Abstract
Previous studies suggest that the novel protein kinase C (PKC) isoforms initiate a mitogen-activated protein kinase (MAPK) signaling cascade that regulates keratinocyte differentiation. However, assigning these functions has relied on treatment with pharmacologic inhibitors and/or manipulating kinase function using over-expression of wild-type or dominant-negative kinases. As these methods are not highly specific, an obligatory regulatory role for individual kinases has not been assigned. In this study, we use small interfering RNA knockdown to study the role of individual PKC isoforms as regulators of keratinocyte differentiation induced by the potent differentiating stimulus, 12-O-tetradecanoylphorbol-13-acetate (TPA). PKC-δ knockdown reduces TPA-activated involucrin promoter activity, nuclear activator protein-1 factor accumulation and binding to DNA, and cell morphology change. Knockdown of PKC downstream targets, including MEKK-1, MEK-6, MEK-3, or p38-δ, indicates that these kinases are required for these responses. Additional studies indicate that knockdown of PKC-η inhibits TPA-dependent involucrin promoter activation. In contrast, knockdown of PKC-α (a classical PKC isoform) or PKC-ε (a novel isoform) does not inhibit these TPA-dependent responses. Further studies indicate that PKC-δ is required for calcium and green tea polyphenol-dependent regulation of end responses. These findings are informative as they suggest an essential role for selected PKC and MAPK cascade enzymes in mediating a range of end responses to a range of differentiation stimuli in keratinocytes.
INTRODUCTION
The protein kinase C (PKC) kinases comprise a family of enzymes that have a key role in regulating cell growth and differentiation. PKC isoforms are classified into three groups (Newton, 1997). The classical PKC forms (α, β, and γ) are calcium, phospholipid, and diacylglycerol dependent; the novel PKCs (δ, ε η, and θ) are activated by diacylglycerol and phospholipids, but they do not respond directly to calcium; and the atypical PKCs (ζ and λ) are calcium and diacylglycerol independent but undergo allosteric activation (Nishizuka, 1992; Rosse et al., 2010). Epidermal keratino-cytes express the PKC α, βII, δ, ε, η, and ζ isoforms (Osada et al., 1990; Dlugosz et al., 1992; Gherzi et al., 1992; Matsui et al., 1992; Fisher et al., 1993; Shen et al., 2001; Hara et al., 2005). The role of these isoforms has been studied in cultured cells and animal models (Dlugosz and Yuspa, 1994; Acs et al., 2000; Denning et al., 2000; Wheeler et al., 2002; Verma et al., 2006; Aziz et al., 2009; Jerome-Morais et al., 2009). A major ongoing effort is assigning specific functions to individual PKC isoforms in regulating keratinocyte proliferation, differentiation, and apoptosis. This is difficult, as PKC isoforms are activated by common stimuli and share common substrates. For example, the classical (α) and the three novel (δ, ε, and η) PKC isoforms expressed in keratinocytes can be activated by the diacylglycerol analog, 12-O-tetradecanoylphorbol-13-acetate (TPA).
We and others have shown that the novel PKC isoforms stimulate keratinocyte differentiation. This is evidenced by an increase in differentiation-associated responses in the presence of increased nPKC expression (Eckert et al., 2004). These nPKCs, in turn, activate mitogen-activated protein kinase (MAPK) signaling, which results in increased nuclear levels of activator protein-1 (AP-1), C/EBP, and Sp1, and increased binding of these factors to target genes to increase transcription (Efimova and Eckert, 2000; Efimova et al., 2002; Eckert et al., 2004). PKC-δ is the most potent of these activators (Deucher et al., 2002) and its activity can be inhibited by a PKC-δ inhibitor, rottlerin (Efimova and Eckert, 2000; Efimova et al., 2004; Zhu et al., 2008). A complicating feature of these studies is that they rely on PKC isoform overexpression, expression of dominant-negative kinases, or the use of isoform-selective chemical inhibitors. Each of these approaches has deficiencies, including the fact that over-expression of a particular PKC isoform or a dominant-negative form may influence the activity of other isoforms, and the fact that most inhibitors are not specific for the target kinase. An example is dominant-negative PKC-δ that inhibits PKC-δ-, PKC-ε- and PKC-η-dependent human involucrin (hINV) promoter activation (Efimova and Eckert, 2000). This may be because dominant-negative PKC-δ may compete for common substrates with other PKC isoforms (Efimova and Eckert, 2000). Thus, these studies, in spite of their utility, do not adequately address the role of individual isoforms.
Previous studies also suggest that specific MAPK cascade enzymes, including MAPK kinase kinase-1 (MEKK-1), MAPK/extracellular signal-regulated kinase (ERK) kinase 3 (MEK-3), and p38-δ, are required for activation of differentiated gene expression in keratinocytes (Efimova et al., 1998). On the basis of these studies, we proposed that among the three p38 isoforms (α, β, and δ) that are expressed at reasonable levels in keratinocytes (Dashti et al., 2001a, b), p38-δ has the dominant role as a regulator of involucrin gene expression (Efimova et al., 1998, 2003). However, here again, assigning function relied on the use of wild-type and dominant-negative kinase overexpression, and kinase inhibitors (Efimova et al., 1998; Eckert et al., 2003, 2004, 2006).
In this study, as an alternative approach to avoid some of these pitfalls, we use small interfering RNA (siRNA) to reduce the level of individual PKC isoforms and individual MAPK cascade kinases, and then challenge the cells with TPA, a stable analog of diacylglycerol; calcium, a physiologic inducer of keratinocyte differentiation; and (-)-epigallocatechin-3-gallate (EGCG), a chemopreventive agent that stimulates keratinocyte differentiation (Efimova et al., 1998; Efimova and Eckert, 2000; Balasubramanian et al., 2002; Balasubramanian and Eckert, 2004). We examine the effect of reducing individual kinase level on keratinocyte morphology, nuclear transcription factor accumulation, and gene activation. These studies suggest that PKC-δ is a required mediator of the keratinocyte response to several differentiating agents, and that it controls a range of downstream biochemical and morphological end points. PKC-η, and the MAPK cascade enzymes, MEKK-1, MEK-6, and MEK-3, and p38-δ MAPK are also important. In contrast, PKC-α, PKC-ε, p38-α, and p38-β seem not to be the required mediators of these responses.
RESULTS
PKC-δ is required for stimulus-dependent hINV promoter activity
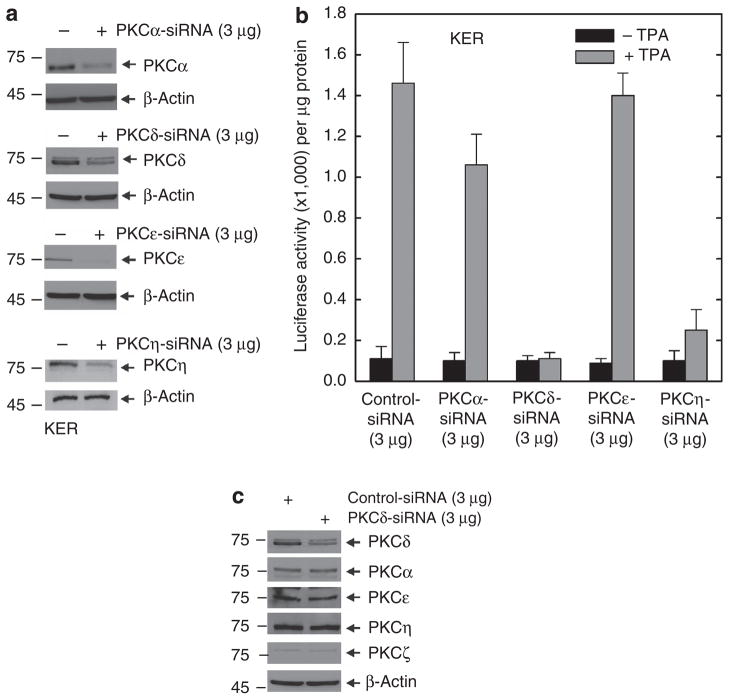
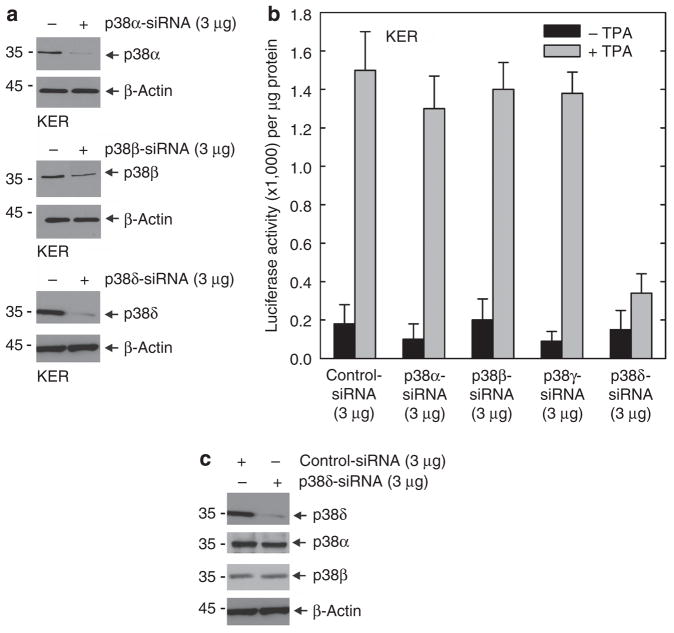
Previous studies show that overexpression of the novel PKC isoforms, δ, ε, or η, leads to increased hINV gene expression (Efimova et al., 2002). To distinguish which of these isoforms is mainly responsible for this regulation, we used siRNA-mediated knockdown. We used TPA as a differentiation stimulus, because TPA has a discrete mechanism of action, as a diacylglycerol mimic (Kazanietz, 2005), and because it is a strong inducer of morphological and biochemical differentiation in keratinocytes (Welter et al., 1995). As shown in Figure 1a and b, knockdown of PKC-δ or PKC-η reduces TPA-dependent hINV promoter activation, but knockdown of PKC-ε is without effect. We also examined the effect of a classical PKC isoform, PKC-α, and show that knockdown has a minimal effect on promoter activity. Thus, among the novel PKC isoforms, only PKC-δ and PKC-η are required as mediators of TPA-dependent hINV promoter activation. In contrast, basal promoter activity (absence of TPA treatment) is not influenced by knockdown of the PKC isoforms.
Figure 1. PKC-δ and PKC-η are required for the TPA-dependent hINV promoter activation.
(a) Knockdown of PKC isoform expression. Keratinocytes were electroporated with 3 μg of control siRNA or siRNA specifying the indicated PKC isoforms per 1 × 106 cells. Total cell extract was prepared at 48 hours after electroporation and 25 μg of protein was electrophoresed for immunodetection of each PKC isoform. For purposes of convenience, we routinely measured PKC levels at 48 hours after application of the siRNA; however, the levels remain reduced for up to 4 days (not shown). This experiment was repeated four times and the siRNA-dependent reduction ranged as follows: PKC-α, 50–70%; PKC-δ, 50–70%; PKC-ε, 80–90%, and PKC-η, 70–90%, as measured by densitometry. (b) PKC-δ is required for TPA-dependent promoter activation. At 48 hours after siRNA electroporation, cells were electroporated with 3 μg of endotoxin-free pINV-2473. After an additional 6 hours, the cells were treated with 50 ng ml−1 TPA and luciferase activity was assessed 18 hours later. The error bars represent the mean±SD of a representative experiment. Similar results were observed in each of four separate experiments. The t-test analysis reveals that PKC-δ and PKC-η siRNAs significantly suppress (P<0.01) TPA-stimulated promoter activity when compared with control siRNA, n = 4. The lesser reduction associated with PKC-α siRNA treatment was not significant (P<0.98). (c) PKC-δ knockdown does not influence the level of other PKC isoforms. Keratinocytes were electroporated with 3 μg of control siRNA or PKC-δ siRNA per 1 × 106 cells. Total cell extract was prepared at 48 hours after electroporation and 25 μg of protein was electrophoresed for immunodetection of each PKC isoform. hINV, human involucrin; PKC, protein kinase C; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
PKC-δ is required for nuclear accumulation of AP-1 transcription factors and change in cell morphology
PKC-δ was selected for detailed study because its mechanism of action in keratinocytes is heavily studied, it has a central role in controlling keratinocyte processes (Deucher et al., 2002; Wheeler et al., 2002; D’Costa and Denning, 2005; D’Costa et al., 2005; Sitailo et al., 2006; Zhu et al., 2008), and it is an effective regulator of involucrin gene expression (Efimova et al., 2002, 2004). We first examined whether the effect of reduced PKC-δ may be mediated through effects on expression of other PKC isoforms. As shown in Figure 1c, PKC-δ knockdown does not alter the level of other PKC isoforms.
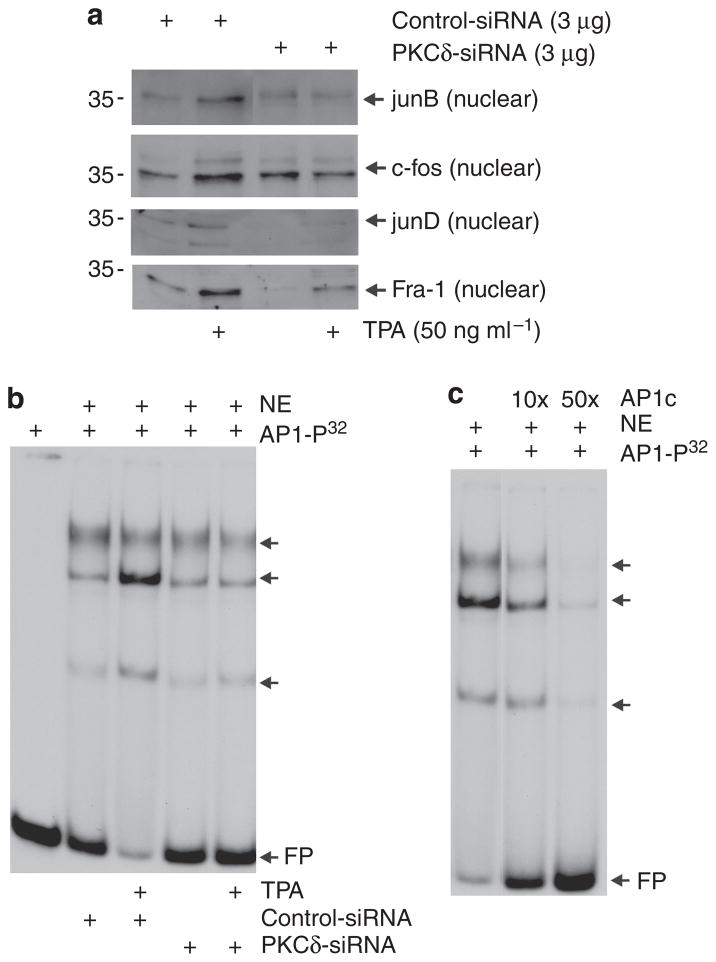
We next examined the effect of reduced PKC-δ level on TPA-dependent downstream responses. Activation of PKC and MAPK kinase signaling has been shown to cause AP-1 factor movement into the nucleus in keratinocytes (Welter et al., 1995). As shown in Figure 2a, TPA-dependent nuclear accumulation of junB, c-fos, junD, and Fos-related antigen-1 (Fra-1) (Welter et al., 1995) is attenuated when PKC-δ level is reduced. A key question is whether this decrease leads to reduced AP-1 factor interaction with binding sites on the hINV promoter. Figure 2b is a gel mobility shift assay that shows increased AP-1 factor DNA binding in response to TPA treatment, and this increase is reduced in extracts from PKC-δ knockdown cells. This is specific binding, as it is competed by radioinert AP-1 site oligonucleotide (Figure 2c).
Figure 2. PKC-δ knockdown reduces TPA-dependent nuclear accumulation of AP-1 factors.
Keratinocytes were electroporated with 3 μg of control or PKC-δ siRNA per 1 × 106 cells. After 24 hours, cells were treated with 0 or 50 ng ml−1 TPA for an additional 48 hours before preparation of nuclear extracts. (a) Nuclear extract (5 μg) was electrophoresed for immunological detection of junB, c-fos, junD, and Fra-1. (b) Reduced PKC-δ level is associated with reduced TPA-induced AP-1 factor DNA binding. Electrophoretic mobility shift assay was performed as outlined in the Materials and Methods. The arrows indicate bands that bind to 32P-AP-1 double-stranded oligonucleotide. (c) Nuclear extracts from cells treated with control siRNA and 50 ng ml−1 TPA were incubated with 32P-AP-1 in the presence of 10- or 50-fold excess of radioinert AP-1 double-stranded oligonucleotide before non-denaturing gel electrophoresis. The arrows indicate migration of the gel-shifted bands and FP indicates migration of the free probe. AP-1, activator protein-1; Fra-1, Fos-related antigen-1; FP, migration of free probe; NE, nuclear extract; PKC, protein kinase C; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
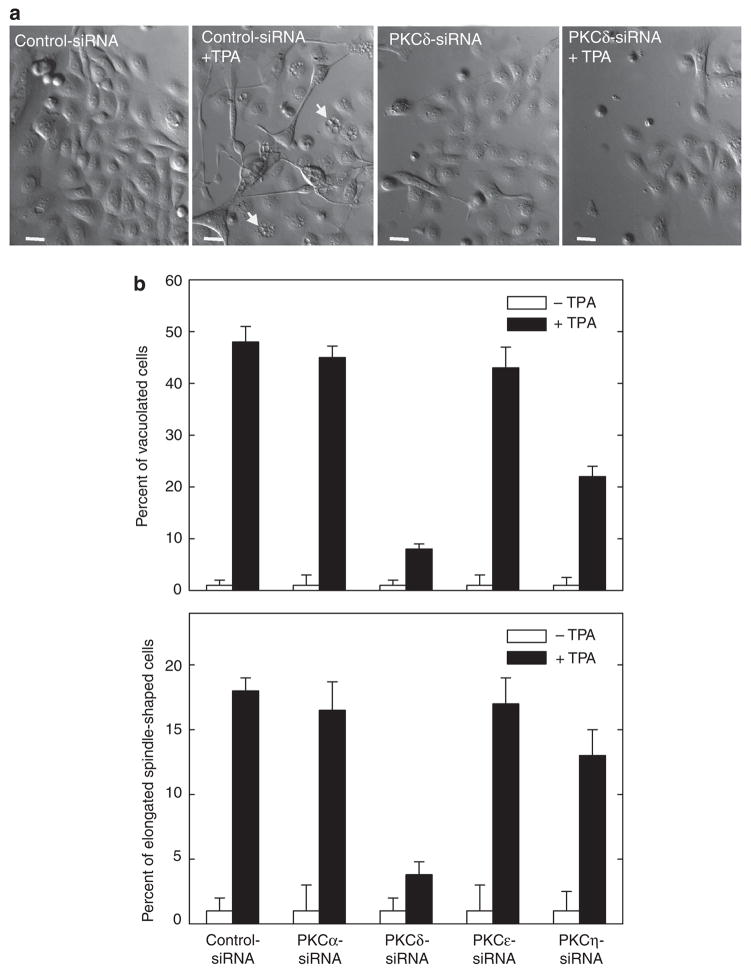
We next explored whether PKC-δ is required for other end responses. TPA treatment is associated with cell elongation and vesicle accumulation (Efimova and Eckert, 2000; Efimova et al., 2002, 2003). Figure 3a shows that these morphological changes are reduced by PKC-δ knockdown. For example, intracellular accumulation of vacuoles is associated with TPA treatment. Untreated cells lack these vacuoles, but approximately 45–50% of TPA-treated cells are vacuole positive (arrows). In the presence of PKC-δ siRNA, TPA-dependent vacuole formation is reduced to <7% of cells (Figure 3b). A similar reduction is observed for the formation of elongated spindle-shaped cells. Thus, these findings indicate that PKC-δ is required for TPA-dependent gene activation, nuclear AP-1 factor accumulation, and morphological response. We also compared the role of PKCs α, ε, and η in mediating TPA-dependent morphology change. Figure 3b shows that PKC-η knockdown partially reduces the morphological response, but that PKC-α or PKC-ε knockdown does not restore normal morphology.
Figure 3. PKC-δ knockdown reduces TPA effect on cell morphology.
(a) Keratinocytes were electroporated with 3 μg of control or PKC-δ siRNA per 1 × 106 cells. After 24 hours, cells were treated with 0 or 50 ng ml−1 TPA for an additional 48 hours before monitoring of cell morphology. Cells expressing normal PKC-δ level seem vacuolated and take on a spindle-shaped morphology, and this response is attenuated in PKC-δ siRNA-treated cells. The arrows indicate vacuolated cells. Bars = 25 μm in length. Phase-contrast images were obtained using an Olympus IX81 motorized inverted microscope (Hamburg, Germany) and a × 20 objective. (b) Keratinocytes were electroporated with 3 μg siRNA encoding the indicated PKC isoform and treated with TPA as indicated above, and after an additional 48 hours morphology was assessed. The plots were generated by determining the percentage of cells containing large intracellular vacuoles or showing an elongated spindle shape (see photographs) out of a minimum of 100 cells on each of three slides. The results are expressed as the mean±SD. This experiment is representative of four repeated experiments. The t-test analysis reveals that PKC-δ and PKC-η siRNAs significantly suppress (P<0.01) TPA-stimulated cell shape change when compared with control siRNA, n = 4. PKC, protein kinase C; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
PKC-δ is required for calcium- and EGCG-dependent regulation
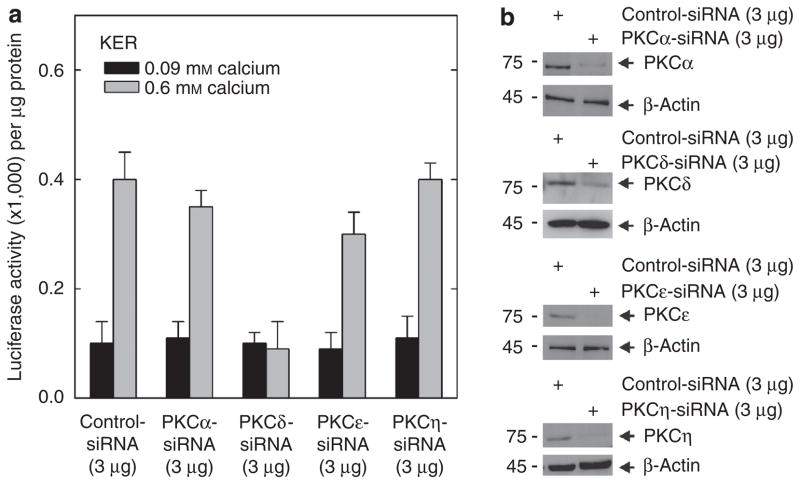
We also explored whether PKC-δ has a role in response to other stimuli. These include calcium, a physiological inducer of keratinocyte differentiation (Li et al., 1995; Denning et al., 2000; Bikle et al., 2001, 2002), and EGCG, a chemopreven-tive agent that is known to enhance keratinocyte differentiation (Balasubramanian et al., 2002; Balasubramanian and Eckert, 2004). Keratinocytes were treated with control or PKC isoform-specific siRNA before challenge with calcium. This resulted, as shown in Figure 4b, in the expected reduction in PKC isoform level. Figure 4a shows that calcium treatment produces a 4-fold increase in hINV promoter activity, and this increase is completely inhibited when PKC-δ level is reduced. In contrast, knockdown of the other PKC isoforms does not markedly attenuate the calcium-dependent increase in promoter activity, except that in some experiments knockdown of PKC-ε did have a minimal effect on promoter activity. Calcium treatment of keratinocytes also promotes morphology change (Kimura et al., 2007). However, the calcium-dependent change in cell morphology, observed in cells grown in high calcium (0.6 mM calcium) conditions, is not inhibited by knockdown of PKCs α, δ, ε, or η (not shown).
Figure 4. PKC-δ is required for the calcium-associated increase in hINV promoter activity.
(a) Keratinocytes were electroporated with 3 μg of control siRNA or the indicated PKC-specific siRNA per 1 × 106 cells. At 2 days after siRNA electroporation, the cells were trypsinized, collected by centrifugation, and electroporated with 3 μg of endotoxin-free hINV promoter-luciferase reporter plasmid. After 6 hours, the cells were treated with KSFM (0.09 mM calcium) or KSFM containing 0.6 mM calcium. After an additional 18 hours, the cells were harvested and extracts were assayed for luciferase activity. The results are expressed as the mean±SD. This experiment is representative of three repeated experiments. The t-test analysis reveals that PKC-δ siRNA significantly suppresses (P<0.01) calcium-stimulated promoter activity below the activity observed with control siRNA, n = 3. (b) Keratinocytes were treated with siRNA as indicated above and PKC isoform level was monitored after 48 hours. The experiment was repeated three times and the siRNA-dependent reduction in PKC isoform level ranged as follows: PKC-α, 50–70%; PKC-δ, 50–70%; PKC-ε, 80–90%, and PKC-η, 70–90%. hINV, human involucrin; KSFM, keratinocyte serum-free medium; PKC, protein kinase C; siRNA, small interfering RNA.
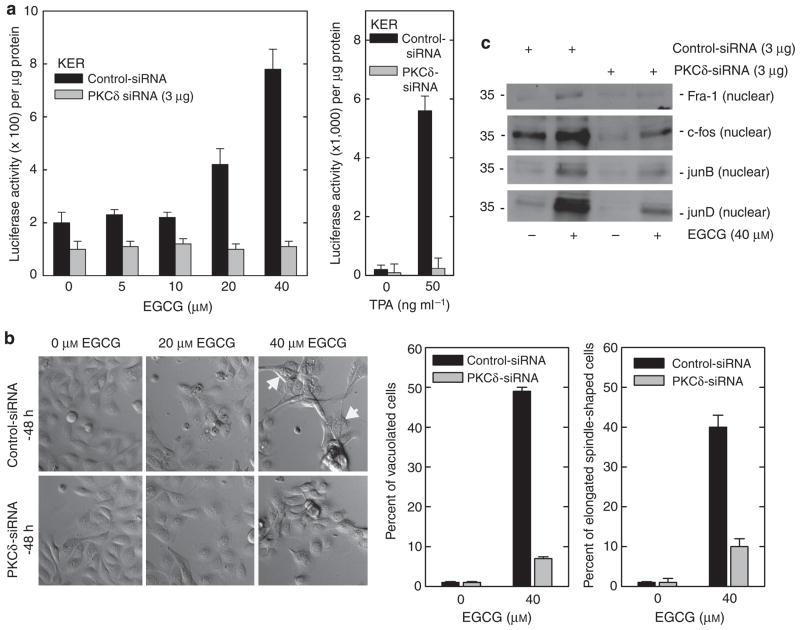
We have previously shown that EGCG increases hINV promoter activity through activation of MAPK signaling (Balasubramanian et al., 2002, 2005; Balasubramanian and Eckert, 2004). We therefore examined whether PKC-δ is required for this response. As shown in Figure 5a, PKC-δ knockdown completely eliminates EGCG-dependent promoter activation. Moreover, this is associated with a reduction in EGCG-dependent movement of AP-1 factors (Fra-1, c-fos, junB, and junD) into the nucleus (Figure 5c) and inhibition of EGCG-associated morphology change (Figure 5b). We also examined the effect of PKCs α, ε, and η siRNA on the EGCG-dependent promoter activity, cell vacuole, and cell spindle-shaped formation, and found no effect (Figure 6), suggesting that only the PKC-δ isoform is required for these responses.
Figure 5. PKC-δ is required for the EGCG-dependent hINV promoter activity, nuclear AP-1 factor accumulation, and morphology change.
(a) Keratinocytes were electroporated with 3 μg of control or PKC-δ specific siRNA per 1 × 106 cells. At 48 hours after siRNA electroporation, cells were electroporated with 3 μg of endotoxin-free pINV-2473. After an additional 6 hours, the cells were treated with 0–40 μM EGCG (left panel) or 50 ng ml−1 TPA (right panel) and luciferase activity was assessed 18 hours later. The results are expressed as the mean±SD. The t-test analysis reveals that PKC-δ siRNA significantly suppresses 20 and 40 μM EGCG-dependent promoter activity (P<0.01) below the activity observed with control siRNA, n = 3. (b) PKC-δ is required for EGCG-dependent morphological change. Cells expressing normal PKC-δ levels show a spindle-like shape and accumulate intracellular vacuoles, a response that is attenuated in PKC-δ-knockdown cells. The plots were generated by determining the percentage of cells containing large intracellular vacuoles or showing an elongated spindle shape (see photographs) out of a minimum of 100 cells on each of three slides. The results are expressed as the mean±SD. This experiment is representative of three repeated experiments. PKC-δ siRNA significantly suppresses (Po0.01) EGCG-stimulated cell shape change when compared with control siRNA, n = 3. Bar = 25 μm. (c) PKC-δ is required for EGCG-dependent nuclear accumulation of AP-1 factors. Nuclear extract (5 μg), prepared from cells treated with 3 mg of control or PKC-δ siRNA for 48 hours, was electrophoresed and nuclear levels of Fra-1, c-fos, junB, and junD were detected by immunoblot. AP-1, activator protein-1; EGCG, (-)-epigallocatechin-3-gallate; Fra-1, Fos-related antigen-1; PKC, protein kinase C; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
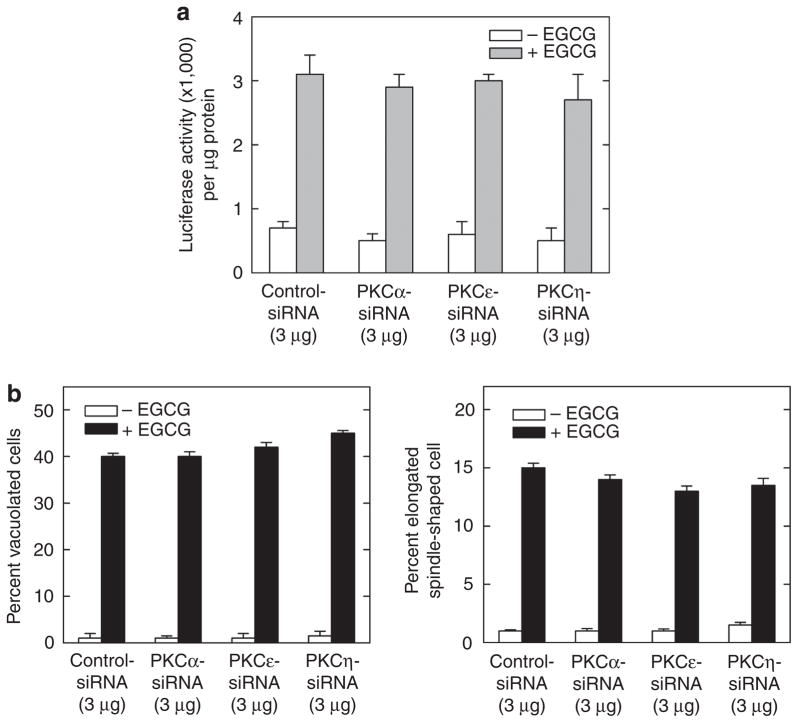
Figure 6. PKCs α, ε, and η are not required for EGCG-dependent hINV promoter activity or morphology change.
(a) PKC α, ε, and η isoforms are not required for EGCG-stimulated hINV promoter activity. Keratinocytes were electroporated with 3 μg of control or PKC-δ-specific siRNA per 1 × 106 cells. At 48 hours after siRNA electroporation, cells were electroporated with 3 μg of endotoxin-free pINV-2473. After an additional 6 hours, the cells were treated with 40 μM EGCG and luciferase activity was assessed 18 hours later. The results are expressed as the mean±SD. This experiment is representative of four repeated experiments. (b) PKC α, ε, and η isoforms are not required for EGCG-dependent morphology change. Keratinocytes were electroporated with siRNA encoding the indicated PKC isoform and treated with EGCG for 48 hours as indicated above and morphology was assessed. The plots were generated by determining the percentage of cells containing large intracellular vacuoles or showing an elongated spindle-shaped morphology out of a minimum of 100 cells on each of three slides. The results are expressed as the mean±SD. This experiment is representative of four repeated experiments. EGCG, (-)-epigallocatechin-3-gallate; hINV, human involucrin; PKC, protein kinase C; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
MAP kinase signaling and keratinocyte response to TPA treatment
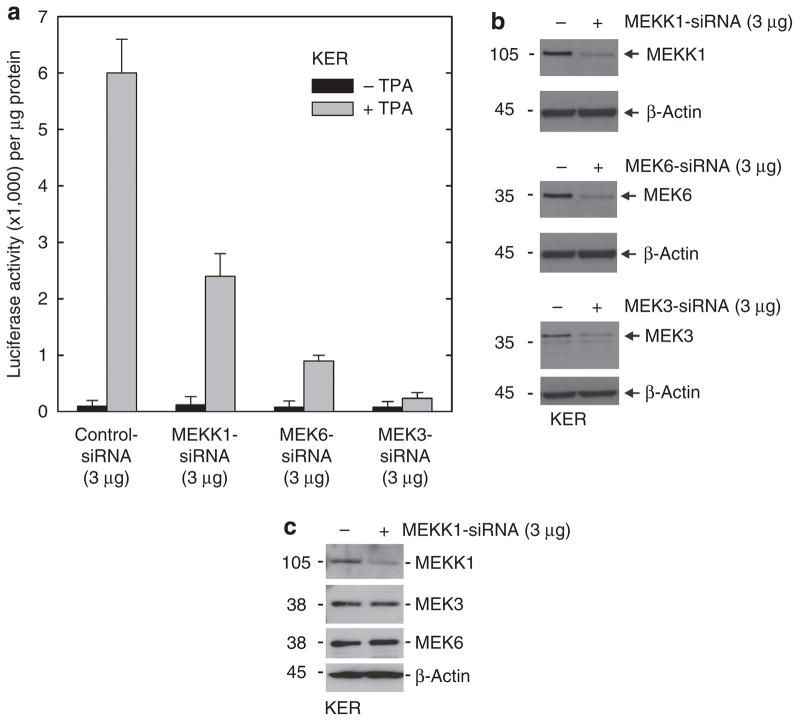
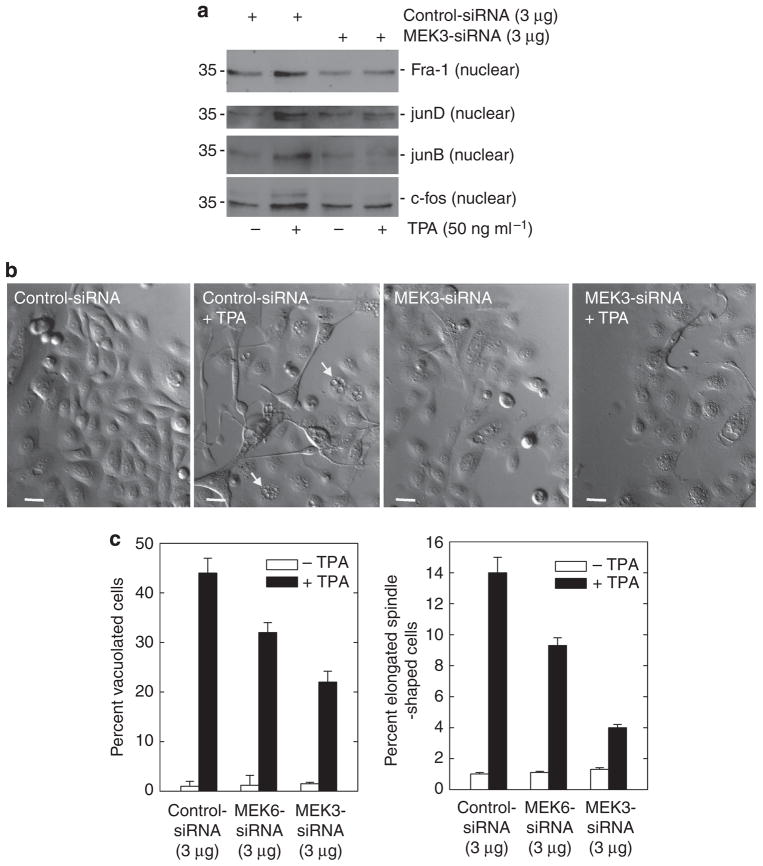
PKC-δ is thought to increase hINV promoter activity through activation of a signaling cascade that includes MEKK-1, MEK-6, and MEK-3 (Dashti et al., 2001a, b; Eckert et al., 2002). To assess the role of these kinases, we used siRNA to reduce MEKK-1, MEK-6, or MEK-3 level before challenge with TPA. MEKK-1 knockdown produces a 50% reduction in TPA-dependent hINV promoter activation (Figure 7a). MEK-6 knockdown produces an 80% reduction, and MEK-3 knockdown completely suppresses activity. Figure 7b confirms that the levels of MEKK-1, MEK-6, and MEK-3 are substantially reduced by the siRNA treatment. We performed extensive checks to assure siRNA specificity and in each case confirmed that the reduction was target specific. As an example, Figure 7c shows that siRNA knockdown of MEKK-1 does not alter MEK-3 or MEK-6 level. Similarly, MEK-3 or MEK-6 knockdown did not influence the level of the other kinases (not shown). Given that MEK-3 knockdown completely suppresses TPA-dependent hINV gene expression, we further studied the effect of MEK-3 knockdown on nuclear AP-1 factor level and cell morphology. As shown in Figure 8a, MEK-3 knockdown reduces TPA-dependent nuclear accumulation of Fra-1, junD, junB, and c-fos. Figure 8c shows that MEK-3 knockdown reduces TPA-dependent increase in cell vacuole formation by 60% and spindle shape by 50%. MEK-6 siRNA also partially attenuates the TPA effect on cell morphology (30–40%), but is not as effective as MEK-3 siRNA.
Figure 7. MEKK-1, MEK-6, and MEK-3 are required for TPA-dependent hINV promoter activation.
(a) Keratinocytes were electroporated with 3 μg of control siRNA or siRNA encoding MEKK-1, MEK-6, or MEK-3 per 1 × 106 cells. After 48 hours, cells were electroporated with 3 μg of endotoxin-free pINV-2473. After another 6 hours, the cells were treated with 50 ng ml−1 TPA and luciferase activity was assessed after 18 hours. The error bars represent the mean±SD of a representative experiment. The t-test analysis reveals that MEKK-1 (P<0.05), MEK-6 (P<0.01), and MEK-3 (P<0.01) siRNAs significantly suppress TPA-stimulated promoter activity when compared with control siRNA, n = 3. (b) Reduced MEKK-1, MEK-6, and MEK-3 levels. Total extract (25 μg) was prepared from cells 48 hours after treatment with siRNA and the indicated proteins were detected by immunoblot. This experiment was repeated four times and the siRNA-dependent reduction in level ranged as follows: MEKK-1, 70–90%; MEK-6, 70–90%; and MEK-3 70–90%, as measured by densitometry. (c) MEKK1 siRNA does not influence the levels of other MAPK cascade kinases. Keratinocytes were electroporated with 3 μg of control siRNA or siRNA encoding the MEKK-1 per 1 × 106 cells. After 48 hours, cells were harvested and the level of the indicated protein was monitored. Similar results were observed in each of three experiments. hINV, human involucrin; MEK, MAPK/ERK kinase; MEKK-1, MAPK kinase kinase-1; PKC, protein kinase C; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
Figure 8. MEK-3 knockdown reduces TPA-dependent nuclear AP-1 factor accumulation and morphological response.
Keratinocytes were electroporated with 3 mg of control or MEK-3 siRNA per 1 × 106 cells. After 24 hours, cells were treated with 50 ng ml−1 TPA for an additional 48 hours. (a) Nuclear extract (5 μg) was electrophoresed for detection of junB, c-fos, junD, and Fra-1. (b) MEK-3 siRNA reduces the effect of TPA treatment on keratinocyte morphology. Keratinocytes were electroporated with 3 μg of MEK-3 siRNA and then treated for an additional 48 hours with TPA before assessment of morphology. Bars = 25 μm. The arrows indicate cells containing large intracellular vacuoles. (c) Effect of MEK-6 and MEK-3 knockdown on TPA-induced keratinocyte morphology change. Keratinocytes were electroporated with 3 μg of specific siRNA and then treated with 50 ng ml−1 TPA for 48 hours before assessing the effect on morphology. The plots were generated by determining the percentage of cells containing large intracellular vacuoles or showing an elongated spindle shape (see pictures) out of a minimum of 100 cells on each of three slides. The results are expressed as the mean±SD. MEK-6 (P<0.05) and MEK-3 (P<0.01) siRNAs significantly suppress TPA-stimulated morphology change when compared with control siRNA, n = 3. AP-1, activator protein-1; Fra-1, Fos-related antigen-1; MEK, MAPK/ERK kinase; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
p38-δ is required for TPA-dependent regulation
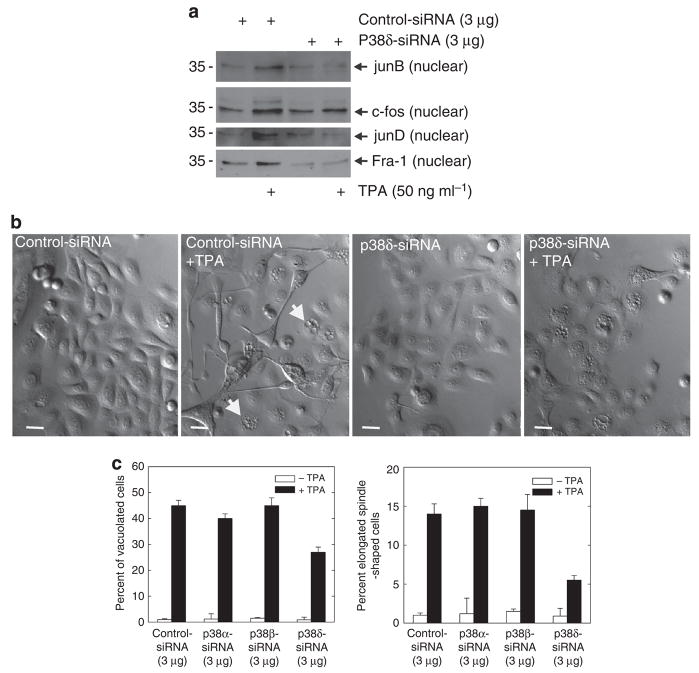
p38 MAPKs comprise a family of four structurally related kinases, α, β, δ, and γ, that have been implicated in regulating keratinocyte differentiation (Cobb, 1999; Chen et al., 2001). Previous studies suggest that p38-δ has an important role in regulating involucrin gene expression (Efimova et al., 2002, 2003). In the present experiments we use siRNA-directed knockdown of p38-δ to confirm this role. Figure 9a shows the successful knockdown of p38 α, β, and δ. p38-γ protein is not expressed in normal keratinocytes (Dashti et al., 2001b; Eckert et al., 2004). Figure 9b shows that p38-δ knockdown results in reduced TPA-dependent hINV promoter activity, but that reducing the level of the p38-α or p38-β isoforms is without effect. As expected, as p38-γ is not readily detected in keratinocytes, p38-γ siRNA did not influence promoter activity. Figure 9c confirms that the effect of p38-δ knockdown is not because of an effect on the level of other p38 isoforms. We next monitored the effect of p38-δ knockdown on TPA-dependent nuclear AP-1 factor accumulation and morphological change. Figure 10a shows that p38-δ knockdown reduces the TPA-dependent accumulation of junB, c-fos, junD, and Fra-1 in the nucleus. Figure 10b and c shows that p38-δ knockdown partially reverses the TPA effect on morphology. In addition, Figure 10c shows a graphical analysis that indicates that knockdown of p38-α or p38-β does not attenuate TPA-dependent changes in cell morphology, but that p38-δ knockdown partially reverses these changes.
Figure 9. p38-δ is required for the TPA-dependent hINV promoter activation.
(a) Knockdown of p38 isoform expression. Keratinocytes were electroporated with 3 μg of control siRNA or siRNA encoding the indicated p38 isoforms per 1 × 106 cells. Total cell extract was prepared at 48 hours after electroporation and 25 μg protein was electrophoresed for immunodetection of each p38 isoform. This experiment was repeated four times and the siRNA-dependent reduction in level ranged as follows: p38-α, 80–90%; p38-β, 70–90%; and p38-δ, 80–90%, as measured by densitometry. (b) p38-δ is required for TPA-dependent hINV promoter activation. At 48 hours after siRNA electroporation, cells were electroporated with 3 μg of endotoxin-free pINV-2473. After an additional 6 hours, the cells were treated with 50 ng ml−1 TPA and luciferase activity was assessed 18 hours later. The results are expressed as the mean±SD. p38-δ siRNA significantly suppresses (P<0.01) TPA-stimulated promoter activity below the activity observed with control siRNA, n = 3. (c) p38-δ knockdown does not influence the level of other p38 isoforms. Keratinocytes were electroporated with 3 μg of control or p38-δ isoform-specific siRNA per 1 × 106 cells. Total cell extract was prepared at 48 hours after electroporation and 25 μg of protein was electrophoresed for immunodetection of each p38 isoform. This experiment was repeated twice with similar results. hINV, human involucrin; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
Figure 10. p38-δ knockdown affects nuclear AP-1 factor level and cell morphology.
(a) p38-δ is required for TPA-dependent nuclear localization of AP-1 factors. Keratinocytes were electroporated with 3 mg of control or p38-δ siRNA per 1 × 106 cells. After 24 hours, cells were treated with 50 ng ml−1 TPA for an additional 48 hours. Nuclear extract (5 μg) was electrophoresed for detection of junB, c-fos, junD, and Fra-1. (b) p38-δ knockdown reduces the effect of TPA on keratinocyte morphology. Keratinocytes were electroporated with 3 mg of control or p38-δ siRNA per 1 × 106 cells. After 24 hours, cells were treated with 50 ng ml−1 TPA for an additional 48 hours. Bars = 25 μm. The arrows indicate vacuolated cells. (c) p38-α and p38-β knockdown does not reduce the effect of TPA on keratinocyte morphology. Keratinocytes were treated with siRNA and TPA as indicated above before assessment of cell morphology. The plots were generated by determining the percentage of cells containing large intracellular vacuoles or showing an elongated spindle-shaped morphology out of a minimum of 100 cells on each of three slides. The results are expressed as the mean±SD. p38-δ siRNA significantly suppresses (P<0.01) TPA-stimulated morphology change below the activity observed with control siRNA, n = 3. AP-1, activator protein-1; Fra-1, Fos-related antigen-1; siRNA, small interfering RNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
DISCUSSION
PKC-δ is required for TPA-dependent responses in keratinocytes
Keratinocytes express six PKC isoforms, including PKCs α, βII, δ, ε, η, and ζ (Osada et al., 1990; Dlugosz et al., 1992; Gherzi et al., 1992; Matsui et al., 1992; Fisher et al., 1993; Shen et al., 2001; Hara et al., 2005). On the basis of their primary sequence, response to stimuli, and unique cofactor dependence, individual PKC isoforms are expected to have different roles (Newton, 1997; Mellor and Parker, 1998). However, individual PKC isoforms can be activated by common ligands, use common substrates, and share common downstream targets. For this reason, identifying the role of individual PKC isoforms in keratinocytes is complicated. To assign functions for these kinases, we previously used the approach of overexpressing dominant-negative or wild-type PKC isoforms and then monitored the effect on downstream end points (Deucher et al., 2002; Efimova et al., 2002, 2004). We have also used pharmacologic inhibitors of individual PKC isoforms (Deucher et al., 2002; Efimova et al., 2002, 2004). These studies show that overexpressed novel PKC isoforms (δ, ε, and η) increase hINV protein and mRNA level, and hINV promoter activity, and that treatment with chemical inhibitors or dominant-negative PKC isoforms attenuates these responses (Efimova et al., 1998, 2002; Efimova and Eckert, 2000). However, analysis of this type of data is complicated because of potential cross-effects and the fact that the inhibitors are not necessarily specific.
PKC-δ is required for TPA-mediated responses
To gain additional insight regarding which PKC isoforms are required for stimulus-dependent regulation, we treated keratinocytes with TPA in the presence of siRNA designed to block expression of individual PKC isoforms. TPA was selected because it is a potent inducer of keratinocyte differentiation (Sharkey et al., 1984; Ashendel, 1985; Ono et al., 1989; Nishizuka, 1992; Gschwendt et al., 1994), and is an analog of the physiological regulator of classical and novel PKC isoform, diacylglycerol, and thereby has a defined action (Nishizuka, 1992). Treating human keratinocytes with TPA increases cell differentiation; increases hINV gene expression, promoter activity, and mRNA level; and alters cell morphology (Yaar et al., 1993; Welter and Eckert, 1995). Our knockdown studies indicate that PKC-δ is required for a range of these TPA-induced responses, including AP-1 factor accumulation in the nucleus, AP-1 factor binding to the hINV promoter AP-1 site, hINV promoter activation, and morphology change. This is particularly interesting, because it implicates PKC-δ in a wide range of cell responses. Moreover, our data suggest that we have only achieved a partial (approximately 80%) reduction in PKC-δ level. This suggests that a threshold level of PKC-δ may be required for response.
We also observed that PKC-η knockdown attenuates TPA-stimulated hINV promoter activity and morphology change. Regarding hINV promoter activation, both PKC-η and PKC-δ are required for this response. Given that this is the case, it is not clear why, when PKC-δ is reduced, the response is not mediated by PKC-η and vice versa. It is possible that two independent pathways (one PKC-δ dependent and the other PKC-η dependent) may be required to activate hINV gene expression or that PKC-δ and PKC-η exist in a single common pathway—perhaps through direct interaction. PKC-η is also required for the morphological response to TPA; however, it seems to be less important than PKC-δ with respect to regulation of cell morphology. In contrast, PKC-α or PKC-ε knockdown does not attenuate any of the responses.
PKC-δ is required for response to other agents
An important question is whether PKC-δ is also a required mediator of response to other agents that regulate keratino-cyte differentiation. Our previous studies show that EGCG treatment stimulates hINV promoter activity and cell morphological change (Balasubramanian et al., 2002; Balasubramanian and Eckert, 2004). These responses are mediated through a MEKK-1, MEK-3, and p38-δ pathway that stimulates increased AP-1 factor level and AP-1 factor binding to the hINV promoter, leading to increased hINV gene expression (Balasubramanian et al., 2002). In this study we show that PKC-δ knockdown eliminates EGCG-dependent responses, including hINV promoter activation, nuclear AP-1 factor accumulation, and formation of elongated and vacuolated cell morphology. These findings suggest that PKC-δ is required for keratinocyte response to two agents, TPA and EGCG, which regulate differentiation. We also monitored the effect of PKC-δ knockdown on calcium-dependent involucrin promoter activity. We and others have shown that calcium treatment increases endogenous hINV level and promoter activity (Bikle et al., 2001; Deucher et al., 2002). These studies indicate that PKC-α inhibits (Deucher et al., 2002) or activates (Bikle et al., 2001) hINV promoter activity, and that PKC-δ increases hINV protein level and promoter activity (Efimova and Eckert, 2000). These knockdown experiments indicate that PKC-δ is required for the calcium-dependent increase in hINV promoter activity, but suggest that PKC-α is not required. Although PKC has been reported to regulate desmosome formation (Sheu et al., 1989; Amar et al., 1998; Kitajima et al., 1999; Alt et al., 2001), we noted no effect of knockdown of any PKC isoform on calcium-associated morphology change.
MEKK-1 and MEK-3 are required for TPA-dependent regulation
The MAPKs are important PKC targets. These kinases are divided into families based on shared sequence homology and function, and include ERK kinases (ERK1/2), p38 kinases (p38 α, β, δ, and γ), and c-Jun N-terminal kinase (JNK) kinases (JNK1/2) (Cobb, 1999; Cobb and Goldsmith, 2000; Raman et al., 2007). MAPKs are regulated by a three-kinase module that includes a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK), and a MAPK (Chang and Karin, 2001; Eckert et al., 2003; D’Costa and Denning, 2005). These kinases are important regulators of keratinocyte proliferation, differentiation, and survival. In general, activation of p38-δ MAPK increases keratinocyte differentiation, whereas activation of ERK1/2 promotes survival (Eckert et al., 2002, 2003). Our previous studies, using overexpression of wild-type and dominant-negative kinases and pharmacologic inhibitors, suggest a role for MEKK-1, MEK-6, and MEK-3 in regulating hINV gene expression (Efimova et al., 1998, 2003; Eckert et al., 2002; Kraft et al., 2007). These studies show that treatment with TPA, overexpression of activated Ras (RasG12V), or overexpres sion of wild-type MEKK-1 increase hINV gene expression. Moreover, these events are inhibited by dominant-negative MEKK-1 (Efimova et al., 1998). However, these studies are complicated by the fact that they rely on overexpression. This study provides evidence that MEKK-1 is important in this pathway, as knockdown of MEKK-1 attenuates hINV promoter activation in response to TPA treatment. Thus, MEKK-1 seems to be a key part of the TPA-triggered signaling cascade that leads to increased hINV promoter activity. It will be interesting in future studies to examine the role of other MEKK kinases (e.g., MEKK-2, MEKK-3, etc.) in this regulation, as various studies suggest a role for these kinases as alternate upstream activators of p38 MAPK (Uhlik et al., 2004).
Our previous study shows that dominant-negative MEK-3 inhibits TPA-dependent hINV promoter activity (Efimova et al., 1998). Our present MEK-3 knockdown experiments confirm a required role for MEK-3 in TPA-dependent hINV promoter activation. We also show a substantial reduction in TPA-dependent hINV promoter activity in MEK-6 knockdown cells. Knocking down MEK-3 or MEK-6 also reduces TPA-dependent morphology change. These results suggest that both MEK-3 and MEK-6 have a role in activating p38-δ that leads to downstream changes in gene expression and cell morphology. It is interesting that knockdown of either MEK-3 or MEK-6 reduces hINV promoter response. This is surprising because MEK-3 could be expected to compensate for the loss of MEK-6 and vice versa. Such a finding suggests that these kinases operate in parallel essential signaling cascades or are part of the same signaling cascade.
p38-δ mediates TPA-associated responses
p38 MAPKs respond to a wide range of extracellular cues, particularly cellular stressors such as UV radiation, osmotic shock, hypoxia, pro-inflammatory cytokines, and less often growth factors. There are four p38 family members (α, β, δ, and γ), and among them p38-α is the most widely studied (Raman et al., 2007). p38 isoforms are activated by MEK-3 and MEK-6 through dual phosphorylation of p38 active motif serine and threonine residues. It is interesting that although p38 isoforms are 40% identical to other MAPKs (ERK1/2, etc.), they share only approximately 60% identity among themselves. This suggests diverse functions. p38-α and p38-β are ubiquitously expressed and are inhibited by pyridinyl imidazole compounds, whereas expression of p38-δ and p38-γ are not inhibited by these agents and have a more limited tissue distribution.
Keratinocytes express p38 α, β, and δ (Dashti et al., 2001a, b), and qPCR studies reveal that the mRNA encoding these isoforms is present in the following ratio: p38-α 1.0, p38-β 0.5, p38-δ 1.0, and p38γ 0.05 (not shown). Although p38-γ-encoding RNA is present, the level is low and, as previously described, the p38-γ protein is not detected (Efimova et al., 2003, 2004; Kraft et al., 2007). Thus, the present studies focus on p38 α, β, and δ. Previous studies suggest that p38-δ activation is associated with increased hINV promoter activity and keratinocyte differentiation (Efimova et al., 2003, 2004; Kraft et al., 2007). This regulation involves a p38-δ-ERK1/2 complex, and overexpressed p38-δ inhibits ERK1/2 activity without reducing ERK1/2 level, suggesting that p38-δ may directly suppress ERK1/2 function (Eckert et al., 2002, 2003). We were able to rule out a role for p38-α or p38-β based on inhibitor studies (Efimova et al., 1998). We also used dominant-negative p38-α as a tool to study the response and showed that dominant-negative p38-α inhibits hINV promoter activation, in response to treatment with TPA or because of overexpression of constitutive active Ras (RasG12V) or wild-type MEKK-1 (Efimova et al., 1998). However, this analysis is problematic, as dominant-negative p38-α may inhibit the function of all p38 isoforms. The present knockdown studies provide additional clarity, as we show that p38-δ knockdown suppresses TPA-dependent increase in hINV promoter activity and nuclear accumulation of AP-1 factors, but that p38-α and p38-β knockdown does not attenuate these responses. These findings suggest that p38-δ is an important mediator of these events.
In summary, our findings suggest that PKC-δ, and to a lesser extent, PKC-η, are key regulators of the keratinocyte response to TPA, EGCG, and calcium. Moreover, downstream kinases, including MEKK-1, MEK-6, MEK-3, and p38-δ, are also important mediators in this signaling cascade, but p38-α and p38-β do not seem to have a role. A striking finding is that these kinases are required mediators for a range of responses, including gene activation events, nuclear transcription factor accumulation and binding to target sites, and morphology change, and are also required for response to a range of agents (calcium, green tea, and phorbol ester). Taken together, these findings suggest that these enzymes are central controllers of normal keratinocyte function.
MATERIALS AND METHODS
Chemicals and Reagents
Keratinocyte serum-free medium and trypsin was purchased from Invitrogen (Carlsbad, CA). Phorbol ester (TPA) and dimethylsulfoxide were obtained from Sigma-Aldrich (St Louis, MO). pGL2-basic plasmid and the chemiluminescent luciferase assay system were purchased from Promega (Madison, WI), and chemiluminescence was measured using a Berthold luminometer (Wildbad, Germany). The involucrin-specific polyclonal antibody was previously described (LaCelle et al., 1998). Anti-β-actin was purchased from Sigma-Aldrich (A5441). Rabbit polyclonal antibodies specifying c-fos (sc-52X), fosB (sc-48X), Fra-1 (sc-605X) JunB (sc-46X) c-jun (sc-1694X), and JunD (sc-74X) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for PKC-α (sc-208), PKC-δ (sc-937), PKC-ε (sc-214), PKC-ζ (sc-7262), PKC-η (sc-215), MEKK-1 (sc-252), MEK-6 (sc-6073), MEK3 (sc-960), p38-α (sc-7972), p38-β (sc-6176), p38-γ (sc-33690), and p38-δ (sc-7587) were obtained from Santa Cruz Biotechnology. Peroxidase-conjugated anti-mouse IgG (NXA931) and peroxidase-conjugated anti-rabbit IgG (NA934V) were obtained from GE Healthcare (Buckinghamshire, UK). The hINV gene expression reporter plasmids were previously described (Welter et al., 1996; Efimova et al., 1998). Gene-specific siRNA were purchased from Santa Cruz as follows: control (sc-37007), PKC-α (sc-36243), PKC-δ (sc-36253), PKC-ε (sc-36251), PKC-η (sc-44019), MEKK-I (sc-35898), MEK-6 (sc-35913), MEK-3 (sc-35907), p38-α (sc-29433), p38-β (sc-39116), p38-δ (sc-36456), and p38-γ (sc-39118). Additional siRNA, including PKC-δ (J-003524–08), p38-β (J-003972–12), and p38-δ (J-003591–09), were from Dharmacon (Lafayette, CO). EGCG was obtained from Sigma.
Immunological methods
For immunoblot, equivalent amounts of protein was electrophoresed on denaturing and reducing 8% polyacrylamide gels and transferred to nitrocellulose membrane. The membrane was blocked by 5% nonfat dry milk and then incubated with appropriate primary and secondary antibody. Antibody binding was visualized using chemiluminescence detection reagents.
Keratinocyte electroporation
Keratinocytes, prepared from human foreskin samples, were electro-porated with siRNA or plasmids using the Amaxa electroporator and the VPD-1002 nucleofection kit (Amaxa, Cologne, Germany). For electroporation, keratinocytes were harvested with trypsin and replated 1 day before use. On the day of electroporation, 1 3 106 of the replated cells were harvested with trypsin and resuspended in keratinocyte serum-free medium. The cells are collected at 2,000 3 g, washed with 1 ml of sterile phosphate-buffered saline (pH 7.5), and suspended in 100 μl of keratinocyte nucleofection solution. The cell suspension, which included 3 μg of gene-specific siRNA, was mixed by gentle up and down pipetting and electro-porated using the T-018 settings. Warm keratinocyte serum-free medium (500 μl) was added and the suspension was transferred to a 9.6 cm2 cell culture plate containing 1.5 ml of keratinocyte serum-free medium. Reduced level of the siRNA targeted protein was confirmed by immunoblot. In all cases, maximal reduction was observed by 48 hours and the reduced level was maintained until at least 96 hours.
Promoter activity
At 2 days after siRNA electroporation, cells were trypsinized, collected by centrifugation, and electroporated with 3 mg of endotoxin-free hINV promoter-luciferase reporter plasmid (pINV-2473) (Welter et al., 1995). After 6 hours, the cells were treated with 50 ng ml−1 TPA and after an additional 18 hours the cells were washed with phosphate-buffered saline (pH 7.5) and scraped into 120 μl of cell lysis buffer before assay of luciferase activity. All assays were performed in duplicate, and each experiment was repeated a minimum of three times. Luciferase activity was normalized as previously described (Efimova et al., 2002).
Nuclear extract preparation and gel mobility shift assay
Keratinocytes were electroporated with 3 μg of siRNA and plated in 58 cm2 surface area (100 mm) dishes. After 24 hours, cells were incubated with EGCG (0–40 μM) or TPA (0–50 ng ml−1) for 48 hours. The cells were then washed with phosphate-buffered saline and nuclear extract was prepared (Welter et al., 1995). Transcription factors binding to the AP-1 site was assayed by gel electrophoretic mobility shift (Welter et al., 1995). Nuclear extract protein (3 μg) was incubated for 25 minutes at 25 ºC in a total volume of 20 μl containing 20 mM HEPES (pH 7.5), 10% glycerol, 50 mM KCl, 2 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 1 μg ml−1 poly (dI-dC), 0.1 mg ml−1 bovine serum albumin, and 50,000 c.p.m. of 32P-labeled AP-1 site (5′-CGCTTGATGAGTCAGCCGGAA-3′) (Welter et al., 1995). The bold nucleotides indicate the consensus AP-1 DNA-binding site. For gel mobility supershift assay, AP-1 factor-specific antibodies (2 μg) were added to the reaction mixture and incubated at 4 ºC for 45 minutes. The 32P-labeled AP-1 probe was then added and the mixture was incubated for 30 minutes at room temperature. Protein–DNA complexes were then separated in a non-denaturing 6% polyacrylamide gel and band migration was detected by autoradiography.
Acknowledgments
This research was supported by the National Institutes of Health grant R01 AR046494 to R Eckert.
Abbreviations
- AP-1
activator protein-1
- EGCG
(-)-epigallocatechin-3-gallate
- ERK
extracellular signal-regulated kinase
- Fra-1
Fos-related antigen-1
- hINV
human involucrin
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- PKC
protein kinase C
- siRNA
small interfering RNA
- TPA
12-O-tetradecanoylphorbol-13-acetate
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Acs P, Beheshti M, Szallasi Z, et al. Effect of a tyrosine 155 to phenylalanine mutation of protein kinase cdelta on the proliferative and tumorigenic properties of NIH 3T3 fibroblasts. Carcinogenesis. 2000;21:887–91. doi: 10.1093/carcin/21.5.887. [DOI] [PubMed] [Google Scholar]
- Alt A, Ohba M, Li L, et al. Protein kinase Cdelta-mediated phosphorylation of alpha6beta4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res. 2001;61:4591–8. [PubMed] [Google Scholar]
- Amar LS, Shabana A, Oboeuf M, et al. Desmosomes are regulated by protein kinase C in primary rat epithelial cells. Cell Adhes Commun. 1998;5:1–12. doi: 10.3109/15419069809005594. [DOI] [PubMed] [Google Scholar]
- Ashendel CL. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985;822:219–42. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Sundling KE, Dreckschmidt NE, et al. Protein kinase Cepsilon inhibits UVR-induced expression of FADD, an adaptor protein, linked to both Fas- and TNFR1-mediated apoptosis. J Invest Dermatol. 2009;129:2011–21. doi: 10.1038/jid.2008.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Eckert RL. Green tea polyphenol and curcumin inversely regulate human involucrin promoter activity via opposing effects on CCAAT/enhancer-binding protein function. J Biol Chem. 2004;279:24007–14. doi: 10.1074/jbc.M314331200. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J Biol Chem. 2002;277:1828–36. doi: 10.1074/jbc.M110376200. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Sturniolo MT, Dubyak GR, et al. Human epidermal keratinocytes undergo (-)-epigallocatechin-3-gallate-dependent differentiation but not apoptosis. Carcinogenesis. 2005;26:1100–8. doi: 10.1093/carcin/bgi048. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Oda Y, et al. The vitamin D response element of the involucrin gene mediates its regulation by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2002;119:1109–13. doi: 10.1046/j.1523-1747.2002.19508.x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Tu CL, et al. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–71. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, et al. MAP kinases. Chem Rev. 2001;101:2449–76. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. Dimerization in MAP-kinase signaling. Trends Biochem Sci. 2000;25:7–9. doi: 10.1016/s0968-0004(99)01508-x. [DOI] [PubMed] [Google Scholar]
- D’Costa AM, Denning MF. A caspase-resistant mutant of PKC-δelta protects keratinocytes from UV-induced apoptosis. Cell Death Differ. 2005;12:224–32. doi: 10.1038/sj.cdd.4401558. [DOI] [PubMed] [Google Scholar]
- D’Costa AM, Robinson JK, Maududi T, et al. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2005;25:378–86. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- Dashti SR, Efimova T, Eckert RL. MEK6 regulates human involucrin gene expression via a p38alpha- and p38delta-dependent mechanism. J Biol Chem. 2001a;276:27214–20. doi: 10.1074/jbc.M100465200. [DOI] [PubMed] [Google Scholar]
- Dashti SR, Efimova T, Eckert RL. MEK7-dependent activation of p38 MAP kinase in keratinocytes. J Biol Chem. 2001b;276:8059–63. doi: 10.1074/jbc.C000862200. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Cheng C, et al. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp Dermatol. 2000;9:192–9. doi: 10.1034/j.1600-0625.2000.009003192.x. [DOI] [PubMed] [Google Scholar]
- Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)alpha and PKCdelta. J Biol Chem. 2002;277:17032–40. doi: 10.1074/jbc.M109076200. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Mischak H, Mushinski JF, et al. Transcripts encoding protein kinase C-alpha, -delta, -epsilon, -zeta, and -eta are expressed in basal and differentiating mouse keratinocytes in vitro and exhibit quantitative changes in neoplastic cells. Mol Carcinog. 1992;5:286–92. doi: 10.1002/mc.2940050409. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Yuspa SH. Protein kinase C regulates keratinocyte transglutaminase (TGK) gene expression in cultured primary mouse epidermal keratinocytes induced to terminally differentiate by calcium. J Invest Dermatol. 1994;102:409–14. doi: 10.1111/1523-1747.ep12372171. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, et al. Opposing action of curcumin and green tea polyphenol in human keratinocytes. Mol Nutr Food Res. 2006;50:123–9. doi: 10.1002/mnfr.200500125. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, et al. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Efimova T, Balasubramanian S, et al. p38 Mitogen-activated protein kinases on the body surface – a function for p38delta. J Invest Dermatol. 2003;120:823–8. doi: 10.1046/j.1523-1747.2003.12120.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Efimova T, Dashti SR, et al. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Invest Dermatol Symp Proc. 2002;7:36–40. doi: 10.1046/j.1523-1747.2002.19634.x. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–85. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. Protein kinase Cdelta regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Mol Cell Biol. 2004;24:8167–83. doi: 10.1128/MCB.24.18.8167-8183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova T, Deucher A, Kuroki T, et al. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:31753–60. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Eckert RL. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J Biol Chem. 2000;275:1601–7. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- Efimova T, LaCelle P, Welter JF, et al. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–95. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Tavakkol A, Leach K, et al. Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: reduced expression of protein kinase C-beta II in psoriasis. J Invest Dermatol. 1993;101:553–9. doi: 10.1111/1523-1747.ep12365967. [DOI] [PubMed] [Google Scholar]
- Gherzi R, Sparatore B, Patrone M, et al. Protein kinase C mRNA levels and activity in reconstituted normal human epidermis: relationships to cell differentiation. Biochem Biophys Res Commun. 1992;184:283–91. doi: 10.1016/0006-291x(92)91190-2. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Kielbassa K, Kittstein W, et al. Tyrosine phosphorylation and stimulation of protein kinase C delta from porcine spleen by src in vitro. Dependence on the activated state of protein kinase C delta. FEBS Lett. 1994;347:85–9. doi: 10.1016/0014-5793(94)00514-1. [DOI] [PubMed] [Google Scholar]
- Hara T, Saito Y, Hirai T, et al. Deficiency of protein kinase Calpha in mice results in impairment of epidermal hyperplasia and enhancement of tumor formation in two-stage skin carcinogenesis. Cancer Res. 2005;65:7356–62. doi: 10.1158/0008-5472.CAN-04-4241. [DOI] [PubMed] [Google Scholar]
- Jerome-Morais A, Rahn HR, Tibudan SS, et al. Role for protein kinase C-alpha in keratinocyte growth arrest. J Invest Dermatol. 2009;129:2365–75. doi: 10.1038/jid.2009.74. [DOI] [PubMed] [Google Scholar]
- Kazanietz MG. Targeting protein kinase C and “non-kinase” phorbol ester receptors: emerging concepts and therapeutic implications. Biochim Biophys Acta. 2005;1754:296–304. doi: 10.1016/j.bbapap.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Kimura TE, Merritt AJ, Garrod DR. Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J Invest Dermatol. 2007;127:775–81. doi: 10.1038/sj.jid.5700643. [DOI] [PubMed] [Google Scholar]
- Kitajima Y, Aoyama Y, Seishima M. Transmembrane signaling for adhesive regulation of desmosomes and hemidesmosomes, and for cell-cell detachment induced by pemphigus IgG in cultured keratinocytes: involvement of protein kinase C. J Investig Dermatol Symp Proc. 1999;4:137–44. doi: 10.1038/sj.jidsp.5640197. [DOI] [PubMed] [Google Scholar]
- Kraft CA, Efimova T, Eckert RL. Activation of PKCdelta and p38delta MAPK during okadaic acid dependent keratinocyte apoptosis. Arch Dermatol Res. 2007;299:71–83. doi: 10.1007/s00403-006-0727-4. [DOI] [PubMed] [Google Scholar]
- LaCelle PT, Lambert A, Ekambaram MC, et al. In vitro cross-linking of recombinant human involucrin. Skin Pharmacol Appl Skin Physiol. 1998;11:214–26. doi: 10.1159/000029830. [DOI] [PubMed] [Google Scholar]
- Li L, Tucker RW, Hennings H, et al. Chelation of intracellular Ca2+ inhibits murine keratinocyte differentiation in vitro. J Cell Physiol. 1995;163:105–14. doi: 10.1002/jcp.1041630112. [DOI] [PubMed] [Google Scholar]
- Matsui MS, Chew SL, DeLeo VA. Protein kinase C in normal human epidermal keratinocytes during proliferation and calcium-induced differentiation. J Invest Dermatol. 1992;99:565–71. doi: 10.1111/1523-1747.ep12667411. [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(Part 2):281–92. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–7. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, et al. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA. 1989;86:3099–103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada S, Mizuno K, Saido TC, et al. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990;265:22434–40. [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Rosse C, Linch M, Kermorgant S, et al. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–12. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Sharkey NA, Leach KL, Blumberg PM. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci USA. 1984;81:607–10. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Alt A, Wertheimer E, et al. PKCdelta activation: a divergence point in the signaling of insulin and IGF-1-induced proliferation of skin keratinocytes. Diabetes. 2001;50:255–64. doi: 10.2337/diabetes.50.2.255. [DOI] [PubMed] [Google Scholar]
- Sheu HM, Kitajima Y, Yaoita H. Involvement of protein kinase C in translocation of desmoplakins from cytosol to plasma membrane during desmosome formation in human squamous cell carcinoma cells grown in low to normal calcium concentration. Exp Cell Res. 1989;185:176–90. doi: 10.1016/0014-4827(89)90047-5. [DOI] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF. The protein kinase C delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J Biol Chem. 2006;281:29703–10. doi: 10.1074/jbc.M607351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlik MT, Abell AN, Cuevas BD, et al. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol. 2004;82:658–63. doi: 10.1139/o04-114. [DOI] [PubMed] [Google Scholar]
- Verma AK, Wheeler DL, Aziz MH, et al. Protein kinase Cepsilon and development of squamous cell carcinoma, the nonmelanoma human skin cancer. Mol Carcinog. 2006;45:381–8. doi: 10.1002/mc.20230. [DOI] [PubMed] [Google Scholar]
- Welter JF, Crish JF, Agarwal C, et al. Fos-related antigen (Fra-1), junB, and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J Biol Chem. 1995;270:12614–22. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- Welter JF, Eckert RL. Differential expression of fos and jun family members c-fos, fosB, Fra-1, Fra-2, c-jun, junB and junD during human epidermal keratinocyte differentiation. Oncogene. 1995;11:2681–7. [PubMed] [Google Scholar]
- Welter JF, Gali H, Crish JF, et al. Regulation of human involucrin promoter activity by POU domain proteins. J Biol Chem. 1996;271:14727–33. doi: 10.1074/jbc.271.25.14727. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Reddig PJ, Dreckschmidt NE, et al. Protein kinase Cdelta-mediated signal to ornithine decarboxylase induction is independent of skin tumor suppression. Oncogene. 2002;21:3620–30. doi: 10.1038/sj.onc.1205451. [DOI] [PubMed] [Google Scholar]
- Yaar M, Gilani A, DiBenedetto PJ, et al. Gene modulation accompanying differentiation of normal versus malignant keratinocytes. Exp Cell Res. 1993;206:235–43. doi: 10.1006/excr.1993.1143. [DOI] [PubMed] [Google Scholar]
- Zhu L, Brodie C, Balasubramanian S, et al. Multiple PKCdelta tyrosine residues are required for PKCdelta-dependent activation of involucrin expression-a key role of PKCdelta-Y(311) J Invest Dermatol. 2008;128:833–45. doi: 10.1038/sj.jid.5701103. [DOI] [PMC free article] [PubMed] [Google Scholar]