Abstract
CD4 counts increase during the postpartum period and may not correctly identify HAART-eligible HIV-positive women. HAART eligibility when defined by two CD4 cutoffs (<200 and <350 cells/μl) measured at two time points (within 96 h of delivery and 6 weeks) in postpartum HIV-positive women was compared. Among HIV-positive women who had CD4 at delivery and 6 weeks (n = 423), time to Stage 3 or 4 opportunistic infection or death was compared using Cox regression between three groups of women: (1) CD4 <200 cells/μl at delivery and 6 weeks, (2) CD4 <200 cells/μl at delivery but ≥200 cells/μl at 6 weeks, and (3) CD4 ≥200 cells/μl at delivery and at 6 weeks. The analysis was repeated using the CD4 <350 cells/μl cut-off. CD4 counts increased by a median (IQR) of 70 (1–178) cells/μl between delivery and 6 weeks and decreased thereafter to approximately delivery levels at 12 months. Only 60% and 61% who had CD4 <200 cells/μl and CD4 <350 cells/μl, respectively, at delivery also had those levels at 6 weeks. Among those with CD4 <350 cells/μl at both delivery and 6 weeks, the risk of death or Stage 3 or 4 disease was 5.27 (95% CI 1.85–14.96) times higher than those with CD4 <350 at delivery but ≥350 cells/μl at 6 weeks. The use of CD4 counts immediately postpartum to define HAART eligibility may lead to substantial misclassification.
Introduction
In the course of HIV infection, plasma CD4 cell counts decrease on average 75 cells/μl per year.1 Conversely, CD4 cell counts rise in women following pregnancy, due to resolution of physiologic hemodilution in both HIV-uninfected and HIV-infected women.2–4 The WHO guidelines currently recommend CD4 <200 cells/μl as an absolute indication of highly active antiretroviral therapy (HAART) initiation and CD4 <350 cells/μl as a benchmark to consider treatment.5,6
A recent publication reported that using CD4 counts at 32 weeks of gestation as HAART eligibility criteria leads to substantial misclassification of HAART eligibility when compared to CD4 values at 1 month postpartum.7 Using WHO clinical staging and CD4 counts, 28.3% of women in this study were HAART eligible according to their baseline CD4 values whereas only 17.2% were eligible according to their postpartum CD4 values. The authors pointed out that CD4 percentage may be a more accurate indicator of immune status in pregnant and postpartum women than absolute CD4 since the former is less affected by hemodynamic changes associated with pregnancy and postpartum. Misclassification of HAART eligibility or premature initiation of HAART may lead to increased viral resistance, waste of resources, noncompliance, and unnecessary adverse events.8–12 In this article, we describe the change in absolute CD4 cell count postpartum up to 12 months and the potential misclassification of HAART eligibility if using CD4 cell counts measured immediately postpartum.
Materials and Methods
ZVITAMBO trial
Details of the ZVITAMBO trial have been previously published.13–15 Briefly, 14,110 mother–infant pairs were recruited within 96 h of delivery in greater Harare, Zimbabwe between November 1997 and January 2000. Mothers were eligible if they did not have a life threatening condition and had planned to stay in Harare after enrollment. Written informed consent was obtained. Baseline characteristics were collected from hospital records and a questionnaire. Follow-up was conducted at 6 weeks and 3 months and 3-monthly intervals through 12–24 months. At delivery, women were tested for HIV by an algorithm incorporating two parallel ELISAs and Western blot. CD4 cells were counted by FACScount (Becton Dickinson). Among a randomly selected subgroup of approximately 10% of HIV-positive women, CD4 cells were also counted at 6 weeks and at 3, 6, 9, and 12 months. Weight was measured at each follow-up visit but not at delivery. At each visit, a 7-day morbidity history was elicited that included oral thrush and chronic diarrhea, and mothers were asked if they had been hospitalized or visited a clinic for treatment of an illness since their previous visit.
The causes and dates of these health care visits were determined from medical records, if available, or by maternal history. Data were available to identify the following Stage 3 opportunistic infections: chronic diarrhea, recurrent or persistent oral candidiasis (presence of at least two oral thrush episodes during follow-up with a ≥14 days interval between the two episodes), pulmonary tuberculosis (TB), and severe bacterial infections (e.g., pneumonia, meningitis, and sepsis). All severe bacterial infections were diagnosed at a clinic or hospital. Data were available to identify the following Stage 4 opportunistic infections: HIV wasting syndrome (simultaneous presence of ≥10% weight loss relative to any previous weight measurement during study and chronic diarrhea as defined in Stage 3), recurrent severe pneumonia (≥2 episodes of pneumonia during follow-up with ≥14 days interval between the two episodes), esophageal candidiasis, extrapulmonary TB, and Kaposi's sarcoma (all diagnosed during a clinic visit or hospitalization). Antiretroviral therapy (ART) was not available during the trial either as prophylaxis or treatment.
Statistical analysis
Statistical analysis was conducted using Stata Version 9.2 (StataCorp LP, Texas). Overall, 14,110 women were recruited and 4495 women were HIV positive at delivery. CD4 was counted at follow-up in a random subsample of approximately 10% of the HIV-positive women. To describe accurately the postpartum trajectory of CD4 counts in the first year, we restricted analyses that involved delivery, 6 week, and 12 month data to those with CD4 data at all those time points. Wilcoxon signed rank tests were used to test change in CD4 distribution between delivery and 6 weeks or 12 months and between 6 weeks and 12 months. McNemar's test was used to test pairwise changes in the proportion of those with CD4 <200 or <350 cells/μl at delivery, 6 weeks, and 12 months.
We also calculated the sensitivity, specificity, positive and negative predictive value, and 95% confidence intervals using information on 423 women who had CD4 measured at both delivery and 6 weeks. We considered CD4 counts at 6 weeks to be the gold standard indicator of immune status at delivery and used CD4 cut-off points of 150, 175, and 200 cells/μl and 300, 325, and 350 cells/μl at delivery to assess its correlation with, and sensitivity, specificity, and predictive values in identifying CD4 counts with cut-off points of 200 and 350 cells/μl at 6 weeks, respectively, among 423 women who had CD4 data available at both time points. Finally, Kaplan–Meier methods were also used to estimate cumulative risk of Stage 3 or 4 disease or death by CD4 count at delivery and 6 weeks.
The 423 women who had CD4 counts at delivery and 6 weeks were divided into three groups: (1) CD4 <200 cells/μl at delivery and 6 weeks, (2) CD4 <200 cells/μl at delivery and CD4 ≥200 cells/μl at 6 weeks, and (3) CD4 ≥200 cells/μl at both delivery and 6 weeks. The censoring date was the last date of follow-up, the first date in which Stage 3 or 4 disease was identified, or the date of death, whichever occurred earlier. We assessed the time to Stage 3 or 4 disease or death between 6 weeks and 24 months using the Cox proportional hazards model and present the 95% confidence intervals. The analysis was repeated by using the CD4 <350 cells/μl cut-off point.
Ethical approval
Ethical approval was granted from the Medical Research Council of Zimbabwe, Medicines Control Authority of Zimbabwe, the Committee on Human Research of the Johns Hopkins University Bloomberg School of Public Health, and the Ethics Committee of the Research Institute of the McGill University Health Center.
Results
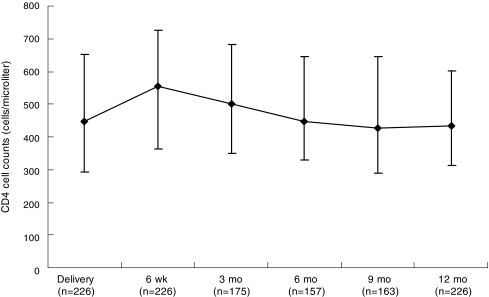
A total of 226 women had complete CD4 count information at delivery, 6 weeks, and 12 months. The median (IQR) number of days between delivery and blood sampling at 6 weeks and 12 months was 43 (42–46) days and at 12 months was 365 (365–368). As illustrated in Fig. 1, the median CD4 cell count increased between delivery and 6 weeks but gradually decreased by about the same magnitude between 6 weeks and 12 months such that the counts at delivery and 12 months did not significantly differ. Among the 27 women who had CD4 <200 cells/μl at delivery, 12 (44.4%) had CD4 ≥200 cells/μl at 6 weeks and among these 12 women, 7 (58.3%) still had CD4 ≥200 cells/μl at 12 months. Among the 78 women who had CD4 <350 cells/μl at delivery, 34 (43.6%) had CD4 ≥350 cells/μl at 6 weeks and among these 34 women, 21 (61.8%) still had CD4 ≥350 cells/μl at 12 months (Table 1).
FIG. 1.
Median and interquartile ranges of CD4 cell counts at delivery, 6 weeks, and 3, 6, 9, and 12 months postpartum. Only those who had information on CD4 counts at delivery, 6 weeks, and 12 months are included.
Table 1.
Postpartum CD4 Change in HIV-Positive Womena
| N = 226 (total) | Delivery | 6 weeks | 12 months |
|---|---|---|---|
| Median (IQRb) (cells/μl) | 448 (293–651) | 553 (362–727) | 432 (312–602) |
| Median difference from delivery (IQR) (cells/μl) | 70 (1–178)c | −14 (−125–77) | |
| Median difference from 6 weeks (IQR) (cells/μl) | −87 [−187–(−14)]c |
The 226 women who had CD4 count available at delivery, 6 weeks, and 12 months are included.
IQR, interquartile range.
Significantly different from 0 by sign rank test (p < 0.05).
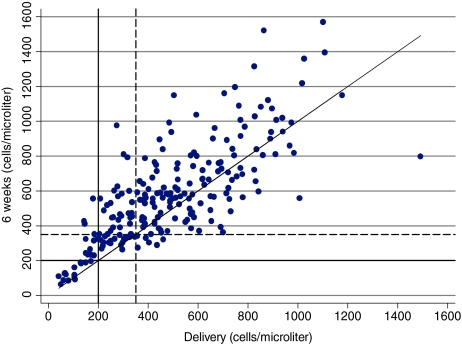
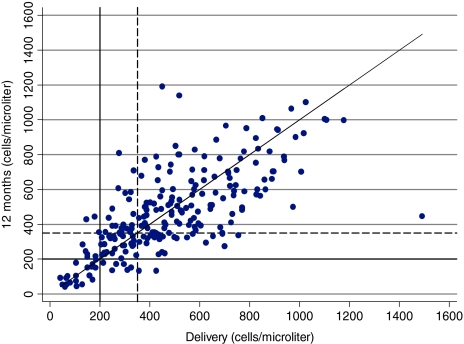
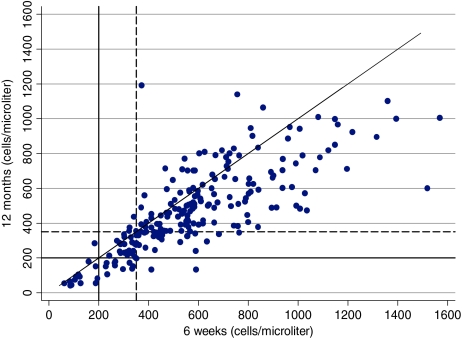
Between delivery and 6 weeks postpartum, CD4 cell count increased in 75.5% (170/226) of the women (Fig. 2). Between delivery and 12 months partum, CD4 cell count increased in 46% (104/226) and decreased in 54% (122/226) of the women. In contrast, 179 (79.2%) women had lower CD4 counts at 12 months than at 6 weeks. At 6 weeks, a smaller proportion of women had CD4 values of <200 cells/μl (6.6% vs. 12.0%; p = 0.0005) or <350 cells/μl (22.1% vs. 34.5%; p = 0.0000) compared to delivery. However, at 12 months, the proportions of women with CD4 <200 cells/μl and CD4 <350 cells/μl were not different compared to delivery (12.0% vs. 12.0%; p = 1.0000 and 35.0 vs. 34.5%; p = 1.0000), respectively. Based on the finding that CD4 counts on average increase between delivery and 6 weeks, we investigated the utility of using lower CD4 cut-off points at delivery than the conventional cut-off points of 200 and 350 cells/μl to identify HAART eligible women. Among those who had CD4 counts <150 cells/μl at delivery, 83% of the women also had CD4 counts <200 cells/μl at 6 weeks but only 60% of the women who had CD4 <200 cells/μl at delivery also had CD4 <200 cells/μl at 6 weeks (Table 2, see PPV values). However, CD4 <150 cells/μl at delivery identified only 79% of women who had CD4 <200 cells/μl at 6 weeks whereas CD4 <200 cells/μl at delivery correctly identified 91% (Table 2, see Sensitivity values). The positive predictive values of all delivery cut-off points in identifying women with CD4 <350 cells/μl at 6 weeks were low (61–68%).
FIG. 2.
Scatter plot of plasma CD4 counts at delivery, 6 weeks, and 12 months. The diagonal line corresponds to y = x. The dashed line is a reference line for CD4 350 cells/μl. It is restricted to the 226 women who had CD4 count at delivery, 6 weeks, and 12 months.
Table 2.
Number and Percentage of Women by CD4 Cutoffs 200 and 350 Cells/μl and Time Postpartuma
|
CD4 at delivery (cells/μl) |
CD4 at 6 weeks (n) (cells/μl) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPVb(95% CI) |
NPVb(95% CI) |
|
|---|---|---|---|---|---|---|
| <200 | ≥200 | |||||
| <200 | 39 | 26 | 91 (78–97) | 93 (90–95) | 60 (47–72) | 99 (97–100) |
| ≥200 | 4 | 354 | ||||
| <175 | 39 | 15 | 91 (78–97) | 96 (94–98) | 72 (58–84) | 99 (97–100) |
| ≥175 | 4 | 365 | ||||
| <150 | 34 | 7 | 79 (64–90) | 98 (96–99) | 83 (68–93) | 98 (96–99) |
| ≥150 | 9 | 373 | ||||
| <350 | ≥350 | |||||
|---|---|---|---|---|---|---|
| <350 | 102 | 64 | 90 (83–95) | 79 (74–84) | 61 (54–69) | 96 (92–98) |
| ≥350 | 11 | 246 | ||||
| <325 | 97 | 53 | 86 (78–92) | 83 (78–87) | 65 (56–72) | 94 (91–97) |
| ≥325 | 16 | 257 | ||||
| <300 | 88 | 41 | 78 (69–85) | 87 (82–90) | 68 (59–76) | 91 (88–94) |
| ≥300 | 25 | 269 |
423 women who had CD4 cell counts available at delivery and 6 weeks are included.
PPV, positive predictive value; NPV, negative predictive value.
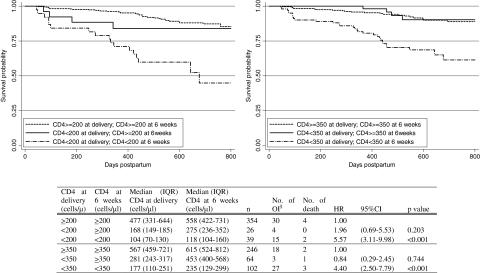
As shown in Fig. 3, women with CD4 counts that were <200 cells/μl at delivery and remained so at 6 weeks had the highest risk of death or Stage 3 or 4 opportunistic infection (HR 5.57; 95% CI 3.11–9.98; p = 0.000) compared to those who had CD4 ≥200 cells/μl at both time points. Compared to women who started with CD4 counts <200 at delivery and then had CD4 counts ≥200 cells/μl at 6 weeks, the risk of death or Stage 3 or 4 disease in women with persistently low CD4 counts at both delivery and 6 weeks was higher but not statistically significant (HR 2.84; 95% CI 0.96–8.45; p = 0.060). Having CD4 counts <350 cells/μl at both time points was also associated with a significantly higher risk (HR 5.27; 95% CI 1.85–14.96; p = 0.002) compared to those with CD4 <350 cells/μl at delivery and CD4 ≥350 cells/μl at 6 weeks.
FIG. 3.
Kaplan–Meier survival curves of time to first Stage 3 or 4 opportunistic infection or death after 42 days postpartum by CD4 count at delivery and at 6 weeks. The 423 women who had CD4 count available at delivery and 6 weeks are included. §Opportunistic infections. Refer to the text for definitions.
Discussion
We have demonstrated that the majority of HIV-infected women in this cohort had higher CD4 counts at 6 weeks postpartum than they did within 4 days of delivery. Forty percent (26/65) of women with CD4 counts <200 cells/μl shortly after delivery had counts of >200 cells/μl at 6 weeks postpartum indicating that using CD4 count immediately postpartum as HAART eligibility criteria could lead to substantial misclassification of HAART eligibility. The magnitude of CD4 increase observed between delivery and postpartum was similar to other studies conducted in Africa.2–4,7 In our study, the proportion with CD4 counts <200 and <350 cells/μl at delivery was 15.4% and 39.2%, respectively, and this decreased to 10.2% and 26.7%, respectively, by 6 weeks.
A study conducted in Ivory Coast with similar sample size also found similar results where 17.8% and 48.3% of the women had CD4 <200 and <350 cells/μl at 32 weeks gestation but at 1 month postpartum the proportion decreased to 9.5% and 28.9%, respectively.7 Our study showed that CD4 200 and 350 cells/μl cut-off points at delivery correctly identified only 60% (95% CI 47–72) and 61% (95% CI 54–69) of women who had CD4 counts less than the same cut-off at 6 weeks. Of note, our study provides additional information regarding the association between CD4 counts obtained early during the postpartum period and risk of progression of HIV. We demonstrated that those who were HAART eligible based on CD4 counts at delivery but no longer so based on values at 6 weeks had a lower risk of Stage 3 or 4 opportunistic infections or death compared to those who were persistently HAART eligible at both time points.
The initiation of HAART is based on clinical, immunologic, and virologic indications, which in turn are associated with risk of progression of HIV. The consequences of initiation of HAART in those who are not in need of it for their health include potential unnecessary adverse events and depletion of scarce resources in settings with limited treatment options.10,11,16 In our study, the proportion of women who were HAART eligible by the CD4 200 and 350 cells/μl cut-off points was almost identical at delivery and 12 months, meaning that values at delivery are a reflection of CD4 counts after 1 year. This finding was not surprising since it is known that CD4 counts decrease on average 75 cells/μl per year1 and the median increase in CD4 counts between delivery and 6 weeks that we observed was 70 cells/μl. Also, more than half of the women who were HAART eligible at delivery but no longer so at 6 weeks were not HAART eligible even by 12 months.
The following may be proposed as possible solutions to correctly identify women during the postpartum period who would be in need of long-term HAART. First, although predictive values are known to be influenced by prevalence, we investigated the possibility that use of lower CD4 values than the conventional CD4 cut-off points for HAART eligibility at delivery might lead to less misclassification or higher positive predictive values. Compared to the conventional cut-off point of CD4 200 cells/μl, use of 175 cells/μl increased the positive predictive value from 60% to 72% while maintaining the same sensitivity of 91%. However, compared to the 350 CD4 cell/μl cut-off point, the positive predictive value increased only from 61% to 68% by using a 300 CD4 cell/μl cut-off point at the expense of lowering the sensitivity from 90% to 78%. Because this low level of sensitivity is not acceptable, we could not identify any cut-off point that might be of any benefit for the CD4 350 cells/μl cut-off point. Since women generally return to the clinic at 6 weeks for a postnatal health care visit for themselves and a vaccination for their infant, this would be a convenient time to check their CD4 count and then make a treatment decision. Finally, it has been reported that CD4 percentage rather than absolute CD4 value is a better indicator of immune status as it remains stable even in the presence of hemodilution.2,7 CD4 percentage is used to decide HAART eligibility in children17 and further studies on the utility of this indicator in pregnant and postpartum women in deciding HAART eligibility are warranted.
Acknowledgments
The ZVITAMBO project was supported by the Canadian International Development Agency (CIDA) (R/C Project 690/M3688), United States Agency for International Development (USAID) (cooperative agreement number HRN-A-00-97-00015-00 between Johns Hopkins University and the Office of Health and Nutrition–USAID), and a grant from the Bill and Melinda Gates Foundation, Seattle, WA. Additional funding was received from the Rockefeller Foundation (New York) and BASF (Ludwigshafen, Germany). Members of the ZVITAMBO Study Group, in addition to the named authors, are Henry Chidawanyika, John Hargrove, Agnes I. Mahomva, Florence Majo, Lucie C. Malaba, Michael T. Mbizvo, Faith Mzengeza, Kusum J. Nathoo, Mary Ndhlovu, Ellen Piwoz, Lidia Propper, Phillipa Rambanepasi, Naume Tavengwa, Brian J. Ward, Lynn S. Zijenah, Clare D. Zunguza, and Partson Zvandasara.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hunter GW. Hunter's Tropical Medicine and Emerging Infectious Diseases. 8th. W.B. Saunders Company; Philadelphia: 2000. [Google Scholar]
- 2.Miotti PG. Liomba G. Dallabetta GA. Hoover DR. Chiphangwi JD. Saah AJ. T lymphocyte subsets during and after pregnancy: Analysis in human immunodeficiency virus type 1-infected and -uninfected Malawian mothers. J Infect Dis. 1992;165(6):1116–1119. doi: 10.1093/infdis/165.6.1116. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim T. Moodley J. Doorasamy T. Lymphocyte changes in pregnancy: A comparison of the human immunodeficiency virus infected and non-infected women. J Obstet Gynecol. 2004;24(5):498–503. doi: 10.1080/01443610410001722509. [DOI] [PubMed] [Google Scholar]
- 4.Temmerman M. Nagelkerke N. Bwayo J. Chomba EN. Ndinya-Achola J. Piot P. HIV-1 and immunological changes during pregnancy: A comparison between HIV-1-seropositive and HIV-1-seronegative women in Nairobi, Kenya. AIDS. 1995;9(9):1057–1060. [PubMed] [Google Scholar]
- 5.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2006. [PubMed]
- 6.WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings. 2006.
- 7.Ekouevi DK. Inwoley A. Tonwe-Gold B, et al. Variation of CD4 count and percentage during pregnancy and after delivery: Implications for HAART initiation in resource-limited settings. AIDS Res Hum Retroviruses. 2007;23(12):1469–1474. doi: 10.1089/aid.2007.0059. [DOI] [PubMed] [Google Scholar]
- 8.Zachariah R. Teck R. Ascurra O. Humblet P. Harries AD. Targeting CD4 testing to a clinical subgroup of patients could limit unnecessary CD4 measurements, premature antiretroviral treatment and costs in Thyolo District, Malawi. Trans R Soc Trop Med Hyg. 2006;100(1):24–31. doi: 10.1016/j.trstmh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Lynen L. Thai S. De Munter P, et al. The added value of a CD4 count to identify patients eligible for highly active antiretroviral therapy among HIV-positive adults in Cambodia. J Acquir Immune Defic Syndr. 2006;42(3):322–324. doi: 10.1097/01.qai.0000221682.37316.d5. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano M. Guidotti G. Andreotti M, et al. Triple antiretroviral prophylaxis administered during pregnancy and after delivery significantly reduces breast milk viral load: A study within the drug resource enhancement against AIDS and malnutrition program. J Acquir Immune Defic Syndr. 2007;44(3):286–291. doi: 10.1097/QAI.0b013e31802c5441. [DOI] [PubMed] [Google Scholar]
- 11.Colebunders R. Hodossy B. Burger D, et al. The effect of highly active antiretroviral treatment on viral load and antiretroviral drug levels in breast milk. AIDS. 2005;19(16):1912–1915. doi: 10.1097/01.aids.0000188428.33280.41. [DOI] [PubMed] [Google Scholar]
- 12.Van de Perre P. Manigart O. Meda N. Long-term reduction of HIV transmission from mother to breastfed child by antiretroviral agents: Are more drugs better than less? AIDS. 2001;15(5):658–659. doi: 10.1097/00002030-200103300-00021. [DOI] [PubMed] [Google Scholar]
- 13.Iliff PJ. Piwoz EG. Tavengwa NV, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19(7):699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey JH. Iliff PJ. Marinda ET, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193(6):860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- 15.Zvandasara P. Hargrove JW. Ntozini R, et al. Mortality and morbidity among postpartum HIV-positive and HIV-negative women in Zimbabwe: Risk factors, causes, and impact of single-dose postpartum vitamin A supplementation. J Acquir Immune Defic Syndr. 2006;43(1):107–116. doi: 10.1097/01.qai.0000229015.77569.c7. [DOI] [PubMed] [Google Scholar]
- 16.Bulterys M. Weidle PJ. Abrams EJ. Fowler MG. Combination antiretroviral therapy in African nursing mothers and drug exposure in their infants: New pharmacokinetic and virologic findings. J Infect Dis. 2005;192(5):709–712. doi: 10.1086/432490. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Antiretroviral therapy for HIV infection in infants and children: Towards universal access. 2006. [PubMed]