloric requirement, and desiccation stress while potentially increasing longevity. In fact, reduced MR is an important adaptation to harsh environmental climates in a number of species. For example, rodents living in desert environments exhibit reduced MR relative to their non-desert counterparts (Lovegrove, 1986). Several diapausing insects have a reduced MR as an adaptation for overwintering (e.g. Rivers and Denlinger, 1994; Denlinger, 2002; Canzano et al., 2006; Ragland et al., 2009; Ragland et al., 2010) and in at least one case, MR is reduced below that expected by temperature alone (Canzano et al., 2006).
There are several examples of studies that directly compare MR between sibling species in an evolutionary context. For example, when MR was measured under stressful environmental conditions, sibling polychaete species in the genus Capitella were shown to have slight but significant differences in MR that were attributed to the evolution of pollution tolerance in one of the incipient species. Interestingly, this difference in MR was not apparent when measured under non-stressful conditions (Gamenick et al., 1998; Linke-Gamenick et al., 2000). Tieleman et al. measured MR across several related genera of birds and found a strong relationship between native aridity and metabolic rate (Tieleman et al., 2003). In contrast, when Haim et al. measured MR across a range of photoperiods and times of the day of two sibling species of mice, one of which was arid adapted, there was not a significant difference in MR for most intervals tested (Haim et al., 2008). The authors concluded that a difference in MR was not responsible for the adaptation. Thus, the results of these and similar studies show that changes in MR can evolve as an adaptation to differing environmental conditions in certain cases.
Because the process of gas exchange is a major source of water loss (Joos et al., 1996; Lighton, 1996; Bjerke and Zachariassen, 1997; Tieleman et al., 2003), a lower MR should thus increase aridity tolerance by reducing water loss due to gas exchange; indeed, reduced resting MR has been previously proposed as a mechanism for desiccation resistance in anophelines (Gray and Bradley, 2005) and other insects (e.g. Bjerke and Zachariassen, 1997). Because M-form A. gambiae predominates (>95%) during the dry season in our study area (Lehmann et al., 2010; Adamou et al., 2011), and thus is assumed to be more arid tolerant than either the S form or A. arabiensis, we predict that M-form A. gambiae would have a lower MR than the other two taxa.
Importantly, whole-organism MR is affected by many factors, including body size, activity level, feeding or digestion status, and temperature (Lighton, 1996; Lighton, 2008; Gray and Bradley, 2003; Gray and Bradley, 2006). For example, larger individuals of a given species typically have a higher MR than smaller individuals, though larger individuals may have a lower relative MR per unit of body mass. Thus, the relationship between mass and MR is rarely truly linear across a large range of body sizes (Lighton, 1996) and is almost invariably logarithmic (Tieleman et al., 2003). Additionally, for ectotherms such as insects, body temperature is affected by the environmental temperature that the organism experiences, and increasing body temperature also increases MR (Chappell, 1983; Lighton, 1996; Bjerke and Zachariassen, 1997; Clarke and Fraser, 2004). Similarly, flight activity increases MR, as energy is used by the flight muscles (Reinhold, 1999), and flying insects are known to have the highest mass-specific MR in the animal kingdom (Joos et al., 1996; Suarez, 2000). Flying depletes circulating carbohydrate resources, requiring the insect to free up additional energy stores (Nayar and Van Handel, 1971; Clements, 1992; Foster, 1995). Additionally, previous studies on several mosquito species have shown that MR increases after blood feeding, during digestion and during egg maturation (Kurtti et al., 1979; Clements, 1992; Gray and Bradley, 2003; Gray and Bradley, 2006).
To explore physiological differences related to differences in aridity tolerance of these species, we measured metabolic rates of wild anopheline mosquitoes during the late wet season in the Sahelian village M'Piabougou, Mali. Because the MR of mosquitoes is affected by many factors, we also measured the effects of temperature, body size, flight activity, sex and female gonotrophic status in each assay. This is the first study of MR conducted in the field and utilizing wild field-collected mosquitoes.
MATERIALS AND METHODS
Study location and mosquito collections
Wild mosquitoes were collected in M'Piabougou, Mali (13.60°N, 7.19°W), a small village with approximately 1500 inhabitants, located within the southern part of the African Sahel region. Most rains fall between June and September, but pools of surface water for larval sites are available from June to November. Surface waters are not present in the vicinity of the village (up to 30 km radius) from December to May. The present study was conducted at the end of the wet season, from late October to mid-November 2009.
Indoor resting mosquitoes were collected by mouth aspiration as previously described (Lehmann et al., 2010). Mosquitoes were transferred to small paper cups and provided access to a cotton ball saturated with water for a minimum of 30 min before assays. The sex of each mosquito and the gonotrophic status of females (recently blood-fed, semi-gravid, gravid or unfed) were determined prior to the assay.
Metabolic rate measurements
Metabolic rate was measured using the constant-volume technique, following Lighton (Lighton, 2008). Metabolic chambers were constructed using 5 ml plastic syringes fitted with three-way stopcocks, each with a small hole punctured above the 5 ml mark to allow air to flow out of the syringe when flushing (see below). A small microphone was connected with a small piece of airtight plastic tubing to one port of the stopcock, allowing for sound recording of mosquito flight during the assay (see below). The other two ports allowed air to flow into and out of the syringe. For each assay, individuals were gently aspirated into six identical chambers, and one additional chamber was used as a negative control. Each set of seven chambers was simultaneously connected to a pump, which gently pushed scrubbed air into each syringe to replace the atmospheric air and any CO2 initially produced by the mosquito. The air was scrubbed through a canister containing Ascarite® and Drierite® (Sigma-Aldrich, St Louis, MO, USA) to remove CO2 and water vapor, respectively. The scrubbed air was pumped through the chambers for 3–5 min to flush out all original air, at which time the chambers were sealed by depressing the plunger to the 4 ml mark and closing the stopcock. Sound recorders were started and the assays were initiated once the stopcocks were closed, sealing the chambers from outside air. The negative control was identical to the assay chambers except that it did not contain a mosquito and sound was not recorded; this chamber was used as a baseline for the chemically scrubbed air and to control for any diffusion of outside air into the chambers during the assay.
The metabolic chambers were left undisturbed for 2–2.5 h, allowing mosquitoes to respire and CO2 to accumulate in the chambers. After the assay was complete, the syringes were individually attached to a stream of chemically scrubbed air being pumped into a FoxBox-C gas analyzer (Sable Systems International, Las Vegas, NV, USA) at a rate of 30–35 ml min–1. Once securely attached, the stopcock was opened, 3 ml of air from the chamber was injected into the gas analyzer and fractional CO2, fractional O2, flow rate and barometric pressure were recorded automatically via ExpeData software (Sable Systems International). After injection, the chamber containing the remaining 1 ml of air and the mosquito was removed, atmospheric air was pulled into the syringe and the mosquito was transferred gently out of the chamber for further analyses (see below). The control chamber without a mosquito was similarly analyzed, and provided a baseline CO2 measurement to account for any diffusion of outside air into the chambers during the assays, incomplete scrubbing of atmospheric CO2 and any CO2 introduced by the small deadspace at the tip of the syringe.
The amount of CO2 produced by the mosquito over the assay duration was calculated using ExpeData software by first adjusting the baseline of the curve to zero CO2 and then integrating the area under the curve produced during the injection of the air sample over the time of the injection using the formula VCO2=flow rate × fractional CO2 in sample (Lighton, 2008). The baseline CO2 measurement from the negative control chamber was also calculated using the above method, and subsequently subtracted from each measurement to compensate for the factors described above. The resulting total CO2 production calculated for each mosquito was scaled by a factor of 4/3 to account for the 1 ml of air remaining in the chamber with the mosquito. Finally, this value was divided by the duration of the assay in minutes and converted from ml to μl, yielding a measurement of mean μl CO2 produced per minute. Thus, for the duration of this paper, the term MR is used to refer to this measurement (mean μl CO2 min–1) per mosquito.
Flight activity analysis
Sound recordings were made during each individual metabolic assay using microphones (ME-15, Olympus America Inc., Center Valley, PA, USA) attached to portable voice recorders (VN-5200PC, Olympus America Inc.). Because mosquito flight produces a very characteristic sound signature, we were able to identify bouts of flight by analyzing the spectrogram of each assay using Audacity 3.1 (Mazzoni, 2009). Sound signatures characteristic of mosquito flight were confirmed by listening to the recording. The total time each mosquito spent flying during its metabolism assay was determined, and the proportion of time spent flying was calculated by dividing the time the mosquito spent flying by the total time the mosquito was subjected to the metabolism assay.
Mosquito species identification and body size measurement
After the metabolism assay, mosquitoes were killed and preserved in silica gel. To determine each specimen's species and molecular form identity, PCR on one to two legs from each specimen was conducted followed by a restriction enzyme digest with Hha I on the resulting PCR product using standard methods (Fanello et al., 2002). Individuals not identified conclusively were excluded from the analysis.
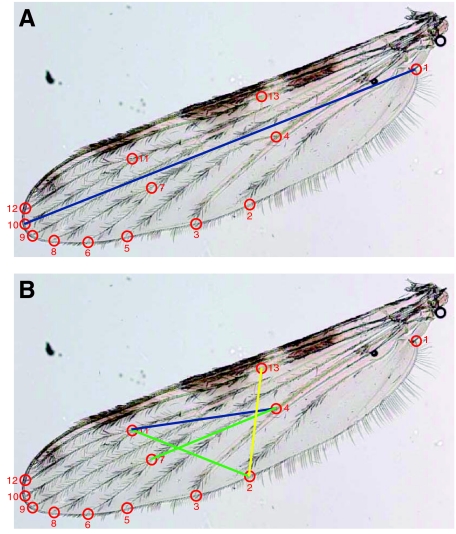
As metabolic rate depends on body size (Lighton, 1996; Schmidt-Nielsen, 1997; Gray and Bradley, 2003), we measured body size of each mosquito. Because (1) the weight of females that have taken a blood meal or are gravid likely does not reflect their true body size, (2) dry mass of female A. gambiae increases with age regardless of feeding status (Gray and Bradley, 2005) and (3) varying nutritional conditions during larval development can drastically change the allometric relationships between mosquito body mass and other parameters (Aboagye-Antwi and Tripet, 2010), we used wing length (from the alular notch to the intersection of the radius 3 vein and the outer wing margin; WL) as the primary measure of body size throughout this analysis. Both wings were removed, rehydrated in 80% ethanol and mounted under a coverslip with glycerol. Digital photographs were taken using a DM-4500B microscope with a DC-500 digital camera (Leica Microsystems, Wetzlar, Germany) at 25× magnification at a resolution of 33 dpi using Photoshop (Adobe Systems Inc., San Jose, CA, USA). For each wing, 13 specific landmarks (see Fig. A1 in Appendix 1) were mapped using the tps-DIG 2.15 software package (Rohlf, 2010). The WL measurements for right and left wings of individuals were highly correlated (R2>0.998); thus mean WL was used for further analyses for individuals with two intact wings. Only one wing was used for those with only one intact wing. Because the wings of our mosquitoes were sometimes damaged, WL was predicted for each mosquito with both wings damaged (lacking one or both points). Predicted WL was generated using a regression analysis based on distances between other wing landmarks (see Appendix 1) after stepwise regression models identified the best predictors using wings that had all 13 landmarks intact.
Fig. A1.
Anopheles gambiae s.l. wing. (A) Thirteen digitized points and straight-line wing-length measurement are used here as a measure of body size. (B) Intermediate distances used to estimate wing length for damaged wings for females (green), males (blue) and both sexes (yellow).
To test for any differences between WL and dry body mass (BM) in our models, we weighed individuals to the nearest 0.001 mg using a Cahn C-31 electrobalance (Cahn Instruments, Cerritos, CA, USA) after first removing all remaining legs and/or wings from the desiccated specimens to standardize the measurements. However, because of damage in the field and/or poor preservation, only ∼60% were intact and thus this resulting subset was used in subsequent analyses.
Statistical analyses
ANOVA models were used to identify factors that significantly affected the MR of mosquitoes and estimate the magnitude of their effects. Temperature during the assay, mosquito body size and flight activity were continuous variables whereas sex/gonotrophic status and species/form were categorical factors; all were considered fixed factors. Initially, a full model with all pairwise and third-order interactions was used, and non-significant interaction terms (P>0.05) were sequentially removed until only significant terms or main-order effects remained. Post hoc comparisons were used to test for differences between gonotrophic stages. All statistical procedures were done using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
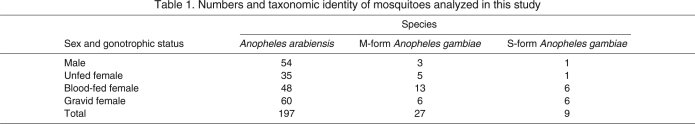
Metabolism measurements were conducted on a total of 300 mosquitoes between 25 October and 15 November 2009. Of these, 233 individuals were identified as either A. arabiensis or A. gambiae s.s. and were classified by sex and female gonotrophic status (Table 1); semi-gravid females or those with partially digested blood meals were excluded from all analyses. During the time the measurements were conducted, temperature varied between 25.1 and 32.8°C, with a mean assay temperature of 28.6°C. Mosquito WL varied from 2.79–3.64 mm for females (mean=3.23 mm) and 2.75–3.21 mm for males (mean=2.96 mm). Mean BM varied greatly between gonotrophic states (male, 0.214 mg; unfed, 0.290 mg; fed, 0.736 mg; gravid, 0.543 mg), but was not significantly different between species within each gonotrophic state (data not shown). Over half of the mosquitoes flew less than 6 s during the assay, representing less than 0.1% of the total assay time, and approximately 25% of the mosquitoes did not fly during the assay. However, four individuals flew for as much as 3–6% of the assay duration, up to approximately 8 min, although flight was not continuous.
Table 1.
Numbers and taxonomic identity of mosquitoes analyzed in this study
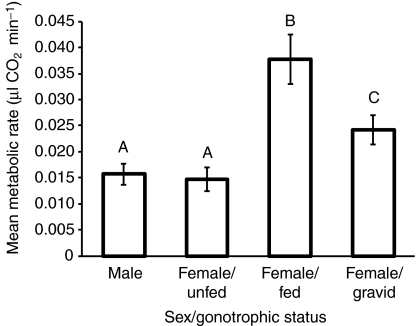
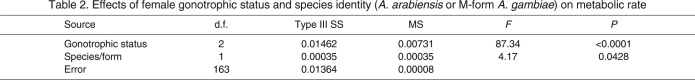
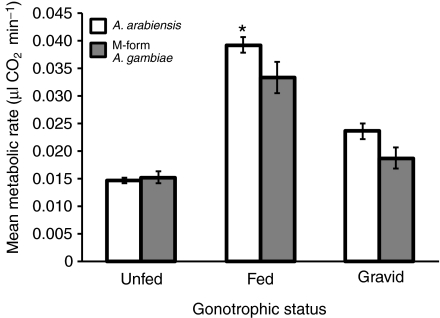
To test the hypothesis that a difference in MR has the potential to contribute to differences in arid tolerance between A. gambiae s.s. and A. arabiensis, we chose to evaluate the effect of species and molecular form on metabolic rate. However, we were unable to obtain enough males from each species, as only four of the males were A. gambiae s.s. (Table 1). Further, of the females measured, appropriate sample sizes were only obtained for A. arabiensis and M-form A. gambiae, as the S form was represented by only eight females (Table 1). Thus, we could only perform a female-only comparison between A. arabiensis and M-form A. gambiae in the following analyses. Because gonotrophic status was identified as a significant factor (Fig. 1; see below), we used a two-way ANOVA with gonotrophic status and species. Initially, a full-model ANOVA with the interaction term was used, but as the interaction was non-significant (P>0.46), it was removed. Both gonotrophic status and species significantly affected metabolic rate (R2=0.52, F3,163=58.27, P<0.0001; Table 2). Specifically, the changes due to gonotrophic status were similar to those found in the global analysis (see below), whereas the mean metabolic rate of A. arabiensis was significantly higher than that of the M-form A. gambiae (Fig. 2; Table 2). Thus, not taking into account other factors such as flight activity or body size, the mean metabolic rate for A. arabiensis is higher than that of M-form A. gambiae.
Fig. 1.
Effect of sex and female gonotrophic status on metabolic rate of Anopheles gambiae s.l.
Table 2.
Effects of female gonotrophic status and species identity (A. arabiensis or M-form A. gambiae) on metabolic rate
Fig. 2.
Effect of gonotrophic status and species identity on metabolic rate of A. gambiae s.l. Asterisk indicates a significant difference in post hoc analyses.
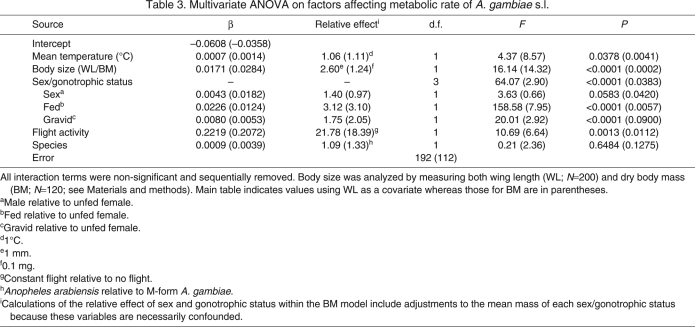
To simultaneously analyze the contributions of all factors relevant to metabolic rate, we included flight activity, assay temperature, body size (WL), gonotrophic status and species in a multi-factor ANOVA. As above, initially the model included all second- and third-order interactions, and non-significant interactions were removed. Using this method, no interactions remained in the final model, but all of the main-order effects were significant except for species (Table 3). The mean MRs of males and unfed females were not significantly different, whereas the mean MR of gravid females was higher than either of these and the MR of blood-fed females was higher than the rest (Fig. 1; Table 3). Increasing levels of flight, temperature and body size were all factors that significantly increased metabolism, as indicated by their positive estimated values (Table 3). When this analysis was repeated expressing body size as BM, the results were similar in both relative effect sizes of the different model parameters and which factors were found to be significant (values in parentheses of Table 3); thus it would appear that both indices of body size give similar results overall.
Table 3.
Multivariate ANOVA on factors affecting metabolic rate of A. gambiae s.l.
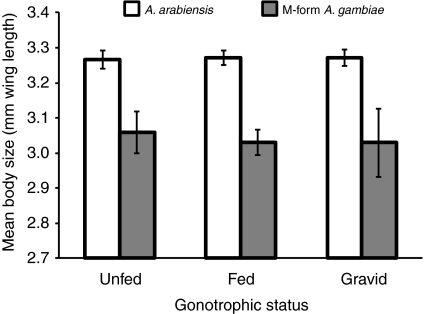
As shown above, for a female-only model using gonotrophic status and species, species was a significant effect (Table 2). However, in the most comprehensive model including body size and flight activity, species was no longer significant (Table 3). To elucidate whether species was confounded by flight activity and/or body size, we compared flight activity and body size between the species. Flight activity did not vary between species (F1,165=1.41, P=0.24) but female body size (WL) of A. arabiensis was on average larger than that of M-form A. gambiae (F1,148=47.47, P<0.0001; Fig. 3). Indeed, only when WL (or BM) was removed from the model did species became significant (not shown). Thus, the difference initially observed between the two species appears to be mediated by a difference in body size, and not by a physiological difference in the intrinsic MR per tissue.
Fig. 3.
Difference in mean body size between female Anopheles arabiensis and the M molecular form of A. gambiae.
We used the model estimates of the effect size (regression slopes) to rank the significant factors by their impact on MR. Flight had by far the highest impact, increasing MR 22-fold relative to the same time interval of rest (Table 3). Blood feeding and gravidity were ranked second and third (Table 3). As for temperature, a 1°C change in the environment would increase (or decrease) MR by approximately 6% (Table 3), indicating that the daily fluctuation observed during our study interval, approximately 4°C, could change MR by approximately 24%. Using the parameters derived by our model and the standard equation for Q10 (Randall et al., 2002), the temperature coefficient, we found that Q10=2.07 for our system. As above, results were similar whether WL or BM was used as the index of body size (Table 3). In summary, flight and gonotrophic status appear to have the highest impact on MR, and a mosquito could reduce her MR by decreasing flight, resting at lower temperatures and reducing the frequency of blood feeding.
DISCUSSION
Anopheles arabiensis and the M and S molecular forms of A. gambiae s.s. often display spatial, seasonal and ecological divergence (Touré et al., 1994; Gimnig et al., 2001; Edillo et al., 2002; Edillo et al., 2006; Koenraadt et al., 2004; Diabaté et al., 2005; Diabaté et al., 2008; Lehmann and Diabaté, 2008; Lee et al., 2009; Lehmann et al., 2010), indicating that they have been adapting to different spatial or temporal climatic niches. In the West African Sahel, M-form A. gambiae persist in low numbers throughout the long dry season, probably via aestivation, and reappear days after the first rain – before a new generation of adults can be produced (Lehmann et al., 2010). In contrast, A. arabiensis and S-form A. gambiae reappear weeks after the onset of rains, suggesting that they arrive by migration from areas where permanent water is available (Lehmann et al., 2010). As physiological or morphological changes often evolve during ecological divergence, we conducted this study to measure variation in MR between these species and molecular forms to determine whether MR could represent an adaptive trait related to the ecological differences among the members of the A. gambiae complex. We hypothesized that a lower MR is an advantage in the arid Sahel because it reduces nutritional demands (and the foraging involved in obtaining new resources), water loss and possibly the rate of cell and tissue aging (Sohal and Weindruch, 1996), and therefore extends survival. We wanted to compare the intrinsic variation in MR between the members of the A. gambiae complex as well as the factors that indirectly modulate the actual MR. However, because the S form was represented by a small sample size (N=8) in our sampling, only M-form A. gambiae and A. arabiensis were included in our analyses.
Although a significant difference was found in overall MR between the M-form A. gambiae and A. arabiensis females, this difference was mediated by a difference in body size. Thus, there was no difference in the intrinsic resting MR per unit of tissue between these species. A previous study comparing laboratory strains of A. arabiensis and the S form of A. gambiae also found no significant difference in resting MR after accounting for the effect of body size (Gray and Bradley, 2005). Because body size is the ultimate determinant of blood-meal size and probably sugar-meal size, a larger mosquito imbibes proportionally greater nutritional resources but burns these resources faster because of its larger mass. Therefore, a mosquito's resources will last for a similar period independent of body size. We conclude that the ecological differences between these species cannot be explained by the difference in their whole-body resting MRs. However, it is important to note that this result does not preclude the possibility of ecologically important differences in metabolism that may only be apparent during the dry season under physiological dormancy.
Species and sex (measured between males and unfed females) did not significantly affect the resting MR, after accounting for body size. However, flight, female gonotrophic status, body size and temperature all were significant. These data highlight the dynamic rather than static nature of MR and suggest that mosquitoes may realize a substantially different MR by changing their behavior. In fact, the magnitude of the difference in intrinsic MR between species adapted to different environments ranges between 9 and 20%. For example, the difference was 8–16% in mice (Haim et al., 2008) and 9–20% in polychaetes (Gamenick et al., 1998; Linke-Gamenick et al., 2000). Even for species with intrinsically different MRs, additional factors are probably necessary to account for their ability to exist in extremely divergent environments. Insect flight can raise the MR 20- to 100-fold over the resting MR (Joos et al., 1996). We found that flight increases MR approximately 18- to 22-fold in A. gambiae s.l. (Table 3), higher than the 10-fold increase in the mosquito Culex tarsalis (Gray and Bradley, 2003) but lower than the 43-fold increase in honeybees (Suarez, 2000). Lastly, because we utilized wild mosquitoes, we were unable to ascertain any effect of mosquito age on MR, which has been found to have a small but significant effect in A. gambiae s.l. (Gray and Bradley, 2005). Overall, our results appear to indicate that our unfed mosquitoes had approximately one-third lower MRs than the S-form A. gambiae previously measured [see fig. 2 in Gray and Bradley (Gray and Bradley, 2005)], which could reflect differences in a variety of factors including body size, temperature or activity.
Implications of these results for alternative strategies used by mosquitoes during the dry season
Based on these results, we can evaluate strategies that may be used by mosquitoes during the dry season. In particular, we evaluate the benefit of behavioral changes to extend survival of aestivating mosquitoes with respect to: (1) inactivity/quiescence versus actively locating and acquiring nutritional resources and (2) using blood or sugar as the primary nutritional resource. Importantly, for the below analysis we chose to use WL and not BM to measure body size, because BM and the gonotrophic status of females are inextricably linked.
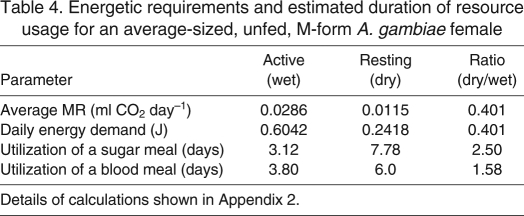
A reproductively active female mosquito forages for sugar and blood sources, locates resting sites and travels to oviposition sites (sometimes over 1 km from the nearest house) approximately every gonotrophic cycle, i.e. approximately every 3 days. An aestivating mosquito can reduce her energy demands by minimizing flight and selecting a cooler resting site. To evaluate the potential benefit of such a strategy, we predicted realized metabolism using the model parameters obtained from mosquitoes under natural conditions and derived their daily caloric requirements (see Appendix 2). These values were determined in an average-sized (WL=3.03 mm), unfed M-form female, flying 1 h day–1, under the mean daily temperature during the study (29°C), and compared with the values of the same female resting in a cooler refugium (25°C) without flight (see details in Appendix 2). The energetic requirements for the resting female were 40% that of the average active female, meaning that a single sugar meal has the potential to sustain the resting female 2.5 times longer than a blood meal (Table 4). According to our calculations (Appendix 2), this alone can increase the interval between sugar-feeding events from approximately 3.1 to 7.8 days (Table 4). Thus, seeking shelter in a cooler refugium (such as in an underground shelter) and avoiding flight activity could be adaptive behavioral changes that contribute to longer survival for an aestivating mosquito because this behavior reduces actual MR and energy requirements while lowering oxidative stress (Sohal and Weindruch, 1996) and the risks associated with foraging for sugar and blood sources, as well as oviposition sites, and further increasing their survival. Additional physiological mechanisms to reduce the resting metabolism during aestivation may further extend the benefits of this strategy. The alternative strategy, however, of a ‘typical’ active mosquito would be required to fulfill substantially higher energetic demands and faces higher risks.
Table 4.
Energetic requirements and estimated duration of resource usage for an average-sized, unfed, M-form A. gambiae female
Utilization of a blood meal to fulfill energetic requirements, rather than reproductive demands, would be a costly strategy for aestivating females, because although the caloric content of blood and nectar (with 15% sugars) is rather similar (Appendix 2), freshly blood-fed and gravid females had mean MRs approximately 2.6- and 1.6-fold higher than that of unfed females, respectively. We presume that many of the unfed females, like the males, had taken sugar meals prior to our MR measurements, but their MRs remained the lowest. Similar MR increases were found in Aedes aegypti (Gray and Bradley, 2006) and Culex tarsalis (Gray and Bradley, 2003) in recently fed and gravid females relative to sugar-fed counterparts. An increase in MR during digestion and oogenesis is not surprising, as digestion requires the synthesis of many enzymes to break down the blood mass and for vitellogenesis and other processes during egg production (reviewed in Clements, 1992; Randall et al., 2002). However, this increase in MR, which results from digestion of blood, but not digestion of sugar, means that although the average caloric value of a blood meal is higher than that of a sugar meal (see Appendix 2), much of the increased value is utilized for digestion and oogenesis. For an active female destined to oviposit, a blood meal will sustain her for a greater interval than a sugar meal (3.8 vs 3.1 d; Table 4), whereas for a resting female, the sugar meal provides a greater return (7.8 vs 6.0 d; Table 4). Thus, foraging on available sugar sources during the dry season may provide a greater benefit to aestivating mosquitoes than a blood meal (in the absence of oviposition sites), and could explain why the density of mosquitoes inside houses is extremely low during the dry season (Lehmann et al., 2010; Adamou et al., 2011). Additionally, during the same time (2–3 days) that a female digests a single blood meal, she could obtain two to three (or more) sugar meals because the digestion of sugar is considerably faster than that of blood (hours instead of days) (reviewed in Clements, 1992). In other mosquito species that undergo a non-reproductive diapause period, a switch from blood feeding to sugar feeding has been observed. For example, female Anopheles messeae in northern Sweden stop blood feeding during winter and instead take sugar before and during the hibernation period (Jaenson and Ameneshewa, 1991). Reduced blood feeding during diapause has also been found in Culex pipiens (Mitchell and Briegel, 1989; Robich and Denlinger, 2005), Culiseta inornata (Barnard and Mulla, 1977; Hudson, 1979) and Anopheles freeborni (Washino, 1970; Washino et al., 1971), and we hypothesize that the greater net caloric return from sugar feeding versus blood feeding may therefore explain the repeated occurrence of this phenomenon across many mosquito taxa.
In conclusion, we found that the resting MRs of A. arabiensis and M-form A. gambiae (during the wet season) were similar, after accounting for variation in body size. Therefore, unless a greater difference exists in the dry season, an intrinsic difference in MR between the species cannot account for the greater aridity tolerance of the M-form A. gambiae. However, behavioral changes, such as reduced flight activity and seeking a cooler resting location, can lower actual MR by 60%. Additionally, sugar feeding during the dry season will provide more energetic resources to mosquitoes than blood feeding (on the same volume) because of the higher metabolism necessary to digest blood as opposed to sugar. Future studies may help determine whether these strategies are used by mosquitoes during the dry season and assess their adaptive value in terms of increased survival and reduced energetic requirements.
APPENDIX 1
Landmarks for wing-length measurements and stepwise regression models to calculate wing length for damaged wings
We used wing length (WL) as a measurement of body size (Lyimo and Koella, 1992; Aboagye-Antwi et al., 2010) because the wet or dry mass of recently blood-fed or gravid females includes mass that is not reflective of their true body size. For most individuals, the measurement was determined by calculating the distance (in pixels) between the X–Y coordinates of the alular notch (point 1) and the intersection of the radius 3 vein and the outer margin (point 10; Fig. A1A), and converting to mm. However, as we measured metabolism on wild-caught individuals, sometimes both wings of an individual were damaged (missing one or both points indicated above), and WL had to be estimated. As 13 landmark points on each wing were digitized (see Fig. A1A), internal distances could be calculated for points located in areas of the wing which were typically undamaged. We used these internal distance calculations in multiple regression models to estimate WL of intact wings, using D2–4, D2–7, D2–11, D2–13, D4–7, D4–11 and D7–11 as possible explanatory variables (‘D’ denotes distance in pixels between the points, with numbers corresponding to landmark numbers in Fig. A1).
Because the wing shapes for females and males are different, separate multiple regression equations were derived for males and females based on these internal distances between key landmarks (Fig. A1B). For females, the best-fitting model (R2=0.886) was: WL (mm)=0.3873 + 0.00464 (D2–13) + 0.00418 (D4–7) + 0.00356 (D2–11). In contrast, the best-fitting model for males (R2=0.613) was: WL (mm)=1.322 + 0.00554 (D2–13) + 0.00277 (D4–11). Thus, we found that the best-fitting equations derived by the stepwise process were not only different for male and female wings, but did not include all of the same internal landmark distances (Fig. A1B). However, we were able to accurately predict WL for intact wings, and used these derived equations to predict WL for damaged wings, allowing more individuals of both sexes to be included in the analyses of metabolism data.
APPENDIX 2
Estimated energetic values of blood and sugar meals
The mean energetic value of blood is 3.56 J μl–1 (assumes normal human average values: hematocrit of 40%, plasma glucose concentration of 0.9 mg ml–1, plasma lipid concentration of 6 mg ml–1, plasma protein concentration of 70 mg ml–1, and hemoglobin concentration of 140 mg ml–1) (Richardson, 1994; Hoffman et al., 2005; Lehane, 2005). To evaluate the energetic content of a sugar meal, we used nectar, which has an average sugar content of 15% by weight and therefore contains 0.15 mg sugar μl–1. As carbohydrates contain 16.74 J mg–1, nectar has an estimated caloric content of 2.51 J μl–1. Assuming an equal meal size of 1.5 μl, a blood meal contains approximately 5.36 J whereas a sugar meal contains approximately 3.77 J. Because the conversion of food consumed to useful energy is not 100%, we will assume a digestive efficiency of 50% for all calculations. Thus, the realized energy of a blood meal is estimated at 2.68 J whereas a sugar meal is estimated at 1.88 J.
Daily energetic requirements for active versus resting females
To estimate the daily caloric requirements of an average M-form A. gambiae female, we used the parameter estimates derived by the ANOVA analysis (Table 3) to calculate both active and resting values. We defined an active mosquito as flying for 1 h day–1 (allowing for host-seeking, locating resting sites, oviposition sites and sugar sources) and experiencing a mean temperature of 29°C (the mean daily indoor temperature during our study interval; see Results). In contrast, we defined a resting mosquito as spending all of its time without flight in a cooler refugium (25°C, approximated by taking underground measurements in our study area). Both calculations assume an unfed, average-sized (3.03 mm; see Results and Fig. 3), M-form A. gambiae female, and yielded a mean MR of 0.01985 μl CO2 min–1 for the active female and 0.00795 μl CO2 min–1 for the resting female. Therefore, this combination of reduced flight activity and sheltering in a cooler location could result in a 2.5-fold reduction in daily MR (Table 4). For an animal utilizing a carbohydrate energy source, as would be the case for a sugar-feeding mosquito, 1 ml of metabolic CO2 is equal to approximately 21.13 J of sugar metabolism (Carpenter, 1921), allowing us to calculate the daily energetic requirements of an active or resting mosquito from the above MR calculations (Table 4). Finally, combining the estimated energetic value of a sugar meal (see above), we estimated that an active mosquito could be sustained for approximately 3.1 days on one average-sized sugar meal, whereas a resting mosquito could survive up to 7.8 days (Table 4).
A blood meal has a higher energetic value than a sugar meal. However, the MR of blood-fed and gravid females was greatly increased over that of an unfed female (Figs 1, 2; Table 3) and semi-gravid females (approximately intermediate between fed and gravid females; data not shown). Therefore, the gross energetic intake of blood feeding differs from the net value after blood digestion. To calculate the approximate net energetic value of a blood meal, we estimated that a female will spend approximately 12 h in the fed metabolic state, the next 36 h in a semi-gravid state (intermediate in MR between fed and gravid females) and finally up to 24–48 h in the gravid state before ovipositing under observed field conditions (Clements, 1992; Lehane, 2005) (A.D., A.S.Y. and T.L., unpublished observations). Utilizing the parameter estimates from Table 3, combined with these lengths of time in each gonotrophic state, we calculated the mean MR (0.0357 ml CO2 day–1) and energetic equivalent (2.26 J) during the full gonotrophic cycle over a period of 72 h for the same average-sized M-form A. gambiae female at 29°C but with no flight (as flight is greatly reduced during this time). Given this increased MR during the gonotrophic cycle, an active blood-fed female could be sustained for an additional 0.8 days, or 3.8 days total, on a single blood meal (Table 4). If, however, a female could utilize the remaining nutritional resources for survival instead of becoming gravid, she could persist for an additional 4.0 days at resting MR, or 6.0 days total on a single blood meal (Table 4). Therefore, in the absence of suitable oviposition sites, an aestivating female mosquito during the dry season may receive a higher benefit from taking sugar meals rather than blood meals. This calculation is conservative, with respect to our conclusion, because we assume a single sugar meal versus a single blood meal (whilst a mosquito could take two or more sugar meals during the time she must digest a single blood meal) and because gravid females in the dry season must resorb the eggs in order to sustain themselves, a process which likely entails additional energetic cost.
ACKNOWLEDGEMENTS
We thank R. Gwadz, T. Wellems and R. Sakai for their assistance in facilitating this work, H. Ackerman for providing average blood chemistry data and K. Hines for use of the electrobalance. We also thank the inhabitants of M'Piabougou and Thierola, Mali, for their assistance and hospitality, without which this work would not have been possible. Comments from P. Armbruster, B. Oppert and two anonymous reviewers improved the quality of this manuscript.
FOOTNOTES
This research was supported by the Intramural Research Program of the NIH, NIAID. Deposited in PMC for release after 12 months.
REFERENCES
- Aboagye-Antwi F., Tripet F. (2010). Effects of larval growth condition and water availability on desiccation resistance and its physiological basis in adult Anopheles gambiae sensu stricto. Malar. J. 9, 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboagye-Antwi F., Guindo A., Traoré A. S., Hurd H., Coulibaly M., Traoré S., Tripet F. (2010). Hydric stress-dependent effects of Plasmodium falciparum infection on the survival of wild-caught Anopheles gambiae female mosquitoes. Malar. J. 9, 243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamou A., Dao A., Timbiné S., Kassogué Y., Yaro A. S., Diallo M., Traoré S. F., Huestis D. L., Lehmann T. (2011). The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar. J. 10, 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber I., Keita M., Sogoba N., Konate M., Diallo M., Doumbia S., Traoré S. F., Ribeiro J. M. C., Manoukis N. C. (2010). Population size and migration of Anopheles gambiae in the Bancoumana region of Mali and their significance for efficient vector control. PLoS ONE 5, e10270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D. R., Mulla M. S. (1977). Effects of photoperiod and temperature on blood feeding, oogenesis, and fat body development in the mosquito Culiseta inornata. J. Insect Physiol. 23, 1261-1266 [DOI] [PubMed] [Google Scholar]
- Bjerke R., Zachariassen E. K. (1997). Effects of dehydration on water content, metabolism, and body fluid solutes of a carabid beetle from dry savanna in East Africa. Comp. Biochem. Physiol. 118A, 779-787 [Google Scholar]
- Canzano A. A., Krockenberger A. A., Jones R. E., Seymour J. E. (2006). Rates of metabolism in diapausing and reproductively active tropical butterflies, Euploea core and Euploea sylvester (Lepidoptera: Nymphalidae). Physiol. Entomol. 31, 184-189 [Google Scholar]
- Carpenter T. M. (1921). Tables, Factors, and Formulas for Computing Respiratory Exchange and Biological Transformations of Energy. Washington, DC: Carnegie Institution of Washington; [Google Scholar]
- Chappell M. A. (1983). Metabolism and thermoregulation in desert and montane grasshoppers. Oecologia 56, 126-131 [DOI] [PubMed] [Google Scholar]
- Clarke A., Fraser K. P. P. (2004). Why does metabolism scale with temperature? Funct. Ecol. 18, 243-251 [Google Scholar]
- Clements A. N. (1992). The Biology of Mosquitoes. Vol. 1, Development, Nutrition, and Reproduction. London: Chapman and Hall; [Google Scholar]
- Collins F. H., Kamau L., Ranson H. A., Vulule J. M. (2001). Molecular entomology and prospects for malaria control. Bull. World Health Organ. 78, 1412-1423 [PMC free article] [PubMed] [Google Scholar]
- Coluzzi M., Sabatini A., Petrarca V., Di Deco M. A. (1979). Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 73, 483-497 [DOI] [PubMed] [Google Scholar]
- Coluzzi M., Petrarca V., Di Deco M. A. (1985). Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Boll. Zool. 52, 45-63 [Google Scholar]
- Coluzzi M., Sabatini A., della Torre A., Di Deco M. A., Petrarca V. (2002). A polytene chromosome analysis of the Anopheles gambiae species complex. Science 298, 1415-1418 [DOI] [PubMed] [Google Scholar]
- Costantini C., Ayala D., Guelbeogo W., Pombi M., Some C., Bassole I., Ose K., Fotsing J.-M., Sagnon N. F., Fontenille D., et al. (2009). Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 9, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- della Torre A., Fanello C., Akogbeto M., Dossou-yovo J., Favia G., Petrarca V., Coluzzi M. (2001). Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol. Biol. 10, 9-18 [DOI] [PubMed] [Google Scholar]
- della Torre A., Costantini C., Besansky N. J., Caccone A., Petrarca V., Powell J. R., Coluzzi M. (2002). Speciation within Anopheles gambiae – the glass is half full. Science 298, 115-117 [DOI] [PubMed] [Google Scholar]
- della Torre A., Tu Z., Petrarca V. (2005). On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem. Mol. Biol. 35, 755-769 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L. (2002). Regulation of diapause. Annu. Rev. Entomol. 47, 93-122 [DOI] [PubMed] [Google Scholar]
- Diabaté A., Dabiré R. K., Kim E. H., Dalton R., Millogo N., Baldet T., Simard F., Gimnig J. E., Hawley W. A., Lehmann T. (2005). Larval development of the molecular forms of Anopheles gambiae (Diptera: Culicidae) in different habitats: a transplantation experiment. J. Med. Entomol. 42, 548-553 [DOI] [PubMed] [Google Scholar]
- Diabaté A., Dabiré R. K., Heidenberger K., Crawford J., Lamp W. O., Culler L. E., Lehmann T. (2008). Evidence for divergent selection between the molecular forms of Anopheles gambiae: role of predation. BMC Evol. Biol. 8, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edillo F. E., Touré Y. T., Lanzaro G. C., Dolo G., Taylor C. E. (2002). Spatial and habitat distribution of Anopheles gambiae and Anopheles arabiensis (Diptera: Culicidae) in Banambani Village, Mali. J. Med. Entomol. 39, 70-77 [DOI] [PubMed] [Google Scholar]
- Edillo F. E., Tripét F., Touré Y. T., Lanzaro G. C., Dolo G., Taylor C. E. (2006). Water quality and immature of the M and S forms of Anopheles gambiae s.s. and Anopheles arabiensis in a Malian village. Malar. J. 5, 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanello C., Santolamazza F., della Torre A. (2002). Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 16, 461-464 [DOI] [PubMed] [Google Scholar]
- Foster W. A. (1995). Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443-474 [DOI] [PubMed] [Google Scholar]
- Gamenick I., Vismann B., Grieshaber M. K., Giere O. (1998). Ecophysiological differentiation of Capitella capitata (Polychaeta). Sibling species from different sulfidic habitats. Mar. Ecol. Prog. Ser. 175, 155-168 [Google Scholar]
- Gimnig J. E., Ombok M., Kamau L., Hawley W. A. (2001). Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J. Med. Entomol. 38, 282-288 [DOI] [PubMed] [Google Scholar]
- Gray E. M., Bradley T. J. (2003). Metabolic rate in Culex tarsalis (Diptera: Culicidae): age, size, activity, and feeding effects. J. Med. Entomol. 40, 903-911 [DOI] [PubMed] [Google Scholar]
- Gray E. M., Bradley T. J. (2005). Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. Am. J. Trop. Med. Hyg. 73, 553-559 [PubMed] [Google Scholar]
- Gray E. M., Bradley T. J. (2006). Malarial infection in Aedes aegypti: effects on feeding, fecundity, and metabolic rate. Parasitology 132, 169-176 [DOI] [PubMed] [Google Scholar]
- Haim A., Zubidat A. E., van Aarde R. J. (2008). Daily rhythms of body temperature and heat production of sibling mastomys species from different ecosystems – the response to photoperiod manipulations. Comp. Biochem. Physiol. 151A, 505-510 [DOI] [PubMed] [Google Scholar]
- Hoffman R., Benz E. J., Jr, Shattil S. J., Furie B., Cohen H. J., Silberstein L. E., McGlave P. (2005). Hematology: Basic Principles and Practice. Philadelphia: Elsevier Inc. [Google Scholar]
- Holstein M. H. (1954). Biology of Anopheles gambiae: Research in French West Africa. Geneva: World Health Organization; [Google Scholar]
- Hudson J. E. (1979). Follicle development, blood feeding, digestion, and egg maturation in diapausing mosquitoes, Culiseta inornata. Entomol. Exp. Appl. 25, 136-145 [Google Scholar]
- Jaenson T. G. T., Ameneshewa B. (1991). Prehibernation diet and reproductive condition of female Anopheles messeae in Sweden. Med. Vet. Entomol. 5, 243-252 [DOI] [PubMed] [Google Scholar]
- Joos B., Lighton J. R. B., Harrison J. F., Suarez R. K., Roberts S. P. (1996). Effects of ambient oxygen tension on flight performance, metabolism, and water loss of the honeybee. Physiol. Zool. 70, 167-174 [DOI] [PubMed] [Google Scholar]
- Koenraadt C. J. M., Githeko A. K., Takken W. (2004). The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Trop. 90, 141-153 [DOI] [PubMed] [Google Scholar]
- Kurtti T. J., Brooks M. A., Wensman C., Lovrien R. (1979). Direct microcalorimetry of heat generation by individual insects. J. Therm. Biol. 4, 129-136 [Google Scholar]
- Lee Y., Meneses C. R., Fofana A., Lanzaro G. C. (2009). Desiccation resistance among subpopulations of Anopheles gambiae s.s. from Selinkenyi, Mali. J. Med. Entomol. 46, 316-320 [DOI] [PubMed] [Google Scholar]
- Lehane M. J. (2005). The Biology of Blood-Sucking in Insects. Cambridge: Cambridge University Press; [Google Scholar]
- Lehmann T., Diabaté A. (2008). The molecular forms of Anopheles gambiae: a phenotypic perspective. Infect. Genet. Evol. 8, 737-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T., Dao A., Yaro A. S., Adamou A., Kassogué Y., Diallo M., Sékou T., Coscaron-Arias C. (2010). Aestivation of the African malaria mosquito, Anopheles gambiae in the sahel. Am. J. Trop. Med. Hyg. 83, 601-606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton J. R. B. (1996). Discontinuous gas exchange in insects. Annu. Rev. Entomol. 41, 309-324 [DOI] [PubMed] [Google Scholar]
- Lighton J. R. B. (2008). Measuring Metabolic Rates: a Manual for Scientists. Oxford: Oxford University Press; [Google Scholar]
- Lindsay S. W., Parson L., Thomas C. J. (1998). Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc. R. Soc. Lond. B 265, 847-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke-Gamenick I., Vismann B., Forbes V. E. (2000). Effects of fluoranthene and ambient oxygen levels on survival and metabolism in three sibling species of Capitella (Polychaeta). Mar. Ecol. Prog. Ser. 194, 169-177 [Google Scholar]
- Lovegrove B. G. (1986). The metabolism of social subterranean rodents: adaptation to aridity. Oecologia 69, 551-555 [DOI] [PubMed] [Google Scholar]
- Lyimo E. O., Koella J. C. (1992). Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology 104, 233-237 [DOI] [PubMed] [Google Scholar]
- Mazzoni D. (2009). Audacity 1. 3. 9, a Free Digital Audio Editor. http://audacity.sourceforge.net
- Mitchell C. J., Briegel H. (1989). Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwintering survival. J. Med. Entomol. 26, 8-26 [DOI] [PubMed] [Google Scholar]
- Nayer J. K., Van Handel E. (1971). The fuel for sustained mosquito flight. J. Insect Physiol. 17, 471-481 [Google Scholar]
- Omer S. M., Cloudsley-Thompson J. L. (1968). Dry season biology of Anopheles gambiae Giles in the Sudan. Nature 217, 879-880 [Google Scholar]
- Omer S. M., Cloudsley-Thompson J. L. (1970). Survival of female Anopheles gambiae Giles through a 9-month dry season in Sudan. Bull. World Health Organ. 42, 319-330 [PMC free article] [PubMed] [Google Scholar]
- Petrarca V., Nugud A. D., Ahmed M. A., Haridi A. M., Di Deco M. A., Coluzzi M. (2000). Cytogenetics of the Anopheles gambiae complex in Sudan, with special reference to An. arabiensis: relationships with East and West African populations. Med. Vet. Entomol. 14, 149-164 [DOI] [PubMed] [Google Scholar]
- Ragland G. J., Fuller J., Feder J. L., Hahn D. A. (2009). Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J. Insect Physiol. 55, 344-350 [DOI] [PubMed] [Google Scholar]
- Ragland G. J., Denlinger D. L., Hahn D. A. (2010). Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 107, 14909-14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. J., Burggren W., French K. (2002). Eckert Animal Physiology: Mechanisms and Adaptations, 5th edn. New York: W. H. Freeman and Company; [Google Scholar]
- Reinhold K. (1999). Energetically costly behavior and the evolution of resting metabolic rate in insects. Funct. Ecol. 13, 217-224 [Google Scholar]
- Richardson R. W. (1994). Handbook of Nonpathologic Variations in Human Blood Constituents. London: CRC Press, Inc. [Google Scholar]
- Rivers D. B., Denlinger D. L. (1994). Redirection of metabolism in the flesh fly, Sarcophaga bullata, following envenomation by the ectoparasitoid Nasonia vitripennis and correlation of metabolic effects with the diapause status of the host. J. Insect Physiol. 40, 207-215 [Google Scholar]
- Robich R. M., Denlinger D. L. (2005). Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc. Natl. Acad. Sci. USA 102, 15912-15917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf F. J. (2010). tpsDIG v. 2.15. Stony Brook, NY: SUNY; http://life.bio.sunysb.edu/morph/ [Google Scholar]
- Schmidt-Nielsen K. (1997). Animal Physiology, 5th edn. Cambridge: Cambridge University Press; [Google Scholar]
- Simard F., Lehmann T., Lemasson J. J., Diatta M., Fontenille D. (2000). Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol. Biol. 9, 467-479 [DOI] [PubMed] [Google Scholar]
- Simard F., Ayala D., Kamdem G. C., Pombi M., Etouna J., Ose K., Fotsing J. M., Fontenille D., Besansky N. J., Costantini C. (2009). Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecol. 9, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Weindruch R. (1996). Oxidative stress, caloric restriction, and aging. Science 273, 59-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K. (2000). Energy metabolism during insect flight: biochemical design and physiological performance. Physiol. Biochem. Zool. 73, 765-771 [DOI] [PubMed] [Google Scholar]
- Tieleman B. I., Williams J. B., Bloomer P. (2003). Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc. R. Soc. Lond. B 270, 207-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touré Y. T., Petrarca V., Traoré S. F., Coulibaly A., Maiga H. M., Sankaré O., Sow M., Di Deco M. A., Coluzzi M. (1994). Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.str. in Mali, west Africa. Genetica 94, 213-223 [DOI] [PubMed] [Google Scholar]
- Touré Y. T., Petrarca V., Traoré S. F., Coulibaly A., Maiga H. M., Sankaré O., Sow M., Di Deco M. A., Coluzzi M. (1998). The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia 40, 477-511 [PubMed] [Google Scholar]
- Washino R. K. (1970). Physiological condition of overwintering female Anopheles freeborni in California (Diptera: Culicidae). Ann. Entomol. Soc. Am. 63, 210-216 [DOI] [PubMed] [Google Scholar]
- Washino R. K., Gieke P. A., Schaefer C. H. (1971). Physiological changes in the overwintering females of Anopheles freeborni (Diptera: Culicidae) in California. J. Med. Entomol. 8, 279-282 [DOI] [PubMed] [Google Scholar]